Abstract
OBJECTIVE
Pulmonary arterioles respond to hypoxia with constriction that raises vascular resistance and pulmonary artery blood pressure. The response is sustained indefinitely by the chronic hypoxia of high-altitude residence among highlanders of European and Andean descent, but not Tibetans. The objective of this study was to identify the consequences of lifelong hypoxia exposure for the pulmonary vasculature among Amhara high-altitude natives from Ethiopia.
METHODS
A three-way static group comparison tested for the effect of Amhara ancestry and high residence altitude on pulmonary hemodynamics measured using echocardiography in samples of 76 healthy adult Amhara lifelong residents at 3700m, 54 Amhara lifelong residents at 1200m, and 46 U.S. low-altitude residents at 282m.
RESULTS
Amhara at 3700m had average Doppler-estimated pulmonary artery systolic pressure (tricuspid regurgitant gradient) of 27.9 ± 8.4 (SD) mmHg as compared with 21.9 ± 4.0 among Amhara at low altitude and 16.5 ± 3.6 in the U.S. low-altitude reference sample. However, there was no residence altitude effect on pulmonary blood flow or vascular resistance. Amhara ancestry was associated with greater pulmonary artery systolic pressure and pulmonary blood flow, yet lower pulmonary vascular resistance.
CONCLUSIONS
The Amhara at 3700m had elevated pulmonary artery pressure, but without the elevated pulmonary vascular resistance characteristic of the classic model of the response to long-term hypoxia by the pulmonary vasculature. The elevated pressure among Amhara may be a consequence of high pulmonary blood flow regardless of altitude and represent a newly identified pattern of response.
Keywords: hypoxia, pulmonary circulation, angiotensin converting enzyme genotype
Introduction
At high altitude the entire lung is unavoidably exposed to lowered inspired oxygen. An immediate reaction is the hypoxic pulmonary vasoconstriction reflex, measured as an increase in pulmonary artery blood pressure, that “automatically increases pulmonary vascular resistance in poorly aerated regions of the lungs, thereby redirecting pulmonary blood flow to regions richer in oxygen content” (Fishman, 2004 p. L893). Lifelong exposure would predict unrelieved hypoxic pulmonary vasoconstriction and lead to sustained elevated pulmonary artery pressure and vascular resistancethat could progress to cardiac enlargement (cor pulmonale) or right heart failure.
Classic studies starting in the 1950s (Penaloza and others, 1963; Rotta and others, 1956) found that Andean highlanders resident above 3700m had higher mean pulmonary artery pressure, pulmonary artery systolic pressure and pulmonary vascular resistance than low-altitude residents. The usual fast postnatal decline to adult pulmonary artery pressure was prolonged into the teens (Niermeyer, 2003; Penaloza and Arias-Stella, 2007). The physiological changes were accompanied by anatomical modifications including enlarged hearts and muscularized pulmonary resistance arterioles owing to the additional workload. Studies of second-generation high-altitude natives of European descent in the Colorado Rockies generally confirmed the Andean findings while reporting larger elevations of pulmonary artery pressure among adolescents (Grover and others, 1967; Hultgren and others, 1971) and similar elevation among adults (Grover and others, 1965; Penaloza and others, 1963; Sime and others, 1963). Recent comparisons of children and adults of European descent residing at high altitude in the Andes agreed that children of European descent had higher pulmonary artery systolic pressures than their Andean counterparts, while adults did not differ (Schwab and others, 2008; Stuber and others, 2008). These data indicate a qualitatively similar pattern of elevated pulmonary artery pressure throughout lifelong residence at high altitude in the Andean population exposed to the opportunity for natural selection for millennia and the European population not exposed, although quantitatively the unselected population has a larger hemodynamic response during adolescence.
In contrast, adult Tibetan highlanders above 3500m had minimal elevation of pulmonary artery pressure, no hypoxic pulmonary vasoconstriction (Groves and others, 1993; Hoit and others, 2006) and normal, non-muscularized pulmonary resistance arterioles (Gupta and others, 1992; Sharma, 1990). Exhaled nitric oxide has been associated with lower pulmonary artery pressure among Tibetans, but not Andean highlanders (Hoit and others, 2006; Schwab and others, 2008; Stuber and others, 2008). Collectively, such population differences suggest that the human pulmonary vasculature is not constrained to a single response to lifelong hypoxia.
Angiotensin converting enzyme (ACE) genotype has been associated with indicators of adaptation to high-altitude hypoxia including pulmonary hemodynamics (Aldashev and others, 2002; Bigham and others, 2008; Rupert and others, 1999; Woods and others, 2000). A well-studied ACE polymorphism is comprised of insertion (I) and deletion (D) variants. The I allele is associated with lower ACE protein and activity levels and less conversion of some vasodilators to vasoconstrictors such as angiotensin (Murphey and others, 2000; Rigat and others, 1992; Woods and others, 2000). These actions suggest the potential to modify, alone or in conjunction with other loci, the hypoxic pulmonary vasoconstriction response in the direction of relatively small response among II or ID genotypes. However, some studies report an association of the I allele with low and others-contrary to expectation - an association of the I allele with high pulmonary artery pressure at high altitude (Aldashev and others, 2002; Kumar and others, 2003; Morrell and others, 1999). Those reveal a range of response by the same genotype exposed to the same environmental stress..
The objective of this study was to identify the consequences of lifelong hypoxia exposure for the pulmonary vasculature among adult Amhara high-altitude natives from Ethiopia. It examines the effect of high residence altitude, ACE levels and I/D genotype, and urinary nitrite and nitrate (NOx) levels on Doppler-estimated pulmonary artery systolic pressure, pulmonary blood flow, vascular resistance and right ventricle size by comparing Amhara who are native residents at high (3700m) to Amhara at low (1200m) altitudes in Ethiopia and in U.S. residents at low altitude (282m). It considers the effect of Amhara ancestry to identify any distinctive features of the colonizing population.
