Abstract
Objective
Monocyte activation and migration are crucial events in the development of atherosclerosis and other inflammatory diseases. This study examined the role of protein kinase D (PKD) in monocyte migration.
Method and Results
PKD2 is the predominant isoform of PKD expressed in monocytic THP-1 cells and primary human monocytes. Lysophosphatidylcholine (lysoPC), a prominent component of oxidized low density lipoprotein, induces rapid and marked PKD activation in these cells. Using multiple approaches, including dominant-negative mutants and small interfering RNA knock-down, we found that lysoPC-induced PKD2 activation was required for the activation of both ERK and p38 MAPK. p38 MAPK mediation of lysoPC-induced monocytic cell migration was reported previously; our results reveal that the lysoPC-induced PKD2-p38 pathway controls monocyte migration.
Conclusions
This study provides the first evidence that 1) lysoPC activates PKD, 2) PKD2 has a novel role in p38 activation, and 3) the PKD2-activated p38 pathway is responsible for lysoPC-induced migration of THP-1 cells and human monocytes. Thus, PKD is a novel and functional intracellular regulator in both lysoPC signaling and monocyte migration. These results suggest a new role for PKD2 in the development of atherosclerosis and other inflammatory diseases.
Keywords: protein kinase, signaling pathway, lysophosphatidylcholine, monocyte migration
Monocytes play an important role in many pathophysiological conditions, particularly when the progression of a disease stems from inflammation, as is the case in atherosclerosis. Oxidized low density lipoprotein (oxLDL), as well as many growth factors, cytokines, adhesion molecules, and chemokines have been implicated in monocyte activation, migration, adhesion and differentiation. It is believed that oxLDL, which has been found in atherosclerotic lesions, mediates the development of atherosclerosis.1, 2
Accumulating evidence suggests that many of the cellular effects of oxLDL during early atherosclerosis development are caused by lysophosphatidylcholine (lysoPC), an important bioactive lipid component of oxLDL (for review see 3). These effects include regulation of a broad range of cellular processes such as monocyte chemotaxis,4 monocyte growth factor production5 and gene transcription.6 LysoPC is thought to be responsible for oxLDL-mediated migration of circulating monocytes, both directly4, 7 and indirectly by stimulation of the release of monocyte chemoattractant protein-1 (MCP-1) from smooth muscle cells and endothelial cells.6, 8 A previous study showed that lysoPC-induced THP-1 monocytic cell migration involves activation of p38 mitogen-activated protein kinase (MAPK).7 However, how p38 MAPK is activated by lysoPC remains elusive.
In an effort to better understand lysoPC-induced signaling cascades and cellular responses to lysoPC, we identified protein kinase D (PKD) as a new component in both the lysoPC-signaling pathway and monocyte activation. PKD is a relatively new serine/threonine protein kinase family consisting of three isoforms: PKD1, PKD2, and PKD3 (for review see 9, 10). Because PKD2 has only recently been identified, many of itsbiological functions remain to be explored. Our data demonstrated that lysoPC activates PKD2 in monocytes and the activated PKD2 mediates lysoPC-induced monocyte migration. These results reveal a novel cellular function of lysoPC-activated PKD2 in the regulation of monocyte migration.
Materials and Methods
Materials
LysoPC (1-Palmitoyl-2-hydroxy-sn-Glycero-3-Phosphocholine) was from Avanti Polar Lipids. Antibodies against PKD2 and phospho-PKD2 (Ser876 of human PKD2) were from Upstate Cell Signaling Solutions. Antibodies against PKD1, phospho-PKD-loop (phospho-Ser738/742 of human PKD1, phospho-Ser706/710 of human PKD2, and phospho-Ser731/735 of human PKD3), phospho-PKD1 C-termini (phospho-Ser910 of human PKD1), ERK, phospho-ERK, p38, and phospho-p38 were from Cell Signaling Technology. An antibody against PKD3 was from Bethyl Laboratories. PKCδ antibody was from BD Biosciences. G2A antibody was from MBL International Corporation. Ficoll-Paque premium density gradient medium was from GE Healthcare. Alpha-naphthyl acetate esterase detection kit was from Sigma-Aldrich.
