SUMMARY
Pluripotent stem cells provide a platform to interrogate control elements that function to generate all cell types of the body. Despite their utility for modeling development and disease, the relationship of mouse and human pluripotent stem cell states to one another remains largely undefined. We have shown that mouse embryonic stem (ES) cells and epiblast stem cells (EpiSCs) are distinct, pluripotent states isolated from pre- and post-implantation embryos respectively. Human ES cells are different than mouse ES cells and share defining features with EpiSCs, yet are derived from pre-implantation human embryos. Here we show that EpiSCs can be routinely derived from pre-implantation mouse embryos. The pre-implantation-derived EpiSCs exhibit molecular features and functional properties consistent with bona fide EpiSCs. These results provide a simple method for isolating EpiSCs and offer direct insight into the intrinsic and extrinsic mechanisms that regulate the acquisition of distinct pluripotent states.
Keywords: epiblast stem cells, pluripotency, embryonic stem cells, blastocyst
INTRODUCTION
The in vitro derivation of pluripotent stem cells both from embryonic and adult tissues holds tremendous promise for biology and medicine. Until recently, access to the pluripotent state in vitro was thought to be limited to the derivation of embryonic stem (ES) cells from pre-implantation blastocysts and the reprogramming of cells in the germ cell lineage. Reports of new pluripotent cell types including induced pluripotent stem (iPS) cells and epiblast stem cells (EpiSCs) provided a major impetus for recent studies that evaluate the genetic and epigenetic mechanisms regulating a cell’s acquisition of a pluripotent state (Brons et al., 2007; Takahashi and Yamanaka, 2006; Tesar et al., 2007). However, we still have only a primitive knowledge of the molecular mechanisms controlling the acquisition and maintenance of pluripotency. Equally unclear is how distinct pluripotent states relate to one another as well as to cells in vivo in the developing embryo.
The archetypal pluripotent stem cells, mouse ES cells, are routinely derived from pre-implantation morula and blastocyst stage embryos (Brook and Gardner, 1997; Evans and Kaufman, 1981; Martin, 1981; Tesar, 2005). These cells can be expanded indefinitely in culture while maintaining a stable genome and an undifferentiated state. Mouse ES cells are remarkably able to differentiate into all cell types of the embryo proper both in vitro and in chimeras in vivo. Mouse ES cells, however, do not directly recapitulate the molecular or signaling properties of their tissue of origin and instead rely on some genes and signaling pathways not expressed or required in vivo in the blastocyst including genes such as Nr3b2/Esrrb and Nr0b1/Dax1 and the LIF/Jak/Stat signaling pathway (Chenoweth et al., 2010; Ivanova et al., 2006; Niakan et al., 2006; Niwa et al., 1998; Niwa et al., 2009; Wang et al., 2006; Yu et al., 1998; Zhang et al., 2008). While mouse ES cells have been widely employed in laboratories across the world, their developmental origin and properties still remain unclear.
EpiSCs, on the other hand, are routinely isolated from the epiblast of early post-implantation rodent embryos and recapitulate the defining properties of their in vivo tissue of origin (Bao et al., 2009; Brons et al., 2007; Guo et al., 2009; Tesar et al., 2007). These cells are pluripotent as they are impressively capable of differentiating into cell types of all three embryonic germ layers as well as the germ lineage in vitro (Aoki et al., 2009; Hayashi and Surani, 2009; Tesar et al., 2007; Vallier et al., 2009). EpiSCs can be expanded indefinitely in culture being maintained in an undifferentiated state by activin/Nodal and FGF signaling pathways (Brons et al., 2007; Guo et al., 2009; Tesar et al., 2007). EpiSCs are regulated by a stable pluripotent state that is distinct from that of pre-implantation derived mouse ES cells.
Similar to mouse ES cells, human ES cells are derived from pre-implantation embryos (Thomson et al., 1998). Due to their common origin, the pluripotent state of mouse and human ES cells was generally thought to be identical. We previously showed that human ES cells are distinct from mouse ES cells and, paradoxically, share defining features with the post-implantation epiblast state (Tesar et al., 2007). Unfortunately the molecular and cellular events that promote ES cell derivation remain poorly described. It remains unclear why pluripotent cell lines isolated from explanted mouse pre-implantation embryos can attain a mouse ES cell state, while pluripotent cell lines isolated from explanted human pre-implantation embryos attain a state similar to that of the post-implantation epiblast.
To address these issues, we analyzed the impact of extrinsic and intrinsic mechanisms on the acquisition of in vitro pluripotent states when starting from pre-implantation mouse embryos. Our results show that both the mouse ES cell and EpiSC states can be derived from the pre-implantation blastocyst stage. The acquisition of these states is dependent on extrinsic signals in the culture medium as well as strain-intrinsic genetic elements.
