Abstract
Fluctuations in the endogenous levels of kynurenic acid (KYNA), a potent α7 nicotinic and NMDA receptor antagonist, affect extracellular dopamine (DA) concentrations in the rat brain. Moreover, reductions in KYNA levels increase the vulnerability of striatal neurons to NMDA receptor-mediated excitotoxic insults. We now assessed the role of a key KYNA-synthesizing enzyme, kynurenine aminotransferase II (KAT II), in these processes in the rodent striatum, using KAT II KO mice—which have reduced KYNA levels—and the selective KAT II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (S-ESBA) as tools. S-ESBA (applied by reverse dialysis) raised extracellular DA levels in the striatum of KYNA-deficient mice threefold and caused a much larger, 15-fold increase in wild-type mice. In the rat striatum, S-ESBA produced a 35% reduction in extracellular KYNA, which was accompanied by a 270% increase in extracellular DA. The latter effect was abolished by co-infusion of 100 nM KYNA. Intrastriatal S-ESBA pre-treatment augmented the size of a striatal quinolinate lesion by 370%, and this potentiation was prevented by co-infusion of KYNA. In separate animals, acute inhibition of KAT II reduced the de novo synthesis of KYNA during an early excitotoxic insult without enhancing the formation of the related neurotoxic metabolites 3-hydroxykynurenine and quinolinate. Taken together, these results provide further support for the concept that KAT II is a critical determinant of functionally relevant KYNA fluctuations in the rodent striatum.
Keywords: astrocytes, excitotoxicity, Huntington's disease, kynurenine aminotransferase II, neuroprotection
Kynurenic acid (KYNA), a neuroprotective metabolite of the kynurenine pathway of tryptophan degradation that is present in the mammalian brain in nanomolar concentrations, has unique pharmacological properties. Initially found to block all ionotropic glutamate receptors at high micromolar concentrations (Perkins and Stone, 1982), KYNA was later shown to have higher potency as an inhibitor of the glycine co-agonist site of the NMDA receptor (Kessler et al., 1989). More recently, nanomolar KYNA was found to antagonize α7 nicotinic acetylcholine receptor (α7nAChR) function by blocking the receptor's allosteric potentiating site (Hilmas et al 2001 and Lopes et al 2007). It appears that this site, rather than the NMDA receptor, constitutes the primary target of the metabolite in vivo, and that the anti-glutamatergic efficacy of endogenous brain KYNA is caused at least in part by the α7nAChR-mediated reduction of glutamate release (Carpenedo et al 2001, Rassoulpour et al 2005 and Grilli et al 2006). Modulation of glutamate release by α7nAChRs, in turn, affects additional neurotransmitter systems including monoamines (Kaiser and Wonnacott 2000 and Barik and Wonnacott 2006).
In rats, focal intrastriatal infusion of KYNA reversibly decreases the extracellular concentration of dopamine (DA) (Rassoulpour et al., 2005). This effect can be duplicated by stimulating the endogenous formation of KYNA either pharmacologically by kynurenine 3-hydroxylase inhibition (Rassoulpour et al., 2005), or by administering L-kynurenine, the direct bioprecursor of KYNA (Wu et al., 2007). Since KYNA synthesis in the brain takes place almost exclusively in astrocytes (Roberts et al 1992 and Guidetti et al 2007b), and because newly produced KYNA readily enters the extracellular compartment (Turski et al., 1989), these studies demonstrated a causal link, within the striatum, between enhanced astrocytic KYNA formation and reduced extracellular DA levels. In particular, the data raised the possibility that astrocyte-derived KYNA may exert functionally significant local control over dopaminergic activity.
In line with such a modulatory role of endogenous KYNA, we reported recently that reductions in striatal KYNA formation are associated with increased extracellular DA levels (Wu et al., 2007). These studies used the reversible astrocyte poison fluorocitrate (Paulsen et al 1987) and the non-specific aminotransferase inhibitor aminooxyacetic acid as experimental tools. Notably, DA levels returned to normal values once the effect of fluorocitrate subsided, and the aminooxyacetic acid–induced rise in DA levels was prevented by the co-administration of 100 nM KYNA.
The present study was designed to examine the consequences of a reduction in endogenously produced KYNA in the striatum in greater detail. To this end, we decided to target kynurenine aminotransferase II, a key astrocytic enzyme of KYNA biosynthesis in the mammalian brain (Guidetti et al 2007a and Guidetti et al 2007b), to attenuate KYNA formation. Using two experimental tools, i.e. mice with a genomic deletion of KAT II (“KAT II knockout mice”; Yu et al., 2004) and the synthetic KAT II inhibitor (S)-4-(ethylsulfonyl)benzoylalanine (S-ESBA; Pellicciari et al., 2006), we achieved permanent and acute reductions, respectively, in striatal KYNA levels. We then studied the effects of reduced KYNA synthesis on extracellular DA by in vivo microdialysis and also examined possible changes in the susceptibility of striatal neurons to an excitotoxic insult. Our results revealed that KAT II is a pivotal enzyme in the rodent striatum, governing both dopaminergic activity and neuronal vulnerability.
Experimental procedures
Animals
Twenty-one day-old KAT II knockout mice (generated on a 129 Sv/Ev background; Yu et al., 2004) and 129 Sv/Ev wild-type mice (Taconic, Germantown, NY, USA) were used for the mouse microdialysis studies, and tissues from adult 129 Sv/Ev wild-type mice were used for the partial purification of mouse KAT I, KAT II and mitochondrial aspartate aminotransferase (mitAAT). Adult, male Sprague–Dawley rats (200–250 g; Charles River Laboratories, Kingston, NY, USA) were used in all other experiments.
All animals were housed in a temperature-controlled, AAALAC-approved animal facility on a 12-h light/dark cycle with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine. All experiments were performed so as to minimize the number of animals used and their suffering.
