Abstract
Background Experts estimate that the prevalence of antibiotics use exceeds the prevalence of bacterial acute respiratory infections (ARIs).
Objective To develop, adapt and validate DECISION+ and estimate its impact on the decision of family physicians (FPs) and their patients on whether to use antibiotics for ARIs.
Design Two‐arm parallel clustered pilot randomized controlled trial.
Setting and participants Four family medicine groups were randomized to immediate DECISION+ participation (the experimental group) or delayed DECISION+ participation (the control group). Thirty‐three FPs and 459 patients participated.
Intervention DECISION+ is a multiple‐component, continuing professional development program in shared decision making that addresses the use of antibiotics for ARIs.
Main outcome measures Throughout the pilot trial, DECISION+ was adapted in response to participant feedback. After the consultation, patients and FPs independently self‐reported the decision (immediate use, delayed use, or no use of antibiotics) and its quality. Agreement between their decisional conflict was assessed. Two weeks later, patients assessed their decisional regret and health status.
Results Compared to the control group, the experimental group reduced its immediate use of antibiotics (49 vs. 33% absolute difference = 16%; P = 0.08). Decisional conflict agreement was stronger in the experimental group (absolute difference of Pearson’s r = 0.26; P = 0.06). Decisional regret and perceptions of the quality of the decision and of health status in the two groups were similar.
Discussion and conclusions DECISION+ was developed successfully and appears to reduce the use of antibiotics for ARIs without affecting patients’ outcomes. A larger trial is needed to confirm this observation.
Keywords: acute respiratory infections, continuing medical education, continuing professional development, implementation, randomized control trial, shared decision making
Introduction
The use of antibiotics for acute respiratory infections (ARIs) has contributed to the antibiotics resistance that presently plagues Canadians. 1 ARIs are the most frequently reported motive for primary care consultations in North America. 2 While ARIs have many forms, a large proportion is viral: only 38% of acute rhinosinusitis cases in adults, 5–15% of acute pharyngitis cases in adults, and 6–18% of ARI cases in children are bacterial. 3 , 4 Nonetheless, experts estimate that antibiotics are used for between 63 and 67% cases of ARI 5 , 6 , 7 . This suggests that antibiotics are overused. 8 , 9
Attempts to optimize the use of antibiotics for ARIs in ambulatory settings have proven less effective than anticipated. 10 Various aspects of the provider–patient interaction have been studied. The physician’s perception of the patient’s (or the parent’s) expectations or resistance to a diagnosis of viral infection is one of the strongest predictors of a physician’s decision to prescribe antibiotics. 11 , 12 , 13 , 14 , 15 In patients, a good understanding of the nature of their illness (i.e., that the ARI is viral) is associated with their satisfaction with the consultation. 16 This suggests that the beliefs, concerns and expectations of both physicians and patients should be taken into consideration when developing interventions to reduce the inappropriate use of antibiotics for the treatment of ARIs. Typically, however, interventions have been provider‐oriented: little attention has been paid to patient‐based interventions and even less to interventions combining physicians, patients and public education.
A promising solution can be found in shared decision making (SDM), a process in which a healthcare decision is made by both the clinician and the patient together. 17 , 18 SDM aims to help patients play an active role in decisions concerning their health, the ultimate goal of patient‐centered care. 19 SDM rests on the best evidence of the risks and benefits of all the available options. 17 Thus, the clinician’s ability to communicate with the patient in such a way as to enable him/her to weigh the risks and benefits of various treatment choices is essential. 20 Indeed, SDM takes place in a context in which the patient’s values and preferences are sought out and his/her opinions valued without excluding the values, preferences and opinions of the clinician. 21 It is a partnership in which the responsibilities and rights of each party are articulated, the benefits to each are clear, and the uncertainty associated with the best choice is made explicit (clinical equipoise). 22 SDM holds that ‘mutual acceptance [of a treatment option]… remains a necessary prerequisite’ to agreement between the patient and the provider on a plan of action. 23 Moreover, SDM has been shown to lower the overuse of screening or treatment options not clearly associated with health benefits for all. 24
Conceptual framework
In our published protocol, 25 we argue that teaching family physicians (FPs) about the probabilistic aspect of a diagnosis (in this case, a diagnosis of bacterial versus viral ARI); presenting them with the best evidence of the benefits and the risks associated with the clinical options (e.g., prescribing or not prescribing antibiotics); and giving them strategies to communicate with patients and involve them in decision‐making, leads to SDM during the clinical encounter (Fig. 1). This SDM would optimize FPs’ and patients’ decisions regarding screening or therapeutic options such as antibiotics. Optimal decisions by FPs would translate into optimal prescriptions and optimal decisions by patients would lead to their optimal use of treatment (e.g., taking antibiotics for their ARI if appropriate). In addition, patients would not regret their decision. Ultimately, population health would improve and quality of life would ameliorate. Consequently, one of our main outcomes of interest was the level of agreement between the patient’s decisional conflict score and the decisional conflict score of his/her FP.
Figure 1.
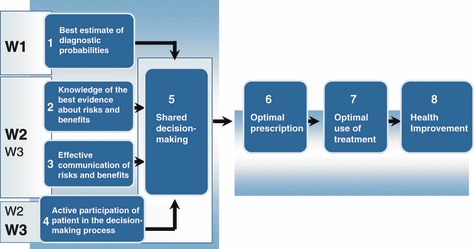
DECISION+ conceptual framework.