Isolation of Human Peripheral Blood Monocytes and Cell Culture
Human monocytes were isolated from heparinized whole blood by sequential centrifugation over a Ficoll-Paque premium density gradient medium and adherence to serum-coated cell culture flasks as described previously.11 Nonadherent cells were then removed. The adherent cells were detached with 5 mM EDTA/PBS. Purity of yielded monocytes was over 95% as determined by an alpha-naphthyl acetate esterase detection kit.11 The isolated human monocytes were then cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 2 mmol/L glutamine for 48 h prior to addition of stimuli. THP-1 cells, a human leukemic monocytoid cell line, were obtained from American Type Culture Collection and were cultured under the same condition as described for monocytes.
Construction of PKD2 and Dominant-Negative PKD2
Full-length human PKD2 (HPKD2) was cloned into the pcDNA3.1A vector (Invitrogen, Carlsbad, CA). The construct of pcDNA3.1A-HPKD2-D695A encoding dominant-negative PKD2 was prepared by mutating an aspartic acid into an alanine at position 695 in human PKD2.12 Detailed information is given in http://atvb.ahajournals.org.
DNA and siRNA Transfection
Cells were transfected with wild-type PKD2 or dominant-negative PKD2 construct with the use of Lipofectamine 2000 from Invitrogen. Transfection of siRNA was performed according to the manufacturer’s instructions (Qiagen). The specific PKD2 siRNA was designed and supplied by Qiagen; the control (nonsilencing) siRNA and PKCδ siRNA were also from Qiagen. G2A siRNA was from Invitrogen.
Western Blot Analysis
Cells were lysed and subjected to Western blot analysis as described previously.13
Cell Migration Assay
The cell migration was performed using a 24-well transwell (6.5-mm diameter, 5-μm pore size; Corning Costar). Briefly, cultured cells were harvested and re-suspended in the medium (RPMI 1640 plus 1 mg/ml BSA) at a cell density of 5 × 106 cells/ml. LysoPC was premixed with the medium in the lower chamber of a transwell and incubated at 37°C for 20 min. Then 100 μl of cells were added to the top chamber of a transwell and incubated for 3 h at 37°C in 5% CO2. Cells passing through the membrane were collected from the lower chamber and counted with a FACS Vantage SE flow cytometry system (BD Biosciences Immunocytometry Systems) and with a hemocytometer.
Statistical Analysis
The means ± SEs were calculated using Excel statistical software, and the statistical significance (P value) was determined by the 2-tailed Student t test. A value of P<0.05 was considered statistically significant.
Results
PKD2 is the Predominant PKD Isoform Expressed in THP-1 Cells and in Primary Human Monocytes
To investigate the biological function of the relatively new protein kinase PKD, we first examined expression of the three isoforms of the PKD family. Cell lysates of THP-1 cells as well as cell lysates of HEK 293 and HeLa cells, which were used as controls, were examined by Western analysis using PKD1-, PKD2-, and PKD3-specific antibodies. As shown in Figure 1A, all 3 isoforms, PKD1, PKD2, and PKD3, were detected in HEK 293 cells. In HeLa cells, PKD2 and PKD3, but not PKD1, were detected. Interestingly, in monocytic THP-1 cells, only PKD2 was detected. Thus, PKD2 is the predominant isoform of PKD expressed in THP1 cells. We also examined the expression pattern of PKD isoforms in freshly isolated primary human monocytes. As shown in Figure 1B, PKD2 is the major isoform detected in human monocytes. Therefore PKD2 is the predominant isoform of PKD expressed in both monocytic THP1 cells and primary human monocytes.
Figure 1.
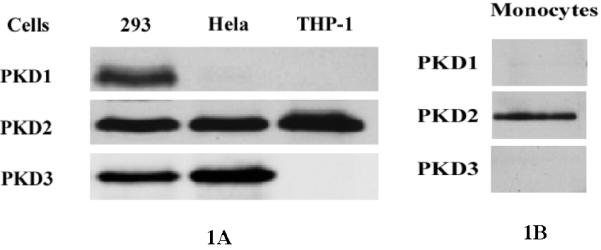
Western blot analysis of expression of PKD isoforms in THP1 cells, A, and primary human monocytes, B. THP1 cells and monocytes were cultured in RPMI 1640 medium containing 10% FBS and 2 mmol/L glutamine for 48 h; HeLa cells and HEK293 cells were cultured in DMEM medium containing 10% FBS for 48 h. Then cell lysates were examined by Western analysis. Specific antibodies against PKD1, PKD2 and PKD3 were used.
