Abstract
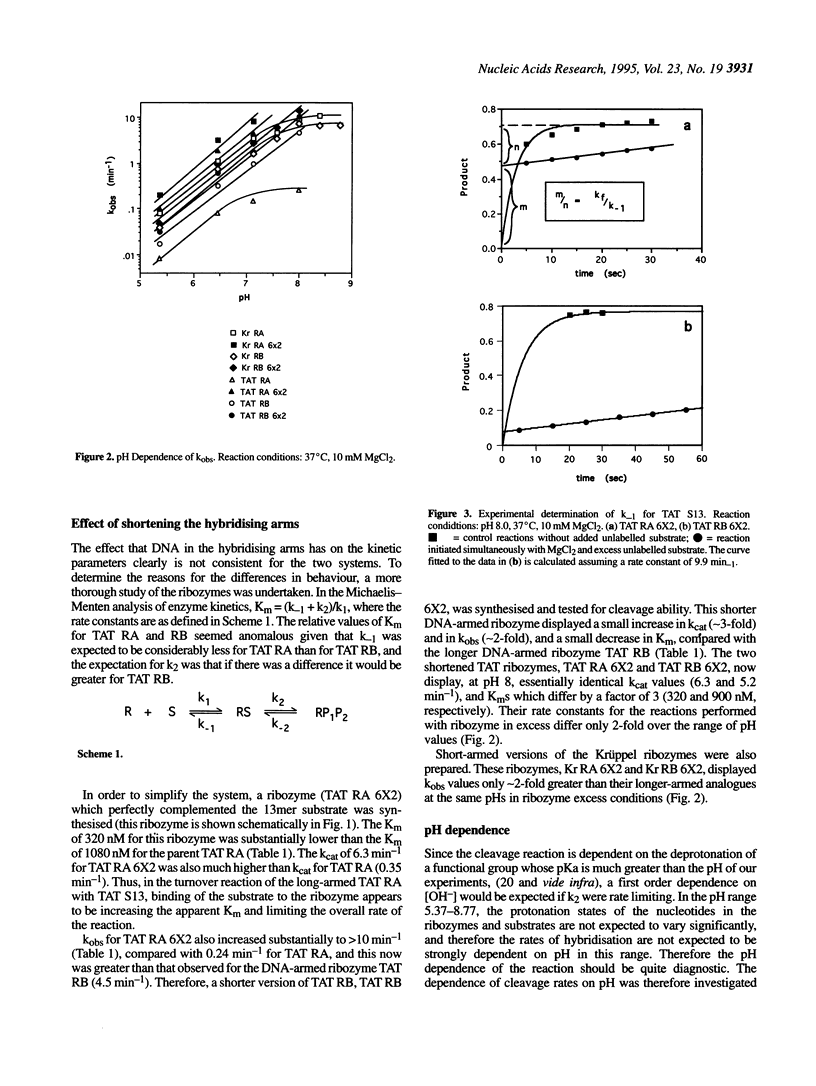
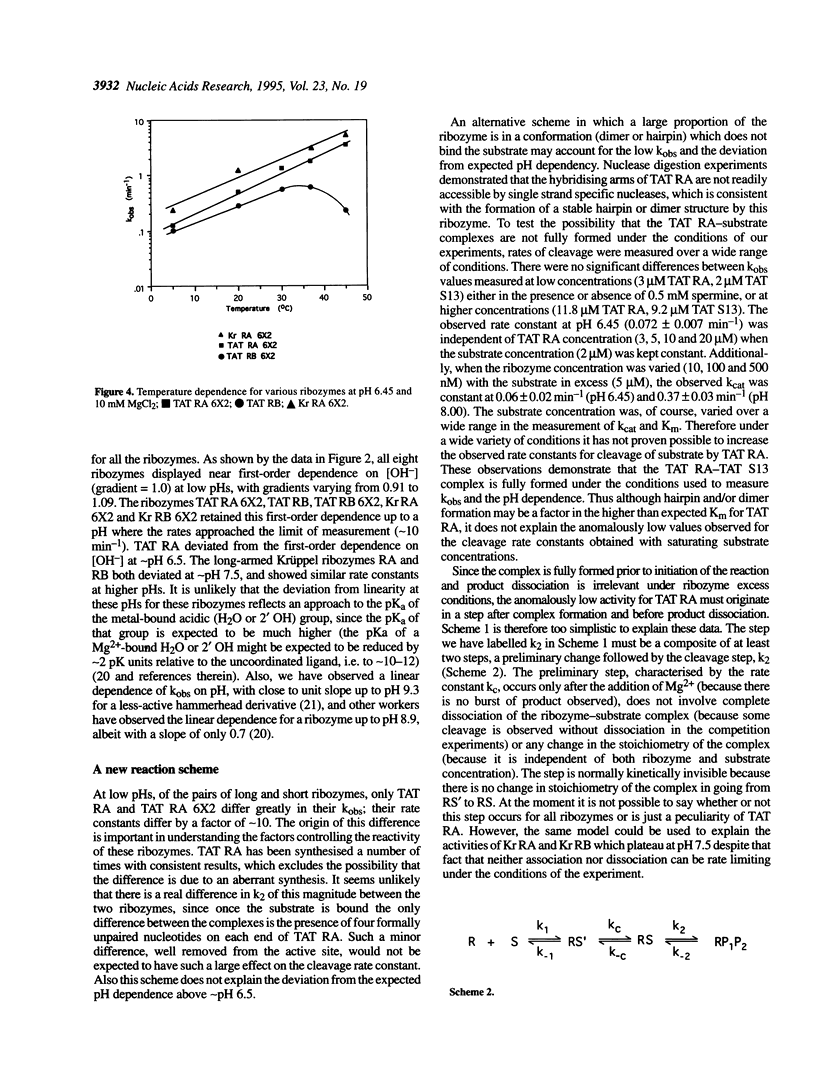
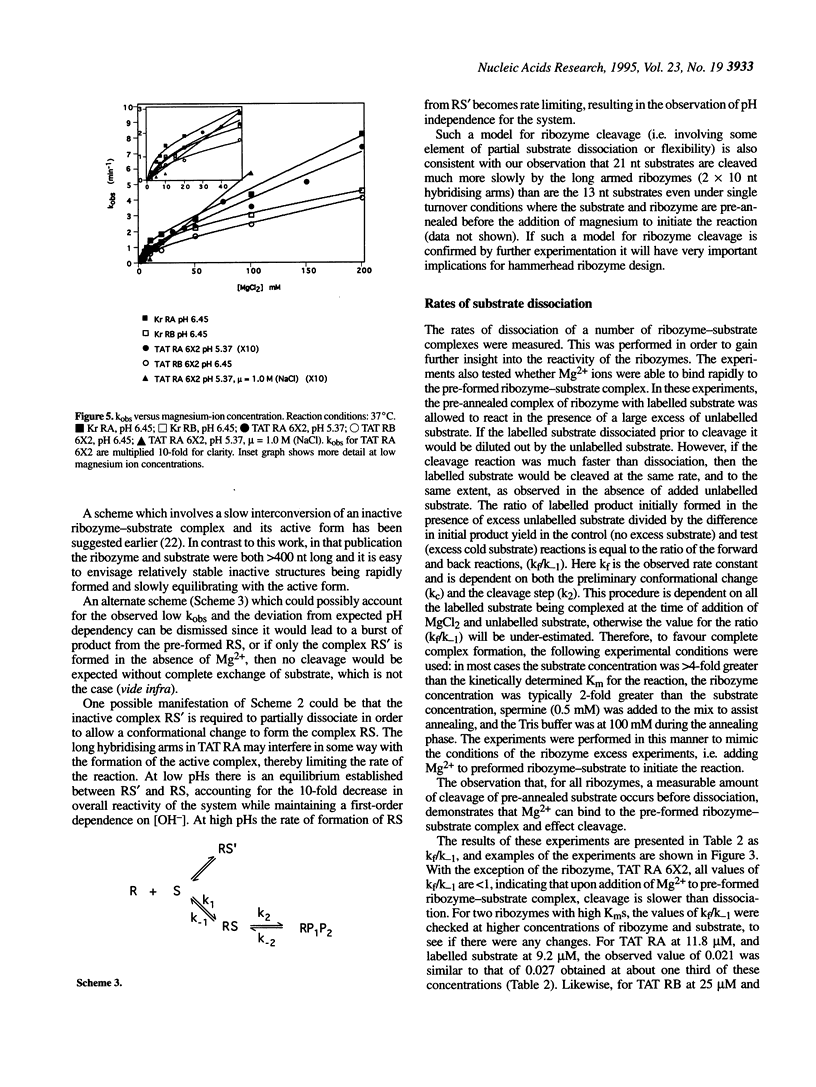
Hammerhead ribozymes targeted against two unrelated RNA substrates have been prepared. For each substrate, four ribozymes, differing in their hybridising arm length and composition (DNA or RNA), have been synthesised and kinetically characterised. The presence of DNA in the hybridising arms had little effect on the overall cleavage rate when the cleavage step was rate determining. Shortening each of the hybridising arms of ribozymes from 10 to 6 nucleotides generally resulted in modest changes in rate constants for cleavage of the same 13mer substrate. In one case the presence of long RNA hybridising arms significantly impeded the cleavage reaction. Cleavage rates displayed first order dependence on hydroxide ion concentration at low pHs. At higher pH, some ribozymes deviated from this first order dependence because of a change in the rate-determining step, possibly due to a requirement for a conformation change in the ribozyme-substrate complex prior to cleavage. Ribozyme cleavage was strongly dependent on temperature in the range 5-45 degrees C, with an activation energy for the reaction of approximately 60 kJ mol-1. The ribozymes displayed biphasic dependence on magnesium ion concentration; evidence of strong apparent binding (Kd approximately 10 mM) as well as a looser interaction was observed for all ribozymes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow R. Kinetics and mechanism in RNA cleavage. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1208–1211. doi: 10.1073/pnas.90.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm S. C., Derrick W. B., Uhlenbeck O. C. Evidence for the role of solvated metal hydroxide in the hammerhead cleavage mechanism. Biochemistry. 1993 Dec 7;32(48):13040–13045. doi: 10.1021/bi00211a013. [DOI] [PubMed] [Google Scholar]