Materials and Methods
The 182 volunteers were 18 to 56 years of age, healthy (by physical exam and self-report in Ethiopia and by self-report in the US), normotensive, non-anemic, non-smoking, not pregnant, without visible goiter; they had normal pulmonary function. Ancestry was established by self-report and by report of the first language learned. The high-altitude residents reported no visits to altitudes below 2000m while the low-altitude residents reported no visits to altitudes above 2000m in the six months prior to measurement. 76 Amhara high-altitude native, lifelong residents of 3530m or higher were measured at 3700m at the Chennek Scout Camp in the Simien Mountains National Park, Amhara Region, Ethiopia. 61 Amhara low-altitude native, lifelong residents were measured at 1200m near Zarima town, Amhara Region, Ethiopia. Zarima was chosen to achieve a marked altitude contrast and remove hypobaric hypoxia yet avoid endemic malaria infection. Seven people with malaria diagnosed using a post-PCR/ligase detection reaction, fluorescent microsphere assay (LDR-FMA) to detect all four human malarial parasites (McNamara and others, 2006) were excluded. 46 U.S. low-altitude residents were measured at 282m in Cleveland, OH. Their self-reported ancestries were European or Caucasian (61%), African or African-American (11%), Middle Eastern (9%), and Hispanic or Asian (8%).
This research adheres to the principles of the Declaration of Helsinki and Title 45 of the U.S. Code of Federal Regulations, Part 46 Protection of Human Subjects. The Institutional Review Boards of University Hospitals of Case Western Reserve University, the Cleveland Clinic, Addis Ababa University Faculty of Medicine, and the Ethiopian Science and Technology Committee approved the protocol. All participants provided written informed consent.
Data were collected in tents erected in the two rural agro-pastoral communities during April and December 2005 and January 2006 and in the General Clinical Research Center of the Cleveland Clinic during September 2006. Ambient conditions at the time of morning calibrations averaged 498 mmHg, 6 °C and 36% relative humidity at the high-altitude site, 659 mmHg, 22 °C, and 36% relative humidity at the low-altitude Ethiopian site, and 754 mmHg, 23 °C, and 29% relatively humidity at the low-altitude U.S. site.
Doppler echocardiography (MyLab30CV, Biosound Esaote, Inc. (Indianapolis, IN, USA) in Ethiopia and Sonos 5500, Philips Medical Systems (Andover, MA, USA) in the U.S) conducted by an experienced echosonographer (ND) provided noninvasive measurement of pulmonary arterial hemodynamics in 193 Amhara and 50 U.S. low-altitude adults. Studies were evaluated blindly as to the source of individual recordings. Standard parasternal, apical, and subcostal 2D views were obtained and color flow-directed pulsed wave Doppler of transvalvular flows and continuous wave Doppler of the tricuspid regurgitant flow were measured. The tricuspid regurgitant jet gradient (TR gradient) measures the backflow into the right atrium from the right ventricle through the tricuspid valve caused by blood pressure in the pulmonary artery. An adequate TR gradient was obtained in 71% of Amhara and in 92% of U.S. low-altitude controls. The higher detection rate in the U.S. was likely due to differences in Doppler sensitivity of the ultrasonographs. The TR gradient and the velocity-time integral of right ventricular outflow to the lungs (RVOTvti) were used as estimators of pulmonary artery pressure and pulmonary blood flow/stroke volume (Pai and others, 2004), respectively. Pulmonary vascular resistance (PVR) was calculated in terms of blood pressure relative to blood flow using the method validated by Abbas and colleagues (Abbas and others, 2003). The interior diameter at the base of the right ventricle at end-diastole (RVbase) was used as a 2D echo-estimator of right heart size (Lang and others, 2005). To control for body size, pulmonary vascular resistance (PVR) and the size of the right ventricle (RVbase) were indexed by body surface area (PVR is multiplied by BSA and RVbase is divided by BSA). Pressure and flow velocity are independent of body size (Liu and Yin, 1987). Doppler echocardiography measurements have been validated by comparison with cardiac catheterization at sea level and high altitude and found to correlate closely and differ little in absolute value at low or high altitude (Allemann and others, 2000; Dorrington and others, 1997; Grunig and others, 2000; McQuillan and others, 2001; Otto, 2004). Thus, non-invasive Doppler-estimates are accurate and appropriate for population studies such as this one.
Intra-observer variability was estimated for TR gradient, RVOTvti, and RVbase using re-measurements after ten days of five Amhara lowlanders and calculating the difference of the two measurements divided by their average and multiplying the result by one hundred. The TR gradient intra-observer variability of 2.7% was consistent with the published range of <4 – 5% (Schwab and others, 2008). The intra-observer variabilities for RVOTvti and RVbase were 10.1 and 3.0%, respectively.
Interestingly, even those with the I allele had more than 50% higher ACE protein level than the average of the US sample. Genotyping for the angiotensin converting enzyme insertion/deletion polymorphism (ACE I/D) was conducted on genomic DNA extracted from blood using primers flanking intron 16 and confirmed with insertion-specific primers (Lindpainter and others, 1995; Rigat and others, 1992).
Hemoglobin concentration was determined in duplicate using the cyanmethemoglobin technique (Hemocue Hemoglobinometer, Hemocue AB, Angelholm, Sweden), immediately after drawing a venous blood sample. Percent oxygen saturation of hemoglobin was determined by pulse oximetry (Masimo Radical, Irvine, CA, USA) as the average of six readings taken ten seconds apart as in previous studies (Beall, 2000). Arterial oxygen content (CaO2, mlO2/dL blood) was calculated as Hb in gm/dL multiplied by percent oxygen saturation of hemoglobin multiplied by 1.39 and divided by 100 (West, 1985). Arterial oxygen and carbon dioxide tensions (paO2, paCO2) were determined from blood gas measurements (Radiometer NPT7, Copenhagen, Denmark) immediately after drawing a blood sample from the radial artery. At high altitude, arterial blood gases were measured in April and echocardiography in December 2005. At low altitude in Ethiopia, both were collected in December.
Serum angiotensin converting enzyme protein level (ng/ml was measured with a solid phase ELISA using the quantitative sandwich enzyme immunoassay technique (R & D Systems, Minneapolis, MN). Urinary nitrite and nitrate (NOx, uM) was measured by placing samples in a reaction chamber with vanadium (III) chloride in HCl and reduced at 95 °C. Released NO-HCl gas was buffered through NaOH and red/infrared emissions were detectedusing thermoelectrically cooled, red-sensitiviey photomultiplier tube (Sievers NOA, Boulder, CO). Samples were processed twice and run in duplicate. Values were standardized for urinary creatinine to control for urine volume.
Pulmonary function was measured as forced vital capacity (FVC) and forced expiratory volume at one second (FEV1) according to American Thoracic Society recommendations (American Thoracic Society, 1995) (Hawk Comprehensive Pulmonary Laboratory, Collins Pulmonary Diagnostics, Ferraris Respiratory, Louisville, CO).