LysoPC Induces Rapid and Marked Activation of PKD
To determine whether lysoPC activates PKD in monocytic cells, we examined PKD phosphorylation in THP-1 cells upon stimulation with lysoPC. Using a specific antibody (Cell Signaling Technology) that recognizes phosphorylated serine residues at the activation loop common to all 3 isoforms of PKD (for human PKD1, at Ser738 and Ser742; for human PKD2, at Ser706 and Ser710; and for human PKD3, at Ser731 and Ser735), we found that lysoPC (15 μmol/L) rapidly and markedly induced PKD phosphorylation in THP-1 cells with a peak (over 8-fold) at 2 min (Figure 2, A and B). Also, lysoPC induced PKD activation in a dose-dependent manner (Figure 2, C and D), and maximum phosphorylation occurred in the lysoPC concentration range of 15-20 μmol/L.
Figure 2.
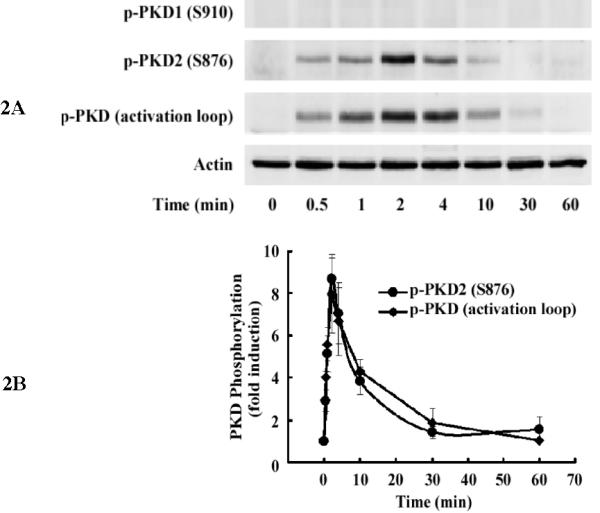
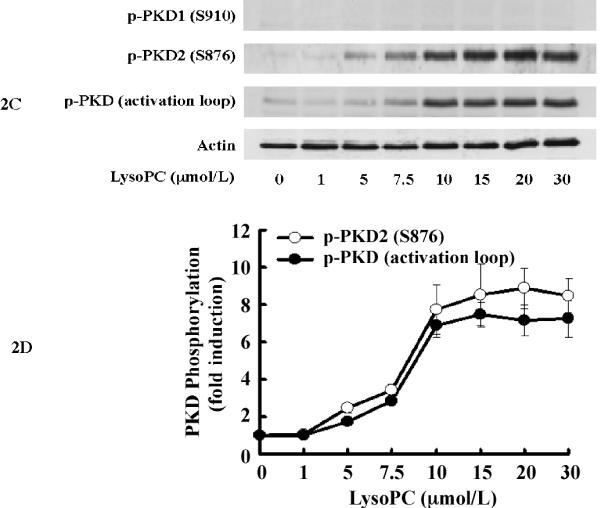
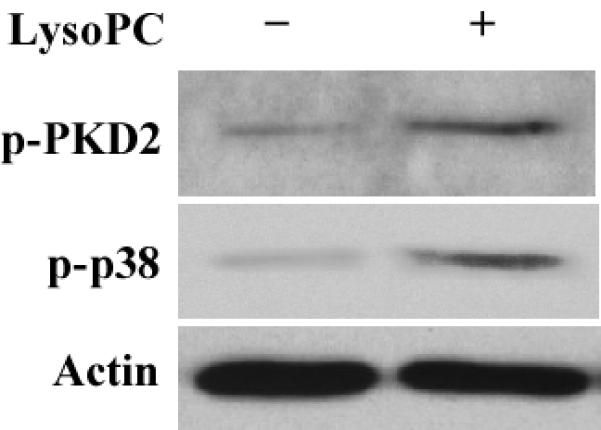
LysoPC induces PKD2 activation in THP-1 cells and monocytes. A, Time course (Western analysis) with LysoPC (15 μmol/L) used to stimulate THP1 cells. Cells were cultured in growth medium for 2 days and then incubated with or without lysoPC (15 μmol/L) for the indicated times. Phospho-specific antibodies against p-PKD1 (S910), p-PKD2 (S876), and p-PKD (activation loop) were used. Actin was used to assess protein loading. B, Time-dependent PKD phosphorylation was quantified by densitometry. C, LysoPC concentration dependence of PKD activation at 2 min in THP1 cells (Western analysis). D, Concentration-dependent PKD2 activation was quantified by densitometry. Data are means ± SE from 4 independent experiments. E, lysoPC (15 μmol/L) induces phosphorylation of PKD2 and p38 MAPK in monocytes (2 min).
