Abstract
It has been proposed that animals that attribute high levels of incentive salience to reward-related cues may be especially vulnerable to addiction. Individual variation has also been observed in the motivational value attributed to aversive cues, which may confer vulnerability to anxiety disorders such as post-traumatic stress disorder (PTSD). There may be a core behavioral trait that contributes to individual variation in the motivational value assigned to predictive cues regardless of emotional valence. To test this hypothesis, we used a Pavlovian conditioned approach procedure to classify rats based on whether they learned to approach and interact with a cue predicting food reward (sign-trackers) or learned upon cue presentation to go to the location of impending food delivery (goal-trackers), and then examined Pavlovian fear conditioning in the same animals. It has recently been proposed that sign-trackers are more vulnerable to substance abuse because they attribute greater incentive motivational value to drug cues. Here we show that sign-trackers also have a tendency to be more fearful of discrete cues that predict footshock. In addition, we found that goal-trackers exhibited greater contextual fear when placed back into the original fear-conditioning context in the absence of temporally discrete cues. These results suggest that there may be a subset of individuals who tend to attribute high levels of motivational salience to predictive cues regardless of emotional valence, which may predispose them to a number of psychiatric comorbidities including PTSD and substance abuse. Other individuals use contexts to appropriately modify their reactions to such salient stimuli.
Keywords: Fear, Pavlovian conditioning, Rat, Comorbidity, Autoshaping, Vulnerability
1. Introduction
Cues in the environment that have been associated with emotionally salient events often themselves come to trigger complex emotional and motivational states that can powerfully influence behavior. For example, eating behavior can be triggered in humans and other animals by exposure to cues associated with food (e.g. the sight of a fast-food restaurant), even when the subject is sated [1, 2, 3]. This basic psychological process can have undesirable consequences, for example, spurring excessive eating that can contribute to obesity. Similarly, in addicts, drug-associated cues can induce craving, which often leads to continued drug use or relapse [4, 5]. In other circumstances, relatively innocuous cues that were associated with a previous trauma can induce extreme fear and avoidance behaviors, e.g., a fireworks display inducing panic in a war veteran [6].
Preclinical studies have shown, however, that there is considerable individual variation in the degree to which animals attribute incentive motivational properties (“incentive salience”) to reward cues. For example, when a food reward is paired with a localizable cue the cue itself becomes attractive, eliciting approach towards it, only in some rats (“sign-trackers,” STs); other rats direct their behavior away from the cue towards to location of reward delivery (“goal-trackers,” GTs) [7, 8, 9]. Furthermore, a food cue is more “desired” in STs than GTs, in that they will work harder to get it [10]. Drug cues also acquire greater control over behavior in STs than GTs. For example, STs are more susceptible to cue-induced reinstatement of cocaine self-administration behavior, suggesting they may be more vulnerable to develop addiction-like behaviors than GTs [11]. Thus, the extent to which individuals attribute predictive reward cues with incentive motivational properties may confer vulnerability to disorders of impulse control.
There is also considerable individual variation in the extent to which cues associated with aversive stimuli acquire control over behavior [12], and this trait may contribute to vulnerability to anxiety disorders [13, 14]. For example, variation in the magnitude of conditioned fear responses in animals has been proposed as a model of vulnerability to post-traumatic stress disorder (PTSD) [12, 15]. Indeed, pathological hyper-reactivity to environmental cues is a central feature common to several psychiatric disorders. In the case of substance abuse, “cue reactivity” is well-documented and includes psychological cravings, physiological responses, and engagement in actual drug use behaviors in response to drug-related cues or contexts [16, 17]. Similarly, PTSD is defined in part by excessive emotional, physiological, and behavioral responses to trauma-related stimuli [18, 19]. From this perspective, one difference between substance abuse and PTSD is the emotional valence of the triggering stimulus, one being “positive” and eliciting approach, the other being “negative” and eliciting avoidance. It is possible that some individuals are prone to attribute excessive emotional and/or motivational significance to environmental cues regardless of valence, which could therefore make them more vulnerable to a number of different psychiatric disorders. We explore this idea here using preclinical animal models to estimate the emotional and/or motivational significance individual rats attribute to both an attractive food cue and to a fearful aversive cue.