One-way analyses of variance provided descriptive statistics and tested for the presence of differences in mean values between any pair of the three samples. When the one-way analysis of variance was significant, Scheffé’s tests identified which pair(s) differed significantly (SPSS version 16, SPSS, Inc., Chicago, IL, USA). Means and standard deviations are reported. Chi-square analyses compared ACE I/D genotypic frequencies. A significance level of p < 0.05 was used.
Sample characteristics
Amhara highlanders were shorter and lighter than Amhara and U.S. lowlanders (Table 1). Amhara highlander males, but not females, had larger Forced Vital Capacities (FVC) and ratio of Forced Expiratory Volume at 1 second (FEV1) to Forced Vital Capacity (FEV1/FVC) than lowland Amhara. Amhara had smaller FVC and FEV1 than the low-altitude U.S. sample, consistent with shorter stature, but did not differ in FEV1/FVC (Table 1). Amhara males had lower systolic blood pressure than U.S. low-altitude males while Amhara females had higher diastolic blood pressures (Table 1).
Table 1.
Characteristics of Amhara samples at high and low altitudes and a U.S. low altitude sample. Values are mean and standard deviation.
| 3700m Amhara | 1200m Amhara | 282m U.S. | ||||
|---|---|---|---|---|---|---|
| Trait | Male, n=53 | Female, n=22 | Male, n=45 | Female, n=9 | Male, n=21 | Female, n=25 |
| Age, yr * | 32 ± 9 | 30 ± 8 | 34 ± 9 | 28 ± 9 | 32 ± 9 | 38 ± 12 |
| Height, cm †, ‡, § | 166 ± 6 | 161 ± 5.7 | 172 ± 7 | 155 ± 4.1 | 180 ± 6 | 164 ± 8.2 |
| Weight, kg †, ‡, a | 52 ± 5 | 49 ± 5.2 | 58 ± 6 | 51 ± 4.0 | 86 ± 13 | 66 ± 13.7 |
| BMI, Kg/m2†,b, c | 18.9 ± 1.5 | 18.8 ± 1.7 | 19.5 ± 1.8 | 23.6 ± 7.4 | 26.6 ± 3.6 | 24.6 ± 4.5 |
| BSA, m2†, ‡, a | 1.55 ± 0.10 | 1.49 ± 0.09 | 1.70 ± 0.10 | 1.50 ± 0.05 | 2.05 ± 0.18 | 1.72 ± 0.17 |
| Hb gm/dL †, e, c, d | 16.3 ± 1.24 | 15.3 ± 1.18 | 15.5 ± 1.25 | 13.7 ± 1.05 | 15.5 ± 1.22 | 13.4 ± 0.99 |
| O2 sat, % †, f, d | 91.8 ± 3.4 | 92.9 ± 3.9 | 98.4 ± 1.2 | 99.0 ± 0.7 | 97.0 ± 1.0 | 98.0 ± 1.0 |
| CaO2, ml O2/100ml blood †, * | 20.9 ± 1.7 | 19.7 ± 1.5 | 21.2 ± 1.7 | 18.9 ± 1.4 | 21.1 ± 1.6 | 18.3 ± 1.4 |
| FVC, L btps, ‡, d | 4.1 ± 0.6 | 3.4 ± 0.6 | 4.5 ± 1.0 | 3.2 ± 0.3 | 5.3 ± 0.8 | 3.8 ± 0.5 |
| FEV1/FVC % e | 84.4 ± 7.9 | 81.1 ± 6.7 | 80.4 ± 7.8 | 83.0 ± 8.6 | 83.6 ± 5.0 | 84.3 ± 4.4 |
| Systolic BP, mm Hg b | 117 ± 8.4 | 116 ± 8.2 | 119 ± 9.4 | 117 ± 5.0 | 126 ± 8.7 | 115 ± 10.0 |
| Diastolic BP, mm Hg | 76 ± 5.8 | 74 ± 5.7 | 77 ± 6.5 | 74 ± 5.1 | 77 ± 5.5 | 70 ± 6.6 |
| Heart rate, bpm b, d | 63 ± 12 | 78 ± 13 | 68 ± 9 | 80 ± 10 | 67 ± 8 | 76 ± 9 |
Females: Amharic no altitude difference, Amharic high altitude significantly different from U.S. Low altitude
sex differences within group(s)
Males: all pairwise differences significant
Females: Amharic no altitude differences, Amharic low significant different from U.S.
Females: Amharic no altitude differences, both significantly different from U.S.
Males: Amharic high and low altitude both significantly different than U.S.
Females: Amharic high altitude significantly different from low altitude only
Females: Amharic high altitude significantly different from low altitude and U.S.
Males: Amharic high altitude significantly different from low altitude only.
Males: Amharic high altitude significantly different from both Amharic and U.S. low altitude
Males: Amharic high altitude and US low altitude males both significantly different than Amharic low altitude
Average hemoglobin concentration among Amhara highlander men was 0.8 gm/dL higher than the lowlanders; average percent oxygen saturation was 5–7% lower. Average hemoglobin concentration among Amhara highlander women was 1.6–1.9 gm/dL higher than the lowlanders; average percent oxygen saturation was 6–7% percent lower (Table 1). Elevated hemoglobin concentration offset lower oxygen saturation at high altitude resulting in average calculated arterial oxygen content that did not vary with altitude or population. The hemoglobin concentration was slightly higher (0.3 – 0.4 gm/dL) and the oxygen saturation slightly lower (2–3%) than the values reported for an Amhara sample at the slightly lower altitude of 3530m (Beall and others, 2002). The sample at 3530m was noteworthy for its similarity with normal low-altitude values. The present sample at 3700m differs from low-altitude and indicates that a slightly greater hypoxic stress elicits responses. The average arterial oxygen tension (paO2) was 53.9 ± 4.7 mm Hg (n=29) among Amhara highlanders as compared with 82.5 ± 4.7 (n=27) among Amhara lowlanders. The average arterial carbon dioxide tension (paCO2) was 36.2 ± 3.7 mm Hg (n=29) among highlanders as compared with 37.3 ± 2.8 mm Hg (n=27) among lowlanders. Those values correspond to alveolar oxygen tensions (pAO2) (Crapo and others, 1999) of 60 and 93 mm Hg at 3700 and 1200m respectively.