Chemicals
DA, KYNA, quinolinic acid (QUIN), L-kynurenine (sulfate salt), pyridoxal-5′-phosphate, Trizma base and acetate salt, and all other fine biochemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). (S)-4-(Ethylsulfonyl)benzoylalanine (S-ESBA) was synthesized at the University of Perugia as described by Pellicciari et al. (2008). All other chemicals were of the highest available purity and were purchased from various commercial suppliers. 3H-Kynurenine (specific activity 12.0 Ci/mmol) was custom-synthesized by Amersham Corporation (Arlington Heights, IL, USA). Before use, 3H-kynurenine was routinely purified by high performance liquid chromatography (HPLC) as described by Guidetti et al. (1995).
Partial enzyme purification
KAT I and KAT II were partially purified from mouse or rat liver according to the procedures described by Guidetti et al. (1997). mitAAT was partially purified from mouse or rat brain (Guidetti et al 2007a).
KAT activity
Routinely, KAT activity was measured using minor modifications of the procedure developed by Okuno et al. (1991). The reaction mixture contained the enzyme preparation (80 µl), 20 µl aliquots of the S-ESBA solution or water, 3H-kynurenine (50 nCi; diluted with unlabeled kynurenine to reach a final concentration of 2 µM), 1 mM pyruvate, 70 µM pyridoxal-5′-phosphate and 150 mM Tris-acetate (pH 7.4) in a total volume of 200 µl. The enzyme preparation consisted of partially purified KAT appropriately diluted with a buffer containing 500 mM Tris-acetate, 50 nM pyridoxal 5′-phosphate and 1.1 mM 2-mercaptoethanol (pH 7.4). Blanks were prepared by omitting the enzyme preparation. Active samples and blanks were incubated for 2 h at 37 °C, and the reaction was terminated by adding 20 µl of 50% (w/v) trichloroacetic acid and 1 ml of 0.1 M HCl. The denatured proteins were removed by centrifugation, and newly synthesized 3H-KYNA was recovered from Dowex 50W cation exchange columns and quantified by liquid scintillation spectrometry.
Microdialysis
Microdialysis was performed as described by Wu et al. (1992). Briefly, rats were anesthetized with chloral hydrate (360 mg/kg, i.p.) and placed in a David Kopf stereotaxic frame. A guide cannula (outer diameter 0.65 mm) was positioned over the striatum (AP: 1 mm anterior to bregma, L: 2.5 mm from midline, V: 3.5 mm below the dura) and secured to the skull with acrylic dental cement and anchor screws. On the next day, a microdialysis probe (CMA/10, membrane length: 2 mm, Carnegie Medicin, Stockholm, Sweden) was inserted through the guide cannula and connected to a microperfusion pump set to a speed of 1 µl/min. The freely moving animals were perfused with Ringer solution containing (in mM) NaCl, 144; KCl, 4.8; MgSO4, 1.2; CaCl2, 1.7; pH 6.7. After the establishment of a stable baseline, S-ESBA (alone or combined with KYNA) was applied by reverse dialysis for 2 h. Subsequently, perfusion with Ringer solution continued.
Analogous to the rats, 21 day-old mice were anesthetized with chloral hydrate and placed in a stereotaxic frame. With the exception of the stereotaxic coordinates (AP: 0.5 mm anterior to bregma, L: 1.2 mm from midline, V: 2.1 mm below the skull) and the membrane length of the microdialysis probe (1 mm), the microdialysis procedure followed was identical to that described for rats.
Microdialysis samples were collected every 30 min for up to 8 h. When both KYNA and DA were measured, the analytes were determined in the same samples. Data were not corrected for recovery from the microdialysis probe. Proper probe placement was verified in every experimental animal.
KYNA and DA determination
KYNA was determined by HPLC with fluorescence detection (excitation wavelength: 344 nm; emission wavelength: 398 nm). To this end, 15 µl of the microdialysate were applied to a 3 µm C18 reverse phase column (80×4.6 mm, ESA, Bedford, MA, USA), and KYNA was isocratically eluted using a mobile phase containing 200 mM zinc acetate and 5% acetonitrile (pH 6.2) at a flow rate of 1 ml/min. The retention time of KYNA under these conditions was ~5 min (S200 fluorescence detector; Perkin Elmer, Beaconsfield, UK) (Wu et al 1992 H.Q.).
DA was measured by HPLC analysis with electrochemical detection. Briefly, 15 µl of the microdialysate was acidified with 10 µl 0.1 N HCl, and 15 µl of the resulting mixture was injected onto a PP-ODS column (30×4.6 mm, EICOM Corporation, Kyoto, Japan). DA was eluted with a mobile phase containing 0.1 M sodium phosphate buffer (pH 6.0), 500 mg/l sodium dodecyl sulfate, 50 mg/l EDTA and 1% methanol at a flow rate of 0.5 ml/min. The retention time of DA under these conditions was ~2 min. DA was detected electrochemically at 450 mV (HTEC 500, EICOM Corporation).
Intrastriatal infusions
Rats were anesthetized with chloral hydrate (360 mg/kg, i.p.) and placed in a stereotaxic frame. At a speed of 1 µl/min, 5 µl of either phosphate-buffered saline (PBS; pH 7.4) or S-ESBA (1 mM, dissolved in PBS) was then injected unilaterally into the striatum (AP: 1 mm anterior to bregma, L: 2.7 mm from midline, V: 4.5 mm below the dura) using a CMA/100 microinjection pump (Carnegie Medicin), and the injection needle was slowly withdrawn. One hour later, 1 µl of QUIN (20 mM), either alone or in combination with KYNA (10 µM), was infused into the same striatum over 10 min, using the same stereotaxic coordinates. The animals were euthanized 4 days later, and their brains were quickly removed, immediately frozen on dry ice and stored at −80 °C until they were processed.
Histology
Brains were allowed to warm to −18 °C, and 30 µm cryostat sections were cut coronally throughout the entire rostro-caudal extent of the striatum. Every fourth section was mounted on a poly-l-lysine-coated microscope slide for cytochrome oxidase staining. Consecutive sections were collected separately for Nissl staining.