2. Material and Methods
All procedures were approved by the University Committee on the Use and Care of Animals.
2.1. Experiment 1
Thirty-six male Sprague-Dawley rats (Harlan) weighing 250-300g were individually housed and kept on a 12:12-hr light/dark cycle. All training/testing took place during the light cycle between the hours of 1300 and 1800. Food and water were available ad libitum for all portions of the experiment.
2.1.1. Pavlovian conditioned approach: apparatus
Sixteen standard MED Associates test chambers (24.1 × 20.5 cm floor area, 29.2 cm high; MED Associates, St. Albans, VT) were used for Pavlovian training. Each conditioning chamber was located in a sound-attenuating enclosure and white masking noise was supplied by a ventilating fan. Each chamber was equipped with a food receptacle, which was located in the center of the 24.1-cm-wide wall, 3 cm above the stainless steel grid floor. A retractable lever was located approximately 2.5 cm to the left or right of the food receptacle, 3 cm above the floor. A white LED was flush-mounted on the inside of the retractable lever and used to illuminate the slot through which the lever protruded. Contacts with the lever were recorded as a “lever press.” A red house light was located on the wall opposite the food receptacle and remained on throughout the training sessions. A pellet dispenser delivered banana-flavored food pellets into the food receptacle. Head entry into the food receptacle was recorded each time a rat broke the infrared photobeam located inside the receptacle (approximately 1.5 cm above the base of the food cup).
2.1.2. Pavlovian conditioned approach: procedure
For two days prior to the start of training banana-flavored food pellets were placed into the rats' home cages to familiarize the animals with the food to be used as reward. Rats were then placed into the operant chambers for two pre-training sessions during which the red house-light remained on but the lever was retracted. Fifty food pellets were delivered on a variable interval (VI) 30-s schedule (i.e., one presentation of the lever occurred on average every 30 s, but the actual time between lever presentations varied randomly between 0 and 60 s), resulting in a pre-training session of approximately 25 min. All rats consumed all the food pellets by the end of the second pre-training session. Each trial during a test session consisted of presentation of the illuminated lever (conditioned stimulus, CS) into the chamber for 8 s on a VI 90-s schedule. Retraction of the lever was immediately followed by the response-independent delivery of one food pellet (unconditioned stimulus, US) in the food receptacle. The beginning of the next intertrial interval (ITI) commenced immediately after pellet delivery. Each test session consisted of 25 trials, wherein the lever (CS) and the food (US) were presented in a paired fashion, resulting in a 35-40 min test session each day for 5 days. All rats consumed all the food pellets that were delivered.
2.1.3. Pavlovian conditioned approach: data analysis
Rats were categorized based on an “Approach Index” score that was derived from the number, latency and probability of lever contacts and magazine entries during CS presentation according to the following formula: [Response bias (lever contacts − magazine entries)/(lever contacts + magazine entries) + Probability (lever contact probability − magazine entry probability) + Contact Latency (lever contact latency − magazine entry latency)/(8 s)] /3. The final “Approach Index” was obtained by averaging scores from sessions 4 and 5. With this index a score of +1 means all responses were directed towards the lever-CS, a score of −1 that all responses were directed away from the lever-CS and towards the food cup, and a score of zero that responses were directed equally to both places. Rats with an Approach Index of less than − 0.5 were designated goal-trackers (GTs; twice as likely to direct behavior towards the magazine), those above +0.5 as sign-trackers (STs; twice as likely to direct behavior towards the CS − lever), and those between −0.5 and +0.5 as intermediate responders (IRs). Between-group comparisons were performed using linear mixed-effects models [20].
