Abstract
Plasmalogens (Plg) are phospholipids containing vinyl ether linkage at the sn-1 position of the glycerophospholipid backbone. In spite of being quite abundant in humans, the biological role of plasmalogens remains speculative. It has been postulated that plasmalogens are physiological antioxidants with the vinyl ether functionality serving as sacrificial trap for free radicals and singlet oxygen. However, no quantitative data on the efficiency of plasmalogens to scavenge these reactive species are available. In this study, rate constants of quenching of singlet oxygen, generated by photosensitized energy transfer, by several plasmalogens and, for comparison, by their diacyl analogs, were determined by time-resolved detection of phosphorescence at 1270 nm. Relative rates of the interaction of singlet oxygen, with plasmalogens and other lipids in solution and liposomal membranes were measured by electron paramagnetic resonance oximetry and product analysis, employing HPLC-EC detection of cholesterol hydroperoxides and iodometric assay of lipid hydroperoxides. Results show that singlet oxygen interacts with plasmalogens significantly faster than with the other lipids, with he corresponding rate constants being by one-two orders of magnitude greater. The quenching of singlet oxygen by plasmalogens is mostly reactive in nature and results from its preferential interaction with the vinyl ether bond. The data suggest that plasmalogens could protect unsaturated membrane lipids against oxidation induced by singlet oxygen, providing that the oxidation products are not excessively cytotoxic.
Keywords: plasmalogens, plasmenylcholine, singlet oxygen quenching, singlet oxygen phosphorescence, lipid peroxidation, EPR-oximetry, cholesterol hydroperoxides
INTRODUCTION
Plasmalogens (Plg) are widely distributed mammalian phospholipids that contain a Z-1’-alkenyl-ether linkage at the sn-1 position of the glycerophospholipid backbone [1]. They comprise as much as 18% of the total phospholipid mass in humans and are particularly abundant in the brain and cardiac tissue [2]. In spite of their prevalent distribution in mammalian tissues, the biological functions of Plg remain unclear. Nevertheless, it is known that a group of inherited diseases with an impaired function or absence of peroxisomes is correlated with a reduced level of cellular Plg. Thus in fibroblasts from the Zellweger cerebro-hepato-renal syndrome (CHRS) patients, reduction of Plg levels varies between 60 and 80%, and in fibroblasts from the rhizomelic form of chondrodysplasia punctata (RCDP), the level of plasmalogens can be reduced by 90% [3] A selective deficiency of ethanolamine plasmalogen was identified in brain samples from patients with senile dementia [4] or in Alzheimer disease [5]. Plg are believed to play a crucial role in ocular development and function [6]. Indeed a variety of ocular abnormalities have been observed in Plg deficiency [7].
It has been postulated that plasmalogens are involved in arachidonate storage and signal transduction [8, 9]. Plg could be responsible for unique membrane protein activities and formation of lipid raft microdomains [10]. The second main hypothesis, seemingly supported by a growing body of experimental evidence, suggests that Plg are physiological antioxidants, with the vinyl-ether bond serving as a sacrificial trap for reactive oxygen species that would otherwise attack the PUFA moieties at the nearby sn-2 position or in other lipid molecules [11–16]. Significantly, it was demonstrated that Plg-deficient mutants, derived from macrophage-like cell line (RAW 264.7), exhibited hypersensitivity to chemical hypoxia [17]. The mutants were also much more susceptible to cytotoxicity induced by rose Bengal-photosensitized reactions. Restoration of the Plg level resulted in wild-type-like resistance to chemical hypoxia and oxidative stress. Although the vinyl-ether bond at the sn-1 position is relatively resistant to enzymatic degradation, it is quite susceptible to oxidation induced by a variety of oxidizing reagents [18–21]. As a result, plasmalogens undergo oxidative decomposition more readily than their fatty acid ester analogs [22]. The interaction of the vinyl-ether bond with oxidizing radicals and singlet oxygen may be responsible, at least in part, for the postulated antioxidant action of plasmalogens. It was suggested that plasmalogens might interfere with the propagation step rather than initiation step of lipid peroxidation and, unlike α-tocopherol, plasmalogens did not induce a lag phase during peroxidation of lipids [15]. It appears that products of vinyl-ether oxidation do not effectively propagate the oxidation of polyunsaturated lipids [14], and plasmalogens, being present in the membrane bilayer, may reduce the total membrane oxidizability [16,23].
The product profile of plasmalogen oxidation strongly depends on the type of estrified fatty acids at the sn-1 and sn-2 positions, and the nature of oxidative stress initiators [24–26]. During Plg oxidation, hydrocarbon chains at both the sn-1 and sn-2 positions can undergo degradation. Free radical induced oxidation of plasmalogens yields sn-1-lyso-lipids, sn-1-formyl-lipids, chain-shortened ω-aldehydes, γ-hydroxy α–, β– unsaturated aldehydes [27].
The reactivity of singlet oxygen with enol-ether bonds, of relatively simple model compounds, was studied by product analysis and indirect measurements of singlet oxygen lifetime some thirty years ago [28–30]. The results can be summarized as follows: i. Enol-ethers quench singlet oxygen with the rate constant in the range: 104 − 4.2×107 M−1s−1, with the rate constant generally being higher in polar solvents compared to non-polar solvents. ii. In polar solvents, photooxidation of enol-ethers predominantly occurs via cycloaddition leading to the formation of dioxetanes. iii. The activation energy for the interaction of enol-ethers with singlet oxygen is very low, while the corresponding entropy change is higly negative, indiacting a rapid reversible formation of an exciplex, which then decays via the rate determining formation of oxidative products.
Products of zinc phthalocyanine photosensitized oxidation of palmitoyl plasmenylcholine in liposomes were determined by MALDI-MS, 1H-NMR and HPLC-EC [26]. Primary products included lysolipid species, along with its hydrolysis product, and an allylic hydroperoxide species. Secondary products were ascribed to degradation of allylic hydroperoxides either via Hock rearrangement or by alkenyl ether epoxidation. In spite of this extensive research effort, there are no reliable data on rate constants of the interaction of plasmalogens with singlet oxygen, and it is unclear whether the hydrocarbon chain in the sn-2 position affects the interaction of Plg with singlet oxygen and to what extent the interaction is modified by the microenvironment.
In this study, we determined rate constants of the interaction of singlet oxygen, generated by photosensitized energy transfer, with different plasmalogens and, for comparison, with their diacyl analogs, employing time-resolved detection of singlet oxygen phosphorescence at 1270 nm and electron paramagnetic resonance (EPR) oximetry. The kinetic measurements were supplemented by determination of lipid hydroperoxides using the iodometric assay and HPLC-EC for detection of cholesterol hydroperoxides with cholesterol employed as a unique mechanistic reporter molecule. To address the issue of how microenvironment affects the interaction of Plg with singlet oxygen, the measurements were carried out in different model systems – in homogeneous solutions of the lipids in organic solvents and organic solvent mixtures, in Triton X-100 micelles and in liposomal membranes.
MATERIALS AND METHODS
Reagents
Phospholipids (PlgPC 16:0/18:1, PlgPC 18:0/18:1, PlgPC 18:0/20:4, POPC, SOPC, SAPC, DMPC, DPPC) and cholesterol were purchased from Avanti Polar Lipids Inc., Alabaster, USA and were used without further purification. Saturated plasmenylcholine, 1-O-1'-(Z)-hexadecenyl-2-oleoyl-sn-glycero-3-phosphocholine was synthesized as described elsewhere [31]. Organic solvents were as follows: UvasolR carbon tetrachloride, LiChrosolvR isopropanol, LiChrosolvR methanol, LiChrosolvR acetonitrile from Merck, Darmstadt, Germany, carbon disulfide (Riedel-de-HaenR) from Sigma-Aldrich, Steinheim, Germany and chloroform from POCH, Gliwice, Poland. Photosensitisers – rose Bengal and merocyanine 540 were from Sigma-Aldrich, St.Louis, MO, USA, and tetraphenylporphyrin was from Aldrich Chem. Co., Milwaukee, WI, USA. 4-Protio-3-carbamoyl-2,2,5,5-tetraperdeuteromethyl-3-pyrroline-1-yloxy (mHCTPO) was a gift from Prof. H. J. Halpern (University of Chicago, Chicago, IL) and used as received.