Cytochrome oxidase staining was performed as described by Poeggeler et al. (1998). Briefly, the sections were first incubated for 75 min in a solution containing 0.1 M Hepes buffer (pH 7.4), sucrose (4.5 g/100 ml), cytochrome c (22.4 mg/100 ml), diaminobenzidine (115 mg/100 ml) and 1% nickel ammonium sulfate (12.5 ml/100 ml). The slides were post-fixed for 10 min in neutral buffered formaldehyde, dipped in distilled water, left in distilled water for 5 min, and dehydrated in ethanol (EtOH; 2 min in 70% EtOH, 2 min in 95% EtOH, 2×2 min in 100% EtOH) and xylene (2×2 min). Subsequently, the slides were coverslipped with Permount© and allowed to dry before analysis.
Cytochrome oxidase–stained tissue sections were analyzed by an investigator who was unaware of the experimental protocol. To quantify the lesion, the sections were photographed with a digital video camera and analyzed using the image-processing program Image J (application freely downloadable at http://rsb.info.nih.gov). Lesioned areas from each striatum were added and multiplied by the inter-section distance (120 µm) to yield the corresponding lesion volume. Nissl-stained sections were used to confirm the proper location of the track of the injection needle.
Assessment of striatal 3H-kyurenine metabolism
Rats were anesthetized with chloral hydrate (360 mg/kg, i.p.) and placed in a stereotaxic frame. At a speed of 1 µl/min, 5 µl of PBS, pH 7.4, and 5 µl of S-ESBA (1 mM, dissolved in PBS) were injected bilaterally into the right and the left striatum, respectively (AP: 1 mm anterior to bregma, L: ±2.7 mm from midline, V: 4.5 mm below the dura) using a CMA/100 microinjection pump (Carnegie Medicin). The injection needles were then slowly withdrawn. One hour later, 6 µl of 3H-kynurenine (2.5 µCi), in combination with QUIN (20 mM), was infused over 10 min into both the right and the left striatum, using the same stereotaxic coordinates. The animals were euthanized 2 h later, their striata were quickly dissected out, immediately frozen on dry ice and stored at −80 °C until processed for the quantification of tritiated KYNA, 3-hydroxykynurenine (3-HK) and QUIN (Guidetti et al., 1995).
Statistics
Two-way analysis of variance (ANOVA) with an appropriate post hoc test was used in all microdialysis studies and to compare lesion volumes. A paired Student's t-test was used for two-group comparisons in the evaluation of newly formed KYNA, 3-HK and QUIN. In all cases, a P value of <0.05 was considered significant.
Results
S-ESBA inhibition of mouse KAT I, KAT II and mitAAT
To characterize the potency and specificity of S-ESBA as an inhibitor of mouse KAT, the effect of the compound (1–10 mM) was tested in vitro at physiological pH, using partially purified preparations of KAT I, KAT II and mitAAT. As shown in Table 1, S-ESBA dose-dependently inhibited all three enzyme activities, but showed preferential efficacy as a KAT II inhibitor, especially at the lower concentrations used.
Table 1.
In vitro inhibition of KAT activity using partially purified preparations of mouse KAT I, KAT II and mitAAT
| S-ESBA (mM) | % Inhibition |
||
|---|---|---|---|
| KAT I | KAT II | mitAAT | |
| 1 | 10±4 | 57±1 | 19±4 |
| 2 | 23±2 | 70±1 | 39±4 |
| 5 | 34±4 | 86±1 | 69±2 |
| 10 | 50±3 | 91±0 | 80±1 |
S-ESBA lacks clear-cut specificity, although KAT II is preferentially blocked at 1 and 2 mM. Assays were performed as described in the text. Data are the mean±S.E.M. of three separate experiments.
Effect of S-ESBA on extracellular KYNA and DA levels in the mouse striatum
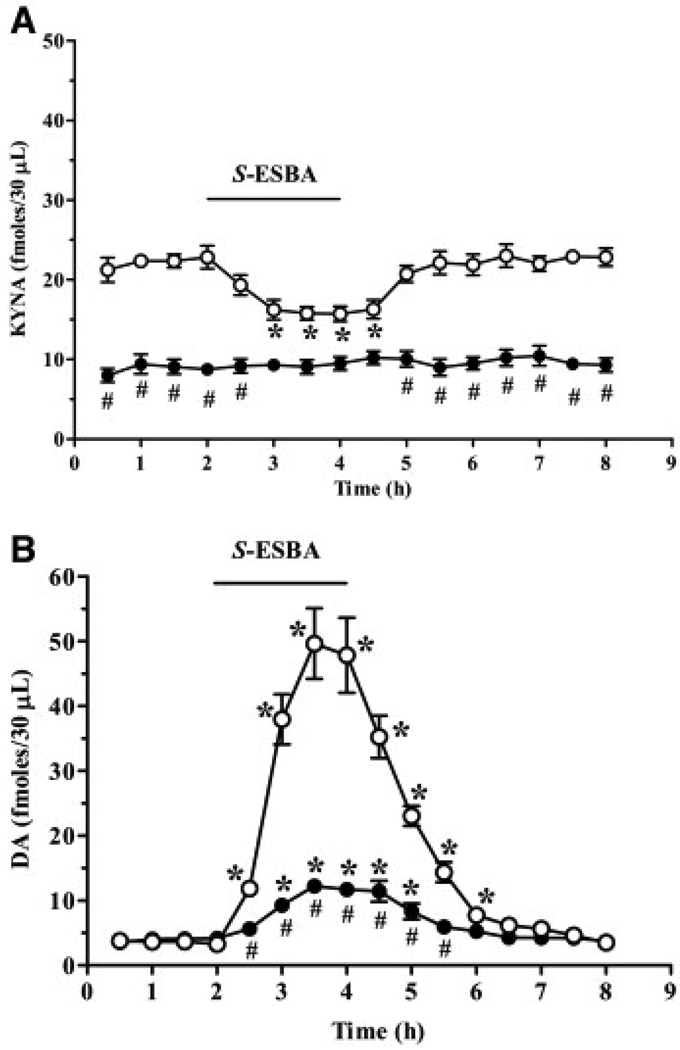
In spite of the compound's inability to target mouse KAT II exclusively, we next examined the effects of S-ESBA on the basal, ambient levels of extracellular KYNA and DA (i.e. the average of the four collection periods before the introduction of S-ESBA) in the striatum of KAT II knockout and age-matched wild-type mice. Confirming the relevance of KAT II as a biosynthetic enzyme of KYNA in the mammalian brain (Guidetti et al., 2007a), the basal extracellular levels of KYNA in mutant mice (8.8±1.0 fmol/30 µl) were 60% lower than in age-matched wild-type controls (22.2±1.2 fmol/30 µl; P<0.05; n=6 per group). In the same microdialysis samples, extracellular DA levels in the knockout mice (4.0±0.6 fmol/30 µl) were slightly higher than in control animals (3.6±0.4 fmol/30 µl; cf. also Wu et al., 2007) (Fig. 1).
