Abstract
Extinction of classically and instrumentally conditioned behaviors, such as conditioned fear and drug-seeking behavior, is a process of active learning, and recent studies indicate that potentiation of glutamatergic transmission facilitates extinction learning. In this study we investigated the effects of the type 5 metabotropic glutamate receptors (mGluR5) positive allosteric modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) on the extinction of cocaine-seeking behavior in rats with a history of intravenous cocaine self-administration. To assess its effects on acquisition and consolidation of extinction learning, CDPPB (60 mg/kg) or vehicle was administered either 20 min prior to, or immediately following, each of 10 extinction sessions, respectively. When administered prior to each extinction session, CDPPB produced a significant reduction in the number of active lever presses on all 10 days of extinction training as compared to vehicle-treated animals. When administered following each extinction session, a significant reduction in the number of active lever presses was observed on the 2nd through 10th day of extinction. Both treatment regimens also reduced the number of extinction training sessions required to meet extinction criteria. Pre- or post-extinction training administration of CDPPB did not alter responding on the inactive lever and had no effects on open field locomotor activity. These data indicate that positive allosteric modulation of mGluR5 receptors facilitates the acquisition and consolidation of extinction learning following cocaine self-administration, and may provide a novel pharmacological approach to enhancing extinction learning when combined with cue exposure therapy for the treatment of cocaine addiction.
Keywords: cocaine, CDPPB, mGluR5, extinction, learning, self-administration, acquisition, consolidation
One of the most problematic issues in the treatment of drug-addiction is the persistence of drug craving and drug-seeking behavior long after the symptoms of acute drug withdrawal have subsided. As a result of chronic and repetitive drug use, drug-associated cues and contexts acquire an excessive degree of motivational salience. This “hypersalience” of drug-associated cues and contexts is a result of associative overlearning produced by the drug’s ability to create strong and lasting associations between its euphorigenic effects and the cues and contexts that are present when the drug is administered. As a result, these cues and contexts can often trigger memories of prior drug use and drug craving (an intense desire for the drug that is often evoked by drug-associated contexts and cues), which may result in drug-seeking behavior and ultimately relapse. In addition to associative overlearning, the ritualistic nature of chronic drug-taking behavior transitions from self-regulated to compulsive and habitual, representing a form of maladaptive instrumental overlearning. The two forms of overlearning result in behavioral inflexibility and perseverance of drug-seeking that is resistant to change during the course of treatment, and are often a cause for relapse and noncompliance with different treatment regimens (Childress et al., 1999; Cleva & Gass, 2010; Kalivas, Volkow, & Seamans, 2005).
Extinction is defined as the gradual reduction of a conditioned response (CR) when the CR is no longer reinforced or the unconditioned stimulus (US) is no longer presented in the presence of conditioned stimulus (CS). Repeated pairings of a discrete CS (i.e., a light and/or tone) with a US (i.e., drug infusion) enables the CS to evoke specific behaviors since the CS is associated with and predicts the availability of the US. Extinction can be defined as a decline in the magnitude and/or frequency of the CR either within a single extinction training session as well as over successive extinction training sessions conducted on a routine basis (i.e., daily or weekly). Extinction is a process of new and active learning (Bouton, 2000, 2004), and as with other forms of learning and memory, it consists of separate phases including acquisition, consolidation, recall, and reconsolidation (Lee, 2009; Myers & Carlezon, 2010b; Taylor, Olausson, Quinn, & Torregrossa, 2009). In behavioral terms, acquisition of extinction learning occurs when the organism first begins to learn that the CS no longer results in the presentation of the US. Enhancement of the acquisition of extinction learning can be achieved via experimental manipulations performed prior to extinction training sessions. Consolidation, on the other hand, is the strengthening and storage of new CS-US expectancies and relationships that carry forward from one extinction training session to the next. Enhancement of the consolidation of extinction learning can be achieved via experimental manipulations performed following extinction training sessions. Finally, reconsolidation is a process by which prior extinction memories are recalled and then reconsolidated back into long-term memory. However, recent evidence suggests that memories that are recalled and reconsolidated are labile and amenable to modification (Lee, 2009; Taylor, Olausson, Quinn, & Torregrossa, 2009).
Cellular hallmarks of learning and memory and associated synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) of synaptic efficacy require glutamatergic transmission in order to occur (Miyamoto, 2006; Rao & Finkbeiner, 2007; Reis et al., 2009; Riedel, Platta, & Micheaub, 2003; Robbins & Murphy, 2006), suggesting that potentiation of glutamate-mediated neural plasticity could serve as an effective adjunct for facilitating extinction learning. Recent studies have shown that potentiation of glutamatergic transmission with ligands such as the N-methyl-D-aspartate (NMDA) partial agonist D-cycloserine (DCS), the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor potentiator 2-[2,6-difluoro-4-({2-[(phenylsulfonyl)amino]ethyl}thio)phenoxy]acetamide (PEPA), or the cystine prodrug N-acetylcysteine (NAC, which stimulates the cystine-glutamate exchanger and normalizes drug-induced deficits in extracellular glutamate during drug withdrawal), reduce extinction responding following active drug self-administration, reduce re-acquisition of drug intake, or facilitate the extinction of a conditioned place preference (CPP) produced by drugs of abuse (Botreau, Paolone, & Stewart, 2006; LaLumiere, Niehoff, & Kalivas, 2010; Nic Dhonnchadha et al., 2010; Thanos, Bermeo, Wang, & Volkow, 2009; Torregrossa, Sanchez, & Taylor, 2010; Zhou & Kalivas, 2008). DCS has also been shown to facilitate the extinction of a naloxone-induced conditioned place aversion in morphine-dependent rats (Myers & Carlezon, 2010a), suggesting that this compound may facilitate the extinction of both appetitive and aversive drug-associated memories. Collectively, these findings suggest that extinction learning can be augmented through pharmacological manipulation of glutamate transmission (Cleva, Gass, Widholm, & Olive, in press; Myers & Carlezon, 2010b; Myers, Carlezon, & Davis, in press).