- Dahm S. C., Uhlenbeck O. C. Role of divalent metal ions in the hammerhead RNA cleavage reaction. Biochemistry. 1991 Oct 1;30(39):9464–9469. doi: 10.1021/bi00103a011. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Kinetics of intermolecular cleavage by hammerhead ribozymes. Biochemistry. 1992 Dec 8;31(48):12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Thermodynamic and structural properties of pentamer DNA.DNA, RNA.RNA, and DNA.RNA duplexes of identical sequence. Biochemistry. 1991 Nov 5;30(44):10606–10613. doi: 10.1021/bi00108a002. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Hendry P., McCall M. J., Santiago F. S., Jennings P. A. A ribozyme with DNA in the hybridising arms displays enhanced cleavage ability. Nucleic Acids Res. 1992 Nov 11;20(21):5737–5741. doi: 10.1093/nar/20.21.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry P., McCall M. J., Santiago F. S., Jennings P. A. In vitro activity of minimised hammerhead ribozymes. Nucleic Acids Res. 1995 Oct 11;23(19):3922–3927. doi: 10.1093/nar/23.19.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel K. J., Herschlag D., Uhlenbeck O. C. A kinetic and thermodynamic framework for the hammerhead ribozyme reaction. Biochemistry. 1994 Mar 22;33(11):3374–3385. doi: 10.1021/bi00177a031. [DOI] [PubMed] [Google Scholar]
- Hertel K. J., Pardi A., Uhlenbeck O. C., Koizumi M., Ohtsuka E., Uesugi S., Cedergren R., Eckstein F., Gerlach W. L., Hodgson R. Numbering system for the hammerhead. Nucleic Acids Res. 1992 Jun 25;20(12):3252–3252. doi: 10.1093/nar/20.12.