Fig. 1.
Effect of S-ESBA on extracellular KYNA (A) and DA (B) levels in the striatum of 21 day-old wild-type (open circles) and KAT II knockout (solid circles) mice. S-ESBA (10 mM) was perfused by reverse dialysis for 2 h (bar), and KYNA and DA were measured in the same dialysates. Data are the mean±S.E.M. (n=6 per group). * P<0.05 vs. baseline, # P<0.05 vs. wild-type animals (two-way ANOVA with Bonferroni's post hoc test).
Applied for 2 h by reverse dialysis, 10 mM S-ESBA failed to significantly reduce extracellular KYNA levels in mice lacking KAT II (n=6; Fig. 1A). However, in the same samples, the compound caused a reversible, threefold increase (to 322±33% of basal levels; P<0.05) in extracellular DA (Fig. 1B).
In contrast to the mutant animals, S-ESBA significantly decreased extracellular KYNA levels in wild-type mice, reaching a nadir of −30±4% of basal levels after 1 h (n=6; Fig. 1A). Once the drug was removed from the perfusion solution, the concentration of KYNA gradually reverted to baseline values. As illustrated in Fig. 1B, the S-ESBA-induced reduction in KYNA levels was accompanied by a dramatic 15-fold increase in extracellular DA. In both absolute and relative terms, this drug-induced increase in DA levels was significantly greater than in mutant mice.
S-ESBA inhibition of rat KAT I, KAT II and mitAAT
In contrast to its less selective effects as an inhibitor of mouse KATs, S-ESBA showed a highly specific inhibition of KAT II over the other two isoforms when tested in vitro against partially purified rat KATs. In agreement with a previous study, which had reported an IC50 of ~6 µM for S-ESBA as an inhibitor of rat KAT II (Pellicciari et al., 2006), the compound completely blocked KAT II activity at concentrations of 1 mM and above. Specificity was especially pronounced when compared with KAT I activity, which was not inhibited by S-ESBA up to 10 mM (Table 2).
Because of the considerably higher potency and specificity of S-ESBA in rats, all subsequent in vivo studies were performed in rats, using 1 mM of the compound as the experimental concentration.
Effect of S-ESBA on extracellular KYNA and DA in the rat striatum
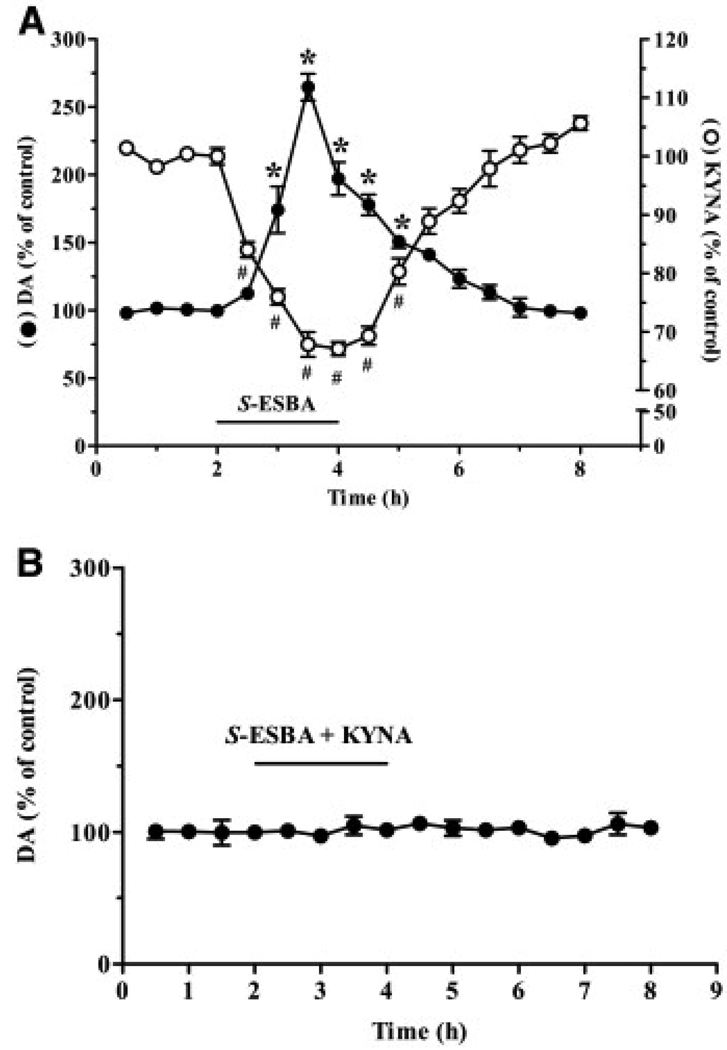
When infused by reverse microdialysis into the rat striatum, 1 mM S-ESBA caused a significant reduction in extracellular KYNA levels compared with baseline values (78.6±3.2 fmol/30 µl), with a nadir of −32±1% after 1.5 h. KYNA levels gradually returned to control values once S-ESBA infusion was discontinued after 2 h (n=6; Fig. 2A). In the same dialysate samples, we observed a significant, reversible increase in extracellular DA (from a baseline of 4.5±0.3 fmol/30 µl), reaching a maximum of 265±11% of baseline values after 1.5 h (Fig. 2A). To examine possible causality between the two neurochemical effects of the compound, namely to assess whether the rise in DA was secondary to the reduction in extracellular KYNA, 1 mM S-ESBA was co-infused with 100 nM KYNA in separate animals (n=5). In these rats, extracellular DA levels remained unchanged throughout the 2 h perfusion of the two test compounds (Fig. 2B).