An alternative strategy to enhancing glutamatergic transmission, and therefore possibly extinction learning, is by allosteric potentiation of the function of type 5 metabotropic glutamate receptors (mGluR5). These receptors are predominantly localized to the perisynaptic annulus of postsynaptic dendritic spines, where they are positively coupled to NMDA receptor function and mediate various forms of synaptic plasticity (Ayala et al., 2009; Gladding, Fitzjohn, & Molnar, 2009; Luscher & Huber, 2010) and learning and memory (Simonyi, Schachtman, & Christoffersen, 2010). For example, potentiation of mGluR5 function has been shown to enhance the induction of LTP and LTD in CA1 region of the hippocampus and improve performance in a spatial memory task (Ayala et al., 2009; Popkirov & Manahan-Vaughan, in press; Rosenbrock et al., 2010). Potentiation of mGluR5 function has also been shown to facilitate the extinction of a cocaine conditioned place preference (Gass & Olive, 2009), an effect that is reversed by blockade of either mGluR5 or NMDA receptors. In contrast, mice carrying a null mutation in the gene encoding the mGluR5 receptor protein show deficits in extinction learning following cue or contextual fear conditioning (Xu, Zhu, Contractor, & Heinemann, 2009). These data strongly support a role for mGluR5 receptors in extinction learning.
In the present study, we sought to determine if enhancement of mGluR5 receptor function by the mGluR5 positive allosteric modulator (PAM) 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) would enhance extinction learning following intravenous cocaine self-administration, as evidenced by reductions in the number of presses on a lever that previously resulted in intravenous cocaine delivery. We also sought to determine if CDPPB-treated animals would require fewer extinction training sessions to reach pre-defined extinction criteria as compared with vehicle-treated animals. A tertiary aim of the present study was to determine if CDPPB would enhance both the acquisition and consolidation of extinction learning, as assessed by administration of the compound prior to or immediately following extinction training sessions. Finally, to rule out the possibility that any observed reductions in lever pressing during extinction produced by CDPPB might be attributable to non-specific motor effects, we also tested the effects of this compound on open field locomotor activity.
Method
Subjects
Experimental procedures conformed to the 1996 National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, the 2003 Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research, and were approved by an Institutional Animal Care and Use Committee at the Medical University of South Carolina. Male Sprague-Dawley rats (250–275 g upon arrival were obtained from Harlan Laboratories, Indianapolis, IN). A total of 89 rats were pre-implanted with silastic rounded tip jugular vein catheters by Harlan Laboratories prior to arrival in the animal facility, and catheters were filled with HepLock solution to maintain patency during shipment. Rats were housed individually in standard polycarbonate cages and given 20 g of lab chow per day. Rats were maintained on this schedule of mild food restriction throughout the course of the experiments, except prior to and during recovery from surgical procedures, when food was available ad libitum. Water was freely available at all times except during behavioral testing. The animal housing room was maintained on a reversed 12 h light-dark cycle (lights off at 0800 h), with controlled temperature and humidity within NIH guidelines. All experimentation was conducted during the dark phase of the light-dark cycle except for the 16 h overnight training session that commenced at 1600 h and terminated at 0800 h the following morning.
Self-Administration Apparatus
Drug self-administration and extinction testing were conducted in computer-interfaced operant self-administration chambers (ENV-008; Med Associates, St. Albans, VT) that were individually housed in melamine sound-attenuating cubicles equipped with a house light (for general illumination) and exhaust fan designed to mask external noise and odors. Each chamber (28 × 27 × 22 cm) was equipped with a 2.5 cm retractable “active” lever mounted 7 cm above a stainless steel rod floor. Above the active lever was a 2.5 cm diameter white stimulus light that was illuminated for 2 sec following each lever press and a Sonalert speaker that provided an auditory stimulus (~65 dB, 2900 Hz) during drug delivery and extinction procedures. Chambers were also equipped with an “inactive” lever. Each press on the active lever activated a drug syringe infusion pump (located outside of the self-administration chamber) that delivered 0.06 ml of drug solution over 2 sec, while each press on the inactive lever produced no programmed consequences. Located between the two levers was a small 30 × 30 mm receptacle for delivery of food pellets during self-administration training only. Tygon microbore tubing (0.02” ID) connected the syringe containing the cocaine solution to one channel of a single channel liquid swivel mounted on a counterbalanced lever arm located atop the self-administration chamber, and to connect the swivel to the intravenous catheter access port via a stainless steel spring tether.
Vascular Access Port Implantation
Rats with pre-implanted jugular vein catheters were anesthetized with isoflurane (2% v/v) vaporized in medical grade breathing air at a flow rate of 0.4 L/min. The skin area between the scapulae where the catheter exited the dorsum was shaved and scrubbed with betadine and 0.1% v/v H2O2. A 2 cm incision was then made to connect the catheter to a backmount threaded vascular access port (Plastics One, Roanoke, VA). The catheter was secured to the surrounding tissue with sutures and a polyethylene mesh collar. The wound was then treated with 2% bacitracin/polymixin B/neomycin and 5% xylocaine (Henry Schein Veterinary Supply, Melville, NY), and sutured closed with 3-0 Vicryl sutures. The HepLock solution was then evacuated from the catheter and the catheter was then flushed with 0.2 ml heparin (70 U/ml). The access port was then sealed with a piece of Tygon tubing closed at one end and a protective cap. Following surgical procedures, rats were allowed at least 5 days of recovery, during which they received daily intravenous infusions of 0.1 ml antibiotic solution (100 mg/ml cefazolin) and 0.2 ml heparin (70 U/ml) to minimize post-surgical infection and maintain catheter patency. Rats were also administered carprofen (2.5 mg/kg s.c.) once daily to minimize post-surgical pain and discomfort. Catheter patency was tested periodically throughout the experiment by infusion of 0.2 ml of sodium methohexital (Brevital sodium, 10 mg/ml) into the catheter and observation of brief loss of postural muscle tone.