Results
Pulmonary hemodynamics
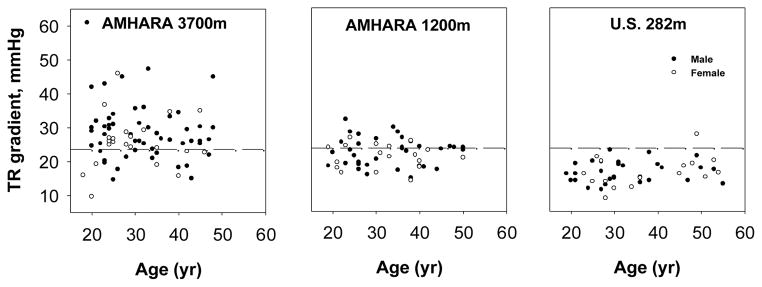
The average TR gradient (Doppler estimator of pulmonary artery systolic pressure) was 27.9 ± 8.4 mm Hg for the sample of Amhara highlanders as compared with 21.9 ± 4.0 among the Amhara lowlanders and 16.5 ± 3.6 for the U.S. sample (Figure 1, Table 2, F (2, 174)=50, p < 0.05). TR gradient did not vary with age or sex. Amhara men at 3700 showed a trend toward an association of higher TR gradient with higher hemoglobin concentration while women showed the opposite (r=+0.27, p = 0.05 and r=−0.45, p < 0.05, respectively).
Figure 1.
Tricuspid regurgitant gradient estimate of pulmonary artery systolic pressure in samples of Amhara at 3700m and 1200m and a U.S. low-altitude sample. All pairwise differences in means were significant. Dashed lines indicate the sea-level mean plus two standard deviations.
Table 2.
Pulmonary blood flow characteristics of Amhara samples at high and low altitudes and a U.S. low altitude sample. Values are mean and standard deviation.
| Trait | 3700m Amhara | 1200m Amhara | 282m U.S. |
|---|---|---|---|
| TR gradient, mm Hg * | 27.9 ± 8.4 (75) | 21.9 ± 4.0 (54) | 16.5 ± 3.6 (46) |
| RVOT vti, cm † | 18.3 ± 3.1 (75) | 17.8 ± 2.8 (54) | 15.3± 1.8 (46) |
| Pulmonary Vascular Resistance Index, Wood units/m2,† | 2.5 ± 0.5 (74) | 2.5 ± 0.4 (54) | 2.8 ± 0.6 (46) |
| Right Ventricle Base Index, cm/m2,§ | 2.0 ± 0.3 (74) | 1.5 ±0.2 (54) | 1.5± 0.2 (46) |
All pairwise differences significant
Amhara no altitude difference, both significantly different than U.S.
Females: Amhara no altitude difference, Amhara high altitude significantly different from U.S. Low altitude
Amhara high altitude significantly different from others
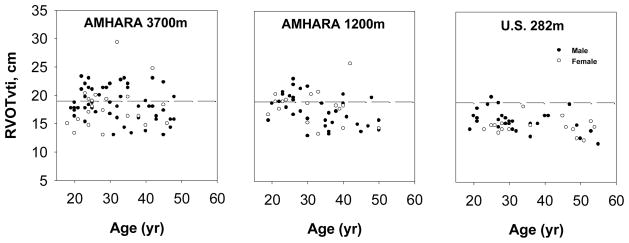
The higher pulmonary artery pressure could result from increased blood flow through the lungs or increased vascular resistance. The RVOTvti (Doppler estimator of pulmonary blood flow/stroke volume) did not vary with altitude while both Amhara samples had 17–20% greater RVOTvti than the U.S. low-altitude sample (Figure 2, Table 2; F (2, 173) = 18, p < 0.05). RVOTvti did not vary with sex; it decreased with age in the Amhara samples (r = −0.44 at 3700m and r = −0.36 at 1200m, both p < 0.05). Higher pressure and blood flow among Amhara suggest lower vascular resistance.
Figure 2.
Right ventricular outflow velocity-time integral estimate of pulmonary blood flow in samples of Amhara at 3700m and 1200m and a U.S. low-altitude sample. Amhara samples had significantly higher flow. Dashed lines indicate the sea-level mean plus two standard deviations.
The Amhara samples had about ten percent lower pulmonary vascular resistance index than the U.S. sample (Table 2; F (2, 173) = 7, p < 0.05). There were neither altitude nor sex differences; pulmonary vascular resistance increased with age in the low-altitude Amhara sample (r=+0.48, p < 0.05). The combination of higher pressure and flow with lower resistance suggests a larger volume of moving blood.
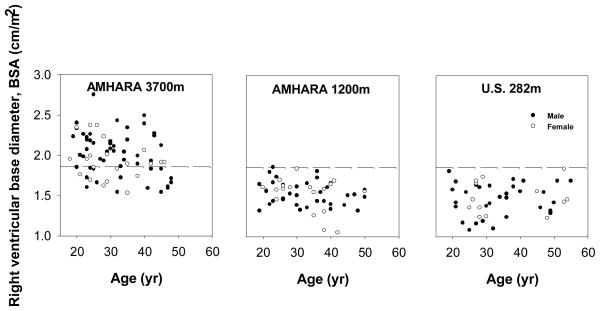
That volume was estimated with the right ventricle index, the diameter at the base of the right ventricle divided by body surface area. The Amhara highlanders had one-third larger right ventricle index than either low-altitude group (Figure 3, Table 2, F (2,173) = 34, p < 0.05). There were no sex differences. Older age was associated with relatively smaller right ventricle index among Amhara at 3700m and a similar trend occurred at 1200m (r = −0.28, p < 0.05 and r = −0.26, p = 0.06, respectively).
Figure 3.
Right ventricle size estimated as the diameter of the ventricle base relative to body surface area in samples of Amhara at 3700m and 1200m and a U.S. low-altitude sample. Amhara highlanders had significantly larger right ventricles. Dashed lines indicate the sea-level mean plus two standard deviations.
Higher flow could result from relatively low concentrations of vasoconstrictors or high concentrations of vasodilators. With respect to potential vasoconstrictors, the two Amhara samples had ACE protein levels markedly higher than the U.S. low-altitude sample. The average serum ACE protein level among the Amhara at 3700m was 202.8 ± 57.5 pg/mL (n=58) while Amhara at 1200m was 181.2 ± 55.0 (n=39) as compared with 106.4 ± 28.9 (n=34) in the U.S. low-altitude sample (F(2,128) = 39.6, p < 0.05). ACE levels did not correlate with measures of pulmonary hemodynamics in any of the three samples. The high and low-altitude Amhara samples had similar ACE I/D genotype and allele frequencies (Table 3, Chi-square = 0.2, p > 0.5). The average ACE protein levels for the II, ID and DD genotypes at 3700m were 183 ± 30, 182 ± 62, 220 ± 45 pg/mL (F(2,50) = 3.3, p < 0.05). At 1200m the values were 161 ± 40, 160 ± 54 and 212 ± 63 pg/ml, respectively 1200m (F (2, 28) = 3.0, p = 0.07). Because ACE converts some vasodilators to vasoconstrictors, these findings suggest that there may be higher concentrations of those vasoconstrictors among the Amhara.