Fig. 2.
(A) Effect of 1 mM S-ESBA on the extracellular levels of KYNA (open circles) and DA (solid circles) in the rat striatum. S-ESBA was applied by reverse dialysis for 2 h (bar), and KYNA and DA were measured in the same dialysates. Data are expressed as % of baseline values and are the mean±S.E.M. (n=6). # and * P<0.05 vs. the respective baseline (two-way ANOVA with Bonferroni's post hoc test). (B) Co-perfusion of KYNA (100 nM) prevents the S-ESBA-induced increase in extracellular DA in the rat striatum. Both compounds were administered for 2 h by reverse dialysis (bar). Data are expressed as % of baseline values and are the mean±S.E.M. (n=5).
Effect of S-ESBA on QUIN-induced lesions of the rat striatum
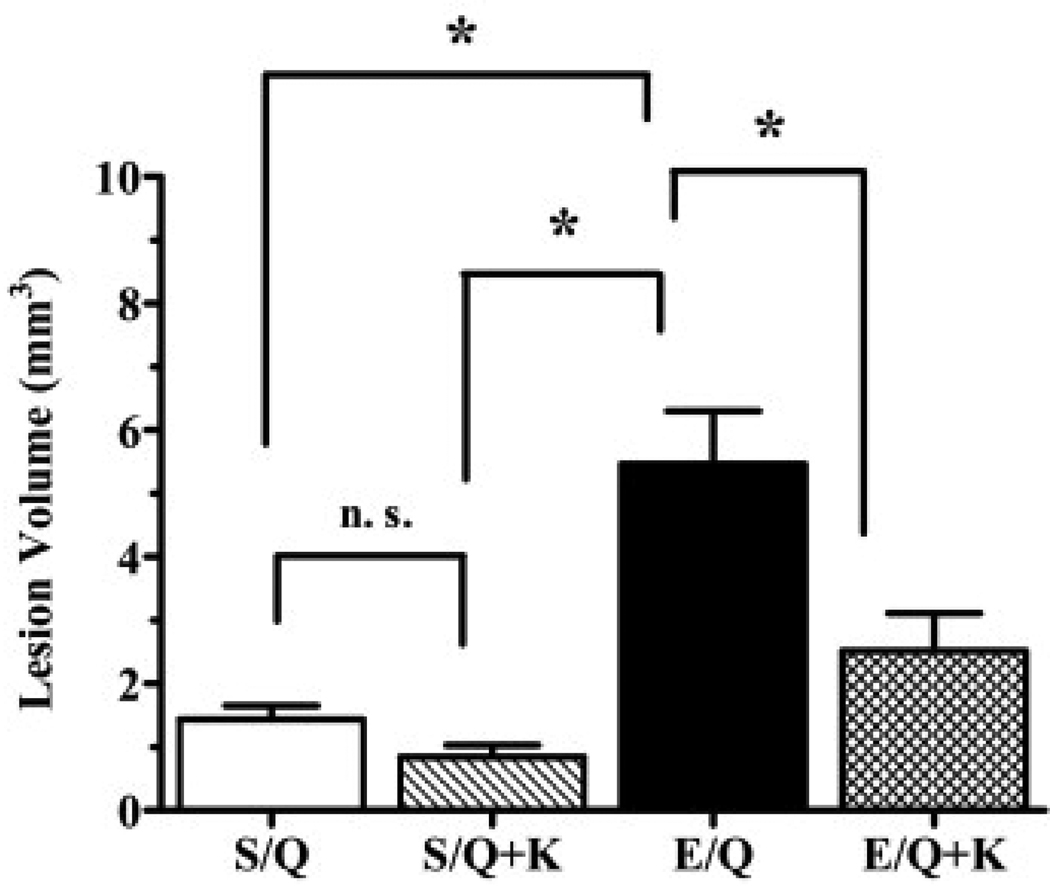
We next used neuronal susceptibility to an excitotoxic insult as an outcome measure to assess the functional implication of the S-ESBA-induced decrease in striatal KYNA formation (Fig. 3). Intrastriatal infusion of QUIN (20 mM) resulted in a lesion with an average volume of 1.4±0.7 mm3 (n=7). Infusion of 1 mM S-ESBA 1 h prior to QUIN caused a significant enlargement of the lesion volume to 5.5±0.6 mm3 (n=10).
Fig. 3.
Effect of S-ESBA (E; 1 mM) on QUIN-induced neurodegeneration in the rat striatum. S-ESBA was infused intrastriatally 1 h before QUIN (Q; 20 mM) or QUIN+KYNA [Q (20 mM)+K (10 µM)]. Control animals were pre-treated with PBS (S). Rats were sacrificed 4 days after the QUIN injection, and the lesion volume was quantified as described in the text. Data are the mean±S.E.M. (n=7–10 per group). * P<0.05 (one-way ANOVA with Bonferroni's post hoc test). n.s.: Not significant.
In order to evaluate if the exacerbation of QUIN toxicity by S-ESBA pre-treatment was causally related to a decrease in KYNA synthesis, separate rats were co-infused with QUIN (20 mM)+KYNA (10 µM) 1 h after receiving an intrastriatal infusion of 1 mM S-ESBA (Fig. 3). In these animals, the exogenously supplied KYNA significantly attenuated the S-ESBA-induced potentiation of QUIN excitotoxicity (lesion volume: 2.5±0.6 mm3; n=10). In control rats (n=7), the size of the lesion caused by co-infusion of a solution containing 10 µM KYNA and 20 mM QUIN was not significantly different from that induced by QUIN alone (Fig. 3).