RESULTS & DISCUSSION
Epiblast stem cells can be derived from pre-implantation mouse embryos
The pre-implantation blastocyst stage embryo consists of two overtly distinct tissues types, the trophectoderm and the inner cell mass (ICM) (Gardner and Rossant, 1979; Rossant et al., 1978). The ICM contains precursors that will generate the epiblast which, after implantation, will differentiate into all of the somatic and germ cell lineages of the embryo proper (Lawson et al., 1991). The ICM of the blastocyst has therefore served as a source of cells for the in vitro derivation of pluripotent ES cells both from mice and man (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998). Unexpectedly, blastocyst-derived mouse ES cells exhibit a mix of molecular features consistent with the epiblast precursors in the ICM and early germ cell state in vivo, while blastocyst-derived human ES cells share defining features with the post-implantation epiblast state. These results suggest that the blastocyst may serve as a source for multiple, distinct pluripotent states.
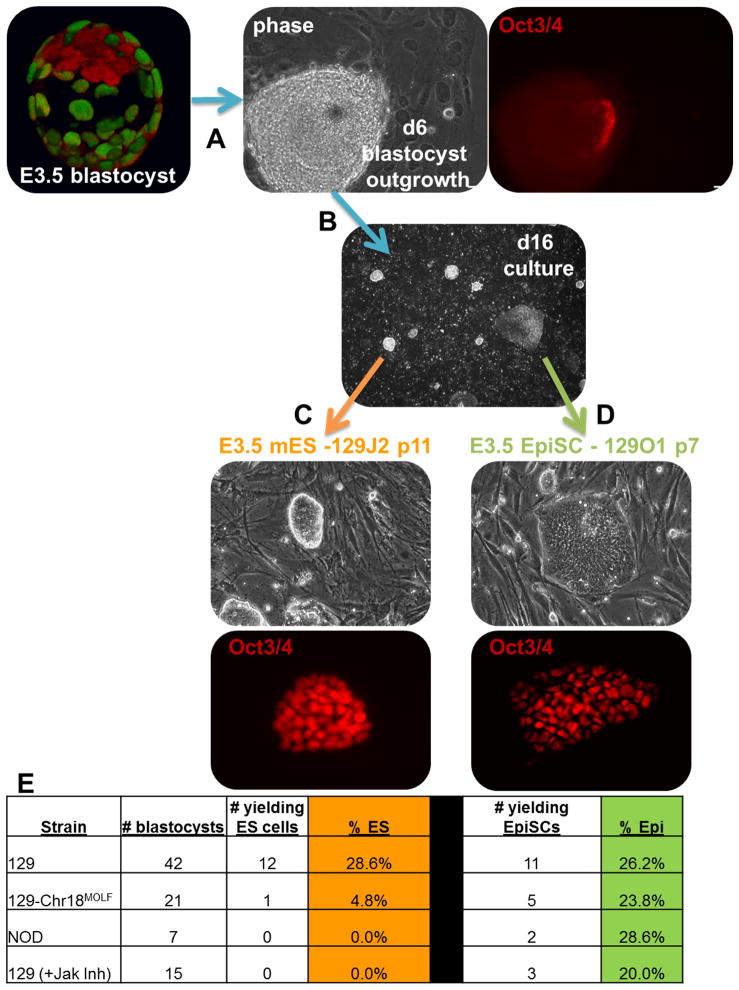
We modified the classical mouse ES cell derivation conditions to test the ability of pre-implantation mouse blastocysts to directly generate EpiSCs, which are normally only derived from post-implantation mouse embryos. Strain 129 blastocysts at embryonic day 3.5 (E3.5) were explanted into separate wells onto a feeder layer in K15F5 medium (see methods) which was optimized to support both mouse ES-like and epiblast-like pluripotent states (Figure 1A). Embryos hatched from their zona pellucidae and attached to the feeder layer overnight. The ICM was allowed to outgrow for 6 days at which time it was dissociated into small clusters and replated. By day 16 two distinct colony types predominated in the cultures; mouse ES-like (domed) or EpiSC-like (flat) (Figure 1B). These colonies were easily distinguished from differentiating extraembryonic cells such as trophectoderm or extraembryonic endoderm. The origin of the mouse ES-like and EpiSC-like colonies appeared to diverge early as conversion of morphology was never observed. When domed colonies were picked and individually expanded by dissociation to a single cell suspension in mouse ES cell growth conditions, they could be readily propagated as mouse ES cell lines (Figure 1C). Flat colonies exposed to the same treatments failed to expand as they did not typically survive after single cell dissociation. We were, however, able to propagate the flat colonies by employing conditions developed for the growth of post-implantation derived EpiSCs and human ES cells (Figure 1D). In experiments where all media and feeder cells were identical and carefully controlled, strain 129 blastocysts (n=42) yielded 12 mouse ES cell lines (28%) and 11 EpiSC lines (26%) (Figure 1E). In only one instance were both an EpiSC line and a mouse ES cell line derived from the same blastocyst (shown in Fig. 1B). These results show that both mouse ES-like and epiblast-like pluripotent states can be derived from the pre-implantation blastocyst and maintained as stable cell lines.