2.1.4. Fear conditioning: apparatus
Pavlovian fear conditioning and testing was conducted in a different room using a different set of standard MED Associates behavioral test chambers one week after Pavlovian conditioned approach testing was completed. The floor of each chamber consisted of stainless steel rods connected to a shock source and solid-state grid scrambler for the delivery of a footshock US. The boxes were also equipped with a houselight and tone generator. Each chamber rested on a load-cell platform to record chamber displacement in response to the rat's motor activity.
2.1.5. Fear conditioning: procedure
Rats were exposed to an auditory fear conditioning session which began 3 min after being placed in the chamber. The conditioning session consisted of 5 trials in which a tone CS (30 s, 4 KHz, 80 dB SPL) co-terminated with a footshock US (2 s, 1 mA). The ITI was fixed at 4 min during fear conditioning. Rats remained in the chamber for 3 min after the last tone-shock pairing. The day after fear conditioning, the rats underwent extinction training and were placed into a different context from that used for training, with different levels of ambient light, noise, and odor, and then exposed to 20 CS-alone presentations (2-min mean ITI). This procedure was repeated on 2 subsequent days.
2.1.6. Fear conditioning: data analysis
Freezing behavior was the dependent variable used to analyze conditioned fear. Load-cell activity generated by displacement of the chamber was digitized at 5 Hz, and freezing behavior was scored if the rat was immobile for at least one second. For each session, freezing behavior was expressed as a percentage of total observations. On each extinction day, fear to the tone CS was indexed by averaging freezing across the first six CS trials of each session and subtracting that value from the pre-CS baseline. This measure therefore provided an index of the level of conditioned fear (on test day 1) and the degree to which that fear exhibited between-session extinction (on test days 2 and 3). Repeated measures data were compared using linear mixed-effects models. Freezing during the tone was also compared using planned unpaired t-tests. Correlation analysis was performed on data from all rats using Fischer's test, and Pearson's correlation coefficient was calculated.
2.2. Experiment 2
Thirty-six male Sprague-Dawley rats (Harlan) weighing 250-300g were housed under the same conditions as described in Section 2.1.
2.2.1. Pavlovian approach and operant conditioning
The Pavlovian approach training procedures were as described in Section 2.1.2, with the exception that only 25 pellets were delivered during each pre-training session. After Pavlovian training was complete, rats were food-restricted to 16g of standard rat chow per day for one week. Rats were then placed in a different set of standard MED Associates behavioral test chambers for appetitive operant conditioning. The chambers were configured such that there was no lever, and nose-poke holes with infrared photobeam sensors to detect head entries were located approximately 2.5 cm to the left and right of the food receptacle, 3 cm above the floor. The right nose-poke hole was designated as inactive such that nose pokes were recorded but had no programmed consequences. Each day, nose pokes in the active hole were reinforced with a chocolate-flavored food pellet under a fixed-ratio (FR) 1 schedule (i.e., only 1 response was required for reinforcement) for the first 5 pellets, then an FR 7 schedule for the next 5 pellets, then a variable-ratio (VR) 20 schedule (i.e., an average of 20 responses were required before reinforcement). Rats received daily 50-min sessions until responding stabilized after 6 days at a rate of ~1 response/sec.
2.2.3. Fear conditioning
Rats were placed on ad libitum feeding for one week after operant conditioning was complete. Rats then underwent fear conditioning using the same procedures as described in Section 2.1.5. However, rather than undergoing extinction training to the tone, the next day rats were placed back into the original context that had been used during initial fear conditioning and behavior recorded for 10 min. No CS or US presentations were given during the contextual fear test session.
2.2.4. Conditioned suppression
Rats were again food-restricted for one week following fear conditioning, and then placed back in the operant conditioning chambers. For the conditioned suppression test, operant conditioning continued as before, with the same reinforcement schedule, but beginning 30 min into the session, six 30-s presentations of the tone CS were superimposed on the ongoing operant behavior using a pseudorandom VI 420-s schedule. Conditioned suppression sessions continued for four days.