As shown in Figure 2, A-D, when the blot was probed with antibodies specific for phosphorylated PKD1 (S910) and PKD2 (S876), phosphorylation of PKD2 was detected following the same time- and concentration-dependent pattern of the phosphorylation of the common activation loop of all 3 PKD isoforms. In contrast, phosphorylation of PKD1 (S910) was not detected. These results demonstrate that PKD2 is activated upon stimulation by lysoPC in THP-1 cells.
LysoPC also markedly induced phosphorylation of PKD2 in freshly isolated human monocytes, and the level of phosphorylation of PKD2 was similar as that of phosphorylation of p38 MAPK (Figure 2E).
PKD2 Is a Key Upstream Regulator of ERK and p38 MAPK in Response to LysoPC
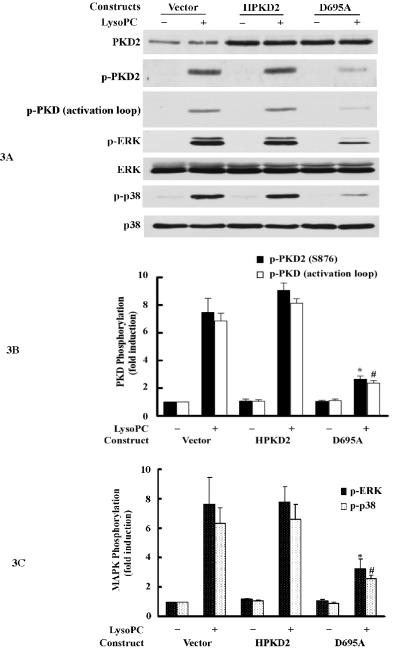
LysoPC-induced ERK and p38 MAPK activation in THP-1 monocytic cells has been reported previously.7 However, the identity of the upstream signaling molecules that mediate ERK and p38 activation, in response to lysoPC, is still unknown. We explored the possible role of PKD2 in ERK and p38 activation by examining whether a dominant-negative PKD2 affects lysoPC-induced ERK and p38 activation. THP-1 cells were transfected with an empty vector or plasmids expressing either wild-type PKD2 or dominant-negative PKD2 mutant. As shown in Figure 3A, wild-type PKD2 and dominant-negative mutant PKD2 were expressed at equal levels in cells transfected with recombinant plasmids. It was found that upon stimulation with lysoPC, phosphorylation of PKD2, PKD activation loop, ERK and p38 was detected in cells and that overexpression of wild-type PKD2 had no significant effect on the degree of lysoPC-induced phosphorylation of PKD2, PKD activation loop, ERK and p38. However, in cells overexpressing dominant-negative PKD2, the degree of the phosphorylation of PKD2, PKD activation loop, ERK and p38 was markedly reduced (PKD2 phosphorylation reduced by 85.7%, PKD activation loop phosphorylation by 83.0%, ERK phosphorylation by 66.2% and p38 phosphorylation by 71.0%) (Figure 3, A, B and C). These results indicate that dominant-negative PKD2 inhibits lysoPC-induced ERK and p38 activation and strongly suggest that PKD2 is an upstream mediator of lysoPC-induced activation of both ERK and p38.
Figure 3.
