Summary
PURPOSE
Quadriceps strength deficits are common in patients with knee osteoarthritis, and have been attributed to central activation failure. Methodological differences may have contributed to previous reports of extensive central activation failure. The purpose of this study was to determine the extent of quadriceps muscle strength deficits and activation failure in middle aged patients with symptomatic medial knee osteoarthritis.
METHODS
Measurements of isometric quadriceps strength and voluntary activation were made in twelve subjects with knee osteoarthritis and twelve similarly aged uninjured subjects. Voluntary activation was tested by superimposing a train of electrical stimulation on a maximal effort volitional contraction of the quadriceps muscle.
RESULTS
The group of subjects with knee osteoarthritis had significantly less quadriceps strength relative to body mass index (BMI) than the group of control subjects (p = 0.010) yet did not demonstrate significantly lower levels of central activation (p = 0.233). However, fifty percent of the OA group, and only 25% of the control group failed to fully activate the quadriceps.
DISCUSSION
The finding of quadriceps weakness is consistent with past literature. Providing adequate instruction, feedback, and several attempts to maximally contract the muscle likely yielded a lack of a significant difference in activation between groups. This finding has implications for the rehabilitation of weakened quadriceps in patients with knee osteoarthritis.
Keywords: Quadriceps strength, Knee Osteoarthritis, Central activation failure, practice
Introduction
Quadriceps weakness is common in patients with knee osteoarthritis (4-6, 17) and may contribute to the substantial functional deficits that occur with disease progression. Quadriceps weakness may result from the pain of osteoarthritis (7, 10), however, some have suggested that quadriceps weakness precedes the onset of knee osteoarthritis (OA), and is itself a risk factor for the development of knee OA, particularly in women (2, 24). The involvement of quadriceps weakness in the development of knee OA may be linked to the role of the quadriceps during gait, where an eccentric contraction of the quadriceps is responsible for providing shock absorption at the knee. The inability to adequately attenuate large compressive forces at the knee can result in impulsive loading, which has been attributed to quadriceps weakness (18) and inactivity (12), and may be responsible for osteoarthritic changes (23).
The gradual decline in quadriceps strength associated with knee OA has been attributed, in part, to impairment in the central nervous system’s ability to fully volitionally activate the muscle (termed central activation failure or arthrogenous muscle inhibition (AMI)) (5, 6, 21). It is theorized that AMI can result from progressive joint degeneration, resulting in abnormal articular afferent information being sent to the α-motorneurons, thereby reducing their activation (10). Such a failure in central activation suggests that the motoneuron pool is not being maximally activated, and may occur as a consequence of joint pain, effusion, joint damage, a decrease in motivation, or fear of further joint injury or pain (7), all of which are common in individuals with symptomatic knee OA.
The two primary methods of testing a muscle for AMI are the twitch interpolation and the burst superimposition techniques, both of which superimpose an electrical stimulus on a volitional muscle contraction (13, 14). The underlying premise of both techniques is that if the muscle is not maximally activated, the electrical stimulus would be capable of generating additional force above that of the volitional force. Volitional muscle force increases as a result of increases in discharge rate and increases in motor unit recruitment. Greater motor unit recruitment leaves fewer inactive motor units to be stimulated by the superimposed electrical stimulus, which would yield a smaller electrically induced force during the stimulation (1). When all available motor units are volitionally activated, no augmentation in muscle force should be elicited by the electrical stimulus. The twitch and burst superimposition techniques diverge in the type of stimulus that is superimposed on the volitional force. The twitch interpolation technique is performed by superimposing a single pulse on various levels of muscle contractions, as well as on the resting muscle (1). Quantification of central activation using twitch interpolation is calculated as (1 – superimposed twitch force at MVC/twitch force at rest) (11). In contrast, the burst-superimposition technique superimposes a train of pulses (100 Hz) on a maximal volitional effort, and the maximum volitional force is compared to the total force produced by the electrical stimulus on the volitional contraction (13). A ratio of 1.0 would therefore imply full activation of the muscle. Both protocols can accurately measure AMI if the contraction is a maximal effort and the muscle is fully potentiated (3, 19, 20, 25).