Although, in this pilot trial, decisional conflict was not one of our main outcomes of interest, its assessment in patients may be valuable. A Cochrane systematic review of 55 studies indicates that patient decision aids known to increase patients’ involvement in decision‐making are associated with reduced decisional conflict in patients. 26 This suggests that increasing patients’ involvement in the decision‐making process may help lower their decisional conflict. This finding is congruent with a meta‐analysis of 10 studies indicating that decisional conflict is strongly associated with patients’ decisional delay and decisional regret. 27 In turn, decisional regret correlates with overall quality of life ratings. 28
Notwithstanding its potential for optimizing decisions in clinical practice, SDM is not yet widely adopted and tests of its effectiveness are needed. 29 For this reason, we conducted a pilot trial in the clinical context of ARI, whose aims were (1) to assess the feasibility of recruiting family medicine groups (FMGs), FPs and their patients; (2) to develop, adapt and validate DECISION+ training workshops and related material (for a description of DECISION+, see ‘Intervention’); (3) to evaluate physicians’ participation and satisfaction regarding DECISION+; and (4) to estimate the impact of DECISION+ on (i) physicians’ and patients’ decision whether to use antibiotics, (ii) physicians’ and patients’ decisional conflict scores and the level of agreement between those scores, (iii) the prescription profile of antibiotics for ARIs, (iv) FPs’ intention to practice SDM, (v) FPs’ intention to follow clinical practice guidelines for the treatment of ARIs, (vi) FPs’ scores on a script concordance test, and (vii) patients’ decisional regret. 25 This paper covers aims 2 and 4. Aims 1 and 3 are discussed elsewhere. 30
Materials and methods
Trial design and population
Between November 2007 and March 2008, we conducted a pilot, two‐arm parallel clustered randomized clinical trial (RCT) whose main objective was to assess the feasibility of a larger clustered RCT. Details of the trial protocol are reported elsewhere. 25 Briefly, FMGs from the Quebec City, Canada, greater urban area were invited to participate. An FMG is a group of FPs who work closely with nurses to offer family medicine services to registered individuals. Participating FMGs were randomized either to an experimental group that was immediately exposed to the DECISION+ program or to a control group for which the introduction of DECISION+ program was delayed for 6 months (Fig. 2).
Figure 2.
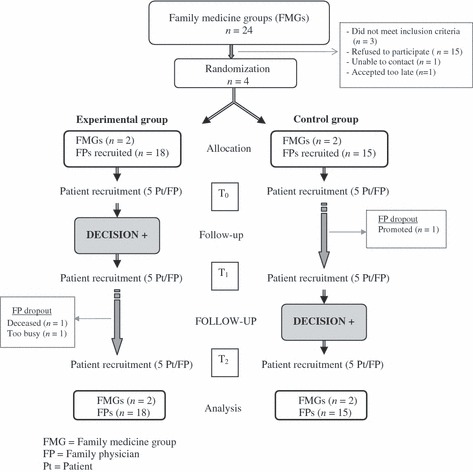
Trial flow diagram.
A biostatistician simultaneously randomized all FMGs and allocated them to groups using Internet‐based software. FPs were the main target population and were recruited through FMGs. The trial’s investigators and research assistants and participating FPs were not blinded to the group allocation. However, codes were attributed to the trial groups and the biostatistician analysed the data blindly. Team members accessed the codes only after having completed the analyses and interpreting the results.
Participants and eligibility criteria
Family practitioners were eligible to participate if they had not previously participated in an implementation trial of SDM and if they planned to remain in clinical practice for the duration of the trial. Patients were eligible to participate if they were consulting their FP for an ARI. No age restrictions were imposed. Patients (or their guardians) had to be able to read, understand, and write French and had to give informed consent to participate in the trial. Patients with a condition requiring emergency care were excluded.
Development, adaptation and validation of the intervention (DECISION+)
Designed on the basis of previous exploratory work, 29 , 31 , 32 , 33 DECISION+ is a theory‐based, continuing professional development program made up of three main components: (i) interactive workshops and related material; (ii) reminders of expected behaviours; and (iii) feedback to FPs on the agreement between their decisional conflict and that of their patients. 25
A series of three interactive workshops was developed with local opinion leaders and experts in medical education from the two continuing medical education offices involved in this trial. 34 A 1.5‐h training workshop introducing FPs to SDM 32 was expanded to include all components of the conceptual framework underlying this pilot trial 25 and to comply with continuing professional development regulations that stated that workshops must last at least 3 h for FPs to be reimbursed for their attendance. The resulting three 3‐h interactive workshops addressed (i) the probability that primary care physicians would encounter bacterial versus viral ARIs (rhinosinusitis, bronchitis, pharyngitis, and otitis media); (ii) scientific evidence of the benefit/risk balance of the various treatment options; (iii) risk communication techniques; and (iv) strategies for fostering patient participation in the decision‐making process. 25 Each workshop was piloted and modified in response to participants’ feedback, and each workshop was attended by four to six people: Family practitioners and residents in family medicine from the research team’s academic network and medical education experts. Each pilot workshop was audiotaped and participants completed an evaluation form. A research assistant acted as a non‐participant observer. After each workshop, the research team debriefed the session with the participants and among themselves in order to address any problems. In the end, four, rather than three, pilot workshops were conducted because the second workshop was completely redesigned and repiloted after feedback on its first testing was received.
Overall, participants found that the pilot workshops were well structured and well documented. They felt that both the workshops and the tools were relevant and potentially useful enough to be implemented in clinical practice. However, they suggested that more time be spent on practical and less on theoretical aspects.
All workshops included videos of simulated patient–FP consultations for each ARI. These videos were produced specifically for this training program and distinguished two approaches: usual care or SDM. First, clinical vignettes depicting usual care were developed with the help of experienced clinicians. The research team then adapted the vignettes to depict a SDM consultation based on our conceptual framework. Each workshop included exercises to facilitate group discussion about facilitators and barriers to SDM in this context. The research team developed five decision support tools: one for each of the four targeted ARIs (rhinosinusitis, pharyngitis, bronchitis and acute otitis media) and one integrating all four ARIs. Each tool was designed to help FPs understand and communicate to their patients the probabilistic nature of the diagnosis of a bacterial ARI as well as the risks and benefits of using or not using antibiotics. During each workshop, FPs received decision support tools and through video examples and group exercises were trained to use them. These tools were provided progressively: one per workshop, one between two workshops, and one after the third workshop. This schedule allowed FPs to experiment with the tools between workshops and provide feedback to the research team during the next workshop. Using this feedback, the research team improved the tools iteratively (e.g., by rounding the number of patients whom physicians should treat from 7 to 10, to make it easier for physicians to remember).