2.2.5. Conditioned suppression: data analysis
Nose pokes were recorded in 2-s bins throughout the training sessions. Repeated measures data were compared using linear mixed-effects models. One-sample t-tests were used to test whether conditioned suppression ratios were different from 0.5. Correlation analysis was performed on data from all rats using Fischer's test, and Pearson's correlation coefficient was calculated.
3. Results
3.1. Exp. 1: Pavlovian conditioned approach
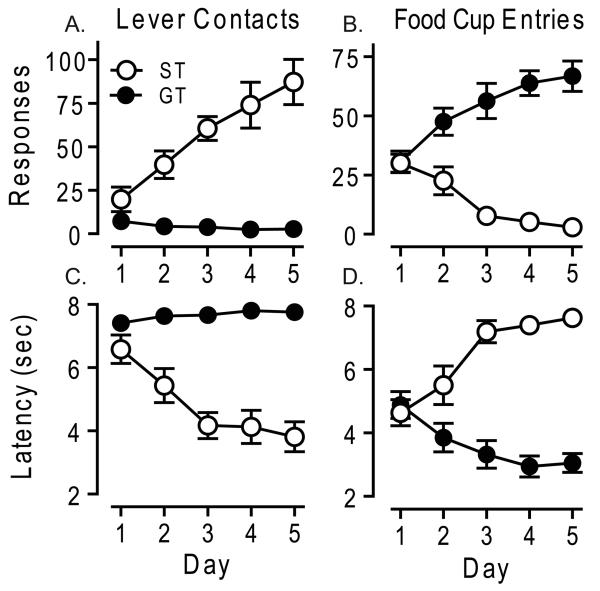
As described previously, rats varied in the topography of the conditioned response (CR) they acquired [7, 10]. Upon presentation of the lever-CS some rats (“sign-trackers”, STs) came to approach and contact the lever, whereas others came to approach and engage the food cup (“goal-trackers”, GTs), even though neither of these responses was necessary for receipt of the food reward. Based on an Approach Index that takes into account the probability and latency of each response type, 10 rats were classified as STs, 12 as IRs, and 14 as GTs. Fig. 1 shows the pattern of Pavlovian conditioned approach behavior in rats classed as STs or GTs as a function of day of training. During the CS period STs contacted the lever more rapidy (Fig 1C; F1, 22 = 266.88, p < 0.001), and avidly than GTs (Fig 1A; F1, 102 = 140.10, p < 0.001), and GTs entered the food cup more rapidly (Fig 1D; F1, 31 = 299.20, p < 0.001) and avidly than STs (Fig 1B; F1, 97 = 114.22, p < 0.001). STs and GTs learned their respective CRs at a comparable rate, as indicated by no significant phenotype-by-day interactions for number of contacts with the lever vs. food cup entries (F4, 110 = 1.37, p = 0.25) or for latency to lever contact vs. food cup entry (F4, 67 = 1.06, p = 0.38).
Figure 1.
Pavlovian Conditioned Approach. Development of different conditioned responses in sign-trackers (ST, n = 10) and goal-trackers (GT, n = 14) during pairing of a lever-CS and a food-US, as a function of day of training. Data points are mean ± SEM for (A) number of lever contacts, (B) latency to first lever contact after lever presentation, (C) number of food cup entries during the lever presentation, (D) latency to first food cup entry after lever presentation.