Liposomes preparation
Lipid films composed of DMPC and additional lipid (PlgPC 16:0/16:0, PlgPC 18:0/18:1, SOPC, POPC or Chol) were dried from chloroform under the stream of nitrogen and left under vacuum until total evaporation of organic solvent. Hydration was done in pH 7.4 phosphate buffered solution A (PBSA) in a heating water bath at temperature over the main phase transition of DMPC. Afterwards, liposomes were pressed through 100 nm diameter extruder membrane to obtain large unilamellar vesicle suspension (LUV). All preparation steps were carried in darkness.
Micelles preparation
Lipids were solubilized in micelles using 2% w/v Triton-X 100 and PBSA (pH 7.4). Final concentration of lipids was 5 mM in molar ratio of 3:2 of DMPC and additional lipid (PlgPC 16:0/16:0, POPC or Chol).
EPR measurements
Photosensitized oxygen consumption was measured by EPR-oximetry employing mHCTPO as a spin probe and using Bruker EMEX-AA spectrometer (Bruker BioSpin, Germany). Spectral parameters of mHCTPO spin probe were calibrated for dissolved oxygen concentration in water solution at room temperature [32]. Micelles solution, in flat quartz cells placed in the EPR resonant cavity, was irradiated with green light derived from a Cermax PE300CE-13FM 300W lamp in air-cooled housing (Perkin Elmer, USA) using a combination of filters (5 cm of aqueous solution of 5 g/l CuSO4, green dichroic and a cut-off < 500 nm filter). Sample irradiance, measured by a calibrated photodiode (Hamamatsu, Photonics, K.K, Japan), was in the range 151–174 W/m2. In experiments with liposomes, sample irradiance was ~46 W/m2.
Singlet oxygen quenching measurements
To determine rate constants of the interaction of singlet oxygen with plasmalogens and other lipids, time-resolved singlet oxygen phosphorescence at 1270 nm was measured as a function of the quencher concentration. Under the experimental conditions employed, singlet oxygen was generated within a short time interval after pulse excitation of 2–3 µM tetraphenylporphyrin used as a photosensitizing dye. The experimental set up, described elsewhere [33], was as follows: a Q-switched Nd:YAG laser (Surelite II; Continuum, USA) was employed as the excitation source, generating 5-ns pulses of a second (532 nm) or a third (355 nm) harmonic. Singlet oxygen phosphorescence was detected with a nitrogen-cooled germanium detector (EO-8179P; North Coast Scientific Corporation, USA). Silicon cut-off filter, transmitted light above 1100 nm and an interference filter transmitted light around 1270 nm for cutting out stray light were used. A home-build sequence generator was used to trigger the sequence of events, and special custom-written software was used for data collection and analyses. Plasmalogens and their diacyl analog phospholipids were dissolved in carbon tetrachloride, carbon disulfide or selected mixtures of these solvents.
HPLC-EC detection of lipid hydroperoxides
HPLC-EC with controlled growth mercury drop electrode was used to detect cholesterol hydroperoxides, namely 7α/β-OOH, 6α-OOH, 6β-OOH and 5α-OOH [34]. Reduction potential was set to −150 mV. The aliquot of lipid extracts dissolved in isopropanol were loaded onto C-18 reverse phase column (Ultrasphere™ XL, Beckman, USA). HPLC high purity solvents were used for eluent mixture: 71% methanol, 11% acetonitrile, 8% isopropanol and 9% water. NaClO4 was used as an electrolyte. Eluent flow was set to 1.5 ml/min. The system was purged with argon during the measurements.