Quadriceps AMI has been demonstrated in patients with knee osteoarthritis using twitch interpolation (5, 9, 10, 15, 16, 21), although the results vary widely. Some authors report severe AMI in control subjects (5) or in the contralateral pain-free knees (15, 16), results that are in sharp contrast to those who have failed to document the same degree of AMI in the quadriceps of healthy subjects using more aggressive stimulation parameters (25, 27). That the twitch revealed substantial AMI in control and uninvolved limbs while trains of stimulation do not suggests that methodological differences may have contributed to the large AMI values in the quadriceps of the osteoarthritic groups as well (19).
Accurate estimation of AMI requires a maximal effort contraction. Producing a maximal contraction requires sufficient practice, motivation, and feedback (11). Often several maximal efforts are required to achieve maximal activation, yet much of the literature refers to testing protocols in which only one attempt is measured (15, 16), or inadequate rest is provided between efforts (9, 10, 21). If adequate attempts are allowed, and visual feedback and appropriate rests are provided, then activation failure can be accurately measured. The purpose of this study was to determine the extent of quadriceps muscle strength deficits and activation failure in middle-aged patients with symptomatic, medial knee osteoarthritis. We hypothesized that the subjects would exhibit deficits in quadriceps strength and activation compared to age-matched subjects without knee OA. We also hypothesized that the quadriceps weakness would be partially accounted for by activation failure.
Methods
Twelve subjects (7 males, 5 females, ranging in age from 39 to 64, mean = 52.6, SD = 7.2 years) with symptomatic, medial compartment knee osteoarthritis were referred by an orthopedic surgeon who made the diagnosis of osteoarthritis from the clinical history, a physical examination, and radiographic changes observed from a standing postero-anterior radiograph with the knees bent to 30° (22). The radiographs of all subjects in the OA group showed definite joint space narrowing; yet no subject demonstrated attrition of the subchondral bone. Each subject was being treated by the surgeon for complaints of knee pain, and had been scheduled for an opening wedge high tibial osteotomy to correct genu varum. A control group of 12 uninjured similarly aged subjects (6 males, 6 females, ranging in age from 40 to 55, mean = 48.9, SD = 4.9 years) was recruited from the community to undergo identical testing. Subjects were not included if they were pregnant, had a history of ligament deficiency, neurological impairment, impaired balance or history of unexplained falls, rheumatoid arthritis, total knee replacement in either knee, any other orthopedic problems in the hips, ankles or spine, or a body mass index (BMI) ≥ 40.0. All subjects gave informed consent that was approved by the Human Subjects Review Board of this institution.