3.2. Exp. 1: Fear Conditioning
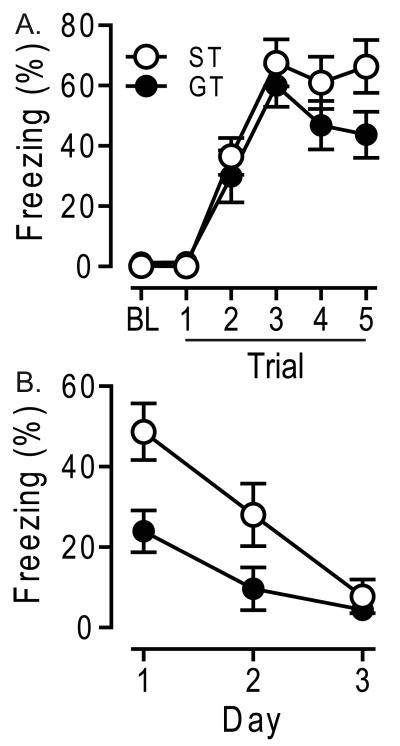
There were no significant differences between STs and GTs in freezing during the tone CS during initial acquisition of fear conditioning (Fig 2A; F1, 61 = 3.43, p = 0.07). During extinction training, baseline freezing before the first CS-alone trial on each day did not differ between STs and GTs (day 1, ST = 24 ± 6%, GT = 27 ± 5%, F1, 70 = 0.20, p = 0.65; day 2, ST = 23 ± 6%, GT = 30 ± 5%, F1, 70 = 0.94, p = 0.34; day 3, ST = 28 ± 6%, GT = 25 ± 5%, F1, 70 = 0.07, p = 0.79). In contrast, freezing to the tone CS was reliably different in the two groups. STs exhibited significantly more freezing to the tone CS than did GTs (Fig 2B; main effect of group, F1, 55 = 12.60, p = 0.001), and planned comparisons showed significant group differences on day 1 (Fig 2B; day 1 ST = 49 ± 7%, GT = 24 ± 5%, t = 2.90, p < 0.01; day 2 ST = 28 ± 8%, GT = 10 ± 5%, t = 2.02, p = 0.056; day 3 ST = 8 ± 4%, GT = 4 ± 3%, t = 0.70, p = 0.49). There was no difference between groups in the rate of extinction across days (Fig 2B; day*phenotype interaction, F2, 54 = 2.14, p = 0.13). Importantly, there was a significant correlation between the Approach Index score derived from the autoshaping procedure and freezing to the tone CS on the initial day of extinction after fear conditioning (r2 = 0.164, p = 0.01).
Figure 2.
Conditioned Freezing. Acquisition and extinction of a conditioned freezing response in sign-trackers (ST, n = 10) and goal-trackers (GT, n = 14). (A) Acquisition. Mean ± SEM % time spent freezing during the baseline period (BL) before tone presentations, and each of five trials, consisting of a 30-sec tone presentation immediately followed by a foot shock. (B) Extinction. Mean ± SEM % time spent freezing during the first six 30-sec tone presentations under extinction conditions, minus baseline % freezing before the tones.
3.3. Exp. 2: Pavlovian Approach and Fear Training
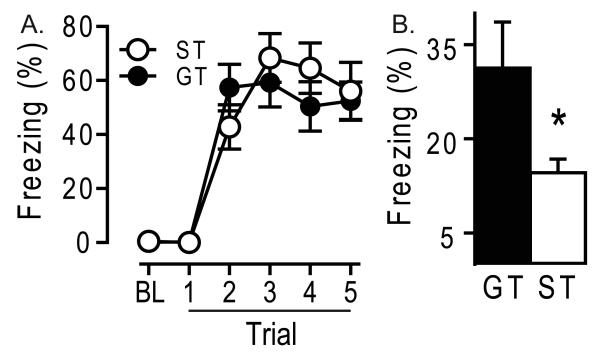
As in Exp. 1, rats were classified as STs and GTs based on their Approach Index score after repeated pairings of the lever CS with the food US over 5 days. In this experiment, 11 rats were classified as STs, 14 as IRs, and 11 as GTs, and their pattern of behavior was similar to that shown in Fig. 1 (data not shown). As in Exp. 1, there were no group differences in the initial acquisition of conditioned fear (Fig 3A; F1, 32 = 0.18, p = 0.68). However, when the rats were placed back into the initial fear-conditioning context in the absence of any tones or shocks to measure contextual fear, GTs exhibited greater freezing than STs (Fig 3B; ST = 14 ± 2, GT = 31 ± 7, t = 2.19, p < 0.05). This reveals that the greater conditioning to discrete CSs in STs relative to GTs is reflected in correspondingly weaker conditioning to the conditioning context.