Determination of lipid hydroperoxides by the iodometric assay
Progress of photosensitised peroxidation of lipids was monitored by measuring accumulation of the corresponding lipid hydroperoxides using the iodometric test described elsewhere [35]. In brief, solution of 0.1 mM plasmenylcholine (PlgPC 16:0/16:0) or 2 mM cholesterol in carbon tetrachloride was irradiated with visible light (13.7 W/m2) in the presence of 5 µM tetraphenylporphyrin. Samples were gently stirred during irradiation and aliquots were taken for lipid hydroperoxides measurements at selected time intervals.
RESULTS
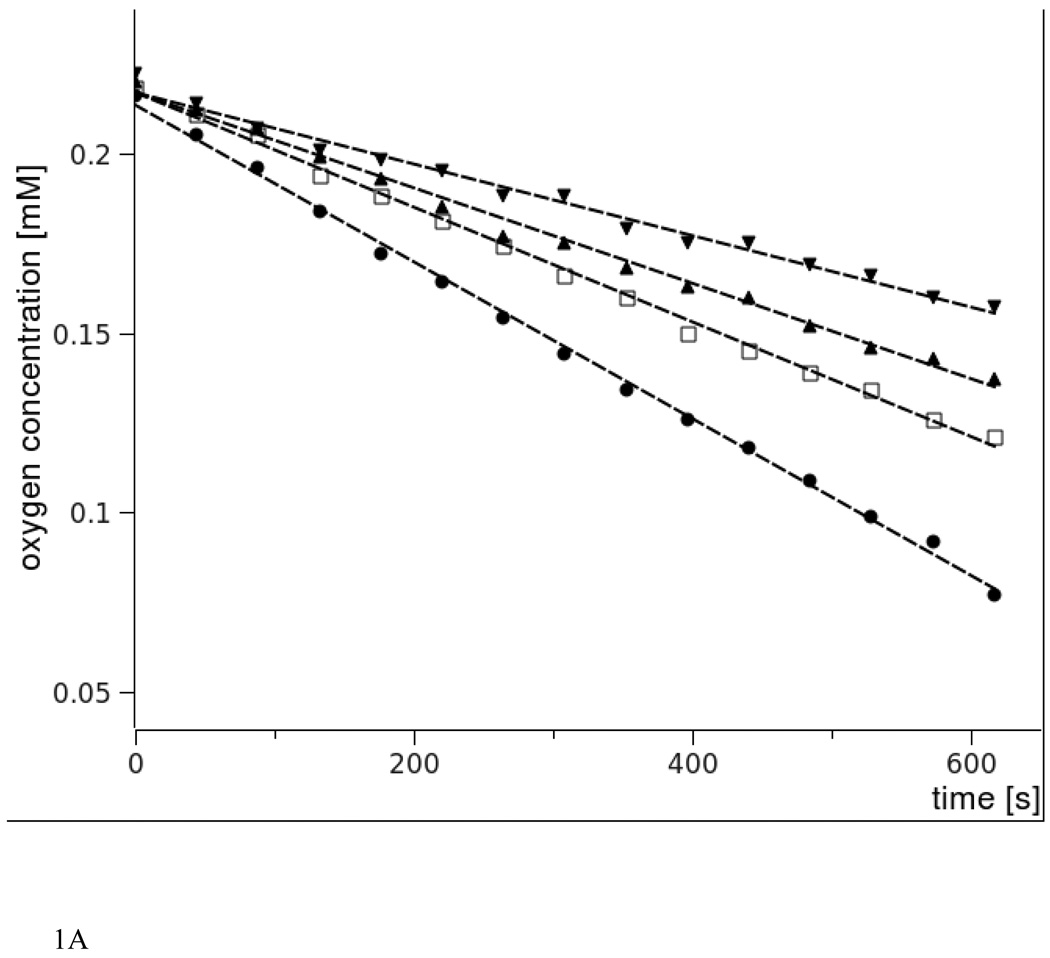
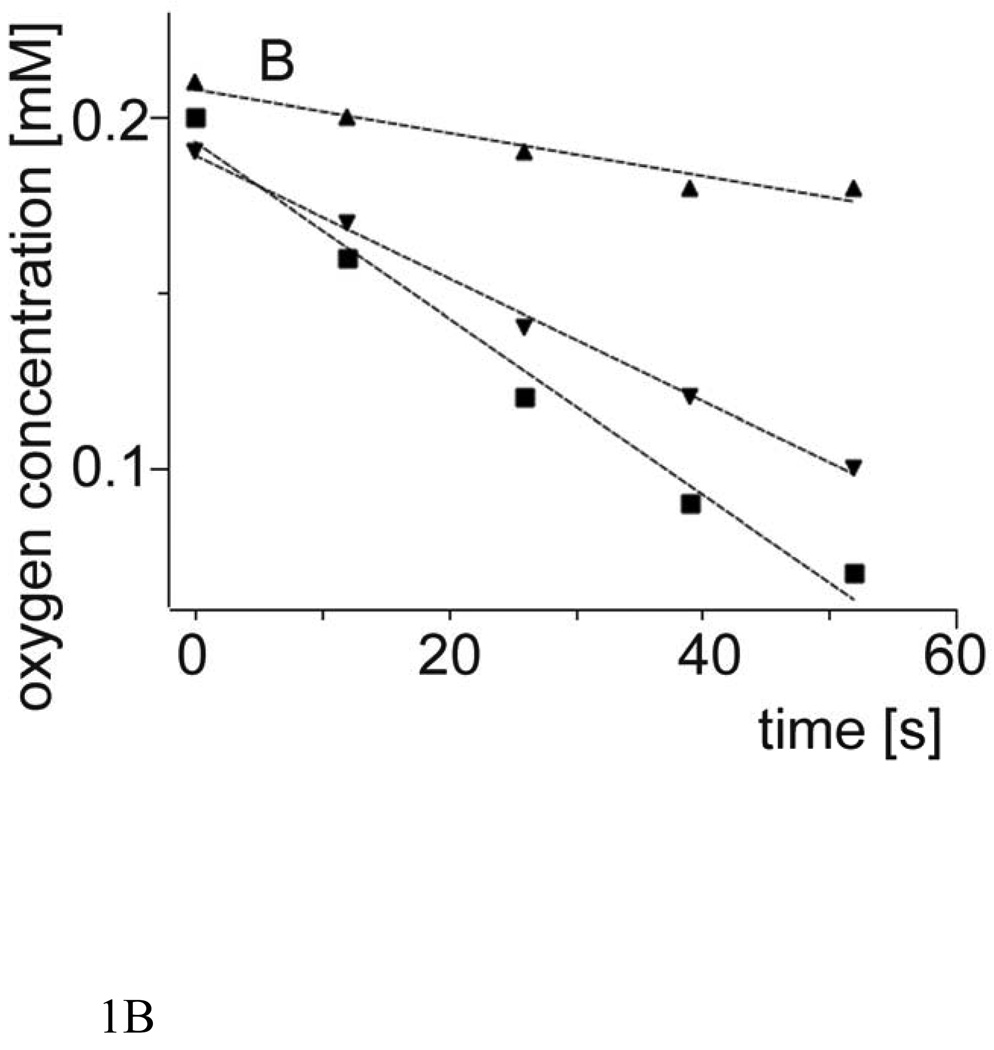
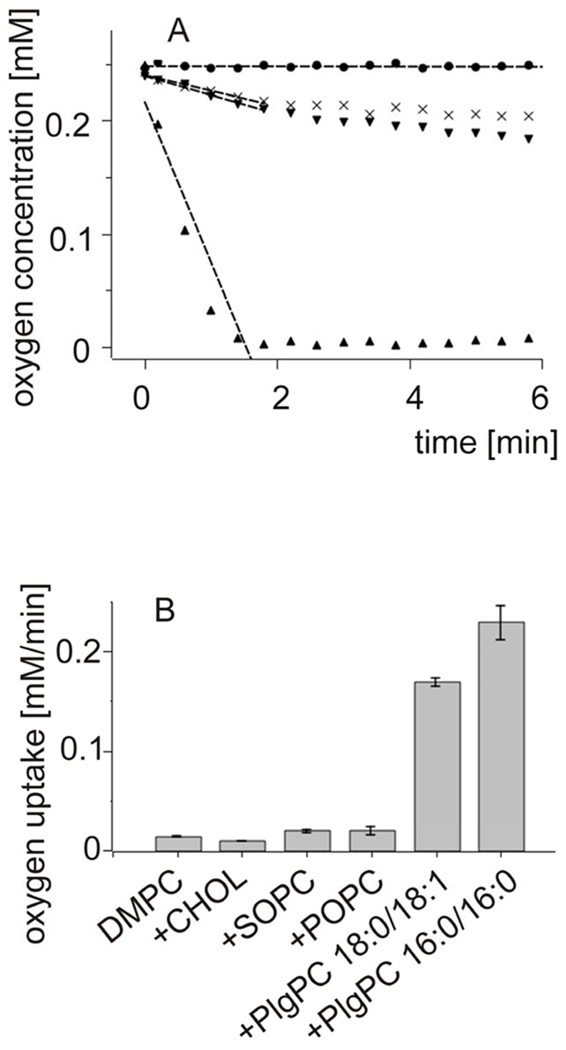
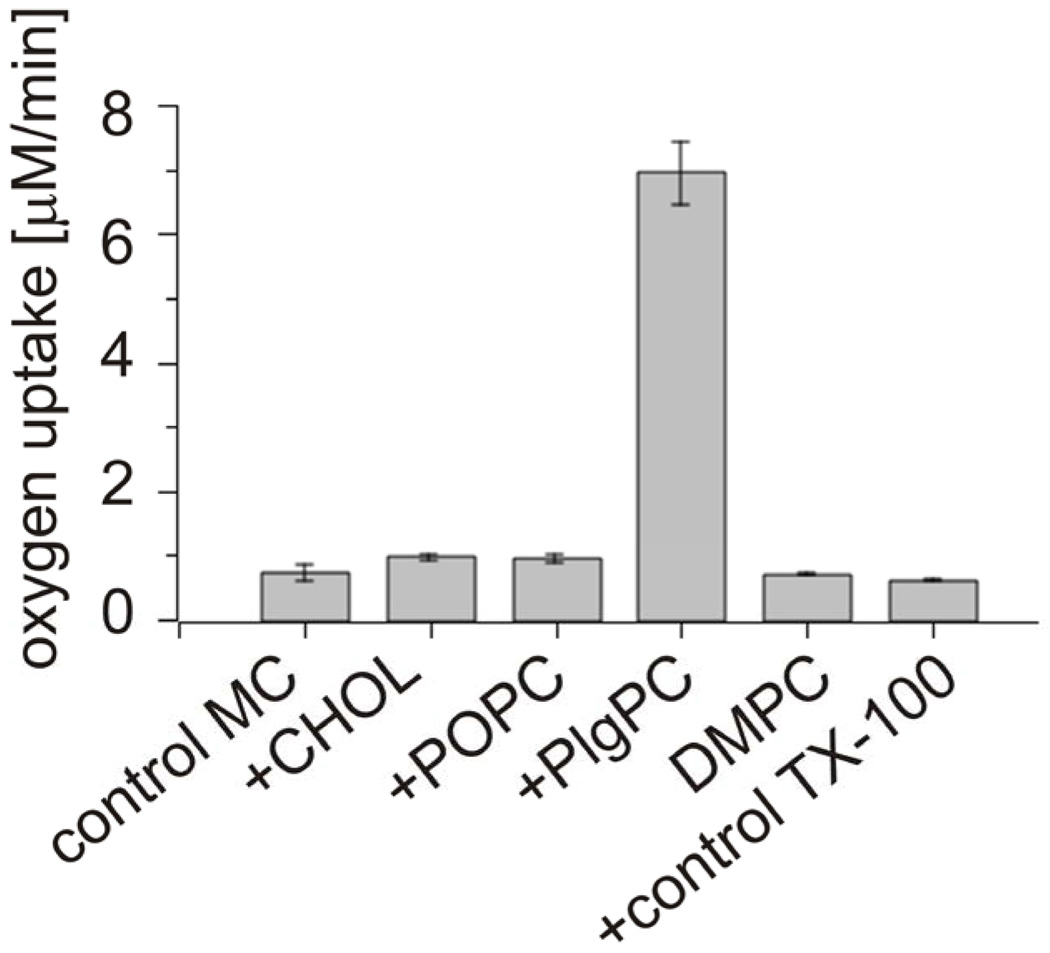
Fig. 1 shows oxygen concentration changes induced by green light irradiation of aqueous suspension of large unilamellar vesicles (LUV) composed of DMPC and selected percentage of either cholesterol or plasmenylcholine, in the presence of rose Bengal (RB), a water-soluble photosensitizer known for high efficiency to photogenerate singlet oxygen [36]. It is apparent that, in comparison with cholesterol, plasmenylcholine is a much better substrate for photosensitized oxidation. Considering the difference in irradiation time and concentration of the two lipids, it is estimated that plasmenylcholine interacts over 30 times faster with singlet oxygen and other reactive oxygen species photogenerated by RB. When the photosensitized oxidation reaction was mediated by tertraphenylporphine (TPP), a lipid soluble photosensitizer with high yield of singlet oxygen generation, the rate of oxygen photoconsumption, in cholesterol-containing DMPC liposomes, was about 15 fold lower than that when liposomes were enriched with PlgPC (data not shown). Using merocyanine 540 (MC 540) that binds avidly to cell membranes [37] and DMPC liposomes with either plasmalogens (PlgPC 18:0/18:1 or PlgPC 16:0/16:0) or their diacyl analogs (POPC or SOPC), the rate of oxygen photouptake was found to be 8.5 – 11 fold greater in samples with plasmalogen-containing liposomes, compared to samples with POPC or SOPC (Fig. 2). Under such conditions, photosensitized oxidation of cholesterol was about 25 times slower than that detected in plasmenylcholine-containing vesicles. At this point it is not clear if the effects observed for different photosensitizing dyes are due to heterogeneity of the liposomal membranes, in which cholesterol and phospholipid molecules might be distributed non-uniformly while the dye molecules exhibit preferential affinity to different membrane domains or to differential photogeneration of singlet oxygen and free radicals by the photosensitizers. When DMPC or DPPC and plasmenylcholine were solubilized in Triton X-100 micelles, the rate of oxygen consumption, photosensitized by MC 540, was over 140 times smaller than that observed in liposomes (Fig. 3). This dramatic difference in the rates of photosensitized oxidation of plasmalogen in liposomes and in Triton X-100 micelles could be explained by significantly higher local concentration of the oxidizable substrate in the liposomal membranes where photosensitizing dye molecules also localize.