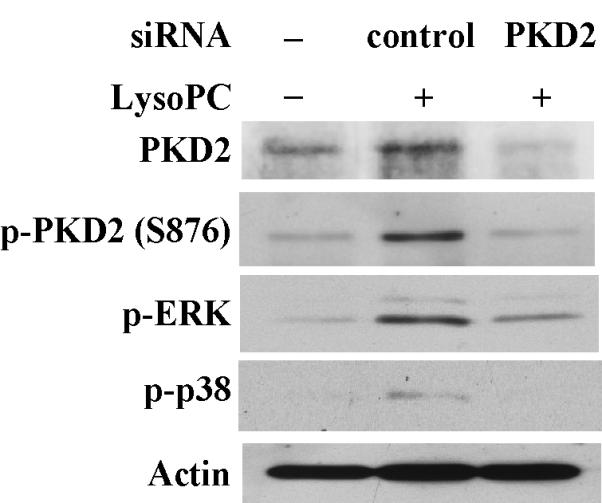
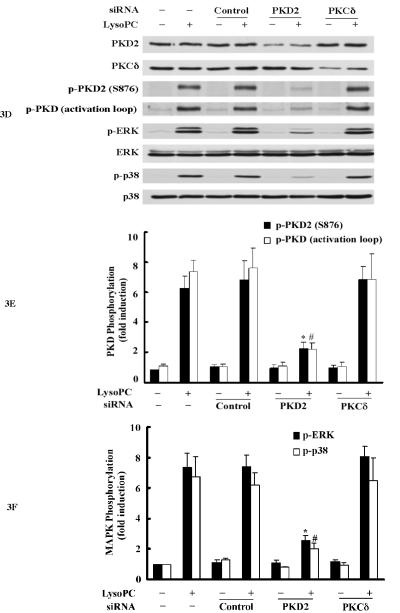
Overexpression of the dominant-negative PKD2 mutant or knockdown of PKD2 expression by siRNA inhibits lysoPC-induced phosphorylation of PKD2, PKD (activation loop), p42/44 ERK and p38 MAPK, A, Western analysis of the effects of overexpression of wild-type and dominant-negative PKD2 mutants on lysoPC-induced phosphorylation of PKD2, PKD (activation loop), p42/44 ERK and p38 in THP-1 cells. The cells were transfected with the following plasmid constructs: vector, wild-type human PKD2 (HPKD2) or dominant-negative human PKD2 (D695A). After transfection, cells were cultured in growth medium for 2 days and then treated with or without lysoPC (15 μmol/L) for 2 min. The cells were lysed, and phosphorylation of PKD2, PKD (activation loop), p42/44 ERK and p38 was assessed by Western analysis. B, Phosphorylation of PKD2 and PKD (activation loop) was quantified by densitometry. Data are means ± SE from 4 independent experiments. *P<0.05; #P<0.05 vs control (vector). C, Phosphorylation of p42/44 ERK and p38 was quantified by densitometry. Data are means ± SE from 4 independent experiments. *P<0.05; #P<0.05 vs control (vector). D, Western analysis of the effects of PKD2 siRNA on lysoPC-induced phosphorylation of PKD2, PKD (activation loop), p42/44 ERK and p38 in THP-1 cells. Cells transfected with non-silencing siRNA (control), PKD2 siRNA or PKCδ siRNA for 2 days were treated with or without LysoPC (15 μmol/L) for 2 min. Cells were lysed, followed by Western analysis. E, Effect of PKD2 siRNA on phosphorylation of PKD2 and PKD (activation loop) was quantified by densitometry. Data are means ± SE from 4 experiments. *P<0.05; #P<0.05 vs non-silencing siRNA. F, Phosphorylation of p42/44 ERK and p38 was quantified by densitometry. Data are means ± SE from 4 independent experiments. *P<0.05; #P<0.05 vs non-silencing siRNA. G, Western blot analysis of the effect of PKD2 siRNA on lysoPC-induced phosphorylation of PKD2, ERK and p38 in monocytes.
To further substantiate the finding that PKD2 functions as an upstream mediator in lysoPC-activated ERK and p38 pathways, we examined the effect of siRNA depletion of endogenous PKD2 on the phosphorylation of ERK and p38 in THP1 cells. As shown in Figure 3, D, E and F, transfection of PKD2 siRNA reduced PKD2 protein expression by 76.1% and blocked PKD2 phosphorylation by 78.3%, PKD phosphorylation (activation loop) by 80.9 %, ERK phosphorylation by 75.7%, and p38 phosphorylation by 82.2% in comparison with cells transfected with control siRNA (nonsilencing siRNA). In contrast, the PKCδ siRNA (an independent kinase control) had no effect on lysoPC-induced phosphorylation of PKD2 and PKD (activation loop) or phosphorylation of ERK and p38. The effect of PKD2 siRNA on lysoPC-induced phosphorylation of PKD2 and p38 in monocytes was also examined. As shown in Figure 3G, depletion of PKD2 by PKD2 siRNA blocked lysoPC-induced phosphorylation of PKD2 and p38. Taken together, the results obtained from the experiments using dominant-negative PKD2 construct (Figure 3, A, B and C) and the siRNA depletion of PKD2 (Figure 3, D, E, F and G) indicate that PKD2 is a new intermediate molecule in lysoPC-triggered signaling and mediates the activation of both ERK and p38 MAPK in monocytes.