Figure 1. Intrinsic and extrinsic factors contribute to the acquisition of distinct pluripotent states from pre-implantation mouse embryos.
To derive pluripotent stem cells, pre-implantation blastocyst stage embryos at E3.5 (shown as a 3D reconstruction immunostained for Oct3/4 (red) and Cdx2 (green)) were (A) explanted and grown for 6 days under specific conditions (see methods) at which point few cells remained positive for the Oct3/4. (B) Using passaging protocols described in this manuscript, both ‘flat’ and ‘domed’ colony morphologies were evident by day 16. (C) ‘Domed’ colonies gave rise to Oct3/4+ mouse ES cell lines while (D) ‘flat’ colonies produced Oct3/4+ EpiSCs. (E) The derivation efficiency for blastocyst-derived mouse ES cells and EpiSCs was 28% and 26% respectively in strain 129 but intrinsic genetic elements present in other strains as well as extrinsic modulation of signaling pathways altered the efficiencies.
Pre-and post-implantation derived EpiSCs share DNA methylation and gene expression signatures
Previous work has elucidated gene expression and epigenetic signatures that define and distinguish pluripotent states (Tesar et al., 2007). Core pluripotency genes such as Oct3/4, Nanog, and Sox2 are expressed in both mouse ES cell and EpiSC states while other, non-overlapping sets embody one or the other state. While global gene expression has proven to be a general indicator of pluripotent state, metastable fluctuations in the mouse ES cell pluripotent state often blur the boundaries (Furusawa et al., 2004; Hayashi et al., 2008). For example, Dppa3, which is not expressed in EpiSCs or human ES cells, is considered a defining marker of the mouse ES cell state but its expression is heterogeneous in mouse ES cell cultures. It is thought that the Dppa3-negative mouse ES cells are more ‘epiblast-like’ but have not been stably committed to this transition since they readily revert to Dppa3-positive. These metastable fluctuations highlight that gene expression patterns are not sufficient to distinguish or define the two pluripotent states. Previous studies have established that global epigenetic differences exist between these two pluripotent states (Hayashi et al., 2008; Tesar et al., 2007). These marks are stable and can be reliably used to ascertain cell state identity. For example, Dppa3 hypermethylation is a defining mark of the EpiSC state, where, regardless of transcriptional status, Dppa3 is hypomethylated in mouse ES cells (Hayashi et al., 2008).
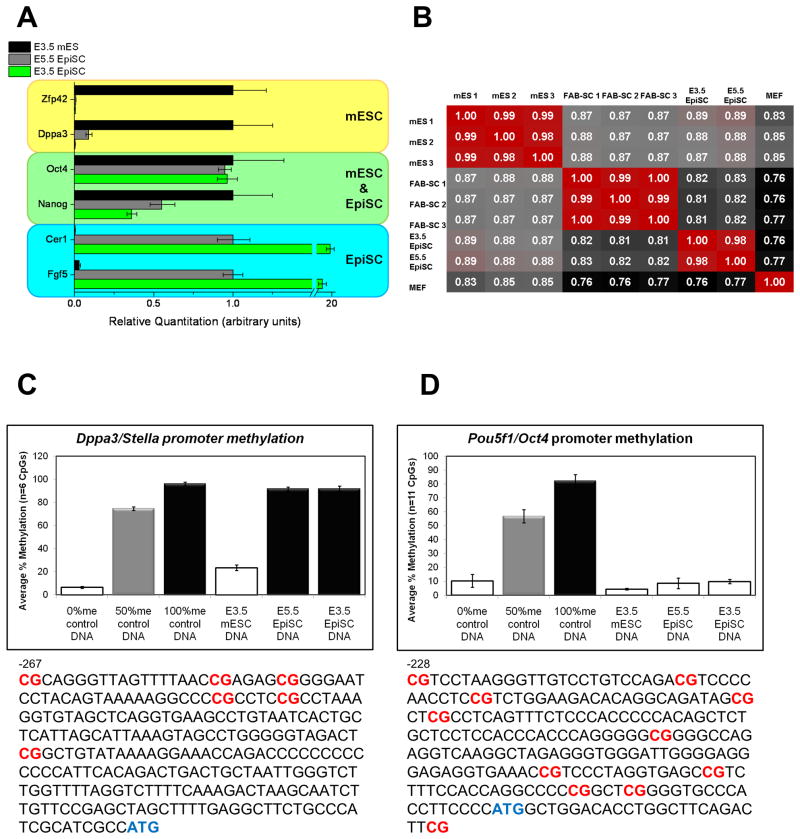
We tested whether pre-implantation derived EpiSCs (E3.5) shared molecular features with pre-implantation derived mouse ES cells (E3.5) or post-implantation derived EpiSCs (E5.5). Using quantitative RT-PCR we tested the expression of core pluripotency genes as well as those previously described to define the mouse ES cell or EpiSC state (Tesar et al., 2007). Pre-implantation-derived EpiSCs shared gene expression patterns consistent with post-implantation-derived EpiSCs and not mouse ES cells (Figure 2A). Using Agilent microarrays, we further analyzed global gene expression patterns. Pre-implantation-derived EpiSCs showed high correlation with post-implantation-derived EpiSCs and a clear distinction from mouse ES cells and other blastocyst-derived stem cells such as FAB-SCs (Figure 2B). To confirm these defining expression patterns, we analyzed DNA methylation at the promoter region of Oct3/4, a core pluripotency gene, and Dppa3, whose methylation pattern provides one of the most reliable markers to differentiate between the mouse ES cell and EpiSC states. Bisulfite pyrosequencing revealed that the Oct3/4 promoter was hypomethylated in pre-implantation-derived EpiSCs consistent with all other known pluripotent cells (Figure 2C). Dppa3 however was hypermethylated in both pre- and post-implantation-derived EpiSCs while being hypomethylated in mouse ES cells (Figure 2D). These results show that pre-implantation derived EpiSCs share molecular features with post-implantation derived EpiSCs.