Effects of S-ESBA on kynurenine pathway metabolism in the rat striatum
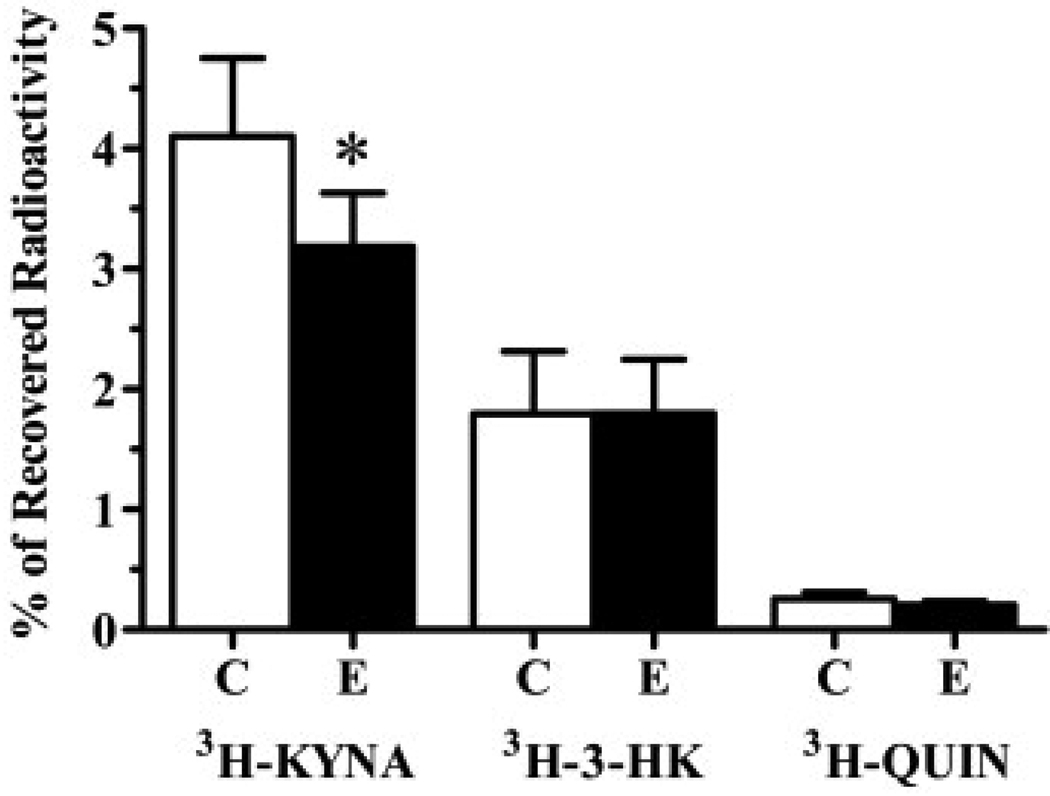
A final study was designed to examine the possibility that an acute shift of kynurenine pathway metabolism toward enhanced production of the endogenous neurotoxins 3-HK and QUIN might have contributed to the enhanced vulnerability of striatal neurons after the application of S-ESBA. To this end, we determined the effects of S-ESBA (1 mM), infused intrastriatally 1 h before the co-infusion of 20 mM QUIN and 3H-kynurenine, on the production of 3H-KYNA, 3H-3-HK and 3H-QUIN in the striatum (Guidetti et al., 1995). Compared with the vehicle pre-treated, contralateral striatum, S-ESBA significantly reduced the synthesis of KYNA without affecting the de novo formation of 3-HK or QUIN (n=8; Fig. 4).
Fig. 4.
Effect S-ESBA (E; 1 mM) pre-treatment on the de novo synthesis of 3H-KYNA, 3H-3-HK and 3H-QUIN following an intrastriatal infusion of QUIN (20 mM) and 3H-kynurenine in the rat striatum. S-ESBA was infused intrastriatally 1 h before QUIN and 3H-kynurenine, as described in the text. Contralateral striata (C) were pre-treated with PBS. Data are expressed as % of recovered radioactivity and are the mean±S.E.M. (n=8). * P<0.05 vs. contralateral controls (paired t-test).
Discussion
Using two complementary experimental tools, i.e. KAT II knockout mice and S-ESBA, the present study supports the notion that reductions in striatal KYNA formation are functionally significant in vivo. In particular, our results show that KAT II, which is only one of several enzymes capable of synthesizing KYNA in the brain (Guidetti et al., 2007a), can regulate extracellular DA levels in the striatum and acutely affect the susceptibility of striatal neurons to excitotoxic injury. We also demonstrated that these effects of KAT II are indeed causally related to the formation of the enzyme product KYNA, since small amounts of exogenously supplied KYNA readily neutralized the effects of KAT II inhibition. These findings, which have implications for basal ganglia physiology and pathology, are especially remarkable in light of the fact that both the genetic and the pharmacological manipulations of KAT II activity caused significant decreases yet did not abolish striatal KYNA levels. In other words, KYNA synthesis catalyzed by KAT II might have a distinct and perhaps disproportionate influence on KYNA-mediated effects in the brain.
Tests using partially purified enzymes, which were performed in preparation of the in vivo studies, revealed that S-ESBA was a less specific and less potent KAT II inhibitor in the mouse than in the rat. Thus, though the compound had efficacy in the millimolar range and showed preference for mouse KAT II (compared with KAT I and mitAAT), both the potency and the selectivity of S-ESBA were more striking in the rat. This difference in the inhibitory properties of S-ESBA, which is also observed between rat and human KAT II, is likely due to species variations in the amino acid sequence of the targeted enzyme domain (Pellicciari et al., 2008). In practical terms, assuming an approximately 20% recovery after application by reverse dialysis (Höcht et al., 2007), this species difference prompted us to use 10 mM S-ESBA in our in vivo experiments in mice, whereas perfusion with 1 mM S-ESBA was deemed suitable in rats.