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M., Tabler M., Tzortzakaki S., Sczakiel G. Extension of helix II of an HIV-1-directed hammerhead ribozyme with long antisense flanks does not alter kinetic parameters in vitro but causes loss of the inhibitory potential in living cells. Nucleic Acids Res. 1994 Sep 25;22(19):3951–3957. doi: 10.1093/nar/22.19.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall M. J., Hendry P., Jennings P. A. Minimal sequence requirements for ribozyme activity. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5710–5714. doi: 10.1073/pnas.89.13.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault J. P., Labuda D., Usman N., Yang J. H., Cedergren R. Relationship between 2'-hydroxyls and magnesium binding in the hammerhead RNA domain: a model for ribozyme catalysis. Biochemistry. 1991 Apr 23;30(16):4020–4025. doi: 10.1021/bi00230a029. [DOI] [PubMed] [Google Scholar]
- Ratmeyer L., Vinayak R., Zhong Y. Y., Zon G., Wilson W. D. Sequence specific thermodynamic and structural properties for DNA.RNA duplexes. Biochemistry. 1994 May 3;33(17):5298–5304. doi: 10.1021/bi00183a037. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sawata S., Shimayama T., Komiyama M., Kumar P. K., Nishikawa S., Taira K. Enhancement of the cleavage rates of DNA-armed hammerhead ribozymes by various divalent metal ions. Nucleic Acids Res. 1993 Dec 11;21(24):5656–5660. doi: 10.1093/nar/21.24.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimayama T. Effects of deoxyribonucleotide substitutions in the substrate strand on hammerhead ribozyme-catalyzed reactions. Gene. 1994 Nov 4;149(1):41–46. doi: 10.1016/0378-1119(94)90410-3. [DOI] [PubMed] [Google Scholar]