Intravenous Self-Administration Procedures
After recovery from vascular access port implantation surgery, rats were initially trained to press the active lever for delivery of a 45 mg food pellet into the receptacle on a continuous fixed ratio 1 (FR1) schedule of reinforcement during a 16 h overnight training session. Approximately 24 h following the end of the initial overnight training session, rats were then allowed to acquire intravenous self-administration of cocaine (0.5 mg/kg per infusion, delivered in a volume of 0.06 ml over a 2 s period) in daily 2 h sessions by substituting cocaine infusions as the reinforcer. This dose of cocaine was chosen since it has been demonstrated that higher doses of cocaine (i.e., 0.5 mg/kg/infusion and above) are more reinforcing than lower doses as measured by breakpoints on a progressive ratio schedule of reinforcement (Arnold & Roberts, 1997; Roberts, Loh, & Vickers, 1989), and therefore potentially more resistant to extinction than lower doses. The start of each self-administration session was signaled by placement of the animal in the self-administration apparatus, connection of the infusion tether to the access port, illumination of the house light, and extension of the levers into the chamber. Each drug infusion was accompanied by concurrent illumination of the stimulus light above the active lever for 2 sec (during infusion of the drug) and presentation of an auditory stimulus (~65 dB, 2900 Hz, 2 sec duration). Each cocaine infusion was followed by a 20-sec timeout period, during which additional active lever presses were recorded but produced no programmed consequences. Catheters were flushed with 0.1 ml heparin solution (70 U/ml) prior to and after each self-administration session, and with 0.1 ml antibiotic solution (100 mg/ml of cefazolin) after each session to maintain patency and prevent infection. Self-administration sessions were conducted daily (minimum 10 days) until maintenance criteria was reached, which required < 15% variability in active lever presses across two consecutive self-administration sessions to be observed.
Extinction Procedures
Extinction procedures and treatment with the mGluR5 positive allosteric modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) commenced after maintenance criteria was reached. During extinction, animals were randomly subdivided into four groups (CDPPB or vehicle pre-session, CDPPB or vehicle post-session). Extinction training was conducted in 2 hr daily sessions in the presence of drug-associated cues (i.e., presentation of the light/tone stimulus complex for 2 sec following each active lever press, followed by a 20 sec timeout), since it has been observed that such procedures produce drug-seeking behavior that is more resistant to extinction than that observed during extinction in the absence of drug-associated cues (Feltenstein & See, 2006; Ranaldi & Roberts, 1996). No drug solution was infused during extinction sessions, and pressing the inactive lever during extinction had no programmed consequences. Twenty min prior to or immediately after each extinction training session, animals were administered vehicle or CDPPB (60 mg/kg s.c.) according to their group assignment and returned to their home cages. The 60 mg/kg dose of CDPPB was chosen since initial findings with a lower (30 mg/kg) dose, which we previous have reported to produce modest and short-lived reductions in extinction responding in rats trained to self-administer a 0.3 mg/kg/infusion dose of cocaine (Olive, 2010a), was found to be ineffective in reducing extinction responding when administered prior to extinction sessions following self-administration of a higher (0.5 mg/kg/infusion) dose of cocaine (n=8, p > 0.05 vs. vehicle). Therefore, a 60 mg/kg dose of CDPPB was used for all subsequent studies. Pre-session animals were placed in the self-administration apparatus 20 min following treatment and lever pressing behavior was recorded. Extinction criteria were considered to have been met when, in 3 of the 10 extinction sessions, the number of active lever presses exhibited by an individual animal was <25% of those observed on the average of the last two days of active drug self-administration for that particular animal. These extinction criteria were based on an average of those used in two previous studies where extinction criteria were set at <20% of the number of active lever presses during last two days of cocaine self-administration (Knackstedt & Kalivas, 2007) and <30% of active lever presses during the last 3 days of cocaine self-administration number (Jin et al., 2010). In addition, this percentage based approach to extinction criteria is more stringent than those that require the number of active lever presses to be less than a set value (i.e., <10 lever presses) since this latter method does take into account whether an animal previously demonstrated high or low levels of active lever pressing during self-administration procedures.
Open Field Locomotor Activity Assessment
To determine if any observed decreases in extinction responding in CDPPB-treated animals could potentially be a result of nonspecific motor impairments, open field locomotor activity was assessed in a separate set of drug-naïve animals. The locomotor activity assessment apparatus consisted of a 30 × 30 cm open field testing arena equipped with infrared photobeams that measured horizontal locomotor activity at 100 ms resolution (Kinder Scientific, Poway, CA). Each open field testing arena was located in a sound-attenuating cubicle equipped with a house light and fan to mask external noise and odors, and was interfaced to a PC computer. Rats were first habituated to the testing apparatus by placement in the testing apparatus for 1 hr per day for two consecutive days. Next, animals (n=8 per group) received either vehicle or CDPPB (60 mg/kg s.c.) 20 min prior to each of an additional 5 subsequent locomotor test sessions. Each test session was 1 hr in length and was conducted on 5 consecutive days. Distance traveled (in cm) was recorded by the computer.
Drugs
Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in sterile saline prior to i.v. administration. CDPPB was custom synthesized by Azopharma Product Development Group (Hollywood, FL) according to previously published methods (Kinney et al., 2005; Lindsley et al., 2004), purified to >95% purity by liquid chromatography-mass spectrometry, and suspended in 10% v/v Tween-80 (Sigma-Aldrich).