Fig.1.
Oxygen uptake monitored by EPR oximetry in liposomes composed of DMPC-Chol (A) or DMPC- PlgPC 16:0/16:0 (B) photosensitized with rose Bengal. 1mM DMPC concentration was held constant whereas Chol or PlgPC concentration was varied.
A. Cholesterol concentration are as follows: 0.4 mM (triangles down), 0.6 mM (triangles up), 0.8 mM ( rectangle),1 mM (circle).
B. Plasmalogen concentration are as follows: 0.1 mM (triangles up), 0.3 mM (triangles down), 0.4 mM (squares).
Fig.2.
Oxygen uptake monitored by EPR oximetry in large unilamellar liposomes photosensitized with MC 540.
4.4 mM SUV were composed of DMPC and one of additional lipid (Chol, SOPC, POPC, PlgPC 18:0/18:1 or PlgPC 16:0/16:0 designated as “+” in Fig.2B) in molar ratio 3:2.
A. The kinetics of oxygen decay in LUV composed of: DMPC-PlgPC 16:0/16:0 dark control (circles), control DMPC (diagonal cross), DMPC-POPC (triangles down), DMPC-PlgPC 16:0/16:0 (triangles up).
B. Calculated oxygen uptake [mM/min] in LUV of different lipid composition. DMPC is control of pure DMPC liposomes.
Fig.3.
Oxygen uptake monitored by EPR oximetry in TX-100 micelles photosensitized with MC 540. 5mM lipid mixtures were composed of DMPC and one of additional lipid (Chol, POPC, or PlgPC 16:0/16:0 designated as “+”) in molar ratio 3:2. Additional controls are as follows: “control MC” is a control of pure photosensitizer in buffer, “DMPC” is a control of pure 5 mM DMPC micelles and “control TX-100” is a photosensitizer in micelles.
Using HPLC-EC, we were able to detect in chloroform extracts of DMPC/plasmenylcholine/cholesterol liposomes, subjected to aerobic irradiation with green light in the presence of RB or MC 540, large amounts of hydroperoxides with the retention time between 20 and 30 minutes. The retention time suggests that plasmenylcholine hydroperoxides were formed [26]. Unfortunately, without an appropriate plasmenylocholine hydroperoxides standard, no quantitative evaluation was possible. At shorter retention times (4–6 min), substantial amounts of singlet oxygen specific cholesterol hydroperoxides: 5α-OOH, 6α-OOH and 6β-OOH were detected (Fig. 4). Although 7α/β-OOH were also formed, particularly when RB was used as the photosensitizer, their amount was significantly lower than that of 5α-OOH, suggesting that singlet oxygen is the main reactive oxygen species responsible for the observed oxidation of liposomal lipids. A small difference in the retention time for the PlgPC oxidation products observed when two different photosensitizers were used, could arise from several independent factors such as difference in the content of organic solvents due to extensive purging of the of the elution media with argon and difference in the column temperature.
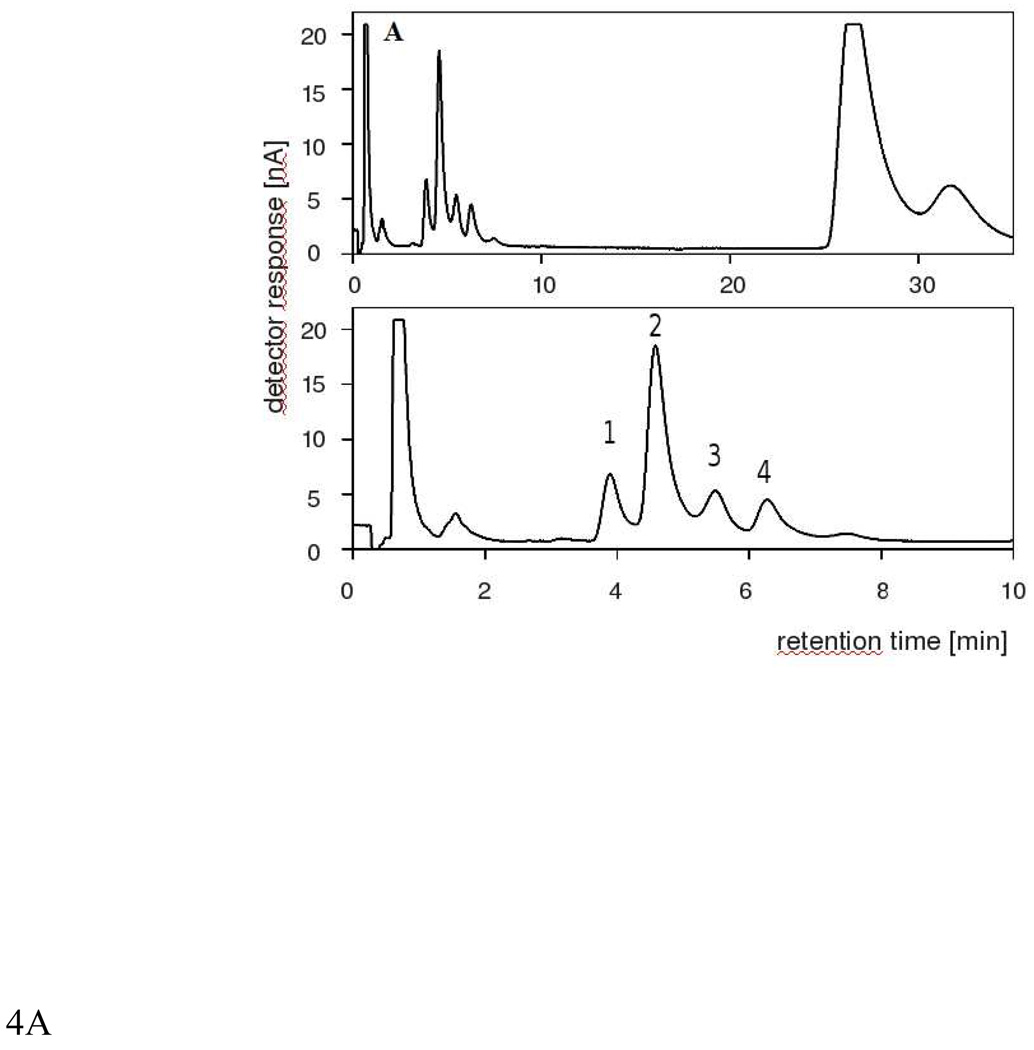
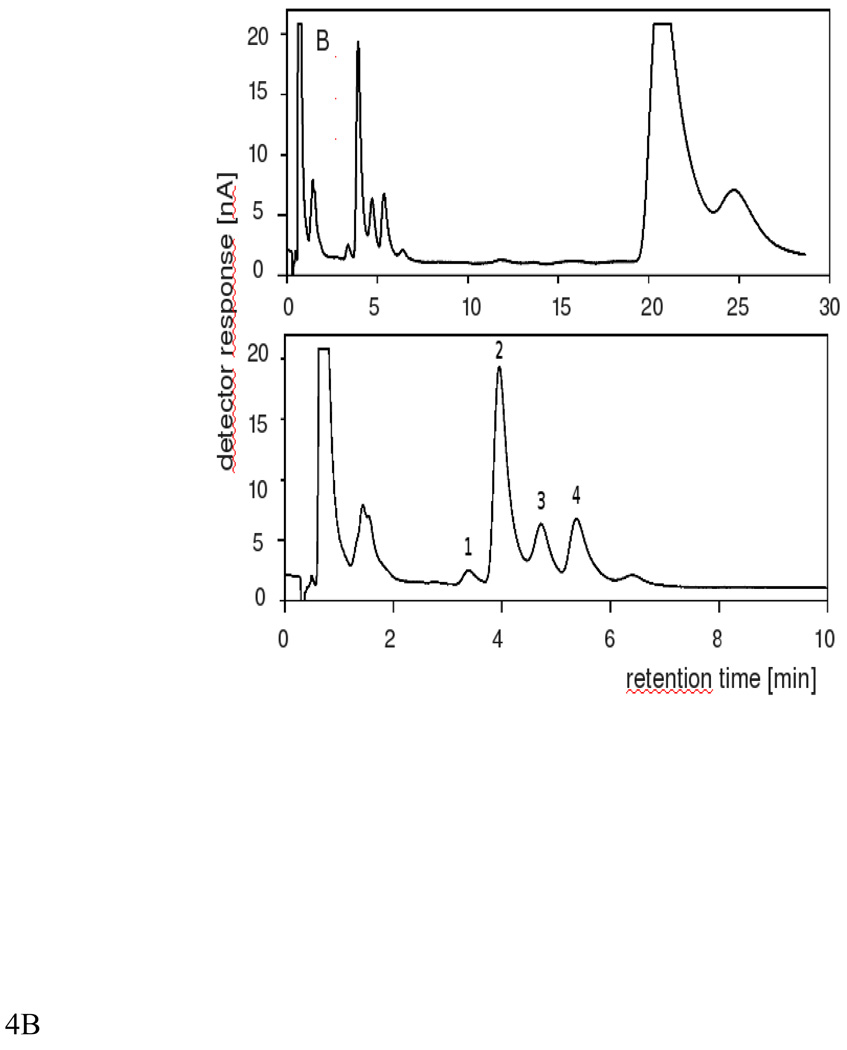
Fig.4.