Figure 3.
Context Fear. Conditioned fear responses in sign-trackers (ST, n = 11) and goal-trackers (GT, n = 11). (A) Acquisition. Mean ± SEM % time spent freezing during the baseline period (BL) before tone presentations, and each of five trials, consisting of a 30-sec tone presentation immediately followed by a foot shock. (B) Context freezing. Mean ± SEM % time spent freezing in the original context in which animals had been shocked. No tones or shocks were administered during this 10-min test. *Significant difference ST vs GT p < 0.05.
3.4. Exp. 2: Conditioned Suppression
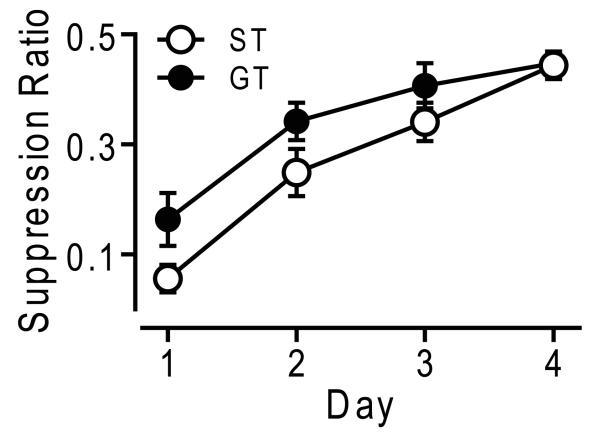
After instrumental training STs and GTs did not differ in rate of responding during 50-min sessions (responses/sec: ST = 1.22 ± 0.11, GT = 1.31 ± 0.05, t = 0.72, p = 0.48). Responses in the inactive hole were minimal for both groups; total inactive nose pokes for the entire 50-min session was 4 ± 1 for STs and 4 ± 1 for GTs. The tone CS suppressed responding in both STs and GTs on all 4 days of testing. However, the CS suppressed responding to a significantly greater extent in STs compared to GTs, as measured by significantly lower mean suppression ratios (Fig 4; main effect of group, F1, 35 = 7.55, p < 0.01; phenotype-by-day interaction, F3, 23 = 0.84, p = 0.49). There was a significant correlation between the Approach Index score, and the suppression ratio on day 1 (r2 = 0.106, p = 0.05).
Figure 4.
Conditioned Suppression. Conditioned suppression ratios (tone responses/[tone responses + pre-tone responses]) in sign-trackers (ST, n = 11) and goal-trackers (GT, n = 11) across 4 days of testing. Data are presented as mean ratio ± SEM.
4. Discussion
We found that rats prone to attribute incentive salience to a reward (food) cue, as assessed by their tendency to approach it (i.e., STs), also tended to attribute greater emotional and/or motivational significance to a fear-provoking aversive cue, assessed by either conditioned freezing behavior or conditioned suppression of ongoing instrumental behavior. Variation in one trait accounted for between 11 to 16 % of the variance in the other, depending on the measure of conditioned fear. In contrast, those rats that did not attribute incentive salience to the discrete reward cue (the lever-CS), but instead, upon CS presentation approached the location of reward delivery (i.e., GTs) showed greater freezing in a context paired with aversive footshock, suggesting that for GTs contextual cues may be more important in regulating their reactions to emotionally significant events.
A number of relatively obvious explanations for the observed correlation between conditioned approach behavior and conditioned fear are not supported by the data. For example, the observed differences might have resulted from individual variation in Pavlovian learning, i.e., forming CS-US associations. However, this is not the case because STs and GTs learned their respective CRs at the same rate, as reported previously [10]. Furthermore, they did not differ in the initial acquisition of conditioned fear (Figs 2A and 3A), and there was also no difference in the rate of extinction of conditioned freezing. The lack of group differences in the initial acquisition of conditioned fear implies that the footshock was equally aversive to STs and GTs, and the lack of differences in baseline freezing in a new context and the increased contextual freezing of GTs in the conditioning context argue that differences in fear conditioning or freezing behavior do not account for the present results. Rather, the results show that specific, cue-induced responses to aversive stimuli correlate with specific, cue-induced appetitive responses.