PKD2 Mediates LysoPC-induced Monocyte Migration
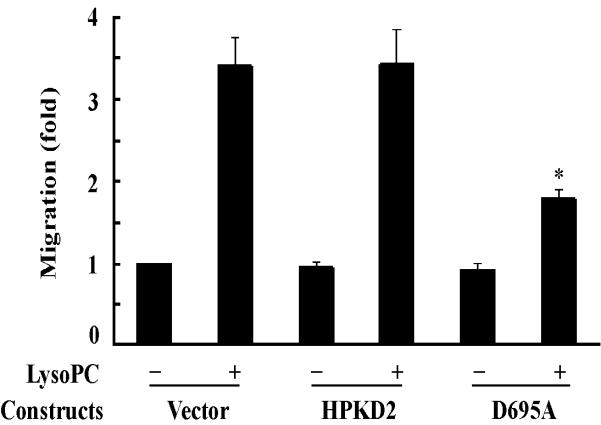
It has been reported that lysoPC possesses a potent chemotactic attraction for monocytes4 and p38 mediates lysoPC-induced monocytic cell migration.7 Based on our observation that PKD2 functions as a novel mediator of lysoPC-induced activation of both ERK and p38 (Figure 3), it became interesting to determine whether PKD2 mediates lysoPC-induced monocyte migration. We first examined the effect of overexpression of dominant-negative PKD2 on lysoPC-induced THP-1 cell migration. The effect of transfection of wild-type PKD2 or the dominant-negative PKD2 on phosphorylation of ERK and p38 is shown in Figure 3A. We observed that overexpression of the wild-type PKD2 had no significant effect on lysoPC-induced THP-1 cell migration; in contrast, overexpression of dominant-negative PKD2 reduced lysoPC-induced cell migration by 67.6% (Figure 4A). These results strongly suggest that PKD2 mediates lysoPC-induced THP-1 cell migration.
Figure 4.
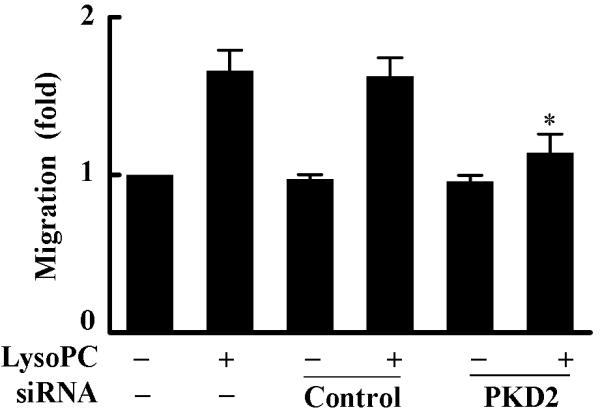
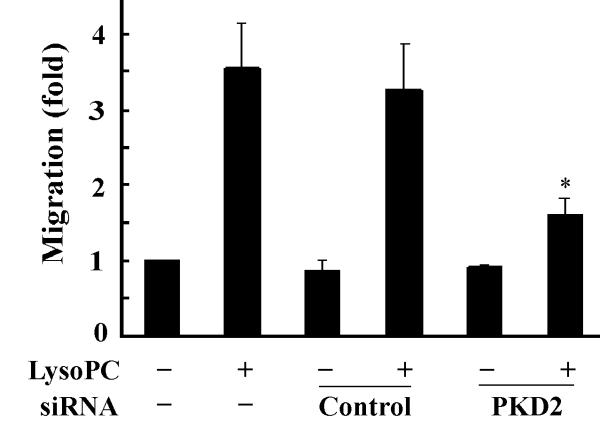
Overexpression of the dominant-negative PKD2 mutant or knockdown of PKD2 expression by siRNA blocks lysoPC-induced monocyte migration. A, Effect of overexpression of dominant-negative PKD2 on lysoPC-induced THP-1 cell migration. After transfection, cells were cultured in growth medium for 2 days and then treated with or without lysoPC (15 μmol/L) for 3 h, and cell migration was determined as described in the Methods section. Data are means ± SE from 6 experiments. *P<0.05 vs control. B, Effect of PKD2 siRNA on lysoPC-induced THP-1 cell migration. The cells, mock transfected or transfected with either nonsilencing siRNA (control) or PKD2 siRNA, were treated with or without lysoPC (15 μmol/L) for 3 h in the migration assay. Data are means ± SE from 8 experiments. *P<0.05 vs control siRNA. C, Effect of PKD2 siRNA on lysoPC-induced monocyte migration. The cells, mock transfected or transfected with either nonsilencing siRNA (control) or PKD2 siRNA, were treated with or without lysoPC (15 μmol/L) for 3 h in the migration assay. Data are means ± SE from 3 experiments. *P<0.05 vs control siRNA.