Statistical Analysis
Data were analyzed using SigmaPlot version 11.0 software (Systat Inc., San Jose, CA, USA). Effects of CDPPB on the number of active and inactive lever presses during self-administration (SA, calculated as an average of the last two days of active drug self-administration) and during extinction were analyzed by a two-way repeated measures analysis of variance (ANOVA) with treatment group and session as factors. The number of sessions required to reach extinction criteria were analyzed by a one-way ANOVA. Effects of CDPPB on locomotor activity were analyzed by a two-way repeated measures ANOVA with treatment group and day as factors. All ANOVAs were followed by pairwise Holm-Sidak multiple comparisons post hoc tests. p < 0.05 was considered statistically significant for all tests performed. All data are presented as mean ± SEM.
Results
Of the 89 rats that were implanted with jugular vein catheters, nine rats were removed from the study prior to the commencement of extinction training due catheter patency issues. Eight animals that completed cocaine self-administration testing in initial studies that were treated with a lower (30 mg/kg) dose of CDPPB administered prior to extinction sessions showed no reductions in extinction responding. Therefore, a 60 mg/kg dose was used for the remainder of the experiments. The remaining 72 rats completed cocaine self-administration and extinction training. However, data from an additional 10 rats (6 that received vehicle treatment, 4 that received CDPPB treatment) were excluded from analysis due to computer malfunction on one day of extinction training, which resulted in loss of data.
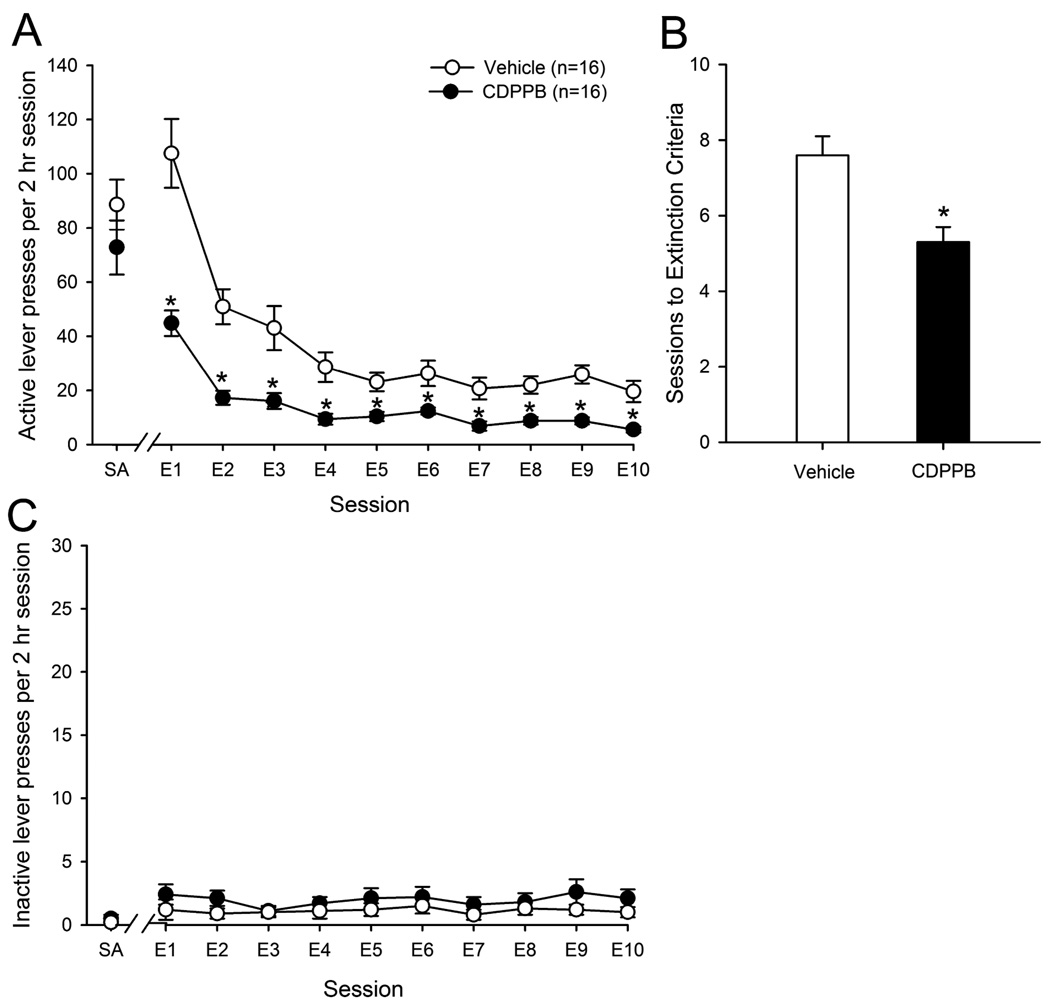
Analysis of the effects of administration of CDPPB prior to daily extinction sessions on the number of active lever presses emitted during the last 2 days of self-administration and during 10 daily extinction sessions revealed a significant effect of treatment group (F(1,30) = 25.6, p < 0.001), session (F(10,300) = 58.5, p < 0.001) as well as a treatment group × session interaction (F(10,300) = 5.4, p <0.001). Post-hoc analyses revealed that animals treated with CDPPB emitted significantly fewer presses on the active lever during each of the 10 extinction sessions as compared with vehicle-treated animals (see Figure 1A). Additionally, as shown in Figure 1B, the number of extinction sessions required to reach extinction criteria was significantly lower in CDPPB-treated animals as compared to vehicle-treated animals (F(1,30) = 11.8, p <0.005). There was no effect of treatment group, session, nor a treatment group × session interaction with regards to the number of inactive lever presses emitted during the last two days of SA and during extinction sessions (all p-values > 0.05; see Figure 1C). Since in these animals CDPPB was administered prior to extinction training sessions, these data indicate that positive allosteric modulation of mGluR5 receptors facilitates the acquisition of extinction learning following cocaine self-administration.