Quadriceps strength was tested on a day when subjects were not fatigued from prior physical activity. Each subject was seated on an isokinetic dynamometer (KinCom, Chattanooga Group, Inc., Chattanooga, TN) with the hips and knees fixed at 90E, and the back supported. The distal tibia was affixed to the dynamometer arm, and Velcro straps secured the thigh and waist. The anterior thigh was cleansed with isopropyl rubbing alcohol, and pre-gelled, self-adhesive 3″ X 5″ electrodes (ConMed Corporation, Utica, NY) were placed over the proximal vastus lateralis and distal vastus medialis. Subjects were asked to perform three near-maximal contractions to familiarize them with the testing procedures. Real-time visual feedback of force generation was made available during the test, and all subjects were encouraged to reach a visual target force that was set 25% above the level achieved during the near-maximal warm up contractions. If the subject reached the target during any contraction, the target was raised to continually provide the motivation for as strong a contraction as possible. During the test, vigorous verbal encouragement was provided as each subject produced a four second maximum isometric contraction. Approximately two seconds into the contraction a supramaximal burst of electrical current (100 pulses/second, 600 Φsecond pulse duration, 10 pulse tetanic train, 130 Volts) was sent through the electrodes to fully stimulate the quadriceps. If the subject was generating maximum force, then no increase in torque was observed during the electrical stimulus. If the subject failed to generate a full volitional force (defined as a volitional force immediately prior to the electrical stimulus being greater than ninety-five per cent of the peak electrically elicited force) the subject was encouraged to kick harder, and the test was repeated, up to three times with a five-minute rest between sessions. Custom written software (Labview, National Instruments, Austin, TX) was used to evaluate the force curve. The volitional force of the quadriceps immediately prior to the electrical stimulus was normalized to the subject’s BMI (weight in kg/height in m2) to allow for comparison between groups. As a measure of activation, the central activation ratio (CAR) was calculated according to equation 1
| 1 |
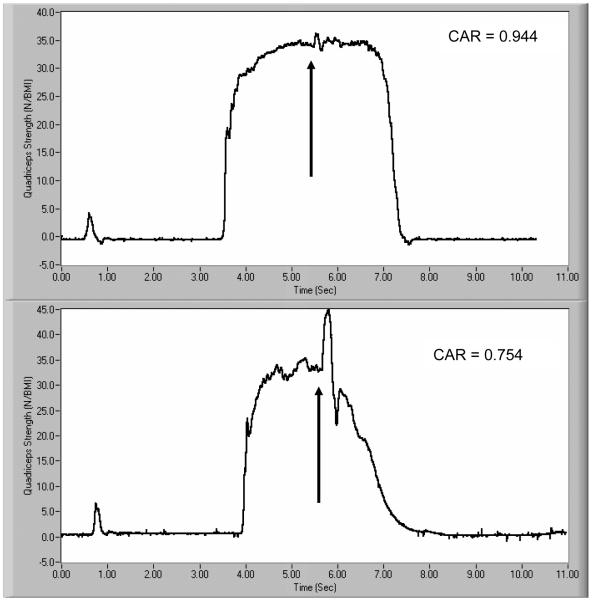
where Fvolitional is the volitional force produced by the quadriceps immediately prior to the electrical stimulus, and Felectrical is the peak force produced by the electrical stimulus superimposed on the volitional effort. A CAR of 1.000, therefore, represents complete activation of the muscle (i.e. no inhibition) (Figure 1). This method has been used in a similar age group for quantifying both strength and activation deficits (26, 27).
Figure 1.
Two examples of a force curve for the calculation of quadriceps strength and CAR. The top curve represents a subject with a CAR of 0.944, while the curve for the bottom subject yields a CAR of 0.754.
The presence of significant group differences was determined using one-way analysis of variance for quadriceps strength and activation deficits (SPSS 11.0, Chicago, IL). Using a CAR ∃ 0.950 as our operational definition of “full activation”, we used the Fisher’s exact test to determine if group differences existed in the frequency of subjects who exhibited activation failure (CAR < 0.950). Regression analysis was performed to determine the relative effect of central activation on quadriceps strength. Significance was set at the α = 0.05 level.
Results
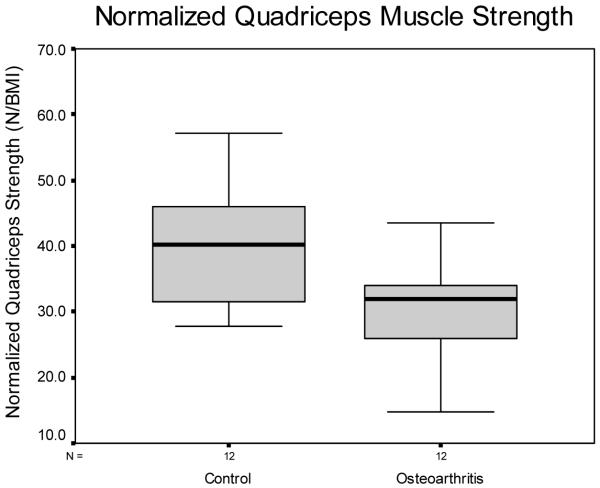
The normalized quadriceps strength of the OA group of 30.1 N/BMI (SD = 7.5; range 14.8-43.6) was significantly less than the normalized quadriceps strength of the control group of 39.7 N/BMI (SD = 9.2; range 27.7-57.2) (p = 0.010) (Figure 2). The OA group had a mean BMI of 31.3 (SD = 5.1; range = 25.1-37.9) and the control group had a BMI of 28.6 (SD = 5.6; range = 20.7-39.2). There was no difference in BMI between the OA and control groups (p = 0.230).