KAT II knockout mice are a useful tool for the study of the role of KAT II, and by extension KYNA, in brain function. At a young age, when both tissue and extracellular levels of brain KYNA are significantly reduced, mutant animals show distinct phenotypic changes, including enhanced locomotor activity, altered α7nAChR function and, of particular relevance to the present study, increased striatal vulnerability to NMDA receptor-mediated excitotoxic injury (Alkondon et al 2004, Yu et al 2004 and Sapko et al 2006). Supporting the idea that extracellular KYNA levels in the brain are in part determined by KAT II, striatal perfusion with S-ESBA did not cause a further decrease in extracellular KYNA levels in the knockout mice (cf. Fig. 2). In contrast, the same treatment resulted in a significant reduction in extracellular KYNA in age-matched wild-type controls. Notably, the rapid return to baseline KYNA values after S-ESBA was removed from the perfusion solution indicates that KAT II, perhaps more so than other KYNA-synthesizing enzymes such as KAT I or mAAT (Guidetti et al., 2007a), is responsible for the rapid mobilization of neuroactive KYNA in the brain.
Analogous to the effects of a down-regulation of KYNA levels by the astrocytic poison fluorocitrate or the non-specific aminotransferase inhibitor aminooxyacetic acid (Wu et al., 2007; cf. introduction), S-ESBA application caused a transient increase in the extracellular levels of striatal DA, determined in the same microdialysis samples as KYNA. This elevation in DA, which essentially paralleled the reduction in extracellular KYNA, was dramatically higher in wild-type mice than in KAT II knockout animals, suggesting that the dopaminergic response in normal tissue was triggered by an acute reduction in KYNA synthesis. This was confirmed in separate animals, where the addition of 100 nM KYNA to the S-ESBA solution totally prevented the rise in DA levels. On the other hand, the functional link between KYNA and DA was apparently impaired in the striatum of knockout mice. Thus, mutant animals showed only a modest, non-significant increase in basal extracellular DA levels. This, as well as the fact that the acute increase in DA following S-ESBA application was far less pronounced in knockout than in wild-type mice, indicates qualitative differences in the dopaminergic modulation by KYNA after chronic attenuation of KYNA synthesis. Ongoing experiments are designed to elaborate the nature and causes of these differences and to examine their (patho)physiological consequences.
In the rat striatum, perfusion with S-ESBA resulted in a ~30% decline in extracellular KYNA levels, similar to the compound's effect in the rat hippocampus (Pellicciari et al., 2006). As confirmed in additional experiments adding 100 nM KYNA to S-ESBA (Fig. 2B), this relatively moderate decrease was nevertheless sufficient to cause a significant increase in the levels of extracellular DA. Thus, in rats as in mice, acute KAT II inhibition promptly translates into a positive dopaminergic signal. Based on our previous studies, which demonstrated reductions in extracellular DA levels when striatal KYNA was moderately elevated (Rassoulpour et al 2005 and Wu et al 2007), it is likely that the reciprocal link between fluctuations in KYNA and DA is mediated by α7nAChRs on cortical glutamatergic afferents (Pakkanen et al., 2005). We propose that these presynaptic receptors, which can significantly modulate striatal glutamate release (Kaiser and Wonnacott 2000 and Marchi et al 2002), are regulated by astrocyte-derived KYNA, possibly through volume transmission (Agnati and Fuxe 2000, Del Arco et al 2003 and Wu et al 2007). Alternatively or in addition, extracellular glutamate may be provided by astrocytes, which can release glutamate by stimulation of α7nAChRs (Sharma and Vijayaraghavan 2001 and Patti et al 2007). In line with these scenarios, local perfusions of KYNA reduce, whereas local perfusions with S-ESBA enhance, extracellular glutamate levels in the rat striatum (Carpenedo et al 2001 and Rassoulpour et al 2005; H.-Q.W. and R.S., unpublished observations). Presumably mainly through presynaptically situated AMPA receptors, these changes in extracellular glutamate in turn affect striatal DA release (Glowinski et al 1988, Westerink et al 1992, Sakai et al 1997 and Segovia et al 1997). In other words, our studies suggest that fluctuations in KYNA, which occur under a number of physiological conditions (Gramsbergen et al 1997 and Hodgkins and Schwarcz 1998), indirectly regulate extracellular DA levels in the striatum by initially targeting α7nAChRs on glutamatergic nerve terminals or astrocytes.
Both glutamate and DA have been prominently linked to the etiology of several neurological and psychiatric diseases affecting the basal ganglia (Carlsson and Carlsson 1990, Calabresi et al 1996, Laruelle et al 2003 and Kelley 2004). In particular, a number of molecular mechanisms have been invoked to explain the deleterious effects of a hyperfunction of glutamatergic and dopaminergic transmission on the viability of striatal neurons (see Gil and Rego, 2008, for a recent review). Since our data demonstrated an acute surge in extracellular DA after KAT II inhibition, and since excessive dopaminergic activity increases the susceptibility of striatal neurons to excitotoxic insults (Jakel and Maragos 2000 and Poeggeler et al 2007), we examined the effects of a local S-ESBA perfusion on QUIN-induced neurodegeneration. In line with a study in KAT II knockout mice (Coyle 2006 and Sapko et al 2006), we observed an exacerbation of neuronal vulnerability when QUIN (20 mM) was applied at the nadir of KYNA reduction. Corroborating the molecular specificity of this potentiation, the effect was not seen when QUIN was co-applied with a low concentration of KYNA. In separate animals, S-ESBA treatment did not shift kynurenine pathway metabolism toward increased QUIN formation, i.e. the aggravation of excitotoxicity by the KAT II inhibitor appears to be directly related to an increase in extracellular DA, perhaps in conjunction with elevated glutamate levels (see above and also Poeggeler et al 2007 B.).
Conclusion
In summary, the present experiments highlight the importance of one specific KYNA-synthesizing enzyme, KAT II, in the modulation of dopaminergic tone and function in the rodent striatum. Since DA homeostasis is essential for a variety of physiological processes involving the basal ganglia, both activation and down-regulation of KAT II activity can be envisioned to play a role in phenomena ranging from motor control to cognition and motivated behaviors (Groenewegen 2003, David et al 2005, Graybiel 2005, Seger 2006, Balleine et al 2007 and Grahn et al 2008). In addition, the local regulation of extracellular DA by KAT II-derived KYNA may be of pathophysiological significance, so that synthetic agents targeting KAT II specifically, including enzyme inhibitors such as S-ESBA, may prove beneficial in the treatment of a variety of disorders involving striatal dopaminergic neurotransmission.