Table 3.
Angiotensin converting enzyme (ACE) genotypes and allele frequencies of Amhara samples at high and low altitudes.
| 3700m Amhara | 1200m Amhara | |
|---|---|---|
| Genotype Frequency * | N (%) | N (%) |
| II | 7 (11) | 5 (14) |
| ID | 23 (36) | 12 (33) |
| DD | 34 (953) | 19 (53) |
| Total | 64 | 36 |
| Allele Frequency * | ||
| I | 29% | 31% |
| D | 71% | 69% |
Genotype frequencies do not differ from Hardy-Weinberg Equilibrium frequencies; genotype frequencies do not differ between altitudes by test of comparison of genotype distribution by Chi-square test (3 × 2 contingency table with 2 df); allele frequencies do not differ between altitudes by test of comparison of allele distribution by Chi-square test (2 × 2 contingency table with 1 df).
With respect to vasodilators, the average ratio of urinary nitrite and nitrate (NOx) to creatinine levels was 0.03 ± 0.03 uM/uM at 3700m and 0.03 ± 0.04 uM/uM at 1200m as compared with 0.01 ± 0.01 uM/uM in the U.S. low-altitude sample. Urinary NOx/creatinine ratio did not correlate with measures of pulmonary hemodynamics in any of these samples.
Discussion
The major finding was elevated Doppler-estimated pulmonary artery systolic pressure among Amhara at 3700m and 1200m. Approximately 73% of Amhara highlanders at 3700m had Doppler-estimated pulmonary artery systolic pressure above the reference range of variation in the US low-altitude sample while approximately 40% of the Amhara lowlanders at 1200m did so (Figure 1). Regardless of altitude, Amhara also had elevated Doppler-estimated blood flow. Approximately 40% of the Amhara had RVOTvti above the normal range of low-altitude variation in the US low-altitude sample (Figure 2). However, the higher pressures and flow were not associated with higher pulmonary vascular resistance: none of the Amhara had pulmonary vascular resistance above the US low-altitude normal range. This pattern of pulmonary vascular response to high altitude differs from the classic model that couples pulmonary artery pressure and vascular resistance (Penaloza and Arias-Stella, 2007). The larger body size of the U.S. reference sample is unlikely to explain these results. The Amhara – U.S. population differences occurred among both males and females even though the size difference was larger for males than females. Furthermore, the relatively high U.S. pulmonary vascular resistance and small diameter of the right ventricular base occur whether or not the correction for body size is applied (data not shown). This is consistent with other findings that pressure and blood flow velocity are little affected by body size (Liu and Yin, 1987).
The modestly higher mean pulmonary artery pressure among the low-altitude Amhara at 1200m relative to the U.S. low-altitude sample was unexpected based on the common convention that 2500m is a threshold for detectable hypoxic stress and response. The Amhara at 1200m had alveolar and arterial oxygen tensions consistent with reports from the US at 1400m (Crapo and others, 1999). The calculated alveolar oxygen tension (pAO2) of 93 mm Hg was well above the 50–60 mm Hg range associated with hypoxic pulmonary vasoconstriction (Moudgil and others, 2005). We are unaware of comparable pulmonary hemodynamic data from other samples at similar altitudes. Future studies of Amhara at very low altitude and Europeans at intermediate altitude are necessaty to establish whether these findings reflect a response to an unexpectedly low altitude or a general characteristic of Amhara.
These results suggest that the elevated pulmonary artery pressure was not due to vasoconstriction across the pulmonary bed (classic model), but rather was due to relative increase of blood volume. The high pulmonary blood flow measured by RVOTvti – the distance a unit of blood travels as it enters the pulmonary artery, and a surrogate measure for stroke volume (Pai and others, 2004) – of both Amhara samples, together with the larger right ventricle at high altitude, implies a larger cross-sectional area for blood flow, perhaps owing to more dilation, more arterioles in the lung, or recruitment of more pulmonary arterioles (Wagner and others, 1979). Approximately 65% of the Amhara at 3700m had right ventricle diameter indices more than two standard deviations above the mean (Figure 3), a finding that classically was attributed to high workload on heart muscle owing to high resistance found among Andean highlanders. The present context suggests that a high workload may result from high blood volume, although that was not measured in this study. (Hemoglobin concentration was not a major contributor to pulmonary hemodynamics.)
Considering that the characteristics of the lowland Amhara likely represent those of the first colonizers of the highland areas, a plausible hypothesis reasons that the high flow-low resistance pattern was augmented under high-altitude hypoxia with an increase in blood volume resulting in the observed elevated pressure.
Despite the enhanced pulmonary blood flow, the Amhara avoid raised vascular resistance although the mechanism is not known. The ACE polymorphism and ACE levels were not influential. A predominance of the ACE I allele might have been expected because it is associated with relatively less vasoconstriction, however it accounted for only about 30% of the alleles at both altitudes. This was consistent with findings in other parts of the world (Rupert and Hochachka, 2001). The effect of the I allele is variable at high altitude. Some studies report an association of the I allele with low and others an association of the I allele with high pulmonary artery pressure at high altitude (Aldashev and others, 2002; Kumar and others, 2003; Morrell and others, 1999). The present study found no association. The Amhara had average ACE protein levels 70-90% higher than the US sample. The I allele was associated with significantly lower levels at 3700m and there was a similar trend at 1200m. The ACE I/D polymorphism is not associated with variation in ACE protein level in some East and South African samples, suggesting that the phenotypic effects of the I/D polymorphism may differ depending on genetic background (Payne and others, 2007; Scott and others, 2005). Interestingly, even those with the I allele had more than 50% higher ACE protein level than the average of the US sample.
Total body synthesis of the vasodilator nitric oxide was quantified as the ratio of urinary nitrite and nitrate (NOx) to creatinine. The Amhara, regardless of residence altitude, had systemic NOx ratios three times higher than the US reference sample. Perhaps this contributes to vasodilation and enables the high flow of the Amhara. Future studies should evaluate a wide array of vasodilators and constrictors.