Figure 2.
Box and whiskers plot of normalized quadriceps strength between groups. The box represents the interquartile range containing the 50% of values. The whiskers are lines that extend from the box to the maximum and minimum values, excluding outliers. The line drawn across the box represents the median. The OA group had a significantly reduced normalized quadriceps strength compared to the healthy age matched control group (p = 0.010).
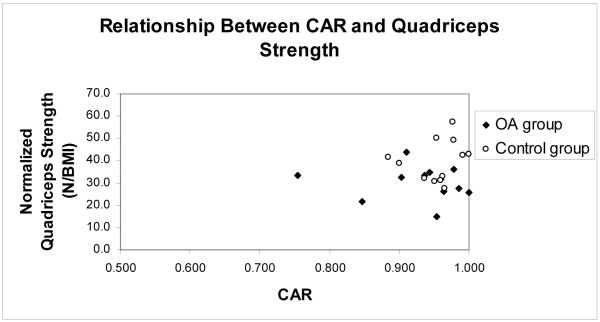
The mean CAR of the OA group was 0.928 ± 0.069 and the control group’s mean CAR was 0.955 ± 0.034 and no significant difference existed between the groups (p = 0.233) (Figure 3). Using our operational definition of 0.950 for the determination of full activation, 3 of the 12 (25%) subjects in the control group failed to maximally activate the quadriceps muscle. In the OA group, 6 of 12 subjects (50%) failed to maximally activate the quadriceps muscle. There was no significant difference in the number of subjects who were unable to fully activate the quadriceps in both groups (p = 0.200). Both groups attempted a mean of 2 ± 1 repetitions to try to achieve a maximum voluntary effort contraction (p = 0.804). Two subjects in the OA group and 1 subject in the control group refused further testing after 2 attempts despite failure to achieve 0.950.
Figure 3.
Relationship between CAR and normalized quadriceps strength. Three of 12 control subjects and 6 of 12 OA subjects had a CAR < 0.950. There was no significant relationship between CAR and the normalized quadriceps strength for the OA group or for the entire group as a whole.
Pearson correlation revealed that there was no relationship between CAR and the subject’s BMI (−0.035). Level of voluntary activation did not account for a significant portion of the variation in the normalized quadriceps strength of the OA group (r2 = 0.018, p = 0.674) or of all of the entire subject pool (r2 = 0.016, p = 0.557).
Discussion
The hypothesis that patients with knee osteoarthritis would exhibit quadriceps weakness was supported by the data. However, the activation failure that was present did not contribute substantially to the reduction in normalized quadriceps strength seen in the subjects with knee osteoarthritis. These data clearly establish that if middle aged patients with symptomatic medial knee osteoarthritis are provided with appropriate practice and several repetitions then the magnitude of activation deficits are small.
One limitation of this research is the small number of subjects tested. Despite this, the quadriceps weakness observed in this group of subjects with knee osteoarthritis is consistent with previously published results. Messier et al. report a 22-36% decrease in isokinetic quadriceps strength relative to body weight in a group of patients with knee osteoarthritis compared to healthy age matched controls (17). Hall et al. tested isometric quadriceps strength in a group of subjects with knee osteoarthritis, and found that they were 20% weaker than an age matched control group (4). The OA group that was tested in this study showed strength deficits of a similar magnitude, demonstrating a 24% average quadriceps strength deficit compared to the asymptomatic, healthy age matched control group. The cause of this weakness, however, remains unclear. We cannot attribute the strength deficits to a natural consequence of aging because the age of the control group was not significantly different from the age of the patients with knee OA. While some have postulated that a lack of motivation, pain in the knee joint, or fear of producing further pain with a maximal contraction, reduces the central nervous system’s input to the α-motorneuron pool and results in impaired activation, the present study suggests that this was not the case in this group of subjects. A regression analysis revealed that the small decline in voluntary activation did not contribute to the reduction in normalized quadriceps strength. This leaves disuse atrophy as the most likely cause of the quadriceps weakness in this group of subjects.