The research team also produced educational material for the participants (a booklet summarizing the content of the workshop, clinical tools) and training manuals for the co‐trainers. Co‐trainers were recruited among the FPs who had participated in the pilot workshops. They were expected to co‐lead the workshops when the principal investigators could not do so. For this trial, all workshops were conducted at each FMG and all were led by the two principal investigators or by co‐trainers.
The second component of DECISION+ consisted of two types of reminders. The first type were different reminders, each of which was printed on one letter‐size sheet of paper, that emphasized the use of the decision support tools discussed in the previous workshop and reiterated the expected SDM‐related behaviours. They also highlighted new studies relevant to the pilot trial topics (e.g., new evidence on the risks and benefits of antibiotics). These reminders were mailed to FPs between each workshop. The second type were postcards that participants were invited to write to themselves at the last workshop in order to remind themselves of what they needed to implement in their practice. The research team collected the postcards and mailed them 6–8 weeks later.
The third component of DECISION+ consisted of the research team informing FPs of the level of agreement between their score on the decisional conflict scale (DCS) with the scores of the first five of their patients recruited in the trial. During the last workshop, FPs were given a personal letter in a sealed envelope. This letter specified the level of agreement between each FP’s decisional conflict scores and those of his/her first five patients. Family practitioners were not informed of their or their patients’ decisional conflict scores per se. However, family practitioners were informed of the performance of their colleagues in the group, for comparison purposes. The research team explained how to interpret the scores. DECISION+ was conducted over a 4–6 months period. Apart from completing data collection forms at their entry into the study, FPs in the control group were not involved in any particular intervention prior to their delayed exposure to DECISION+.
Recruitment
The research team generated a random list of all FMGs in the Quebec City area. Based on that list and in sequence, one of the principal investigators (FL or ML) telephoned each physician in charge of an FMG to seek a meeting with him/her and his/her FMG colleagues to explain the nature of the project. FPs were either recruited at that meeting or by a research professional who individually met eligible FPs. Participating FPs were not involved in recruiting patients. Rather, a research professional waited in the FMG’s waiting room and, with the permission of medical and nursing staff, recruited patients of enrolled FPs during walk‐in clinic hours. Posters about the project were displayed in the waiting room and the medical receptionist handed small promotional cards to patients when the patients checked in. Patients interested in participating in the trial met with the research assistant, who verified their eligibility to participate. Fifteen patients were recruited per FP: five at baseline (T0), five after the FPs in the experimental group were exposed to DECISION+ (T1), and five after the FPs in the control group were exposed to DECISION+ (T2). All participants (i.e., both FPs and patients) signed an informed consent form approved by the Ethics Committee of the Saint‐François d’Assise Hospital of the Quebec University Hospital Center. Participants were not financially compensated.
Outcome measures and collection procedures
Sociodemographic information was recorded at trial entry for FPs and before the consultation for patients. After the consultation, at T0, T1 and T2, both the patient and the FP independently completed a self‐administered questionnaire that assessed the decision about using antibiotics (immediate use, delayed use, or no use) and the respondent’s perception of the quality of the decision (a single item on a 10‐point Likert scale). The participants also completed the DCS. 35 The questionnaire also measured patients’ intention to engage in SDM in future consultations concerning antibiotics for ARIs. Family practitioners’ intentions to engage in SDM and comply with clinical practice guidelines regarding prescribing antibiotics for ARIs were assessed T0, T1 and T2. All intentions were assessed with a three‐item, seven‐point Likert scale, and questionnaires were based on the Theory of Planned Behaviour. 36 Two weeks after the consultation, patients were contacted by phone to complete the Decision Regret Scale 28 and report their perceptions of health changes since the consultation.
Data on the number of prescriptions filled by patients covered by Quebec’s public drug insurance plan were extracted from the Régie de l’Assurance‐Maladie du Québec medication claims database during the 3 months preceding T0 and during the 3 months after FPs in the experimental group were exposed to DECISION+ (T1). A script concordance test was developed with the help of experts in ARIs, in SDM and in general practice. 37 This test probes whether respondents’ knowledge is efficiently organized to take appropriate clinical action. It places respondents in written, but authentic, clinical situations in which they must interpret data to make decisions. More specifically, it measures the concordance between respondents’ scripts and the scripts of a panel of experts. The script concordance test was administered to FPs at each data collection point. Outcome measures and collection procedures were similar for participating FPs from both the experimental and control groups.
Statistical analyses
The data from all randomly allocated units (the FMGs) were analysed on an intention‐to‐treat basis, regardless of the FP’s adherence to the intervention. Because this was a pilot trial, we did not calculate sample size. Missing data on completed questionnaires averaged 8 ± 8% (range 0–19%). To construct a complete dataset, we imputed a random value for all missing data using the maximum likelihood estimation method. 38 For each outcome, we calculated the difference between groups at T1 and the relevant 95% confidence interval. In addition, we assessed the statistical significance of differences between groups using multilevel modeling (Generalized Linear Mixed Models), accounting for the hierarchical structure of the data by specifying random effects at each level (FMG/FP/patient). The potential confounding effect of the baseline characteristics of FPs and patients on the association between the exposition to DECISION+ and the decision to use antibiotics (immediate use versus delayed use or no use) was also assessed using multilevel modeling. We considered an adjusted P‐value of <0.05 as statistically significant. As the agreement between FPs’ and patients’ answers was very high (Kappa = 0.90; P < 0.001), we report only patients’ answers regarding the decision to use antibiotics.