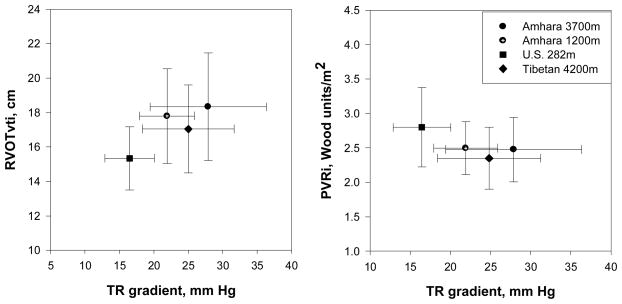
In global perspective, comparing the Amhara highlanders at 3700m with a sample of Tibetans residing at 4200m evaluated by the same echosonographer found the Amhara had higher Doppler-estimated pulmonary artery pressures and blood flow, and a trend toward higher pulmonary vascular resistance index The Tibetan average TR grad was 25.0 ± 6.6 (SD) mm Hg (n=65) (t=2.25, df=138, p < 0.05); average RVOTvti was 17.0 cm ± 2.5 (n=78) (t=2.80, df=150, p < 0.05) and average pulmonary vascular resistance index was 2.3 ± 0.3(n=64) Wood units/m2 (t=1.76, df=138, p=0.08). At the same time all three contrasted with the low altitude U.S> sample that had markedly lower pulmonary blood pressure and higher pulmonary vascular resistance index (Figures 4a and 4b). These comparisons illustrate that there is no simple association of pulmonary hemodynamics with altitude.
Figure 4.
Amhara and Tibetan samples both have higher mean Doppler-estimated pulmonary artery systolic pressure (TR gradient, mm Hg) and Doppler-estimated pulmonary blood flow (RVOTvti, cm) compared with a U.S. low-altitude sample (left panel) yet lower pulmonary vascular resistance (right panel). Means and standard deviations are plotted.
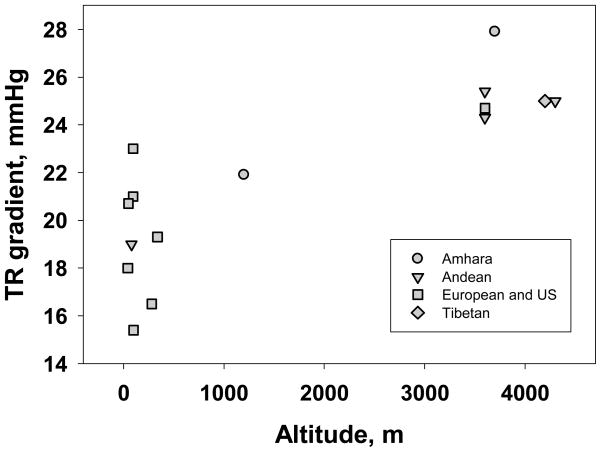
To address this relationship in greater detail, Figure 5 summarizes published data on Doppler-estimated pulmonary artery pressure across a range of altitudes. The range of variation at low altitude is large and actually encompasses the present sample at 1200m. Unexpectedly, based on earlier comparisons (Groves and others, 1993; Penaloza and Arias-Stella, 2007) the Andean samples and the Tibetan resemble one another and have lower TR gradients than the present Amhara sample. The wide range of variation at low altitudes suggests the need to increase the number of samples at high altitude before reaching firm conclusions about population differences or secular trends. The high-altitude Amhara sample of the present study stands out with a particularly high pulmonary artery pressure. One possible mechanism is suggested by measurements across the lifespan. Andean infants and children at 4500m retain high neonatal pulmonary pressures and finally attain relatively low ‘adult’ pressures after 11 years of age or later that remain above low-altitude pressures throughout adulthood. That contrasts with low-altitude Andean infants and children whose pressures achieve ‘adult’ levels by 1–4 years of age (Penaloza and Arias-Stella, 2007). Additional work is required to test the alternative hypotheses that the high pressures of highland Amhara are a function of high blood volume, represents hypoxic pulmonary vasoconstriction or is an outcome of very delayed fall from neonatal to low adult pressures.
Figure 5.
Doppler-estimated average pulmonary artery systolic pressure (TR gradient, mm Hg) of populations with low and high residence altitude illustrates a wide range of variation at low altitude and a distinctively high mean for the Amhara highlanders of the present study. (Aessopos and others, 2000; Grunig and others, 2000; Grünig and others, 2009; Hoit and others, 2006; Huez and others, 2009; Maignan and others, 2009; McQuillan and others, 2001; Schwab and others, 2008; Stuber and others, 2010).
In summary, these findings suggest a need for revising, at least for some indigenous high-altitude populations, the classic model of hypoxic pulmonary vasoconstriction, elevated pulmonary artery pressure and pulmonary vascular resistance. The effect of Amhara ancestry inferred by comparing low-altitude Amhara and U.S. residents is a pattern of elevated pulmonary artery pressure and high flow without elevated pulmonary vascular resistance. The effect of residence altitude on Amhara inferred by comparing samples at 3700m and 1200m is a pattern of high pulmonary artery pressure without elevated pulmonary vascular resistance.
Acknowledgments
GRANTS
Biosound Esaote, Inc. (Indianapolis, IN, USA) and Philips Medical Systems (Andover, MA, USA) loaned echo Doppler equipment. Funding was provided by the National Science Foundation Award NSF0452326 to CMB, HL60917 to SCE, Clinical Translation Science Award RR024989 to Case Western Reserve University and the Cleveland Clinic and an ASE Sonographer Award to ND.
We are grateful to the study participants and communities for their hospitality and cooperation with our field work and to the staff of the CTSA at the Cleveland Clinic (Daniel Laskowski, Kay Stelmach, Melanie Panliashen, Stephanie Slattery) for their hospitality and assistance with collecting the U.S. sample. Sisay Mequanet and Berhanu Mogosho of the Simien Mountains National Park Office and Tekle Yalew of the North Gondar Administrative Zone facilitated local permissions and introductions. Community facilitators included Marew Shimelesh and Setaragew at 3700m, Alem Seged and Getachew at 1200m. Many people contributed to data collection and sample analysis: Daniel Tessema, laboratory technician; Nurse Molla Tafete of the University of Gondar; Nurse Mengistu Bogale; Nurse Telake Azale of the North Gondar Zone Health Department, Gezahegne Fentahun, M.D. of Addis Ababa University and Laurie Gray of Case Western Reserve University. We also thank the officials of the Ethiopian Science and Technology Commission, the Amhara Regional Government, and the staff of the Semien Mountains National Park who gave permissions and facilitated local arrangements as well as the Gondar Red Cross for renting the laboratory and living tents. Anonymous reviewers offered thoughtful and constructive comments.