Acknowledgments
We dedicate this work to our friend Dr. Paolo Guidetti, who sadly passed away on December 28, 2007. The studies were supported by USPHS grant HD16596.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- HPLC
high performance liquid chromatography
- KYNA
kynurenic acid
- mitAAT
mitochondrial aspartate aminotransferase
- PBS
phosphate-buffered saline
- QUIN
quinolinic acid
- S-ESBA
(S)-4-(ethylsulfonyl)benzoylalanine
- α7nAChR
α7 nicotinic acetylcholine receptor
- 3-HK
3-hydroxykynurenine
References
- Agnati LF, Fuxe K. Volume transmission as a key feature of information handling in the central nervous system possible new interpretative value of the Turing's B-type machine. Prog Brain Res. 2000;125:3–19. doi: 10.1016/S0079-6123(00)25003-6. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia-implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glial metabolites of tryptophan and excitotoxicity: coming unglued. Exp Neurol. 2006;197:4–7. doi: 10.1016/j.expneurol.2005.10.021. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington's disease. Eur J Neurosci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Chéramy A, Romo R, Barbeito L. Presynaptic regulation of dopaminergic transmission in the striatum. Cell Mol Neurobiol. 1988;8:7–17. doi: 10.1007/BF00712906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R. Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem. 1997;69:290–298. doi: 10.1046/j.1471-4159.1997.69010290.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grilli M, Raiteri L, Patti L, Parodi M, Robino F, Raiteri M, Marchi M. Modulation of the function of presynaptic alpha7 and non-alpha7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain. Br J Pharmacol. 2006;149:724–732. doi: 10.1038/sj.bjp.0706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem. 1995;65:2621–2632. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höcht C, Opezzo JA, Taira CA. Applicability of reverse microdialysis in pharmacological and toxicological studies. J Pharmacol Toxicol Methods. 2007;55:3–15. doi: 10.1016/j.vascn.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Hodgkins PS, Schwarcz R. Metabolic control of kynurenic acid formation in the rat brain. Dev Neurosci. 1998;20:408–416. doi: 10.1159/000017338. [DOI] [PubMed] [Google Scholar]
- Jakel RJ, Maragos WF. Neuronal cell death in Huntington's disease: a potential role for dopamine. Trends Neurosci. 2000;23:239–245. doi: 10.1016/s0166-2236(00)01568-x. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. Alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lopes C, Pereira EF, Wu HQ, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R. Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. J Neurochem. 1991;57:533–540. doi: 10.1111/j.1471-4159.1991.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Pakkanen JS, Jokitalo E, Tuominen RK. Up-regulation of beta2 and alpha7 subunit containing nicotinic acetylcholine receptors in mouse striatum at cellular level. Eur J Neurosci. 2005;21:2681–2691. doi: 10.1111/j.1460-9568.2005.04105.x. [DOI] [PubMed] [Google Scholar]
- Patti L, Raiteri L, Grilli M, Zappettini S, Bonanno G, Marchi M. Evidence that alpha7 nicotinic receptor modulates glutamate release from mouse neocortical gliosomes. Neurochem Int. 2007;51:1–7. doi: 10.1016/j.neuint.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Rizzo RC, Costantino G, Marinozzi M, Amori L, Guidetti P, Wu HQ, Schwarcz R. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. Chem Med Chem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Venturoni F, Bellocchi D, Carotti A, Marinozzi M, Macchiarulo A, Amori L, Schwarcz R. Sequence variants in kynurenine aminotransferase II (KAT II) orthologs determine different potencies of the inhibitor S-ESBA. Chem Med Chem. 2008;3:1199–1202. doi: 10.1002/cmdc.200800109. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Rassoulpour A, Guidetti P, Wu HQ, Schwarcz R. Dopaminergic control of kynurenate levels and N-methyl-D-aspartate toxicity in the developing rat striatum. Dev Neurosci. 1998;20:146–153. doi: 10.1159/000017309. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Rassoulpour A, Wu HQ, Guidetti P, Roberts RC, Schwarcz R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-D-aspartate neurotoxicity. Neuroscience. 2007;148:188–197. doi: 10.1016/j.neuroscience.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulpour, Wu H-Q, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Du F, McCarthy KE, Okuno E, Schwarcz R. Immunocytochemical localization of kynurenine aminotransferase in the rat striatum: a light and electron microscopic study. J Comp Neurol. 1992;326:82–90. doi: 10.1002/cne.903260107. [DOI] [PubMed] [Google Scholar]
- Sakai K, Akiyama K, Kashihara K, Tsuchida K, Ujike H, Kuroda S, Shohmori T. AMPA receptors modulate dopamine release in the striatum, as measured by brain. doi: 10.1016/s0197-0186(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: implications for Huntington's disease. Exp Neurol. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Mora F. Endogenous glutamate increases extracellular concentrations of dopamine, GABA, and taurine through NMDA and AMPA/kainate receptors in striatum of the freely moving rat: a microdialysis study. J Neurochem. 1997;69:1476–1483. doi: 10.1046/j.1471-4159.1997.69041476.x. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Gramsbergen JB, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Santiago M, De Vries JB. In vivo evidence for a concordant response of terminal and dendritic dopamine release during intranigral infusion of drugs. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:637–643. doi: 10.1007/BF00168736. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Baran H, Ungerstedt U, Schwarcz R. Kynurenic acid in the quinolinate-lesioned rat hippocampus: studies in vitro and in vivo. Eur J Neurosci. 1992;4:1264–1270. doi: 10.1111/j.1460-9568.1992.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, Du F, Whetsell WO, Jr, Guidetti P, Schwarcz R, Tagle DA. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 2004;24:6919–6930. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]