Figure 2. Pre- and post-implantation-derived EpiSCs share gene expression and DNA methylation patterns that are distinct from other pluripotent states.
(A) Quantitative RT-PCR of pre-implantation-derived EpiSCs (E3.5) showed gene expression patterns consistent with post-implantation derived EpiSCs (E5.5) and not pre-implantation derived mouse ES cells (E3.5). (B) Correlations of whole genome microarray data showed a strong correlation between pre- and post-implantation derived EpiSCs and their clear distinction from other pluripotent cells such as FAB-SCs and mouse ES cells. Bisulfite pyrosequencing of pre-implantation-derived EpiSCs (E3.5) reveled hypermethylation of CpGs in the (C) Dppa3 promoter region and hypomethylation in (D) Oct3/4 promoter region. The location of analyzed CpGs is indicated in red.
Pre-and post-implantation derived EpiSCs utilize similar mechanisms to regulate pluripotency and differentiation
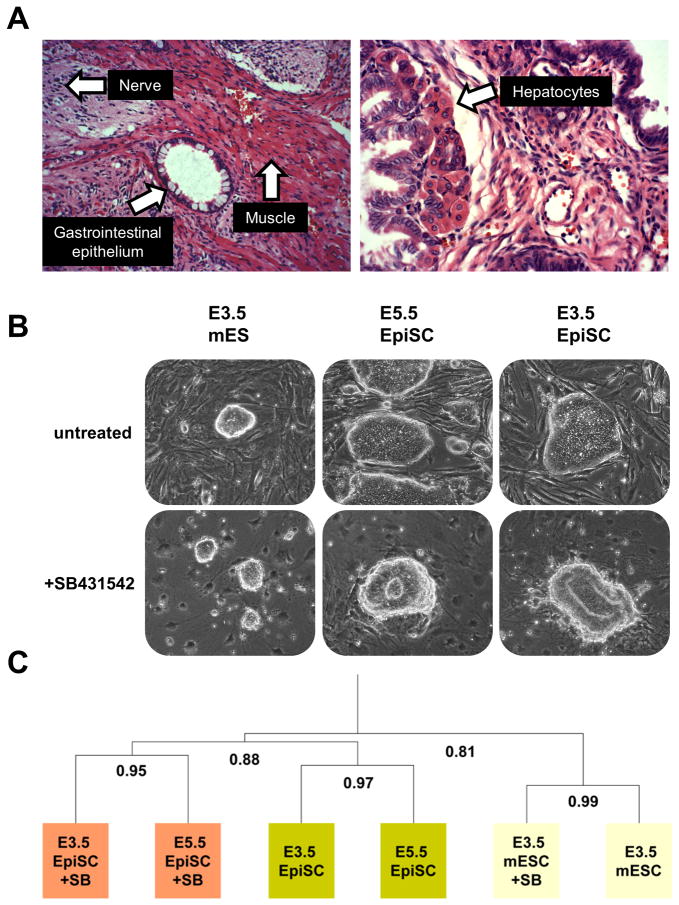
While the expression of specific genes provides a good indicator of cellular state, analysis of the functional regulation of pluripotency affords a better indicator. We first utilized a standard assay of pluripotency by injecting pre-implantation-derived EpiSCs subcutaneously into immunocompromised mice. Pre-implantation-derived EpiSCs readily formed teratomas consisting of a mixture of cells representing all three classical germ layers. Extensive differentiation was evident and included neural rosettes, ganglion cells, gastrointestinal epithelium, hepatocytes, muscle, and adipocytes among others (Figures 3A and S1). These results show that pre-implantation-derived EpiSCs are pluripotent.
Figure 3. Pre- and post-implantation-derived EpiSCs utilize similar & developmentally-relevant mechanisms to regulate pluripotency and differentiation.