Several authors tested subjects who were older than the subjects that we tested, which may prohibit comparisons between studies (5, 9, 10, 15, 21). Age may be a confounding variable because activation is diminished in elderly subjects when compared to younger individuals (27). Nevertheless, the extent of AMI that has been reported previously is so different from our results that we feel that the differences are due primarily to methodological errors. With the exception of Hassan et al (5), who demonstrated 21.4% quadriceps activation in their group of subjects, most authors have reported that the quadriceps muscle in patients with knee osteoarthritis is activated at only 66 – 77% of its maximum (9, 10, 15, 16, 21). In contrast, our subjects demonstrated an average quadriceps activation level of nearly 93% of the maximum capacity of the muscle. This level is still not maximal, however, and clearly there is some degree of activation failure that is present. In studies of younger subjects, approximately 10% fail to achieve a CAR of 0.950 (26, 27). The healthy middle-aged individuals in this study had a 25% incidence of activation failure. In contrast, in groups of older adults, approximately 40% of the subjects failed to reach full activation of the quadriceps muscle (26, 27). Our results of the incidence of activation failure, therefore, are quite consistent with previous reports. However, as a group, the patients with knee osteoarthritis appear to be showing signs of reduced activation. Fifty percent (6 of 12 subjects) of the subjects with knee osteoarthritis were unable to fully activate the quadriceps (CAR ∃ 0.950) indicating that as a group, they are beginning to show some reduction in activation that is beyond that caused by aging.
Although 50% of the OA group failed to fully activate the quadriceps, it is the magnitude of the central activation deficits in this group that is so drastically different from those presented previously (5, 9, 10, 15, 16, 21). Previous research has used a twitch interpolation technique, which has been suggested to be inadequate at estimating central activation failure at high force levels (8, 25). In fact, the assertion that a single pulse was able to elicit additional force in the quadriceps muscle, when a tetanic train of pulses did not, implies that the subjects recruited for previous studies were not providing a maximal effort contraction. In addition, previous researchers have failed to provide visual feedback of force generation (5, 21); provide ample rest between contractions (5, 9, 10, 15, 16), or an adequate warm-up (5, 9, 10, 21), all of which can contribute to an augmentation in central activation failure.
Our subjects already showed both clinical and radiographic signs of knee osteoarthritis, yet they do not exhibit mean deficits in activation that were significantly different from the healthy age matched control group. Although small differences in central activation can be observed, the extent of this disparity is small enough that it would likely be clinically insignificant, or could indicate the start of a problem that could get worse with age or the progression of their knee OA. Therefore, the results of this study have important clinical implications for the rehabilitation of patients with knee osteoarthritis. If activation deficits of the previously reported magnitude (5, 9, 10, 15, 16, 21) could be eradicated through practice in patients with knee osteoarthritis, then there is reason to believe that a trial of early physical therapy can influence the patient’s symptoms. Hurley suggests that individuals without activation deficits will have greater success strengthening the quadriceps (6). Stronger quadriceps have been linked to greater functional ability and a reduction in symptoms (9). A regimen of early aggressive strengthening of the quadriceps may improve the patient’s function; and the earlier that quadriceps strength deficits are addressed, the less likely they are to undergo progressive osteoarthritic changes. Waiting until the patient exhibits bony attrition at the knee, may allow more substantial activation deficits to develop, making it more difficult to reverse the progressing strength deficits, and requiring surgical intervention.
Acknowledgements
The authors would like to thank William Newcomb, MD for his assistance with subject recruitment, and Laura C. Schmitt, PT for her assistance with data collections. Funding was provided by the National Institutes of Health (5R01HD037985-02, 1P20RR016458-010003), and the Foundation for Physical Therapy Promotion of Doctoral Studies Program.
Contributor Information
Michael D. Lewek, Biomechanics and Movement Science Program, Department of Physical Therapy, University of Delaware, Newark, DE 19716.
Katherine S. Rudolph, Department of Physical Therapy and Biomechanics and Movement Science Program, University of Delaware, Newark, DE 19716.
Lynn Snyder-Mackler, Department of Physical Therapy and Biomechanics and Movement Science Program, University of Delaware, Newark, DE 19716.