To evaluate the sustainability of the program’s effect, we calculated the difference between the results at T2 and those at T1 for each outcome in the experimental group. Replicability of the program’s effect was evaluated by first calculating the difference between T2 and T0 results for each outcome in each trial group and then calculating the difference between trial groups. A difference tending towards 0 indicates the sustainability (T2 – T1 in the experimental group) or the replicability [(T0 – T2 in the experimental group) − (T0 – T2 in the control group)] of the effect of DECISION+. As these were exploratory analyses, 95% confidence intervals were only performed to assess the precision of our estimates of differences. All statistical analyses were performed using the SAS statistical package (SAS Institute Inc. 2005. SAS OnlineDoc® 9.1.3; SAS Institute Inc., Cary, NC, USA).
Results
Participants
Figure 2 depicts the flow of the RCT. Four of the 21 eligible FMGs were enrolled and randomized either to the experimental group (FMGs = 2, FPs = 18, patients = 245) or to the control group (FMGs = 2, FPs = 15, patients = 214). A fifth FMG agreed to participate but notified us too late to be randomized. After discussion, we suggested that this FMG be exposed to DECISION+ at the same time as the delayed‐exposure FMGs (the control group). Because the fifth FMG had not been randomized, however, its data were not considered for this paper but are reported elsewhere. 30
Characteristics of FMG and FPs are presented in Table 1 and patient information is presented in Table 2. Otherwise, FP and patient characteristics were comparable between trial groups and no characteristic emerged as a confounding factor of the association between the intervention and the use of antibiotics. Although Quebec’s public prescription drug insurance plan covers 41% of the provincial population, it only covered antibiotics prescriptions for 123 (27%) of the 459 patients recruited for our study. The proportion of FMGs’ FPs who chose to participate in the trial was higher in the experimental group (90%) than in the control group (68%). Out of the 33 enrolled FPs, only three FPs (9%) dropped out of the trial; 20 patients (8%) from the experimental group and 14 patients (5%) from the control group could not be contacted over the 2‐week follow‐up.
Table 1.
Characteristics of participating FMGs and family physicians, by study group
| Characteristics | Experimental group | Control group | ||
|---|---|---|---|---|
| Family Medicine Groups | FMG 1 | FMG 2 | FMG 3 | FMG 4 |
| Number of physicians | 10 | 10 | 12 | 10 |
| Number of female physicians | 7 | 4 | 7 | 5 |
| Number of walk‐in hours per weekday | 12 | 8 | 12 | 8 |
| Approximate number of patients seen per day | 70 | 50 | 110 | 50 |
| Participating family physicians n/N (%) | 18/20 (90) | 15/22 (68) | ||
| Women n/N (%) | 10/18 (56) | 9/15 (60) | ||
| Mean ± SD years of age | 48 ± 9 | 48 ± 7 | ||
| Mean ± SD years of professional experience | 22 ± 9 | 21 ± 10 | ||
| Mean ± SD number of working hours per week | 45 ± 11 | 43 ± 14 | ||
| Mean ± SD number of patients seen per week | 105 ± 47 | 105 ± 29 | ||
| Preferred role in decision making n/N (%) | ||||
| Patient decides | 4/18 (22) | 0/15 (0) | ||
| Patient decides, considering physician’s opinion | 4/18 (22) | 8/15 (53) | ||
| Both parties decide | 3/18 (17) | 1/15 (7) | ||
| Physician decides, considering patient’s opinion | 6/18 (33) | 6/15 (40) | ||
| Physician decides | 1/18 (6) | 0/15 (0) | ||
FMG, family medicine group; SD, standard deviation.
Table 2.
Patient characteristics by study group and data collection period
| Patient characteristics | Experimental group | Control group | Total population n = 459 | ||||
|---|---|---|---|---|---|---|---|
| T0 n = 92 | T1 n = 81 | T2 n = 72 | T0 n = 77 | T1 n = 70 | T2 n = 67 | ||
| Number of women (%) | 62 (67) | 57 (70) | 50 (69) | 57 (75) | 47 (68) | 51 (76) | 324 (71) |
| Number of adults (%) | 55 (60) | 54 (67) | 57 (79) | 61 (79) | 46 (66) | 48 (72) | 321 (70) |
| Mean ± SD years of age | 37 ± 12 | 36 ± 13 | 40 ± 13 | 41 ± 13 | 38 ± 12 | 37 ± 11 | 40 ± 14 |
| Number of children (%) | 37 (40) | 27 (33) | 15 (21) | 16 (21) | 24 (34) | 19 (28) | 138 (30) |
| Mean ± SD years of age | 4 ± 3 | 5 ± 4 | 3 ± 3 | 7 ± 5 | 5 ± 4 | 5 ± 4 | 5 ± 4 |
| Number of participants whose family income ≥ Cdn. $45 000/year (%) | 51 (55) | 43 (56) | 41 (62) | 38 (54) | 42 (63) | 44 (72) | 259 (60) |
| Number of participants currently working (%) | 63 (68) | 58 (72) | 57 (70) | 61 (80) | 57 (83) | 58 (87) | 354 (77) |
| Number of participants with a university or college degree (%) | 51 (55) | 57 (72) | 44 (61) | 44 (58) | 39 (57) | 41 (63) | 271 (60) |
| Number of participants with public drug insurance (%) | 27 (29) | 32 (40) | 18 (25) | 17 (22) | 21 (30) | 8 (12) | 123 (27) |
| Preferred role in decision making, n (%) | |||||||
| Patient decides | 4 (4) | 4 (5) | 5 (7) | 3 (4) | 5 (7) | 2 (3) | 23 (5) |
| Patient decides, considering physician’s opinion | 29 (32) | 35 (43) | 23 (32) | 24 (33) | 25 (36) | 17 (26) | 153 (34) |
| Both parties decide | 31 (34) | 16 (20) | 21 (29) | 14 (19) | 16 (23) | 12 (18) | 110 (24) |
| Physician decides, considering patient’s opinion | 16 (17) | 19 (23) | 14 (19) | 24 (33) | 13 (19) | 21 (32) | 107 (24) |
| Physician decides | 12 (13) | 7 (9) | 9 (13) | 8 (11) | 11 (16) | 13 (20) | 60 (13) |
Because of missing values, the denominator for some characteristics differ from the sample size.