(A) Pre-implantation-derived EpiSCs are pluripotent as evidenced by haematoxylin and eosin stained histological sections of teratomas. Examples of specific cell types are denoted with an arrow. Additional images are found in Figure S1. Both pre- (E3.5) and post- (E5.5) implantation derived EpiSCs exited pluripotency and differentiated into neural rosettes in response to Alk-4/5/7 inhibition while mouse ES cells maintained their pluripotency as evidenced by (B) morphology and (C) hierarchical cluster analysis of whole genome microarray data of treated and untreated samples.
We previously identified the regulation of activin/Nodal signaling as a distinguishing characteristic of the mouse ES cell and EpiSC states (Tesar et al., 2007). EpiSCs rely on activin/Nodal signaling to maintain the pluripotent state and when inhibited rapidly differentiate into the neurectodermal lineage. In sharp contrast, mouse ES cells do not rely on activin/Nodal signaling to maintain pluripotency and its inhibition does not result in differentiation. We tested the functional regulation of activin/Nodal signaling in pre-implantation derived EpiSCs. In response to treatment with a small molecule inhibitor of Alk-4/5/7 (SB431542) to effectively block activin/Nodal signaling, pre-implantation-derived EpiSCs rapidly down regulated core pluripotency genes, up regulated genes specific to the neurectodermal lineage, and formed neural rosettes by day 4 (Figure 3B). This response was indistinguishable from that of post-implantation-derived EpiSCs. Further analysis of global gene expression changes in response to activin/Nodal inhibition confirmed a high correlation of pre- and post-implantation-derived EpiSC lines in both the undifferentiated and differentiating states (Figure 3C). These results show that pre-implantation-derived EpiSCs are molecularly and functionally equivalent to post-implantation-derived EpiSCs.
Intrinsic and extrinsic factors regulate the acquisition of distinct pluripotent states from pre-implantation embryos
Previous studies demonstrated that the efficiency of deriving mouse ES cells is dependent on genetic background (Brook et al., 2003; Brook and Gardner, 1997; Gardner and Brook, 1997). Under standard conditions, one of the only mouse strains to yield ES cell lines with an appreciable frequency is the inbred strain 129. Interestingly, and probably not coincidentally, strain 129 is one of the only mouse strains to show a significant and heritable frequency of pluripotent, testicular germ cell tumors (Stevens and Little, 1954). Most other mouse strains are refractory to both the acquisition of the pluripotent mouse ES cell state in vitro under standard conditions and the acquisition of pluripotent tumors within the germline. Recently, mouse ES cells were reproducibly derived for the first time from the prototypical ‘non-permissive’ non-obese diabetic (NOD) strain blastocysts and through iPS cell-based reprogramming from fibroblasts (Hanna et al., 2009; Nichols et al., 2009). While providing an exciting opportunity to utilize the power of mouse ES cells (gene targeting, chimera generation, etc) in this widely employed model, these new NOD mouse ES cells proved to be highly unstable in that they required continual stimulation of c-myc or Klf4 through inducible transgene over-expression or other exogenous means.
Conversely, the isolation of EpiSCs from post-implantation mouse embryos is not dependent on strain-specific modulators. EpiSCs have been derived from a plethora of mouse strains under standard conditions. We tested the ability to isolate EpiSCs from pre-implantation embryos of strains which do not readily yield mouse ES cell lines such as NOD and the consomic strain 129-Chr18MOLF in which the MOLF/EiJ strain chromosome 18 homozygously replaced the strain 129 chromosome 18 on an inbred 129 background (Anderson et al., 2009). Interestingly, while failing to yield mouse ES cell lines at any appreciable frequency, NOD and 129-Chr18MOLF blastocysts yielded EpiSCs at a frequency equivalent to strain 129, ~25% (Figure 1E). These results show that strain-specific genetic elements restricting access to the mouse ES cell pluripotent state do not impact access to the EpiSC state and suggest that intrinsic genetic elements in a limited number of strains such as 129 modulate entry or stabilization of the mouse ES cell state.
External signals could also modulate access to distinct pluripotent states. In fact, recent work has shown that inhibition of both MAPK and GSK3β (referred to as 2i) and activation of the Jak/Stat3 pathway via LIF can enhance the efficiency of mouse ES cell line derivation and allow for derivation in some previously non-permissive strains (Ying et al., 2008). We tested whether inhibition of Jak/Stat signaling, a pathway required for mouse ES cells but not EpiSCs, would alter the ability to isolate either pluripotent state. Inhibition of Jak/Stat signaling with a small molecule inhibitor of Jak resulted in a failure of strain 129 blastocysts to yield mouse ES cell lines while EpiSC lines could be readily derived (Figure 1E). These results show that extrinsic cues can modulate the acquisition of distinct pluripotent states. Additionally, these results raise interesting questions about the lineage relationship of distinct pluripotent cell types and renews interest in the possibility that mouse ES cells could be of germ cell origin (Zwaka and Thomson, 2005) (see model in Figure S2). Understanding of both intrinsic and extrinsic modifiers will provide critical insight into the acquisition of pluripotency (see Figure 4). In fact, a recent study has shown that simple modulation of the extrinsic signaling conditions determines whether over-expression of Oct3/4, Sox2, Klf4, and c-myc gives rise to either iPS cells or induced EpiSCs (iEpiSCs) (Han et al., 2010). Further knowledge should advance efforts to create defined pluripotent cell lines, whether from embryos, somatic cells, or germ cells, and reliably control their differentiation to functional derivatives for laboratory and clinical use.