References
- [1].Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–5. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- [2].Brandt KD, Heilman DK, Slemenda C, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–7. [PubMed] [Google Scholar]
- [3].Bulow PM, Norregaard J, Danneskiold-Samsoe B, Mehlsen J. Twitch interpolation technique in testing of maximal muscle strength: influence of potentiation, force level, stimulus intensity and preload. Eur J Appl Physiol Occup Physiol. 1993;67:462–6. doi: 10.1007/BF00376464. [DOI] [PubMed] [Google Scholar]
- [4].Hall KD, Hayes KW, Falconer J. Differential strength decline in patients with osteoarthritis of the knee: revision of a hypothesis. Arthritis Care Res. 1993;6:89–96. doi: 10.1002/art.1790060208. [DOI] [PubMed] [Google Scholar]
- [5].Hassan BS, Mockett S, Doherty M. Static postural sway, proprioception, and maximal voluntary quadriceps contraction in patients with knee osteoarthritis and normal control subjects. Ann Rheum Dis. 2001;60:612–8. doi: 10.1136/ard.60.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hurley MV. The effects of joint damage on muscle function, proprioception and rehabilitation. Man Ther. 1997;2:11–17. doi: 10.1054/math.1997.0281. [DOI] [PubMed] [Google Scholar]
- [7].Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–98. vi. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- [8].Hurley MV, Jones DW, Newham DJ. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond) 1994;86:305–10. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- [9].Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol. 1998;37:1181–7. doi: 10.1093/rheumatology/37.11.1181. [DOI] [PubMed] [Google Scholar]
- [10].Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641–8. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–62. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- [12].Jefferson RJ, Collins JJ, Whittle MW, et al. The role of the quadriceps in controlling impulsive forces around heel strike. Proc Inst Mech Eng [H] 1990;204:21–8. doi: 10.1243/PIME_PROC_1990_204_224_02. [DOI] [PubMed] [Google Scholar]
- [13].Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle & Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [14].Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–9. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- [15].Machner A, Pap G, Awiszus F. Evaluation of quadriceps strength and voluntary activation after unicompartmental arthroplasty for medial osteoarthritis of the knee. J Orthop Res. 2002;20:108–11. doi: 10.1016/S0736-0266(01)00068-7. [DOI] [PubMed] [Google Scholar]
- [16].Machner A, Pap G, Krohn A, et al. Quadriceps muscle function after high tibial osteotomy for osteoarthritis of the knee. Clin Orthop. 2002:177–83. doi: 10.1097/00003086-200206000-00021. [DOI] [PubMed] [Google Scholar]
- [17].Messier SP, Loeser RF, Hoover JL, et al. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- [18].Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171–5. doi: 10.1002/jor.1100180202. [DOI] [PubMed] [Google Scholar]
- [19].Miller M, Downham D, Lexell J. Superimposed single impulse and pulse train electrical stimulation: a quantitative assessment during submaximal isometric knee extension in young, healthy men. Muscle & Nerve. 1999;22:1038–1046. doi: 10.1002/(sici)1097-4598(199908)22:8<1038::aid-mus5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [20].Norregaard J, Lykkegaard JJ, Bulow PM, Danneskiold-Samsoe B. The twitch interpolation technique for the estimation of true quadriceps muscle strength. Clin Physiol. 1997;17:523–532. doi: 10.1046/j.1365-2281.1997.05555.x. [DOI] [PubMed] [Google Scholar]
- [21].O’Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–94. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Piperno M, Hellio Le Graverand MP, Conrozier T, et al. Quantitative evaluation of joint space width in femorotibial osteoarthritis: comparison of three radiographic views. Osteoarthritis Cartilage. 1998;6:252–9. doi: 10.1053/joca.1998.0118. [DOI] [PubMed] [Google Scholar]
- [23].Radin EL, Yang KH, Riegger C, et al. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991;9:398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- [24].Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–9. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [25].Stackhouse SK, Dean JC, Lee SC, Binder-MacLeod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23:1706–12. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [26].Stackhouse SK, Stevens JE, Lee SC, et al. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–9. [PubMed] [Google Scholar]
- [27].Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil. 2001;82:973–8. doi: 10.1053/apmr.2001.23995. [DOI] [PubMed] [Google Scholar]