Cdn, Canadian; SD, standard deviation; T0, baseline; T1, after DECISION+ was implemented in the experimental group; T2, after DECISION+ was implemented in the control group.
Outcome measures at T1
Table 3 presents the results at baseline (T0) and after the DECISION + program was implemented in the experimental group (T1). None of the differences between the experimental and the control groups at T1 were statistically significant. However, the magnitude of the difference between the groups on some outcomes nonetheless suggests that the program had a positive effect. While the groups were similar at baseline (T0), the experimental group had, at follow‐up (T1), reduced its immediate use of antibiotics by 16% compared to the control group. This reduction is highly clinically significant. The intracluster correlation coefficient calculated on the decision about using antibiotics as reported by the patient was 0.02 (patients nested within an FP). The reduction in the mean proportion of patients covered by Quebec’s public drug insurance plan who filled a prescription for an antibiotic was 6%.
Table 3.
Outcome measures at baseline (T0) and after the implementation of the DECISION+ program in the experimental group (T1), by study group
| Outcome | Experimental group | Control group | Difference at T1 (95% CI) | P‐value* | ||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||
| Patients who decided to use antibiotics immediately (%) | 56 | 33 | 54 | 49 | −16 (−31 to 1) | 0.08 |
| Mean proportion of patients who filled a prescription (%)† | 79 | 45 | 70 | 51 | −6 (−17 to 6) | 0.35 |
| Correlation of FPs’ and patients’ DCS scores (Pearson’s r) | 0.14 | 0.24 | −0.05 | 0.02 | 0.26 (−0.06 to 0.53) | 0.06 |
| Mean ± SD score of the quality of the decision‡ | ||||||
| FPs | 8.8 ± 1.1 | 8.7 ± 1.2 | 8.3 ± 1.4 | 8.5 ± 1.3 | 0.2 (−0.34 to 0.89) | 0.29 |
| Patients | 8.2 ± 2.1 | 8.7 ± 1.9 | 8.4 ± 1.9 | 8.6 ± 1.9 | 0.1 (−0.88 to 0.94) | 0.57 |
| Mean ± sd score of the intention§ | ||||||
| FPs to engage in SDM | 0.8 ± 0.8 | 1.3 ± 1.2 | 0.3 ± 1.6 | 0.8 ± 1.3 | 0.5 (−0.2 to1.3) | 0.77 |
| FPs to comply with CPGs | 1.9 ± 0.8 | 2.1 ± 0.9 | 1.8 ± 0.8 | 2.2 ± 0.5 | −0.1 (−0.7 to 0.5) | 0.58 |
| Patients to engage in SDM | 1.1 ± 1.4 | 0.7 ± 1.4 | 0.8 ± 1.6 | 0.8 ± 1.4 | −0.1 (−0.6 to 0.4) | 0.16 |
| Patients with decisional regret (%) | 1 | 7 | 1 | 9 | −2 (−12 to 5) | 0.91 |
| Patients who felt they had stable, a little better, or much better health at 2 weeks (%)¶ | 87 | 94 | 91 | 85 | 9 (−2 to 18) | 0.08 |
CI, confidence interval; CPGs, clinical practice guidelines; DCS, decisional conflict scale; FP, family physician; SD, standard deviation; SDM, shared decision‐making, T0, baseline; T1, after DECISION+ was implemented in the experimental group.
*All P values except the difference between correlations were adjusted for baseline values (T0) and the study’s cluster design.
†Among patients covered by Quebec’s public drug insurance plan who consulted a participating physician for an acute respiratory infection (as reported for billing purposes).
‡1 = very low quality to 10 = very high quality.
§−3 = strongly disagree to +3 = strongly agree.
¶Versus not much worse or much worse.
The correlation coefficient for DCS scores among FPs and patients in the experimental group, although low, was higher than in the control group. This indicates better agreement on the decisional process between FPs and patients in the experimental group. The intentions of FPs to engage in SDM in future consultations regarding the use of antibiotics for ARIs increased in both groups, with higher intentions in the experimental group. Most patients considered their health to be either stable or improved 2 weeks after the consultation, with a slightly higher proportion of patients in the experimental group having a positive perception of their health status. There was no difference between trial groups insofar as the following elements were concerned: FPs’ and patients’ decisional conflict scores and perceptions of the quality of the decision, FPs’ intentions to comply with clinical practice guidelines regarding the use of antibiotics for ARIs, patients’ intentions to engage in SDM in future consultations regarding the use of antibiotics for ARIs, and patients’ decisional regret.
Outcome measures at T2
Table 4 presents the outcome measures of both trial groups after the control group was exposed to DECISION+ (T2). The proportion of patients in the experimental group who decided to use antibiotics immediately after the consultation was similar at T2 and T1 (35 vs. 33%; absolute difference 2%). Results for all other outcomes were also similar at T2 and T1 for this group.
Table 4.