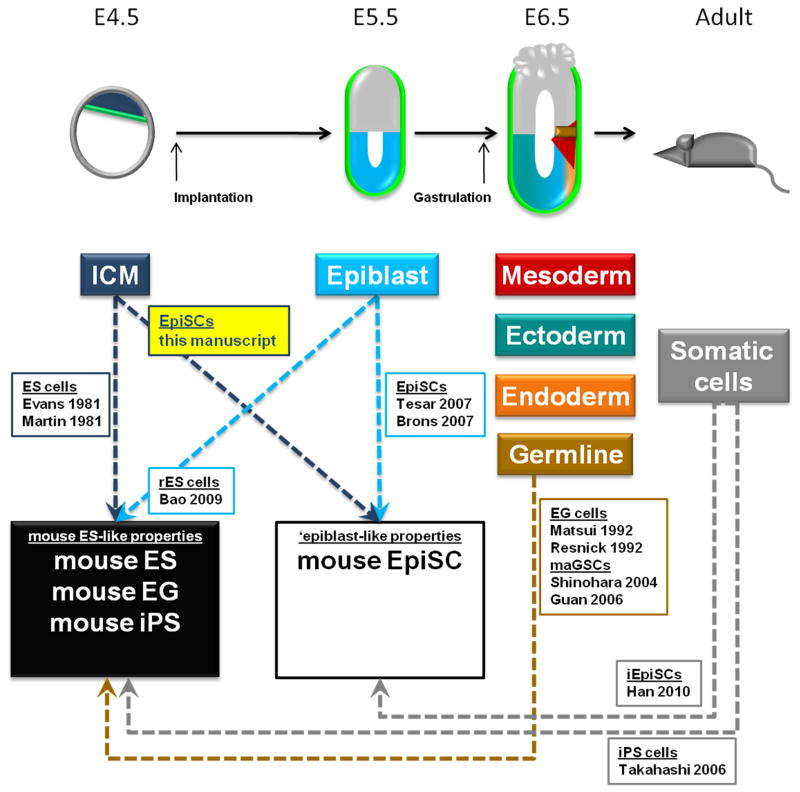
Figure 4. Acquisition of distinct pluripotent states.
Summary diagram depicting the multiple avenues which have provided access to the two pluripotent states (mouse ES-like and epiblast-like) and highlighting the contribution of this manuscript to the field. See also Figure S2.
EXPERIMENTAL PROCEDURES
Embryos
Timed natural matings were used for all experiments. Twelve hours from the middle of the dark period was termed embryonic day 0.5 (E0.5). Mouse embryonic fibroblasts (MEFs) were obtained from E13.5 fetuses from the CF1 strain (Charles River). Pre-implantation blastocyst stage embryos from either 129S1/SvImJ (Jackson Laboratories), 129-Chr18MOLF (Anderson et al., 2009), or non-obese diabetic (NOD) strains were flushed from the uterine horns on E3.5 into FHM Hepes-buffered medium (Millipore).
Derivation of mouse ES cells and EpiSCs from blastocysts
Individual blastocysts were explanted into 1.9cm2 wells containing γ-irradiated MEFs pre-incubated with K15F5 medium which consisted of Knockout DMEM (Invitrogen) supplemented with 15% Knockout Serum Replacement (KSR; Invitrogen), 5% ES cell-qualified fetal bovine serum (FBS; Gibco lot: 52207), 2mM Glutamax (Invitrogen), 1x non-essential amino acids (Invitrogen), and 0.1mM 2-mercaptoethanol (Sigma) with no additional growth factor additives. After 5–6 days the ICMs had reached a suitable size and were picked off the plate with a pulled glass pipette and incubated for 5 minutes at room temperature in an 8μl drop of 0.25% trypsin-EDTA. The ICMs were partially dissociated individually with a pulled glass pipette. Caution was taken not to produce a completely single cell suspension. The partial dissociates were plated individually into a 1.9cm2 well containing a feeder layer of irradiated MEFs and cultured for an additional 6 days in K15F5 medium without additional growth factor additives. At this point each culture was passaged by a brief exposure (2–3 minutes) to 0.25% trypsin/EDTA, inactivation of trypsin with FBS-containing MEF medium, gentle tritration to prevent complete single cell dissociation of any pluripotent clusters, and plating into a 9.6cm2 well containing feeders in K15F5 medium. Morphologically distinct mouse ES cell and/or EpiSC colonies became evident over the next 4–8 days (16–20 total days from initial blastocyst explants). These colony types were separately expanded in medium designed to support either mouse ES cells or EpiSCs. Mouse ES cell colonies were individually picked from the plate and dissociated to a single cell suspension in 8μl drops of trypsin/EDTA and plated into 1.9cm2 wells containing feeders in K15F5 medium supplemented with 103 units/ml leukemia inhibitory factor (LIF; Millipore). EpiSC colonies were manually dissociated into small clusters using a glass needle and plated into 1.9cm2 wells containing feeders in EpiSC cell medium consisting of Knockout DMEM supplemented with 20% KSR, 2mM glutamax, 1x non-essential amino acids, 0.1mM 2-mercaptoethanol, and 10ng/ml FGF2 (R&D Systems). For experiments testing the impact of Jak inhibition on pluripotent stem cell derivation, Jak Inhibitor I (Calbiochem) was provided to the cultures starting at day 0 (blastocyst explant) and added daily until colony picking (day 16–20).