Footnotes
DISCLOSURES BDH received lecture fees from the Philips Medical Systems in 2007 and 2008.
LITERATURE CITED
- Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41(6):1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- Aessopos A, Farmakis D, Taktikou H, Loukopoulos D. Doppler-Determined Peak Systolic Tricuspid Pressure Gradient in Persons with Normal Pulmonary Function and Tricuspid Regurgitation. J Am Soc Echocardiogr. 2000;13(7):645–649. doi: 10.1067/mje.2000.104535. [DOI] [PubMed] [Google Scholar]
- Aldashev AA, Sarybaev AS, Sydykov AS, Kalmyrzaev BB, Kim EVLBM, Maripov R, Kojonazarov BK, Mirrakhimov MM, Wilkins MR, Morell NW. Characterization of high altitude pulmonary hypertension in the Kyrgyz: association with angiotensin coverting enzyme genotype. Am J Respir Crit Care Med. 2002;166:1396–1402. doi: 10.1164/rccm.200204-345OC. [DOI] [PubMed] [Google Scholar]
- Allemann Y, Sartori C, Lepori M, Pierre S, Melot C, Naeije R, Scherrer U, Maggiorini M. Echocardiographic and invasive measurements of pulmonary artery pressure correlate closely at high altitude. Am J physiol Heart Circ Physiol. 2000;279:H2013–H2016. doi: 10.1152/ajpheart.2000.279.4.H2013. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Standardization of Spirometry -- 1994 Update. Am J Respir Crit Care Med. 1995;52(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Beall CM. Oxygen saturation increases during childhood and dcreases during adulthood among high altitude native Tibetans residing at 3800–4200m. High Altitude Med Biol. 2000;1(1):25–32. doi: 10.1089/152702900320658. [DOI] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A. 2002;99(26):17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Kiyamu M, Leon-Velarde F, Parra EJ, Rivera-Ch M, Shriver MD, Brutsaert TD. Angiotensin-Converting Enzyme Genotype and Arterial Oxygen Saturation at High Altitude in Peruvian Quechua. High Altitude Medicine & Biology. 2008;9(2):167–178. doi: 10.1089/ham.2007.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- Dorrington KL, Clar C, Young JD, Jonas M, Tansley JG, Robbins PA. Time course of the human pulmonary vascular response to 8 hours of isocapnic hypoxia. Am J Physiol. 1997;273(3 Pt 2):H1126. doi: 10.1152/ajpheart.1997.273.3.H1126. [DOI] [PubMed] [Google Scholar]
- Fishman AP. Acute hypoxia and pulmonary vasoconstriction in humans: uncovering the mechanism of the pressor response. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L893–894. doi: 10.1152/classicessays.00004.2004. [DOI] [PubMed] [Google Scholar]
- Grover RF, Okin JT, Overy HR, Treger A, Spracklen RHN. Natural History of Pulmonary Hypertension in Normal Adult Residents of High Altitude. Abstract. Circulation: An Offical Journal of the American Heart Association. 1965;31–31(Supplement II):II–102. [Google Scholar]
- Grover RF, Vogel JHK, Blount SGJ. Pulmonary Hypertension in Normal Man Residing at 10,000 Feet. In: Tromp SW, Weike WH, editors. Biometeorology. Oxford: Pergamon Press; 1967. pp. 199–207. [Google Scholar]
- Groves BM, Droma T, Sutton JR, McCullough RG, Cullough REM, Zhuang J, Rapmun G, Sun S. Minimal Hypoxic Pulmonary Hypertension in Normal Tibetans at 3,658 m. J Appl Physiol. 1993;74(1):312–318. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- Grunig E, Mereles D, Hildebrandt W, Swenson E, Kubler W, Kuecherer H, Bartsch P. Stress Doppler Echocardiography for Identification of Susceptibility to High Altitude Pulmonary Edema. J Am Coll Cardiol. 2000;35(4):980–987. doi: 10.1016/s0735-1097(99)00633-6. [DOI] [PubMed] [Google Scholar]
- Grünig E, Weissmann S, Ehlken N, Fijalkowska A, Fischer C, Fourme T, Galié N, Ghofrani A, Harrison RE, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation. 2009;119(13):1747–1757. doi: 10.1161/CIRCULATIONAHA.108.800938. [DOI] [PubMed] [Google Scholar]
- Gupta ML, Rao KS, Anand IS, Banerjee AK, Boparai MS. Lack of smooth muscle in the small pulmonary arteries of the native Ladakhi. Is the Himalayan highlander adapted? Am Rev Respir Dis. 1992;145(5):1201. doi: 10.1164/ajrccm/145.5.1201. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM. Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol. 2006;99:1796–1801. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- Huez S, Faoro V, Guénard H, Martinot JB, Naeije R. Echocardiographic and tissue Doppler imaging of cardiac adaptation to high altitude in native highlanders versus acclimatized lowlanders. American Journal of Cardiology. 2009;103(11):1605. doi: 10.1016/j.amjcard.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Hultgren HN, Grover RF, Hartley LH. Abnormal Circulatory Responses to High Altitude in Subjects with a Previous History of High-Altitude Pulmonary Edema. Circulation. 1971 Nov;XLIV:759–770. doi: 10.1161/01.cir.44.5.759. [DOI] [PubMed] [Google Scholar]
- Kumar R, Qadar Pasha MA, Khan AP, Gupta V, Grover SK, Norboo T, Srivastava KK, Selvamurthy W, Brahamchari SK. Association of high-altitude systemic hypertension with the deletion allele-of the angiotensin-converting enzyme (ACE) gene. Int J Biometeorol. 2003;48(1):10–14. doi: 10.1007/s00484-003-0172-4. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lindpainter K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. A Prospective Evaluatinoof an Angiotensin-Converging-Enzyme Polymorphism and the Risk of Ischemic Heart Disease. New Engl J Med. 1995;332:706–711. doi: 10.1056/NEJM199503163321103. [DOI] [PubMed] [Google Scholar]
- Liu ZR, Yin FC. Normalization of hemodynamic parameters: application to vascular resistance and impedance. Am J Physiol. 1987;252(4 Pt 2):R710–719. doi: 10.1152/ajpregu.1987.252.4.R710. [DOI] [PubMed] [Google Scholar]