Figure 1.
A: Administration of the mGluR5 PAM CDPPB prior to extinction training sessions reduces extinction responding (i.e., responses on the lever that previously resulted in cocaine infusions); *p<0.05 vs. vehicle treated animals on the same day of extinction (E1, etc.). B: Pre-session administration of CDPPB reduces the number of training sessions required to achieve extinction criteria. *p<0.05 vs. vehicle treated animals. C: CDPPB had no effect on the number of inactive lever presses emitted during extinction training. SA represents the average number of lever presses emitted during the last 2 days of active cocaine self-administration.
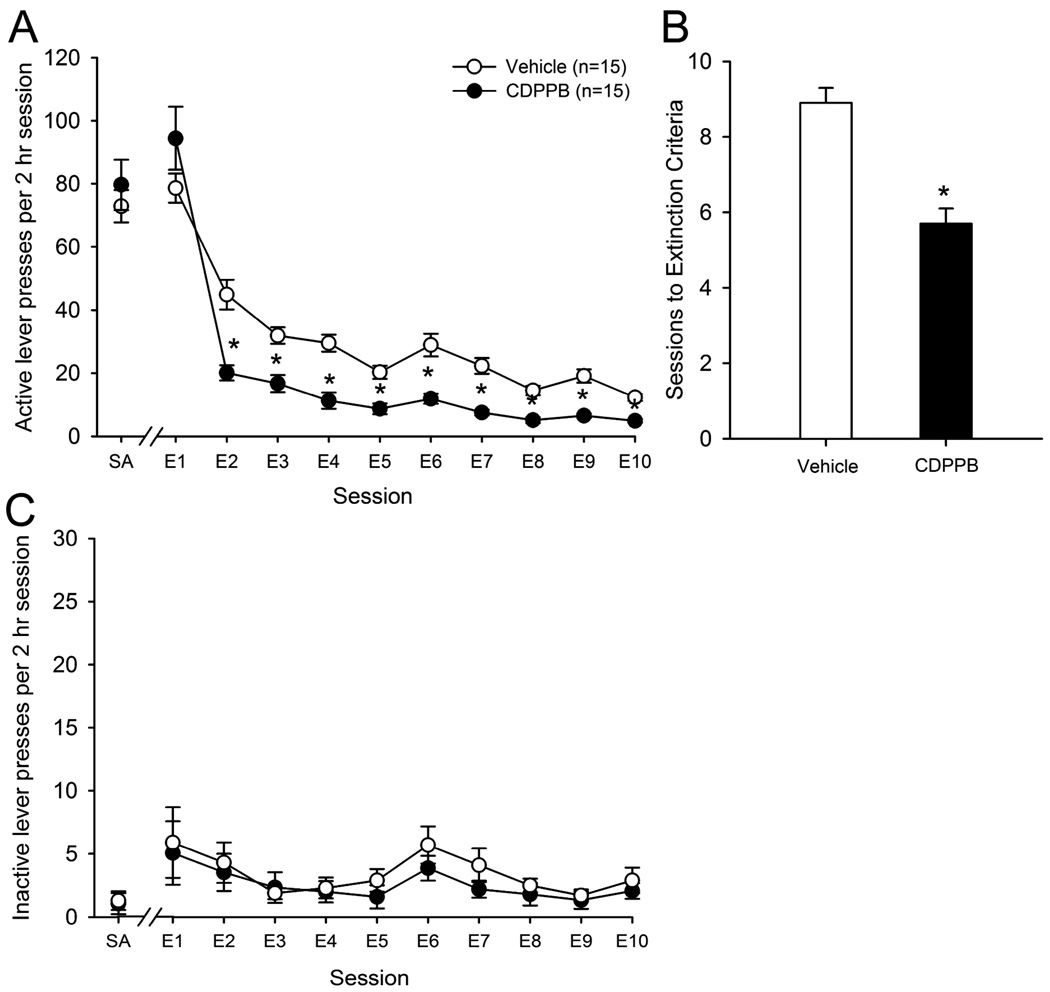
Similarly, the effects of administration of CDPPB immediately following daily extinction sessions on the number of active lever presses emitted during the last 2 days of SA and subsequent extinction sessions revealed a significant effect of treatment group (F(1,28) = 14.2, p < 0.005), session (F(10,280) = 100.5, p < 0.001) as well as a treatment group × session interaction (F(10,280) = 4.1, p < 0.001). Post-hoc analyses revealed that animals treated with CDPPB emitted significantly fewer presses on the active lever during the 2nd through 10th extinction sessions as compared with vehicle-treated animals (see Figure 2A). Additionally, the number of extinction sessions required to reach extinction criteria was significantly lower in CDPPB-treated animals as compared to vehicle-treated animals (F(1,28) = 33.7, p < 0.001; see Figure 2B). Statistical analyses indicated a significant effect of session (F(10,280) = 3.5, p < 0.001) but not treatment group (F(10,280) = 0.4, p > 0.05) nor a treatment group × session interaction (F(10,280) = 0.2, p > 0.05) with regards to the number of inactive lever presses emitted during the last two days of SA and during extinction sessions (see Figure 2C). These results indicate that administration of CDPPB immediately following extinction training facilitates the consolidation of extinction learning following cocaine self-administration.
Figure 2.
A: Administration of the mGluR5 PAM CDPPB immediately following extinction training sessions reduces extinction responding on extinction days E2–E10; *p<0.05 vs. vehicle treated animals on the same day of extinction. B: Post-session administration of CDPPB reduces the number of training sessions required to achieve extinction criteria. *p<0.05 vs. vehicle treated animals. C: CDPPB had no effect on the number of inactive lever presses emitted during extinction training.