Outcome measures in the study groups after the DECISION+ program was implemented in the control group (T2); assessment of the sustainability and replicability of the effect of the program
| Outcomes | Experimental group at T2 | Control group at T2 | Difference in the experimental group between T1 and T2 (95% CI)* | Difference between the change in the experimental group between T0 and T2 and the change in the control group between T0 and T2 (95% CI)* |
|---|---|---|---|---|
| Patients who decided to use antibiotics immediately (%) | 35 | 46 | 2 (−14 to 16) | −13 (−39 to 6) |
| Correlation of FPs’ and patients’ DCS scores (Pearson’s r) | 0.17 | 0.18 | −0.1 (−0.4 to 0.2) | −0.1 (CI cannot be estimated) |
| Mean ± SD score of the quality of the decision† | ||||
| FPs | 8.7 ± 1.1 | 8.5 ± 1.0 | 0 (−0.4 to 0.2) | −0.3(−0.8 to 0.1) |
| Patients | 9.1 ± 2.1 | 8.1 ± 1.8 | 0.4 (−0.2 to 1.1) | 1.2 (0.3–2.3) |
| Mean ± sd score of the intention‡ | ||||
| FPs to engage in SDM | 1.4 ± 0.7 | 0.7 ± 1.0 | 0.1 (−0.5 to 0.7) | 0.05 (−0.9 to 1) |
| FPs to comply with CPGs | 2.1 ± 0.7 | 2.0 ± 0.9 | 0 (−0.5 to 0.5) | 0 (−0.6 to 0.7) |
| Patients to engage in SDM | 1.1 ± 1.5 | 0.7 ± 1.3 | 0.4 (−0.1 to 0.8) | 0.1 (−0.5 to 0.7) |
| Patients with decisional regret (%) | 3 | 9 | −4 (−22 to 7) | −6 (−30 to 22) |
| Patients who felt they had stable, a little better, or much better health at 2 weeks§ (%) | 94 | 91 | 0 (−8 to 8) | 7 (−6 to 21) |
CI, confidence interval; CPGs, clinical practice guidelines; DCS, decisional conflict scale; FP, family physician; SD, standard deviation; SDM, shared decision making; T0, baseline; T1, after DECISION+ was implemented in the experimental group; T2, after DECISION+ was implemented in the control group.
*A difference tending towards 0 indicates the sustainability (T2 – T1 in the experimental group) or the replicability [(T2 – T0 in the experimental group) − (T2 – T0 in the control group)] of the DECISION+ program.
†1 = very low quality to 10 = very high quality.
‡−3 = strongly disagree to +3 = strongly agree.
§Versus a little worse or much worse.
By comparing the differences between T2 and T0 results in the experimental and control groups, we explored the effect of the program once the intervention was replicated. In the experimental group, 21% fewer patients decided to use antibiotics immediately at T2 (35%) than at T0 (56%). In the control group, the reduction was only 8% between T2 (54%) and T0 (46%). This 13% difference indicates that insofar as this outcome is concerned, the DECISION+ program had less effect in the control group than in the experimental group. Comparable results were observed for all other variables, with the exception of FPs’ intentions to engage in SDM in future consultations regarding antibiotics for ARIs: these intentions improved similarly in the two groups. Unanticipated technical difficulties with the script concordance test precluded its use as an outcome measure. Experts could not achieve consensus on the expected answers.
In light of these results and using proposed guidelines for reporting complex behaviour change interventions, 39 we explored the intervention context and process and noted differences. Among FP participating in the trial, the proportion of those who attended the three workshops was similar in both groups (experimental = 9/18, 50% vs. control group = 7/15, 47%). However, we found that not all workshops had been conducted by the same individuals. In the experimental group, five of the six workshops (three per FMG) had been conducted by both principal investigators (FL and ML), whereas only one of the six workshops in the control group were lead by them.
Discussion
In this trial, we successfully developed, adapted and validated DECISION+, a theory‐based, continuing professional development program. This program comprised three main components: (i) interactive workshops and related material; (ii) reminders of expected behaviours; and (iii) feedback to FPs on the agreement between their decisional conflict and that of their patients. Our results suggest that DECISION+ reduces FPs’ and patients’ decision to use antibiotics and increases the level of agreement between their decisional conflict scores without affecting decisional regret and patients’ self‐reported health improvements at 2 weeks. We also observed that the effect of DECISION+ lasted at least until after the control group was exposed to the program (a period of about 8 months). These results provide valuable information regarding three aspects of the future RCT.
The first concerns the development, adaptation and validation of DECISION+. Although our results suggest that the intervention is sustainable over 8 months, we could not reproduce in the control group the results observed in the experimental group. When workshops were not conducted by the principal investigators (ML and FL), they failed to produce the expected change. In addition to the principal investigators’ obvious motivation to have the program succeed, it is possible that either both or one of the principal investigators involved in the training sessions may have been acting as a local opinion leader, that is, an individual perceived by his or her colleagues as likeable, trustworthy, and influential; someone who acts as a persuasive agent of behavioural change. 40 Our findings of the importance of carefully choosing, training and motivating the workshop leaders have therefore caused us to modify our procedure for the future trial. We plan four new strategies: videoconferencing workshops so that the same pair of trainers can train participants at several sites at the same time; fewer trainers; a standardized train‐the‐trainers workshop; and assessment of the trainers before they are allowed to conduct training.
The second aspect of the future trial informed by our results regards outcomes assessment. Based on the estimate and confidence intervals of patients’ immediate decision to use antibiotics (our main outcome) and its related intracluster correlation coefficient, we calculate that with six clusters (FMGs), we will need 360 patients at each data collection point to detect a reduction from 60 to 40% with 80% power and a 5% significance. 41 Given the small proportion of participating patients who were covered by Quebec’s provincial drug plan (27% compared to 41% of the provincial population), we will not collect provincial drug registry information in the future trial. Instead, we will copy the prescription received by participating patients immediately after the consultation. Finally, because of the difficulties associated with developing and scoring the script concordance test, we will not use this test in the larger trial.
Although not statistically significant and originating from a pilot project, our results are somewhat congruent with those of two recently published RCTs. 42 , 43 These two studies showed that FPs trained in communication skills and patient‐centered care prescribed less antibiotics than did FPs without such training, and that prescription rates and absolute use dropped within the ranges observed in our trial. Our observations are also congruent with the conclusions of a Cochrane review on the effectiveness of interventions to improve the use of antibiotics for ARIs in ambulatory settings. 44 Patient‐mediated interventions and physician reminders were found to be potentially effective methods deserving further study. 44 Finally, although DECISION+ trained physicians in SDM and gave them tools to conduct SDM with their patients, we realized that it would be important that the next trial assess patients’ exposure to these tools. Consequently, we have devised a strategy to expose patients to the tools and measure their exposure.