Cell culture
Mouse ES cells were passaged every third day as a single cell suspension using 0.25% trypsin/EDTA and seeded at 3.0×104cells/cm2 for routine culture. EpiSCs were passaged every third day using 1.5mg/ml collagenase type IV (Invitrogen) and trituration into small clumps of ~10–100 cells. MEFs were maintained with DMEM supplemented with 10% FBS, 2mM glutamax, 1x non-essential amino acids, and 0.1mM 2-mercaptoethanol. MEFs were irradiated with 30–60 gray and seeded as feeders at a density of 5.0×104 cells/cm2. All cells were grown on Nunclon Δ-treated dishes or multiwell plates (Fisher Scientific) coated for 2 hours at 37°C with 0.1% (w/v) porcine gelatin (Sigma). Antibiotics at concentrations of 50 units/ml and 50 μg/ml for penicillin and streptomycin, respectively, were used only for primary explants of MEFs and blastocysts.
Small molecule treatment
SB431542 was maintained as a 20mM stock solution in DMSO (vehicle) and was provided at 20uM to the cultures at the time of plating and every day thereafter with the media change. JAK inhibitor I (Calbiochem) was maintained as a 10mM stock in DMSO and provided at 0.6uM every day.
Immunostaining
Cells were prepared for immunostaining by fixation in 4% paraformaldehyde (Electron Microscopy Sciences) for 15 minutes and subsequent permeabilization for 10 minutes with 0.2% Triton-X in PBS. Cells were then blocked for non-specific binding with 10% normal goat or donkey serum (Abcam) in PBS for 1–2 hours at room temperature. Primary antibodies were diluted in blocking solution and incubated with the samples overnight at 4°C. Samples were rinsed with PBS and incubated with the appropriate fluorescently labeled Alexa secondary antibodies (Invitrogen, 1:500) for 1 hour at room temperature. Primary antibodies used were: mouse monoclonal IgG2b Oct3/4 (C-10; Santa Cruz, 1:400), mouse monoclonal IgG1 Cdx2 (CDX2-88; Biogenex, 1:50), and rabbit polyclonal Blimp1 (kindly provided by Reuben Tooze, Leeds, UK, 1:100). Nuclei were visualized with DAPI (Sigma, 1μg/ml).
Bisulphite pyrosequencing
Genomic DNA was isolated from pre-implantation-derived mES cells, pre-implantation-derived EpiSCs, and post-implantation-derived EpiSCs after their separation from the feeders. Bisulfite modification of 500ng of genomic DNA was done using the EZ DNA Methylation kit (Zymo Research). Bisulfite converted DNA was used with gene specific primers (Epigendx) to amplify promoter regions of Pou5f1 (ASY585; 11 CpGs sites analyzed) and Dppa3 (ADS1399; 6 CpG sites analyzed) using HotStar Taq polymerase (Qiagen). Methylation of the amplified regions was quantified by pyrosequencing using a PyroMark Q96 ID platform (Qiagen). Unmethylated and in vitro methylated DNA (Epigendx) served as controls. Percentage of methylation was determined by the percentage of C to T conversion at the analyzed CpG sites.
RNA preparation and qRT-PCR
Total RNA was prepared using TRIzol (Invitrogen) and samples were treated with DNase (turbo DNA-free, Applied Biosystems). 400ng of RNA was reverse transcribed using Superscript III (Invitrogen) with random primers. Real-Time PCR was performed using 8ng of cDNA according to the manufacturer’s protocol using the Applied Biosystems (ABI) 7300 Real-Time PCR System with the ABI TaqMan Gene Expression Master Mix and Taqman pre-optimized gene expression assays: Zfp42 (Mm01194090_g1), Dppa3 (Mm01184198_g1), Oct3/4 (Mm00658129_gH), Nanog (Mm02019550_s1), Cer1 (Mm00515474_m1), FGF5 (Mm00438919_m1). Gapdh (Mm99999915_g1) served as an endogenous control for normalization.