We confirmed this finding by employing another independent approach: siRNA down-regulation of endogenous PKD2 expression. Transfection of PKD2-specific siRNA into THP1 cells efficiently blocked endogenous PKD2 protein expression, PKD2 phosphorylation and MAPK phosphorylation, as shown in Figure 3D. We observed that transfection of the PKD2 siRNA reduced lysoPC-induced THP1 cell migration by 73.5%. In contrast, the nonsilencing siRNA (control) did not have a significant effect on lysoPC-induced cell migration (Figure 4B). Using primary human monocytes, we observed that PKD2 siRNA significantly reduced lysoPC-induced cell migration (by 78.6%, Figure 4C). Taken together, these data clearly indicate that PKD2 functionally mediates lysoPC-induced monocyte migration.
LysoPC Activation of PKD2 is Mediated by Pertussis Toxin (PTX)-Sensitive G-proteins and G2A protein
A PTX-sensitive pathway has been reported to mediate lysoPC-induced MAPK phosphorylation and arachidonic acid release in monocytic cells;7, 14 however, a PTX-insensitive pathway has also been reported to mediate lysoPC-induced calcium mobilization in monocytic cells.15 To our knowledge, whether G-proteins contribute to the newly identified PKD2 activation in intact cells has not been reported. To determine whether and which groups of G-proteins are responsible for lysoPC-induced PKD2 activation, we pretreated THP-1 cells with PTX, an established and specific Gi/o protein inhibitor, and found that PTX concentration-dependently blocked lysoPC-induced PKD2 activity (Figure 5A and 5B). Our data also showed that PTX blocked p38 activation (Figure 5C). These results indicate that the Gi/o group of G-proteins mediates lysoPC-induced activation of PKD2 and p38 in monocytic cells and suggest that a membrane receptor mediated pathway regulates lysoPC-induced PKD2 activation. A recent report demonstrated that receptor G2A mediates lysoPC-induced chemotaxis to monocytic THP1 cells.16 To determine whether G2A mediates lysoPC-induced PKD2 activation in monocytic cells, we investigated the effect of depletion of G2A expression on lysoPC-induced PKD2 activation using the G2A-specific siRNA. Our results showed that depletion of G2A blocked lysoPC-induced PKD2 activation (Figure 5D), indicating that G2A functionally mediates the lysoPC signal leading to PKD2 activation.
Figure 5.