- Maignan M, Rivera-Ch M, Privat C, León-Velarde F, Richalet JP, Pham I. Pulmonary pressure and cardiac function in chronic mountain sickness patients. Chest. 2009;135(2):499. doi: 10.1378/chest.08-1094. [DOI] [PubMed] [Google Scholar]
- McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. The American journal of tropical medicine and hygiene. 2006;74(3):413. [PMC free article] [PubMed] [Google Scholar]
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Sarybaev AS, Alikhan A, Mirrakhimov MM, Aldashev AA. ACE genotype and risk of high altitude pulmonary hypertension in Kyrghyz highlanders. The Lancet. 1999 Mar;353(6):814. doi: 10.1016/S0140-6736(99)00206-8. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. Journal of applied physiology (Bethesda, Md: 1985) 2005;98(1):390. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Murphey LJ, Gainer JV, Vaughan DE, Brown NJ. Angiotensin-converting enzyme insertion/deletion polymorphism modulates the human in vivo metabolism of bradykinin. Circulation. 2000;102(8):829–832. doi: 10.1161/01.cir.102.8.829. [DOI] [PubMed] [Google Scholar]
- Niermeyer S. Cardiopulmonary transition in the high altitude infant. High Altitude Medicine & Biology. 2003;4(2):225. doi: 10.1089/152702903322022820. [DOI] [PubMed] [Google Scholar]
- Otto C. Textbook of Clinical Echocardiography. 3. Philadelphia, PA: Elsevier Saunders; 2004. [Google Scholar]
- Pai RK, Kedia A, Hsu PY, Holmes J, Nawman R, Goens MB, Kusumoto FM. The use of the Doppler pulmonary artery velocity time integral to optimize placement of a ventricular pacing lead in a patient with Ebstein’s anomaly. J Interv Card Electrophysiol. 2004;11(1):55–57. doi: 10.1023/B:JICE.0000035930.88702.69. [DOI] [PubMed] [Google Scholar]
- Payne JR, Dhamrait SS, Gohlke P, Cooper J, Scott RA, Pitsiladis YP, Humphries SE, Rayner B, Montgomery HE. The impact of ACE genotype on serum ACE activity in a black South African male population. Annals of Human Genetics. 2007;71(Pt 1):1. doi: 10.1111/j.1469-1809.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation. 2007;115(9):1132. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- Penaloza D, Sime F, Banchero N, Gamboa R, Cruz J, Marticorena E. Pulmonary Hypertension in Healthy Men Born and Living at High Altitudes. Am J Cardiol. 1963;11(2):150–157. doi: 10.1016/0002-9149(63)90054-7. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20(6):1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotta A, Canepa A, Hurtado A, Velasquez T, Chavez R. Pulmonary Circulation at Sea Level and at High Altitudes. J Appl Physiol. 1956;9:328–336. doi: 10.1152/jappl.1956.9.3.328. [DOI] [PubMed] [Google Scholar]
- Rupert J, Devine D, Monsalve M, Hochachka P. Angiotensin-converting enzyme (ACE) alleles in the Quechua, a high altitude South American native population. Ann Hum Biol. 1999;26(4):375–380. doi: 10.1080/030144699282688. [DOI] [PubMed] [Google Scholar]
- Rupert J, Hochachka P. The evidence for hereditary factors contributing to high altitude adaptation in Andean natives: a review. High Alt Med Biol. 2001;2(2):235–256. doi: 10.1089/152702901750265332. [DOI] [PubMed] [Google Scholar]
- Schwab M, Jayet PY, Stuber T, Salinas CE, Bloch J, Spielvogel H, Villena M, Allemann Y, Sartori C, Scherrer U. Pulmonary-artery pressure and exhaled nitric oxide in bolivian and caucasian high altitude dwellers. High Altitude Medicine & Biology*. 2008;9(4):295. doi: 10.1089/ham.2008.1057. [DOI] [PubMed] [Google Scholar]
- Scott RA, Moran C, Wilson RH, Onywera V, Boit MK, Goodwin WH, Gohlke P, Payne J, Montgomery H, Pitsiladis YP. No association between Angiotensin Converting Enzyme (ACE) gene variation and endurance athlete status in Kenyans. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2005;141(2):169. doi: 10.1016/j.cbpb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sharma S. Clinical, biochemical, electrocardiographic and noninvasive hemodynamic assessment of cardiovascular status in natives at high to extreme altitudes (3000m–5500m) of the Himalayan region. Indian Heart J. 1990;42(5):375–379. [PubMed] [Google Scholar]
- Sime F, Banchero N, Penaloza D, Gamboa R, Cruz J, Marticorena E. Pulmonary Hypertension in Children Born and Living at High Altitudes. Am J Cardiol. 1963;11(2):143–149. doi: 10.1016/0002-9149(63)90054-7. [DOI] [PubMed] [Google Scholar]
- Stuber T, Sartori C, Salm, Golden CS, Hutter D, Thalmann S, Turini P, Jayet PY, Schwab M, Sartori-Cucchia C, et al. Respiratory nitric oxide and pulmonary artery pressure in children of Aymara and European ancestry at high altitude. Chest. 2008;134(5):996. doi: 10.1378/chest.08-0854. [DOI] [PubMed] [Google Scholar]
- Stuber T, Sartori C, Schwab M, Jayet PY, Rimoldi SF, Garcin S, Thalmann S, Spielvogel H, Salmòn CS, et al. Exaggerated pulmonary hypertension during mild exercise in chronic mountain sickness. Chest. 2010;137(2):388–392. doi: 10.1378/chest.09-1355. [DOI] [PubMed] [Google Scholar]
- Wagner W, Latham L, Capen R. Capillary recruitment during airway hypoxia: role of pulmonary artery pressure. J Appl Physiol. 1979;47(2):283–287. doi: 10.1152/jappl.1979.47.2.383. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology - The Essentials. Baltimore, MD: Williams & Wilkins; 1985. [Google Scholar]
- Woods DR, Humphries SE, Montgomery HE. The ACE I/D polymorphism and human physical performance. Trends Endocrinol Metab. 2000;11(10):416–420. doi: 10.1016/s1043-2760(00)00310-6. [DOI] [PubMed] [Google Scholar]