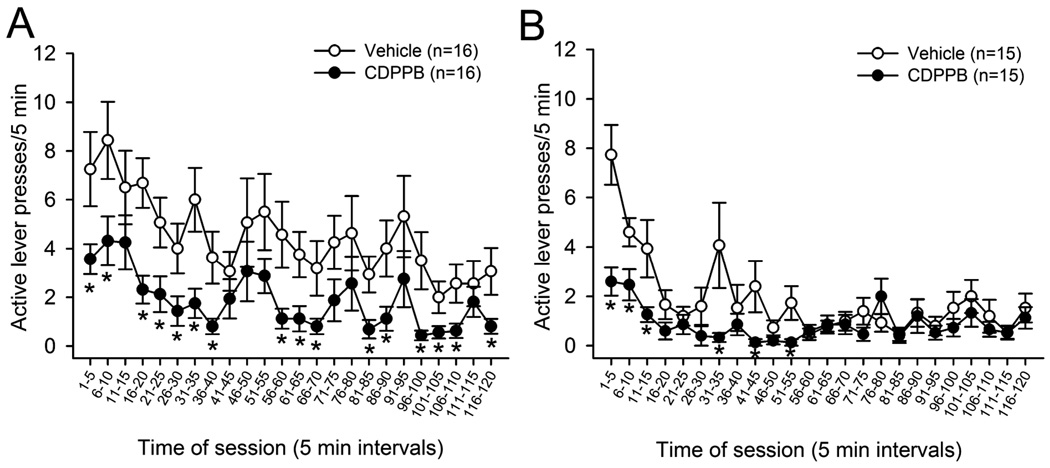
Because analysis of extinction responding over entire 2 hr extinction sessions provides information primarily on general effects of CDPPB, we analyzed the time course of the effects of CDPPB on extinction responding in 5 min time bins. This analysis was performed on the first day of extinction (E1, Figure 3A) in animals receiving CDPPB or vehicle injections prior to extinction sessions (i.e., during acquisition of extinction learning), and on the 2nd day of extinction (E2, Figure 3B) in animals receiving CDPPB or vehicle injections immediately following extinction sessions (i.e., during consolidation of extinction learning). In animals treated with vehicle or CDPPB prior to the first day of extinction training, a significant effect of treatment group (F(1,30) = 21.5, p < 0.001), time interval (F(23,690) = 4.1, p < 0.001), and a treatment group × interval interaction (F(23,690) = 0.5, p < 0.05) were observed. Post-hoc analyses revealed that the number of active lever presses per 5 min time interval was reduced in CDPPB-treated animals during 16 of the 24 time intervals as compared with vehicle-treated animals, and this reduced responding was evident throughout most of the session. In animals treated with vehicle or CDPPB immediately following E1, analysis of extinction responding on the following day (E2) revealed a significant effect of treatment group (F(1,28) = 20.1, p < 0.001), time interval (F(23,644) = 6.8, p < 0.001), and a treatment group × interval interaction (F(23,644) = 2.8, p < 0.001). Post-hoc analyses revealed that the number of active lever presses per 5 min time interval was reduced in CDPPB-treated animals during 6 of the 24 time intervals as compared with vehicle-treated animals, all of which were in the first 60 min of the session. These data indicate that pre-session administration of CDPPB reduces extinction responding throughout most of the subsequent extinction session, whereas post-session administration of CDPPB primarily reduces extinction responding during the first half of the subsequent extinction session. The fact that significant effects of time interval were observed in both analyses provides evidence suggesting that within-session extinction learning occurs regardless of treatment timing.
Figure 3.
A: Patterns of extinction responding in 5-min intervals during extinction day 1 (E1) of animals treated with vehicle or CDPPB prior to each extinction session. B: Patterns of extinction responding in 5-min intervals during extinction day 2 (E2) of animals treated with vehicle or CDPPB immediately following each extinction session. *p<0.05 vs. vehicle treated animals during the same time interval.
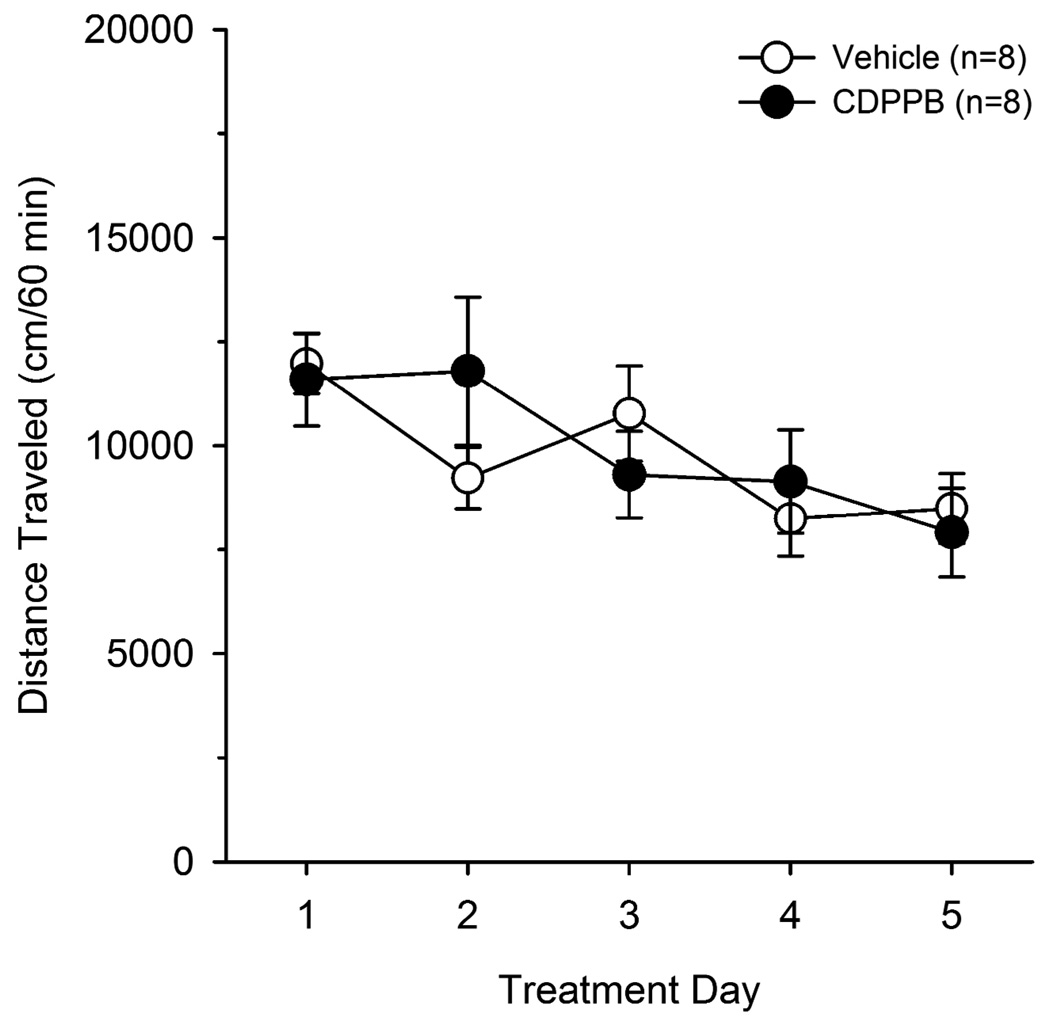
Finally, to rule out the possibility that CDPPB-induced reductions in extinction responding were due to nonspecific motor impairments, we tested the effects of vehicle or CDPPB treatment on open field locomotor activity once daily for 5 consecutive days (see Figure 4). A significant effect of treatment was not observed (F(1,56) = 0.002, p > 0.05), indicating a lack of motor impairing effects of CDPPB. However, a significant effect of treatment day was observed (F(4,56) = 7.9, p < 0.001), and post hoc analysis revealed that locomotor activity was reduced on days 3, 4, and 5 of treatment as compared with day 1. This effect was observed in both vehicle and CDPPB treated animals, suggesting a general habituation to the testing environment rather than a motor impairment effect that was specific to animals treated with CDPPB.