HPLC-EC(Hg) chromatograms of lipids extracted from photosensitised DMPC-Chol-PlgPC 16:0/16:0 liposomes with RB (A) or MC 540 (B). Lipid mol ratio was 5:8:3 of DMPC: Chol: PlgPC 16:0/16:0. The peak identities are as follows: (1) 7α/β-cholesterol hydroperoxide (7α/β-OOH), (2) 5α-cholesterol hydroperoxide (5α-OOH), (3) 6α-cholesterol hydroperoxide (6α-OOH), (4) 6β-cholesterol hydroperoxide (6β-OOH)
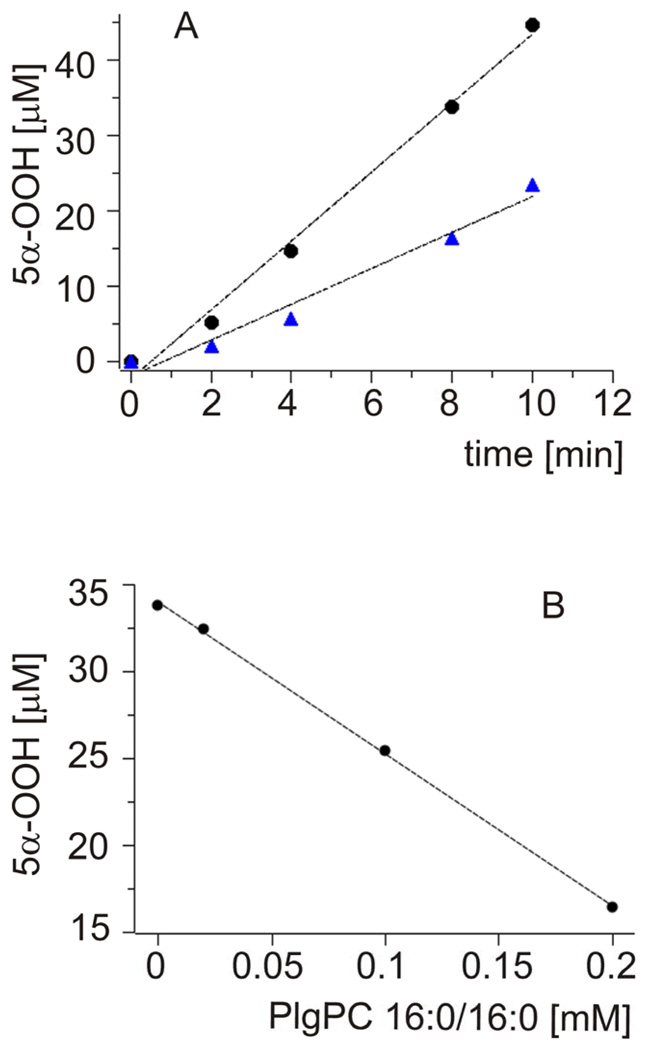
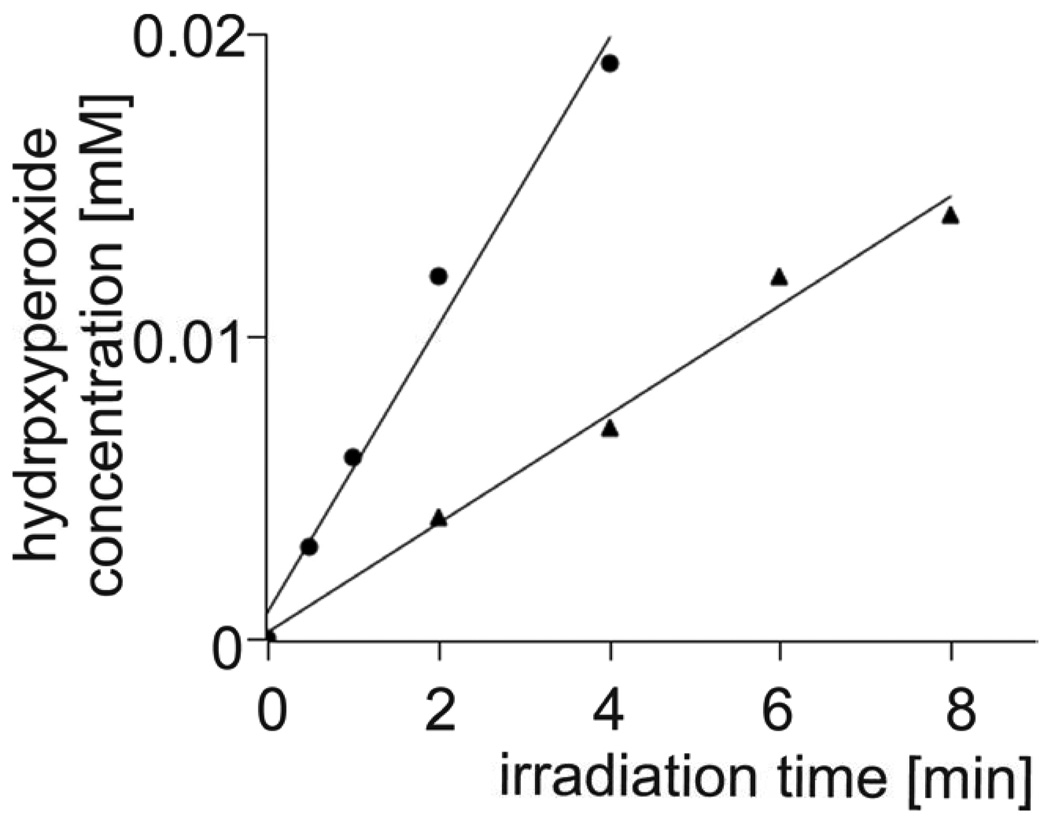
To eliminate, or at least substantially reduce, the effects of site-specific formation and decay of ROS, expected in heterogeneous systems such as liposomal membranes, the formation of cholesterol hydroperoxides was also measured in a homogeneous solution of cholesterol in carbon disulfide, in the absence and presence of increasing concentration of plasmenylcholine. Representative data are shown in Fig. 5A. It is apparent that the rate of TPP-photosensitized accumulation of 5α-OOH decreases in the presence of a small amount of PlgPC. The amount of photoformed 5α-OOH decreased almost linearly with increasing concentration of the added PlgPC (Fig. 5B). To compare quantitatively the rates of photosensitized formation of plasmenylcholine and cholesterol hydroperoxides, these two lipids were dissolved in carbon tetrachloride and subjected to aerobic irradiation with visible light in the presence of TPP, used as a photosensitizer. Total amount of lipid hydroperoxides was determined by the iodometric assay. Data shown in Fig. 6 indicate that at cholesterol concentration 2 mM, the rate of its hydroperoxides accumulation was 1.8 µM/min. On the other hand, if the photosensitized sample contained 0.1 mM plasemenylcholine, the rate of plasemylcholine hydroperoxide formation was 4.8 µM/min. Normalizing to equal concentration of the lipids we can conclude that the rate of photosensitized oxidation of plasemenylcholine is 53 fold greater than that of cholesterol.