- Shimayama T., Nishikawa F., Nishikawa S., Taira K. Nuclease-resistant chimeric ribozymes containing deoxyribonucleotides and phosphorothioate linkages. Nucleic Acids Res. 1993 Jun 11;21(11):2605–2611. doi: 10.1093/nar/21.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Natvig J. B., Førre O. Preformed ribozyme destroys tumour necrosis factor mRNA in human cells. J Mol Biol. 1992 Feb 20;223(4):831–835. doi: 10.1016/0022-2836(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Slim G., Gait M. J. Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991 Mar 25;19(6):1183–1188. doi: 10.1093/nar/19.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Wu Y., Wang J. L., Rossi J. J., Swiderski P., Kaplan B. E., Forman S. J. Ribozyme-mediated inhibition of bcr-abl gene expression in a Philadelphia chromosome-positive cell line. Blood. 1993 Jul 15;82(2):600–605. [PubMed] [Google Scholar]
- Takagi Y., Taira K. Temperature-dependent change in the rate-determining step in a reaction catalyzed by a hammerhead ribozyme. FEBS Lett. 1995 Mar 20;361(2-3):273–276. doi: 10.1016/0014-5793(95)00192-c. [DOI] [PubMed] [Google Scholar]
- Taylor N. R., Kaplan B. E., Swiderski P., Li H., Rossi J. J. Chimeric DNA-RNA hammerhead ribozymes have enhanced in vitro catalytic efficiency and increased stability in vivo. Nucleic Acids Res. 1992 Sep 11;20(17):4559–4565. doi: 10.1093/nar/20.17.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Yang J. H., Usman N., Chartrand P., Cedergren R. Minimum ribonucleotide requirement for catalysis by the RNA hammerhead domain. Biochemistry. 1992 Jun 2;31(21):5005–5009. doi: 10.1021/bi00136a013. [DOI] [PubMed] [Google Scholar]
- van Tol H., Buzayan J. M., Feldstein P. A., Eckstein F., Bruening G. Two autolytic processing reactions of a satellite RNA proceed with inversion of configuration. Nucleic Acids Res. 1990 Apr 25;18(8):1971–1975. doi: 10.1093/nar/18.8.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