Teratomas
Blastocyst-derived EpiSCs were tested for their ability to form teratomas in immunocompromised hosts. Colonies were triturated to produce small cell clusters and cells were resuspended in Knockout DMEM supplemented with 30% growth factor reduced Matrigel (BD Biosciences) at a concentration of 1×107 cells/ml. 100μl of cell suspension (~1×106 cells) was injected subcutaneously into the left flank of female, athymic NCr-nu/nu mice (bred in house; n=4 per line). Tumors were allowed to develop for 4–8 weeks at which time they were removed and fixed in 4% paraformaldehyde for 36 hours. Teratomas were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
Expression microarrays
RNA samples were isolated and analyzed using whole genome Agilent 4×44K Mouse arrays (G4122F) as described previously (Tesar et al., 2007). Publically available samples were downloaded from GEO (NCBI) and used as comparators in Figures 2. Hierarchical clustering and correlation analysis of samples was performed using Genespring software. All data are available on the NCBI Gene Expression Omnibus website through GEO Series accession GSE26814.
Supplementary Material
Acknowledgments
This research was supported by funding from the Case Western Reserve University School of Medicine, the Center for Stem Cell and Regenerative Medicine, the Athymic Animal and Radiation Resources Core facilities of the Case Comprehensive Cancer Center (P30 CA43703), and the NIH/NINDS intramural research program. PJT is a Robertson Investigator of the New York Stem Cell Foundation and a Mt. Sinai Health Care Foundation Scholar. We are grateful to Leslie Cooperman as well as members of the P. Scacheri laboratory for technical assistance and provision of reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson PD, Nelson VR, Tesar PJ, Nadeau JH. Genetic factors on mouse chromosome 18 affecting susceptibility to testicular germ cell tumors and permissiveness to embryonic stem cell derivation. Cancer Res. 2009;69:9112–9117. doi: 10.1158/0008-5472.CAN-09-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Hara A, Niwa M, Yamada Y, Kunisada T. In vitro and in vivo differentiation of human embryonic stem cells into retina-like organs and comparison with that from mouse pluripotent epiblast stem cells. Dev Dyn. 2009;238:2266–2279. doi: 10.1002/dvdy.22008. [DOI] [PubMed] [Google Scholar]
- Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Brook FA, Evans EP, Lord CJ, Lyons PA, Rainbow DB, Howlett SK, Wicker LS, Todd JA, Gardner RL. The Derivation of Highly Germline-Competent Embryonic Stem Cells Containing NOD-Derived Genome. Diabetes. 2003;52:205–208. doi: 10.2337/diabetes.52.1.205. [DOI] [PubMed] [Google Scholar]
- Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. PNAS. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth JG, McKay RD, Tesar PJ. Epiblast stem cells contribute new insight into pluripotency and gastrulation. Dev Growth Differ. 2010;52:293–301. doi: 10.1111/j.1440-169X.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Furusawa T, Ohkoshi K, Honda C, Takahashi S, Tokunaga T. Embryonic stem cells expressing both platelet endothelial cell adhesion molecule-1 and stage-specific embryonic antigen-1 differentiate predominantly into epiblast cells in a chimeric embryo. Biol Reprod. 2004;70:1452–1457. doi: 10.1095/biolreprod.103.024190. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Brook FA. Reflections on the biology of embryonic stem (ES) cells. Int J Dev Biol. 1997;41:235–243. [PubMed] [Google Scholar]
- Gardner RL, Rossant J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979;52:141–152. [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Greber B, Wu G, Tapia N, Arauzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Scholer HR. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 2010 doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Mitalipova M, Cheng AW, Cassady JP, Staerk J, Carey BW, Lengner CJ, Foreman R, Love J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Lopes SM, Tang F, Surani MA. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Surani MA. Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development. 2009;136:3549–3556. doi: 10.1242/dev.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niakan KK, Davis EC, Clipsham RC, Jiang M, Dehart DB, Sulik KK, McCabe ER. Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab. 2006;88:261–271. doi: 10.1016/j.ymgme.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Nichols J, Jones K, Phillips JM, Newland SA, Roode M, Mansfield W, Smith A, Cooke A. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med. 2009;15:814–818. doi: 10.1038/nm.1996. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- Rossant J, Gardner RL, Alexandre HL. Investigation of the potency of cells from the postimplantation mouse embryo by blastocyst injection: a preliminary report. J Embryol Exp Morphol. 1978;48:239–247. [PubMed] [Google Scholar]
- Stevens LC, Little CC. Spontaneous Testicular Teratomas in an Inbred Strain of Mice. Proc Natl Acad Sci U S A. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tesar PJ. Derivation of germ-line-competent embryonic stem cell lines from preblastocyst mouse embryos. Proc Natl Acad Sci U S A. 2005;102:8239–8244. doi: 10.1073/pnas.0503231102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vallier L, Touboul T, Chng Z, Brimpari M, Hannan N, Millan E, Smithers LE, Trotter M, Rugg-Gunn P, Weber A, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2008;283:35825–35833. doi: 10.1074/jbc.M803481200. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.