In addition to assessing FPs’ intention to use SDM in practice, we evaluated the potential effect of DECISION+ on FPs’ intention to use clinical practice guidelines. Physicians sometimes perceive the pressure to apply clinical practice guidelines as a barrier to SDM. 45 Although decision‐makers, researchers, patients’ organizations and other stakeholders are placing increasing emphasis on the need to incorporate patients’ perspectives and SDM within clinical practice guidelines, 46 little, if anything, is known about combining the implementation of a SDM approach with the implementation of clinical practice guidelines. Our results suggest that it is possible to implement SDM in FPs’ practices without damaging FPs’ intentions to comply with clinical practice guidelines.
Nevertheless, our pilot RCT has limitations. With respect to the development, adaptation and validation of DECISION+, we only assessed participants’ exposure to and appreciation of the workshops. We did not assess their exposure to the other two components of DECISION+. It was often difficult to distinguish between the full DECISION+ program (all components) and the series of three workshops (one component). Also, we could not assess the replicability of DECISION+. As discussed above, we plan to reduce the number of co‐trainers and modify their training. With respect to the outcomes assessment, we acknowledge that the DCS alone may not be an adequate outcome measure of the quality of the decisional process regarding the use of antibiotics for ARIs in primary care. Decisional conflict scores were very low in both FPs and patients at T0 and remained so at T1 and T2. However, one of our main outcomes of interest was the agreement between the DCS score of the patient and the score of his/her FP. Although at T1, FPs’ and patients’ DCS scores correlated more closely in the experimental group than in the control group (suggesting stronger agreement among experimental group participants regarding comfort with the decision), the clinical significance of the magnitude of the difference (0.26) is questionable.
Notwithstanding these limitations, we successfully developed, adapted and validated DECISION+, a multi‐component, continuing professional development program in SDM that addresses the use of antibiotics for ARIs. Some of the lessons learned in this pilot trial may be relevant to training FPs in SDM for other clinical contexts and decisions. Our pilot trial – particularly its intervention and its methods – was based on a conceptual framework that is generic enough to be adapted to other contexts. The exploratory assessment of the program’s impact suggests that DECISION+ has the potential to reduce the proportion of FPs and patients deciding to use antibiotics immediately for ARIs. Nonetheless, a large clustered RCT will be necessary to more accurately evaluate the impact of DECISION+ on the decision to use antibiotics for ARIs and to assess the program’s sustainability.
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
FL and ML developed the research protocol and were responsible for the overall conduct of the study. ML, FL and MN supervised the development of the analysis procedures and the statistical analyses themselves. FL, SSJ and ML wrote the first draft of the manuscript. All authors provided a critical review of the manuscript and contributed to the final version. FL is its guarantor.
Acknowledgements
This study was funded by the Fonds de la Recherche en Santé du Québec. FL is Tier 2 Canada Research Chair in Implementation of Shared Decision Making in Primary Care. GG is Tier 1 Canada Research Chair in Health Behaviour. FL, ML, GG AOC and AL are members of Knowledge Translation Canada, a CIHR‐funded national research network. A O’Connor is a Tier 1 Canada Research Chair on Decision Support for Consumers. AL holds a Doctoral Scholarship from the Canadian Institute of Health Research. We thank Jean Rousseau, Nadine Allain‐Boulé and Sylvie Tapp for data collection and Michelle Par for analyses of the prescription plan database. Jennifer Petrela edited this paper.
Re‐use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms.
References
- 1. Patrick DM, Hutchinson J. Antibiotic use and population ecology: how you can reduce your “resistance footprint”. CMAJ, 2009; 180: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Advance Data, 2006; 372: 1–33. [PubMed] [Google Scholar]
- 3. Alberta Clinical Practice Guideline Working Group . Guideline for the Diagnosis and Treatment of Acute Pharyngitis. Alberta, Canada: Alberta Clinical Practice Guidelines Program, 1999. [Google Scholar]
- 4. Agency for Healthcare Research and Quality (AHRQ) . Update on Acute Bacterial Rhinosinusitis. In: Ip S, Fu L, Balk E, Chew P, Devine D, Lau J. (eds) Evidence Report/Technology Assessment. Rockville, MD: U.S. Department of Health and Human Services, 2005: p. 153. [PMC free article] [PubMed] [Google Scholar]
- 5. Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Archives of Pediatrics and Adolescent Medicine, 2002; 156: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 6. Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989–1999. JAMA, 2001; 286: 1181–1186. [DOI] [PubMed] [Google Scholar]
- 7. Dosh SA, Hickner JM, Mainous AG, Ebell MH. Predictors of antibiotic prescribing for nonspecific upper respiratory infections, acute bronchitis, and acute sinusitis. An UPRNet study. Upper Peninsula Research Network. Journal of Family Practice, 2000; 49: 407–414. [PubMed] [Google Scholar]
- 8. McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerging Infectious Diseases, 2003; 9: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kozyrskyj AL, Carrie AG, Mazowita GB, Lix LM, Klassen TP, Law BJ. Decrease in antibiotic use among children in the 1990s: not all antibiotics, not all children. CMAJ, 2004; 171: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database of Systematic Reviews, 2005; 1: CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bauchner H, Pelton SI, Klein JO. Parents, physicians, and antibiotic use. Pediatrics, 1999; 103: 395–401. [DOI] [PubMed] [Google Scholar]
- 12. Palmer DA, Bauchner H. Parents’ and physicians’ views on antibiotics. Pediatrics, 1997; 99: E6. [DOI] [PubMed] [Google Scholar]