The source of the correlation between appetitive and aversive responses reported here may be the tendency to transfer motivational salience from a US to a CS. Motivational salience is a basic property of emotional learning that enables cues to command attention, elicit arousal, and instigate action [21]. In the case of the Pavlovian conditioned approach task, STs transfer motivational salience from the food US to the lever CS, such that STs have a greater tendency to approach and contact the lever (Fig 1A), exhibit consummatory behavior toward the lever [22], and will perform a new instrumental task in order to gain access to the lever [10]. A similar process may be at work in the fear conditioning task, with STs transferring more motivational salience from the footshock US to the tone CS, such that the tone itself becomes aversive and elicits a fear response. After fear conditioning, a neutral stimulus will come to elicit a fear response when paired with a CS associated with footshock, and rats will learn a new instrumental task in order to avoid a CS associated with footshock, demonstrating that the CS in some ways takes on the motivational properties of the US [23, 24].
It is important to note that neither appetitive nor aversive behavioral responses were diminished in GTs; all rats avidly consumed the food pellets during the conditioned approach training, and all rats showed equivalent freezing during fear acquisition. In fact, GTs showed higher levels of freezing to the context associated with footshock than STs. Contextual fear and cue-specific fear are dissociable behaviorally and have separate, though overlapping, neural substrates [25, 26]. Since all the animals tested were “normal” outbred animals, the behavioral differences we detected most likely represent different styles of emotional learning that are both generally adaptive in the natural environment. The strategy employed by GTs appears to rely more heavily on contextual information to modulate behavioral responses to motivationally relevant stimuli, whereas ST behavior is more tightly controlled by specific cues regardless of the context. Lack of contextual control over fear is a particularly disabling feature of several anxiety disorders, producing inappropriate fear responses in safe contexts and thereby interfering with work, family life, and other daily activities.
Individual differences in the intensity of emotional reactions to conditioned cues has been proposed as a key personality trait that can predispose toward or protect against various forms of psychopathology [14, 27]. In particular, the property of transferring motivational salience to appetitive cues is described in theories of addiction as transferring “incentive salience,” and is thought to be a key process in the transition from casual drug use to compulsive, uncontrollable drug use that comprises addiction [28]. Many anxiety disorders, including PTSD but also specific phobias and obsessive-compulsive disorder (OCD), are defined in part by abnormally intense emotional and behavioral responses to relatively mild aversive stimuli [18]. Aberrant attribution of motivational salience to irrelevant stimuli has also been implicated in the etiology of schizophrenia and other psychotic disorders, in which minor coincidences or ordinary internal sensations take on a delusional level of significance [29]. Many of these disorders are frequently comorbid with borderline personality disorder, which is defined in part by exaggerated emotional responses to relatively minor environmental stimuli [18]. The tendency of STs to transfer motivational significance to cues associated with emotionally salient events may be analogous to a core behavioral trait that reaches an extreme in borderline personality disorder, and that can confer vulnerability to a range of psychiatric comorbidities, including PTSD and addiction.
A shared vulnerability factor could at least partially explain the high rates of comorbidity commonly observed between disparate disorders like substance abuse and PTSD [30, 31, 32, 33]. PTSD and substance abuse are both complex disorders with several interacting factors contributing to vulnerability and resilience in each individual. Our finding that only 11-16% of the variance in conditioned fear responses is accounted for by conditioned approach responses (and vice versa) is consonant with the complexity of these two disorders, as is the simple clinical observation that many patients have PTSD without comorbid substance abuse, and many substance abuse patients do not have PTSD. However, the fact that there is a correlation between conditioned approach and conditioned fear indicates that some portion of vulnerability to PTSD and substance abuse could be conferred by a common behavioral trait.