Fig.5.
A. Kinetics of 5α-OOH accumulation detected with HPLC-EC(Hg) system in CS2 solution of either pure 0.5 mM Chol (circles) or 0.5 mM Chol with 0.2 mM PlgPC 16:0/16:0 (triangles) photosensitized with TPP.
B. Effect of different concentration of PlgPC 16:0/16:0 on 5α-OOH accumulation after 8 minutes irradiation with TPP.
Fig.6.
Kinetics of lipid hydroperoxides accumulation determined by iodometric assay in CCl4 solution of either 0.1 mM PlgPC 16:0/16:0 (circles) or 2 mM Chol (triangles). TPP was used as a photosensitizer.
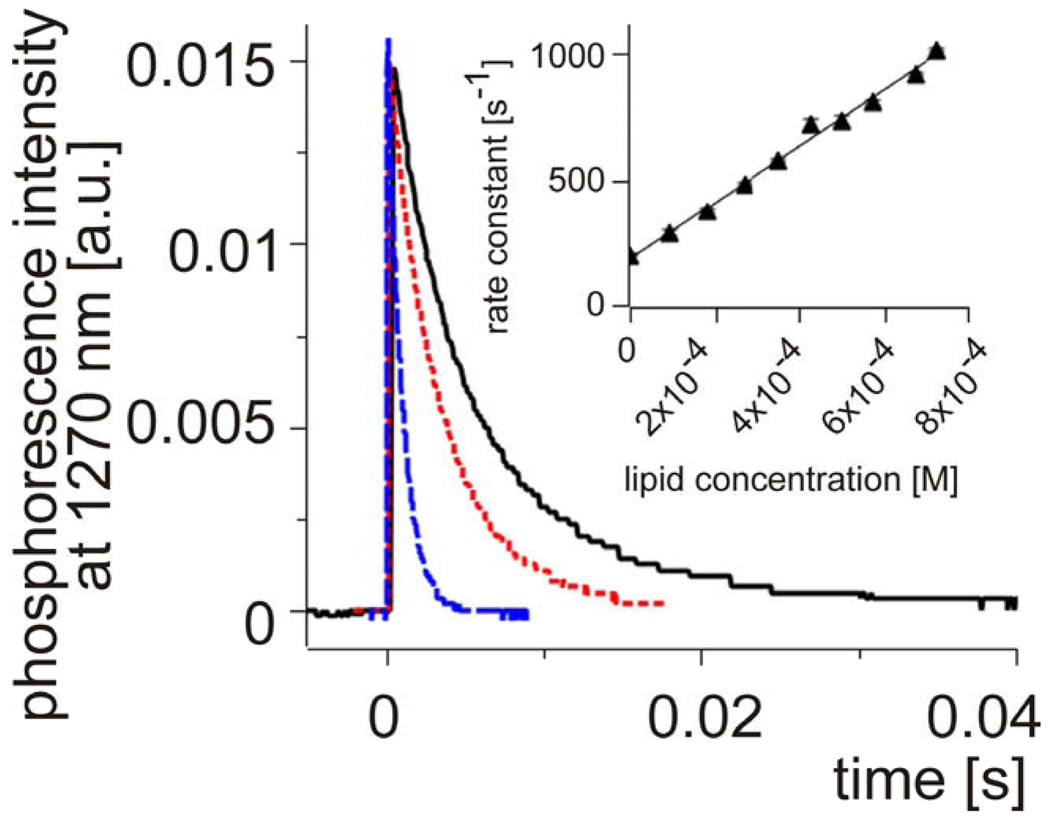
The interaction of plasmalogen molecules with singlet oxygen can be reactive and physical. While the former results in consumption of oxygen and formation of a product, the latter leads to physical quenching of singlet oxygen leaving the interacting molecules chemically unchanged [38]. To determine total rate constants of the interaction of singlet oxygen with Plg and other lipids, time-resolved phosphorescence at 1270 nm was used. This direct method of singlet oxygen detection enables determination of the interaction of a physical and/or chemical quencher with singlet oxygen by measuring reduction of its lifetime as a function of increasing concentration of the quencher [39]. Under realistic experimental conditions, the method works when the “intrinsic” lifetime of singlet oxygen, is long enough. This is primarily determined by the solvent, in which the photosensitizer and lipids are dissolved. In our experiments we used carbon tetrachloride, in which lifetime of singlet oxygen is of the order of milliseconds [40]. Representative data are shown in Fig. 7. It is evident that with increasing concentration of PlgPC 16:0/18:1, the observed lifetime of the singlet oxygen phosphorescence decreases. A plot of the apparent first-order rate constant of singlet oxygen decay as a function of the plasmalogen concentration gives the bimolecular rate constant of singlet oxygen quenching 1.0 (± 0.2) × 106 M−1 s−1. Table 1 summarizes such data for selected plasmalogens and their diacyl analogs in CCl4. The data show that on average, the alkenyl-acyl phospholipids quench singlet oxygen with the rate constant being one order of magnitude higher than that measure for diacyl phospholipids. The highest efficiency of singlet oxygen quenching by plasmenylcholine has been determined in carbon disulfide (Table 2). In this solvent, we observed that the rate constant of singlet oxygen quenching by plasemenylcholine was two orders of magnitude greater than by cholesterol (data not shown). Interestingly, addition of CCl4 to CS2 decreased the quenching rate constant (kQ). Thus addition of 25% CCl4 reduced kQ by a factor of almost three and in the presence of 75% CCl4 the quenching rate constant was over six fold lower than in 100% CS2. The data suggest that the interaction of singlet oxygen with the plasmalogen vinyl ether bond is most efficient in polar organic solvents. Comparison with simple enol-ethers [28–30] suggests that in polar media the interaction of plasmalogens with singlet oxygen predominantly occurs via cycloaddition leading to the formation of dioxetanes.
Fig.7.
Singlet oxygen phosphorescence decay at 1270 nm in an irradiated samples of TPP dissolved in CCl4 (solid line) and after quenching with 0.1 mM PlgPC 16:0/18:1 (doted line) or 1.1 mM PlgPC 16:0/18:1 (dashed line). Inset shows a typical dependence of pseudo-first order rate constant [s−1] of singlet oxygen quenching on concentration of PlgPC 18:0/18:1. From the straight-line slope, the actual bi-molecular rate constants are calculated. Such data are summarized in Table1.
Table 1.
Rate constants for interaction of 1O2 with different plasmalogens and their diacyl analogs in CCl4.
| diacyl phospholipid |
kq [M−1s−1] | alkenyl-acyl phospholipid |
kq [M−1s−1] |
|---|---|---|---|
| POPC | (0.8 ± 0.4) × 105 | PlgPC 16:0/18:1 | (1.0 ± 0.2) × 106 |
| SOPC | (1.8 ± 0.6 ) × 105 | PlgPC18:0/18:1 | (1.3 ± 0.2) × 106 |
| SAPC | (1.3 ± 0.6) × 105 | PlgPC 18:0/20:4 | (1.9 ± 0.8) × 106 |
TPP was used as a photosensitizer and it was excited with 515 nm laser pulses.
Table 2.
Rate constants for interaction of 1O2 with PlgPC 16:0/16:0 in different organic solvents and organic solvent mixtures.
| solvent | kq [M−1s−1] |
|---|---|
| carbon disulfide (CS2) | (6.32 ± 0.33) × 106 |
| CS2 : CCl4 3:1 | (1.99 ± 0.47) × 106 |
| CS2 : CCl4 1:1 | (1.23 ± 0.21) × 106 |
| CS2 : CCl4 1:3 | (0.94 ± 0.01) × 106 |
| carbon tetrachloride (CCl4) | (1.25 ± 0.29) × 106 |
TPP was used as a photosensitizer and it was excited with 532 nm laser pulses.