Figure 4.
CDPPB had no effect on open field locomotor activity in an open field testing environment when administered prior to 1 hour daily sessions for 5 consecutive days. A significant effect of day was observed in both vehicle and CDPPB treated animals, suggesting a general habituation to the locomotor testing environment.
Discussion
Our findings indicate that positive allosteric modulation of mGluR5 receptors by CDPPB facilitates both the acquisition and consolidation of extinction learning following cocaine self-administration. Reductions in extinction responding by CDPPB are not likely due to nonspecific decreases in locomotor activity, since (1) this compound did not alter locomotor activity in an open field testing environment, and (2) extinction responding was decreased on the 2nd through 10th day of extinction in animals that were treated with CDPPB following each extinction training session (i.e, approximately 24 hours following the previous drug administration). The plasma elimination half-life of CDPPB is 4.4 hours (Kinney et al., 2005), and thus the amount of CDPPB administered immediately following the extinction session on one day was largely eliminated by the time the extinction training session on the following day was conducted.
A more detailed analysis of the temporal patterns of extinction responding showed that pre-session treatment with CDPPB produced lasting reductions in responding that lasted for the majority of the extinction training sessions, whereas post-session treatment of CDPPB produced reductions in responding that were primarily confined to the first hour of the extinction session. The reasons for these observed differences are currently unknown, but may reflect differential effects of mGluR5 positive allosteric modulation on the cellular mechanisms underlying acquisition vs. consolidation of extinction learning.
A likely neurochemical mechanism by which CDPPB enhances extinction learning is via indirect stimulation of NMDA receptors, since mGluR5 receptors are structurally and biochemically coupled to NMDA receptor function (Awad, Hubert, Smith, Levey, & Conn, 2000; Doherty, Palmer, Henley, Collingridge, & Jane, 1997; Homayoun, Stefani, Adams, Tamagan, & Moghaddam, 2004; Pisani et al., 2001; Rosenbrock et al., 2010). In addition to potentiating NMDA receptor function, mGluR5 receptor PAMs also reverse cognitive deficits as well as behavioral and electrophysiological changes produced by NMDA receptor antagonists such as MK-801 (Homayoun & Moghaddam, 2008, 2010; Lecourtier, Homayoun, Tamagnan, & Moghaddam, 2007; Stefani & Moghaddam, 2010; Vardigan, Huszar, McNaughton, Hutson, & Uslaner, 2010). Thus, mGluR5 PAMs may represent a novel class of cognition-enhancing compounds that may be of potential benefit in the treatment of schizophrenia and drug addiction (Conn, Lindsley, & Jones, 2009; Gregory, Dong, Meiler, & Jeffrey Conn, 2010; Olive, 2010b; Uslaner et al., 2009).
With regards to drug addiction, we previously showed that administration of CDPPB dose-dependently facilitated the extinction of a previously established conditioned place preference for cocaine (Gass & Olive, 2009), and in this study the effects of CDPPB were blocked by co-administration of antagonists of the mGluR5 or NMDA receptor. However, in the current study we did not examine whether the ability of CDPPB to facilitate extinction learning was due to an indirect potentiation of NMDA receptor function, and future studies are warranted to confirm this likely mechanism.