- 13. Watson RL, Dowell SF, Jayaraman M, Keyserling H, Kolczak M, Schwartz B. Antimicrobial use for pediatric upper respiratory infections: reported practice, actual practice, and parent beliefs. Pediatrics, 1999; 104: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 14. Mangione‐Smith R, Elliott MN, Stivers T, McDonald LL, Heritage J. Ruling out the need for antibiotics: are we sending the right message? Achives of Pediatrics and Adolescent Medicine, 2006; 160: 945–952. [DOI] [PubMed] [Google Scholar]
- 15. Stivers T, Mangione‐Smith R, Elliott MN, McDonald L, Heritage J. Why do physicians think parents expect antibiotics? What parents report vs what physicians believe. Journal of Family Practices, 2003; 52: 140–148. [PubMed] [Google Scholar]
- 16. Ong S, Nakase J, Moran GJ et al. Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Annals of Emergency Medicine., 2007; 50: 213–220. [DOI] [PubMed] [Google Scholar]
- 17. Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ, 1999; 319: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Briss P, Rimer B, Reilley B et al. Promoting informed decisions about cancer screening in communities and healthcare systems. American Journal of Preventive Medicine, 2004; 26: 67–80. [DOI] [PubMed] [Google Scholar]
- 19. Howie J, Heaney D, Maxwell M. Quality, core values and the general practice consultation: issues of definition, measurement and delivery. Family Practice, 2004; 21: 458–468. [DOI] [PubMed] [Google Scholar]
- 20. Edwards A, Elwyn G. How should effectiveness of risk communication to aid patients’ decisions be judged? A review of the literature [see comments]. Medical Decision Making, 1999; 19: 428–434. [DOI] [PubMed] [Google Scholar]
- 21. Atkins D, Best D, Briss PA et al. Grading quality of evidence and strength of recommendations. BMJ, 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competences of involving patients in healthcare choices. British Journal of General Practice, 2000; 50: 892–899. [PMC free article] [PubMed] [Google Scholar]
- 23. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science and Medicine, 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 24. Evans R, Edwards A, Brett J et al. Reduction in uptake of PSA tests following decision aids: systematic review of current aids and their evaluations. Patient Education and Counseling, 2005; 58: 13–26. [DOI] [PubMed] [Google Scholar]
- 25. Légaré F, Labrecque M, LeBlanc A et al. Does training family physicians in shared decision making promote optimal use of antibiotics for acute respiratory infections? Study protocol of a pilot clustered randomised controlled trial. BMC Family Practice, 2007; 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Connor AM, Bennett CL, Stacey D et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 2009; 1: CD001431. [DOI] [PubMed] [Google Scholar]
- 27. O’Connor A, Sun Q, Laupacis A et al. Predicting Downstream Effects of High Decisional Conflict: Meta‐analyses of the Decisional Conflict Scale. Abstract 305. 3rd International Conference on Shared Decision Making‐ Implementing Shared Decision Making In Diverse Health Care Systems And Cultures, University of Ottawa; 2005.
- 28. Brehaut JC, O’Connor AM, Wood TJ et al. Validation of a decision regret scale. Medical Decision Making, 2003; 23: 281–292. [DOI] [PubMed] [Google Scholar]
- 29. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Education and Counseling, 2008; 73: 526–535. [DOI] [PubMed] [Google Scholar]
- 30. LeBlanc A, Légaré F, Labrecque M et al. Training family physicians in shared decision making promoting optimal use of antibiotics for acute respiratory infections: feasibility of a pilot clustered randomised controlled trial (the DECISION+ study). International Shared Decision Making Conference. Boston; 2009. p. A‐3.
- 31. Légaré F, Graham ID, O’Connor AC et al. Prediction of health professionals’ intention to screen for decisional conflict in clinical practice. Health Expectations, 2007; 10: 364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Légaré F, O’Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the Ottawa Decision Support Framework on the Agreement and the Difference between Patients’ and Physicians’ Decisional Conflict. Medical Decision Making, 2006; 26: 373–390. [DOI] [PubMed] [Google Scholar]
- 33. Légaré F, O’Connor AM, Graham ID et al. Primary health care professionals’ views on barriers and facilitators to the implementation of the Ottawa Decision Support Framework in practice. Patient Education and Counseling, 2006; 63: 380–390. [DOI] [PubMed] [Google Scholar]
- 34. Lomas J. Diffusion, dissemination, and implementation: who should do what? Annals of the New York Academy of Sciences, 1993; 703: 226–235, discussion 35–7. [DOI] [PubMed] [Google Scholar]
- 35. O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 36. Ajzen I. Attitudes, Personality and Behavior. Milton‐Keynes: Open University Press, 1988. [Google Scholar]
- 37. Charlin B, van der Vleuten C. Standardized assessment in context of uncertainty: the script concordance approach. Evaluation and the Health Professions, 2004; 27: 304–319. [DOI] [PubMed] [Google Scholar]
- 38. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York: John Wiley & Sons, 2002. [Google Scholar]
- 39. Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implementation Science, 2009; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doumit G, Gattellari M, Grimshaw J, O’Brien MA. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews, 2007; 1: CD000125. [DOI] [PubMed] [Google Scholar]
- 41. Campbell MK, Thomson S, Ramsay CR, MacLennan GS, Grimshaw JM. Sample size calculator for cluster randomized trials. Computers in Biology and Medicine, 2004; 34: 113–125. [DOI] [PubMed] [Google Scholar]
- 42. Cals JW, CC’ B, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ, 2009; 338: b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Francis NA, Butler CC, Hood K, Simpson S, Wood F, Nuttall J. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ, 2009; 339: b2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database of Systematic Reviews, 2005; 4: CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barratt A. Evidence based medicine and shared decision making: the challenge of getting both evidence and preferences into health care. Patient Education and Counseling, 2008; 73: 407–412. [DOI] [PubMed] [Google Scholar]
- 46. Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA, 2008; 300: 436–438. [DOI] [PubMed] [Google Scholar]