DISCUSSION
In this study, we used an array of complementary techniques to monitor the interaction of singlet oxygen with plasmalogens and other lipids. Thus the chemical interaction of Plg with reactive species generated by photosensitized oxidation reaction was monitored by oxygen consumption using EPR oximetry and product analysis employing iodometric assay and HPLC-EC determination for lipid hydroperoxides. Oxygen uptake that accompanies an oxidation process, is one of the most universal and sensitive indicator of the reaction progress. We, and others, have previously demonstrated that photosensitized oxidation reaction can conveniently be monitored by EPR-oximetry using an appropriate spin probe [32, 41]. Although this method is not specific for any particular type of oxidation reaction, under appropriate experimental conditions it could also be used to differentiate Type I from Type II photosensitized oxidation reactions. Thus if the lifetime of singlet oxygen that is responsible for the oxidation process, is determined by the interaction with solvent molecules, exchanging H2O for D2O could greatly accelerate the observable oxygen consumption [42]. In addition, the rate of oxygen consumption should substantially decrease in the presence of adequate concentrations of a singlet oxygen quencher. Unfortunately such experimental conditions are not established in the liposomal system studied. The system is highly heterogeneous, in which the formation and decay of singlet oxygen (and free radicals) is predominantly site-specific. This is due to association of the photosensitizing dye molecules with liposomal membranes. Although significant binding with phospholipid membranes is expected for the hydrophobic TPP and the amphiphillic MC540, it may come as a surprise that also the negatively charged RB molecules exhibit substantial association with liposome vesicles. Indeed, we observed a distinct batochromic shift of the RB absorption maximum after liposomes were added to aqueous solution of the dye (data not shown). A substantial binding of RB molecules with liposomal membranes was evident from control experiments, in which the liposomes were spin down after centrifugation. In such samples less than 5% of the initial RB concentration was found in the supernatant, while most of the dye was in the liposomal fraction. Thus, under the conditions used, oxygen consumption measurements do not provide any specific information about the predominant type of photosensitized reactions mediated by the photosensitizing dyes used.
However, the experiments clearly show that photosensitized oxidation of Plg is one to one and half orders of magnitude faster than that of unsaturataed diacyl phospholipids and cholesterol (Fig.1 and 2). The vinyl ether linkage is responsible for the interaction with singlet oxygen (and/or free radicals) leading to the observable oxygen consumption in plasmalogen samples subjected to photosensitized oxidation. As evident from Fig. 2B, the highest rate of oxygen consumption was found in photosensitized samples that contained liposomes with saturated plasmenylcholine (PlgPC 16:0/16:0), i.e. glycerophospholipid with no double bonds except the vinyl ether bond. The predominant mode of the photosensitized oxidation reaction operating in the system studied could be deduced from experiments, in which cholesterol hydroperoxides were determined by HPLC-EC (Fig 4). In these experiments, cholesterol was used as a mechanistic reporter probe [43]. While 7α/β-OOH are formed via free radical mediated oxidation, they can also result from spontaneous conversion of 5α-OOH [44]. On the other hand 5α-OOH (as well 6α-OOH and 6β-OOH) are specific products of the interaction of cholesterol with singlet oxygen [28]. The data clearly show that the predominant oxidation products of the liposomal cholesterol, detected by HPLC-EC, are singlet oxygen specific 5α-OOH, 6α-OOH and 6β-OOH. Although 7α/β-OOH are also formed, these are minor products of photosensitized reactions mediated by RB and MC 540. Therefore we can conclude that photosensitized oxidation of plasmalogens is predominantly due to the interaction of their vinyl ether functionality with singlet oxygen. Results shown in Fig. 5 support this conclusion; the fact that the accumulation of 5α-OOH is strongly inhibited by Plg suggests an efficient competition of the alkenyl-acyl phospholipid with cholesterol for singlet oxygen.
An efficient quenching of singlet oxygen by Plg has been demonstrated by direct measurements of singlet oxygen lifetimes in selected organic solvents (Fig. 7, Tables 1 and 2). Even though, the PlgPC 18:0/20:4 is a slightly more efficient quencher of singlet oxygen than the PlgPC 16:0/18:1, there are no consistent correlations with either the number of double bonds in the sn-2 position of the phospholipids or with the length of their carbon chains. Thus, the 16:0/16:0 and 18:0/18:1 plasmalogens are equally efficient singlet oxygen quenchers (Table 1 and Table 2). Interestingly, even in the case of diacyl phospholipids, the number of double bonds in the sn-2 position does not appear to be a major factor in determining the lipid ability to quench singlet oxygen (Table 1). This may suggest a significant contribution of physical quenching in the interaction of diacyl phospholipids with singlet oxygen. Based on a comparative analysis of singlet oxygen quenching by saturated and unsaturated fatty acids, it was concluded that the relative contribution of physical and chemical quenching depends on the number of double bonds and CH groups in the fatty acid hydrocarbon chains [45]. Our study clearly demonstrates that the observable quenching of singlet oxygen by different Plg is primarily due to the interaction of singlet oxygen with their vinyl ether functionality. Considering the data on oxygen photoconsumption and accumulation of hydroperoxides, discussed above, it can be concluded that quenching of singlet oxygen by plasmalogens is mostly chemical in nature.
CONCLUSIONS
We have demonstrated that singlet oxygen is quenched by the vinyl-ether functionality of plasmalogen molecules. The interaction is particularly efficient in polar microenvironments. Although both physical and chemical quenching is probably involved, a substantial amount of Plg hydroperoxides that accompanies photosensitized oxidation of plasmalogens in liposomes membranes and in homogeneous solutions, suggests that addition of singlet oxygen to the sn-1 hydrocarbon chain plays an important role. It can be concluded that in biological membranes, containing similar amounts of plasmalogens and diacyl glycerophospholipids, singlet oxygen is likely to be quenched by the former. Whether or not, the interaction of Plg with singlet oxygen results in efficient antioxidant action will depend on the properties of the products formed. Based on previous studies [11, 17], it can be speculated that products of singlet oxygen interaction with plasmalogens have a lower pro-oxidizing potential than those of diacyl phospholipids with unsaturated bonds.
Acknowledgments
We wish to acknowledge contribution of Dr. Mariusz Zareba and Dr. J. Kalinowska-Tluscik at early stages of this study. Supported in part by Poland Ministry of Science and Higher Education (grant 2040/B/P01/2007/33) and by a NIH (FIRCA grant TW01251).