The neurobiological basis of individual differences in motivational salience is far from clear, but several lines of evidence have implicated dopaminergic activity as a possible candidate. In addition to signaling reward, dopaminergic activity seems to play an important role in behavioral responses to all motivationally salient stimuli, including surprising, novel, or even aversive stimuli [21, 34, 35]. It has recently been suggested that some populations of dopamine neurons may encode emotional valence, while others specifically encode motivational salience irrespective of valence [36]. Interestingly, STs show higher levels of dopaminergic activity in the nucleus accumbens than GTs [37, 38]. In addition, the transfer of stimulus-evoked dopamine release from US to CS during Pavlovian conditioned approach occurs preferentially in STs relative to GTs [39]. Further characterization of the precise neurobiology underlying individual differences in the tendency to transfer motivational salience to predictive cues could lead to important insights into some of the vulnerabilities that lead to clusters of comorbid psychiatric disorders commonly seen in clinical practice.
Acknowledgements
We wish to thank Allison Gates for her technical assistance with the conditioned suppression experiment. This research was supported by NIH grants R37DA04294 to TER and grant R01MH065961 to SM.
Abbreviations
- PTSD
post-traumatic stress disorder
- STs
sign-trackers
- GTs
goal-trackers
- VI
variable interval
- CS
conditioned stimulus
- US
unconditioned stimulus
- ITI
intertrial interval
- IRs
intermediate responders
- FR
fixed-ratio
- VR
variable-ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 2.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 3.Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 5.Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–45. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 7.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hearst E, Jenkins HM. Sign-tracking: the stimulus-reinforcer relation and directed action. The Psychonomic Society; Austin, TX: 1974. [Google Scholar]
- 9.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Erlbaum; Hillsdale, NJ: 1977. pp. 66–97. [Google Scholar]
- 10.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20:413–22. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- 13.Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000;61:225–40. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- 14.Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am Psychol. 2000;55:1196–214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- 17.Childress A, Ehrman R, McLellan AT, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 19.Casada JH, Amdur R, Larsen R, Liberzon I. Psychophysiologic responsivity in posttraumatic stress disorder: generalized hyperresponsiveness versus trauma specificity. Biol Psychiatry. 1998;44:1037–44. doi: 10.1016/s0006-3223(98)00182-6. [DOI] [PubMed] [Google Scholar]
- 20.West B, Welch KB, Galecki AT. Linear mixed models : a practical guide using statistical software. Chapman & Hall/CRC; Boca Raton: 2007. [Google Scholar]
- 21.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 22.Davey GC, Cleland GG. Topography of signal-centered behavior in the rat: Effects of deprivation state and reinforcer type. J Exp Anal Behav. 1982;38:291–304. doi: 10.1901/jeab.1982.38-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizley RC, Rescorla RA. Associations in second-order conditioning and sensory preconditioning. J Comp Physiol Psychol. 1972;81:1–11. doi: 10.1037/h0033333. [DOI] [PubMed] [Google Scholar]
- 24.Kim SD, Rivers S, Bevins RA, Ayres JJ. Conditioned stimulus determinants of conditioned response form in Pavlovian fear conditioning. J Exp Psychol Anim Behav Process. 1996;22:87–104. doi: 10.1037//0097-7403.22.1.87. [DOI] [PubMed] [Google Scholar]
- 25.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–6. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 26.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Posner J, Russell JA, Peterson BS. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 2005;17:715–34. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 29.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- 31.Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664–70. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- 32.Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Curr Opin Psychiatry. 2007;20:614–8. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- 33.Brown PJ, Stout RL, Mueller T. Substance use disorder and posttraumatic stress disorder comorbidity: addiction and psychiatric treatment rates. Psychol Addict Behav. 1999;13:115–22. [Google Scholar]
- 34.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–6. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 35.Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–51. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–17. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 38.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 39.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2010 doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]