Another area of further exploration is to determine the brain regions in which CDPPB acts to facilitate the acquisition and consolidation of extinction learning. mGluR5 receptors are expressed in moderate to high levels such as the cerebral cortex, dorsal striatum, nucleus accumbens, amygdala, and hippocampus (Romano et al., 1995; Shigemoto et al., 1993), all of which have been implicated in extinction learning (Cleva & Gass, 2010; Myers & Davis, 2007). One likely site of action of CDPPB in facilitating extinction is the infralimbic region of the frontal cortex (ILC), since electrical or chemical stimulation of this region potentiates extinction learning (Peters, Kalivas, & Quirk, 2009; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006). Exposure to drug-associated cues has been shown to decrease AMPA receptor expression and reduce AMPA/NMDA current ratios in this region, demonstrating plasticity of glutamatergic synapses in the ILC as a result of drug cue exposure (Van den Oever et al., 2008). Importantly, mGluR5 receptors in this region are required for fear extinction memory (Fontanez-Nuin, Santini, Quirk, & Porter, in press). However, in this study direct activation of mGluR5 receptors in the ILC did not increase NMDA-mediated currents, and another study showed that intra-ILC infusions of the NMDA partial agonist D-cycloserine during extinction training did not reduce context-induced reinstatement of cocaine-seeking behavior (Torregrossa, Sanchez, & Taylor, 2010). Together, this evidence suggests that mGluR5 receptors in the ILC contribute to extinction learning, but may do so in an NMDA-independent manner. Other regions such as the nucleus accumbens, where alterations in various aspects of glutamatergic transmission have been observed following extinction of cocaine-seeking behavior (Knackstedt et al., 2010; Suto, Ecke, You, & Wise, 2010; Sutton et al., 2003), may also be potential sites of action of CDPPB in its ability to facilitate extinction learning.
In summary, we have demonstrated that the mGluR5 PAM CDPPB facilitates the acquisition and consolidation of extinction learning following cocaine self-administration. These findings suggest that mGluR5 PAMs may be of potential use in conjunction with cue exposure therapy in facilitating the extinction of drug craving and other subjective responses evoked by drug associated cues, since cue exposure therapy alone has proved to be disappointingly ineffective in reducing relapse in drug addicts (Conklin & Tiffany, 2002; Havermans & Jansen, 2003; Martin, LaRowe, & Malcolm, 2010).
Acknowledgments
This work was supported by a Public Health Service grant from the National Institute on Drug Abuse (DA024355) from the United States National Institutes of Health. We are grateful to Dr. P. Jeffrey Conn of Vanderbilt University for his invaluable input on these studies, and to Dr. Albert Lee of IQsynthesis for assistance in the custom synthesis of CDPPB.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Contributor Information
Richard M. Cleva, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina
Megan P. Hicks, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina
Justin T. Gass, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina
Kelly C. Wischerath, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina
Elizabeth T. Plasters, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina
John J. Widholm, Department of Psychology, College of Charleston
M. Foster Olive, Center for Drug and Alcohol Programs, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, Biochemistry and Behavior. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. Journal of Neuroscience. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behavioral Brain Research. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Journal of Health Psychology. 2000;19:57–63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Gass JT. Neuroanatomical substrates underlying the extinction of drug-seeking behavior. Open Addiction Journal. 2010;3:63–75. [Google Scholar]
- Cleva RM, Gass JT, Widholm JJ, Olive MF. Glutamatergic targets for enhancing extinction learning in drug addiction. Current Neuropharmacology. doi: 10.2174/157015910793358169. (in press). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends in Pharmacological Sciences. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioral Brain Research. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cerebral Cortex. doi: 10.1093/cercor/bhq147. (in press). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biological Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacological Reviews. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Dong EN, Meiler J, Jeffrey Conn P. Allosteric modulation of metabotropic glutamate receptors: Structural insights and therapeutic potential. Neuropharmacology. 2010;60:66–81. doi: 10.1016/j.neuropharm.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermans RC, Jansen AT. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addictive Behaviors. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. European Journal of Pharmacology. 2010;639:33–39. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. Journal of Pharmacology and Experimental Therapeutics. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. Journal of Pharmacolog and Experimental Therapeutics. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. Journal of Neuroscience. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning and Memory. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biological Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: maintaining memory relevance. Trends in Neurosciences. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O'Brien JA, Lemaire W, Williams DL, Jr, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. Journal of Medicinal Chemistry. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, LaRowe SD, Malcolm R. Progress in cue extinction therapy for the treatment of addictive disorders: a review update. Open Addiction Journal. 2010;3:92–101. [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. Journal of Pharmacological Sciences. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biological Psychiatry. 2010a;67:85–87. doi: 10.1016/j.biopsych.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal-paired cues in animal models: relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010b;35:285–302. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. doi: 10.1038/npp.2010.88. (in press). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010a;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. European Journal of Pharmacology. 2010b;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cerebral Cortex. doi: 10.1093/cercor/bhq093. (in press). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Roberts DC. Initiation, maintenance and extinction of cocaine self-administration with and without conditioned reward. Psychopharmacology. 1996;128:89–96. doi: 10.1007/s002130050114. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends in Neurosciences. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Reis HJ, Guatimosim C, Paquet M, Santos M, Ribeiro FM, Kummer A, Schenatto G, Salgado JV, Vieira LB, Teixeira AL, Palotas A. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Current Medicinal Chemistry. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platta B, Micheaub J. Glutamate receptor function in learning and memory. Behavioral Brain Research. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends in Pharmacological Sciences. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. Journal of Comparative Neurology. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schroder UH. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. European Journal of Pharmacology. 2010;639:40–46. doi: 10.1016/j.ejphar.2010.02.057. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neuroscience Letters. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. European Journal of Pharmacology. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. European Journal of Pharmacology. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56 Suppl:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57BL/c mice. Behavioral Brain Research. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. Journal of Neuroscience. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Wan Li K, Van der Schors RC, Binnekade R, Schoffelmeer AN, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacology Biochemistry and Behavior. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. Journal of Neuroscience. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-Acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biological Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]