Abbreviations
- Plg
plasmalogens
- PlgPC
plasmenylcholine
- TPP
5,10,15,20-tetraphenylporphine
- RB
rose Bengal
- MC540
merocyanine 540
- LUV
large unilamellar vesicles
- EPR
electron paramagnetic resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee TC. Biosynthesis and possible biological functions of plasmalogens. Biochim. Biophys. Acta. 1998;1394:129–145. doi: 10.1016/s0005-2760(98)00107-6. [DOI] [PubMed] [Google Scholar]
- 2.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Paltauf F. Ether lipids in biomembranes. Chem. Phys. Lipids. 1994;74:101–139. doi: 10.1016/0009-3084(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 4.Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer's disease and dementia. J. Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Farooqui AA, Rapoport SI, Horrocks LA. Membrane phospholipid alterations in Alzheimer's disease: deficiency of ethanolamine plasmalogens. Neurochem. Res. 1997;22:523–527. doi: 10.1023/a:1027380331807. [DOI] [PubMed] [Google Scholar]
- 6.Acar N, Gregoire S, Andre A, Juaneda P, Joffre C, Bron AM, Creuzot-Garcher CP, Bretillon L. Plasmalogens in the retina: in situ hybridization of dihydroxyacetone phosphate acyltransferase (DHAP-AT)--the first enzyme involved in their biosynthesis--and comparative study of retinal and retinal pigment epithelial lipid composition. Exp. Eye Res. 2007;84:143–151. doi: 10.1016/j.exer.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 8.Wolf RA, Gross RW. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J. Biol. Chem. 1985;260:7295–7303. [PubMed] [Google Scholar]
- 9.Hanahan DJ, Joseph M, Morales R. The isolation and characterization of a third or neutral phospholipase A2 in the venom of Agkistrodon halys blomhoffii. An improved fractionation procedure for all three enzymes. Biochim. Biophys. Acta. 1980;619:640–649. doi: 10.1016/0005-2760(80)90113-7. [DOI] [PubMed] [Google Scholar]
- 10.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim. Biophys. Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J. Biol. Chem. 1988;263:11590–11596. [PubMed] [Google Scholar]
- 12.Morand OH. Reactivity of plasmalogens to singlet oxygen and radicals. Meth. Enzymol. 1994;234:603–620. doi: 10.1016/0076-6879(94)34132-x. [DOI] [PubMed] [Google Scholar]
- 13.Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp. Gerontol. 1998;33:363–369. doi: 10.1016/s0531-5565(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 14.Reiss D, Beyer K, Engelmann B. Delayed oxidative degradation of polyunsaturated diacyl phospholipids in the presence of plasmalogen phospholipids in vitro. Biochem J. 1997;323:807–814. doi: 10.1042/bj3230807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sindelar PJ, Guan Z, Dallner G, Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 1999;26:318–324. doi: 10.1016/s0891-5849(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 16.Maeba R, Ueta N. Ethanolamine plasmalogen and cholesterol reduce the total membrane oxidizability measured by the oxygen uptake method. Biochem. Biophys. Res. Commun. 2003;302:265–270. doi: 10.1016/s0006-291x(03)00157-8. [DOI] [PubMed] [Google Scholar]
- 17.Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 1999;338:769–776. [PMC free article] [PubMed] [Google Scholar]
- 18.Yavin E, Gatt S. Oxygen-dependent cleavage of the vinyl -ether linkage of plasmalogens. 2. Identification of the low-molecular-weight active component and the reaction mechanism. Eur. J. Biochem. 1972;25:437–446. doi: 10.1111/j.1432-1033.1972.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 19.Foglia TA, Nungesser E, Marmer WN. Oxidation of 1-O-(alk-1-enyl)-2,3-di-O-acylglycerols: models for plasmalogen oxidation. Lipids. 1988;23:430–434. doi: 10.1007/BF02535515. [DOI] [PubMed] [Google Scholar]
- 20.Stadelmann-Ingrand S, Favreliere S, Fauconneau B, Mauco G, Tallineau C. Plasmalogen degradation by oxidative stress: production and disappearance of specific fatty aldehydes and fatty alpha-hydroxyaldehydes. Free Radic. Biol. Med. 2001;31:1263–1271. doi: 10.1016/s0891-5849(01)00720-1. [DOI] [PubMed] [Google Scholar]
- 21.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 22.Mangold HK, Weber N. Biosynthesis and biotransformation of ether lipids. Lipids. 1987;22:789–799. doi: 10.1007/BF02535533. [DOI] [PubMed] [Google Scholar]
- 23.Maeba R, Sawada Y, Shimasaki H, Takahashi I, Ueta N. Ethanolamine plasmalogens protect cholesterol-rich liposomal membranes from oxidation caused by free radicals. Chem. Phys. Lipids. 2002;120:145–151. doi: 10.1016/s0009-3084(02)00101-9. [DOI] [PubMed] [Google Scholar]
- 24.Khaselev N, Murphy RC. Susceptibility of plasmenyl glycerophosphoethanolamine lipids containing arachidonate to oxidative degradation. Free Radic. Biol. Med. 1999;26:275–284. doi: 10.1016/s0891-5849(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 25.Khaselev N, Murphy RC. Peroxidation of arachidonate containing plasmenyl glycerophosphocholine: facile oxidation of esterified arachidonate at carbon-5. Free Radic. Biol. Med. 2000;29:620–632. doi: 10.1016/s0891-5849(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 26.Thompson DH, Inerowicz HD, Grove J, Sarna T. Structural characterization of plasmenylcholine photooxidation products. Photochem. Photobiol. 2003;78:323–330. doi: 10.1562/0031-8655(2003)078<0323:scoppp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Zemski Berry KA, Murphy RC. Free radical oxidation of plasmalogen glycerophosphocholine containing esterified docosahexaenoic acid: structure determination by mass spectrometry. Antioxid. Redox Signal. 2005;7:157–169. doi: 10.1089/ars.2005.7.157. [DOI] [PubMed] [Google Scholar]
- 28.Foote CS. Photosensitized oxidation and singlet oxygen: consequences in biological systems. In: Pryor WA, editor. Free Radicals in Biology. volume 2. New York: Academic Press; 1976. pp. 85–134. [Google Scholar]
- 29.Frimer AA, Bartlett PD, Boschung AF, Jewett JG. Reaction of singlet oxygen with 4-methyl-2,3-dihydro-gamma-pyrans. J. Am. Chem. Soc. 1977;99:7977–7986. [Google Scholar]
- 30.Gorman AA, Gould IR, Hamblett I. Time-resolved study of the solvent and temperature dependence of singlet oxygen (1Δg) reactivity toward enol ethers: reactivity parameters typical of rapid reversible exciplex formation. J. Am. Chem. Soc. 1982;104:7098–7104. [Google Scholar]
- 31.Van den Bossche J, Shin J, Thompson DM. Improved Plasmalogen Synthesis Using Organobarium Intermediates. J. Org. Chem. 2007;72:5005–5007. doi: 10.1021/jo0705059. [DOI] [PubMed] [Google Scholar]
- 32.Różanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JB, Sarna T. Blue Light-Induced Reactivity of Retinal Age Pigment: In vitro Generation of Oxygen Reactive Species. J. Biol. Chem. 1995;70:18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 33.Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A. Photocatalytic generation of oxygen radicals by water-soluble bacteriochlorophyll derivative noncovalently bound to serum albumin. J. Phys. Chem. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- 34.Korytowski W, Bachowski GJ, Girotti AW. Chromatographic-separation and electrochemical determination of cholesterol hydroperoxides generated by photodynamic-action. Anal. Biochem. 1991;197:149–156. doi: 10.1016/0003-2697(91)90371-y. [DOI] [PubMed] [Google Scholar]
- 35.Cheeseman KA. Methods of measuring lipid peroxidation in biological systems: an overview. In: Crastes de Paulet A, Douste-Blazy L, Raoletti R, editors. Free Radicals, Lipoproteins and Membrane Lipids. New York: Plenum Press; 1990. pp. 143–152. [Google Scholar]
- 36.Lambert CR, Kochevar IE. Does Rose Bengal Triplet Generate Superoxide Anion? J. Am. Chem. Soc. 1996;118:3297–3298. [Google Scholar]
- 37.Sieber F. Elimination of residual tumor cells from autologous bone marrow grafts by dye-mediated photolysis: preclinical data. Photochem Photobiol. 1987;46:71–76. doi: 10.1111/j.1751-1097.1987.tb04738.x. [DOI] [PubMed] [Google Scholar]
- 38.Di Mascio P, Devasagayam TP, Kaiser S, Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez-Banzo A, Ragàs X, Kapusta P, Nonell S. Time-resolved methods in biophysics. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008;7:1003–1010. doi: 10.1039/b804333g. [DOI] [PubMed] [Google Scholar]
- 40.Hurst JR, McDonald JD, Schuster GB. Lifetime of singlet oxygen in solution directly determined by laser spectroscopy. J. Am. Chem. Soc. 1982;104:2065–2067. [Google Scholar]
- 41.Wrona M, Korytowski W, Rózanowska M, Sarna T, Truscott TG. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic. Biol. Med. 2003;35:1319–1329. doi: 10.1016/j.freeradbiomed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Monroe B. Singlet oxygen in solution: lifetimes and reaction rate constants. In: Frimer A, editor. Singlet O2. Vol. I: Physical-Chemical Aspects. CRC Press; 1985. pp. 177–224. [Google Scholar]
- 43.Geiger PG, Korytowski W, Lin F, Girotti AW. Lipid peroxidation in photodynamically stressed mammalian cells: use of cholesterol hydroperoxides as mechanistic reporters. Free Radic. Biol. Med. 1997;23:57–68. doi: 10.1016/s0891-5849(96)00587-4. [DOI] [PubMed] [Google Scholar]
- 44.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem Photobiol B: Biology. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 45.Krasnovsky AA, Kagan VE, Minin AA. Quenching of singlet oxygen luminescence by fatty acids and lipids. Contribution of physical and chemical mechanisms. FEBS Letters. 1983;155:233–236. [Google Scholar]