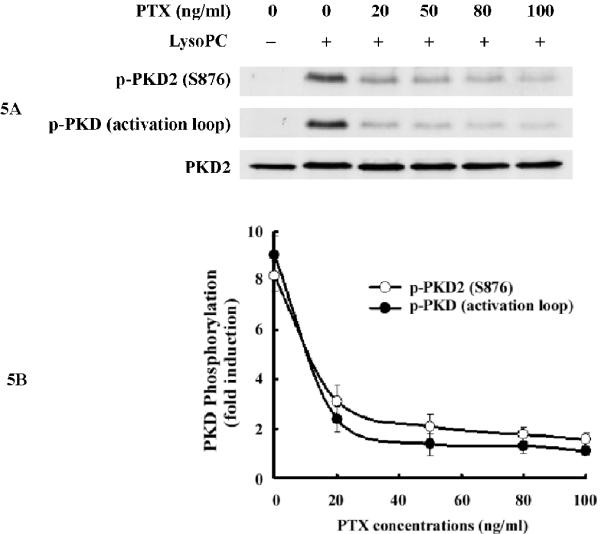
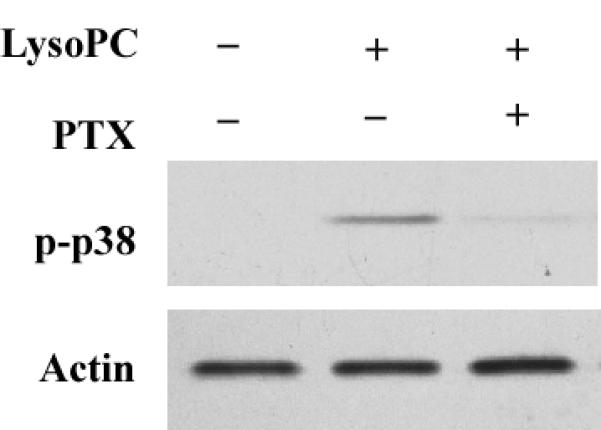
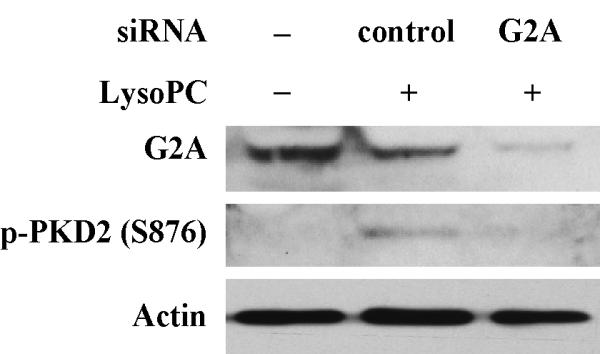
PTX inhibits lysoPC-induced phosphorylation of PKD2 and p38 in monocytic cells and knockdown of G2A expression by G2A siRNA blocks lysoPC-induced PKD2 phosphorylation. A, Effect of PTX on lysoPC-induced PKD2 activation (Western analysis). THP-1 cells were pretreated with PTX at the indicated concentrations for 16 h and then were treated with lysoPC (15 μmol/L) for 2 min. PKD2 activation was analyzed by Western analysis. B, PKD2 phosphorylation was quantified by densitometry. Data are means ± SE from 4 independent experiments. C, PTX blocks lysoPC-induced p38 phosphorylation (Western analysis). D, Knockdown of G2A expression by G2A siRNA reduces lysoPC-induced phosphorylation of PKD2.
Taken together, these results support the conclusion that lysoPC, via a novel PKD2-mediated p38 MAPK signaling pathway, mediates monocyte migration, an important biological function in the early development of atherosclerosis.
Discussion
OxLDL is believed to be an important factor in the development of atherosclerosis. LysoPC is a prominent bioactive component of oxLDL and has been shown to induce monocyte migration,4 an early event in the development of atherosclerosis. Understanding how lysoPC affects monocyte function may be important in understanding the role of oxLDL in the development of atherosclerosis. This study demonstrates for the first time that lysoPC activates PKD in living cells. Our data further indicate that the specific PKD isoform expressed and activated in monocytic THP-1 cells and primary human monocytes is PKD2, which mediates phosphorylation of ERK and p38, providing a regulatory linkage between PKD2 and p38 MAPK. Our results reveal that PKD2 is a novel intermediate molecule in both lysoPC signaling and monocyte activation. Importantly, these results reveal that lysoPC induces monocyte migration via a novel signaling pathway specifically mediated by PKD2 and p38.
Our data provide the first evidence that Gi/o protein mediates PKD2 activation in living cells. The concentration-dependent inhibition of PKD2 activation by the Gi/o inhibitor PTX indicates that a specific G-protein-coupled receptor is involved in mediating lysoPC signaling in monocytic cells. Ovarian cancer G-protein-coupled receptor 1 (OGR1), G2A, GPR4 and T cell death-associated gene 8 (TDAG8) have been reported to bind various lipids.17-20 These G-protein-coupled receptors share high amino acid sequence homologies. Although the functions of G2A and GPR4 are still under debate, a recent article reported that G2A is the crucial receptor mediating lysoPC-induced chemotaxis to THP-1 cells.16 Our results suggest that G2A mediates lysoPC-induced PKD2 activation.
PKDs are a new family of serine and threonine protein kinases. Of the 3 members of the PKD family (PKD1, PKD2 and PKD3)9, 10 only PKD1’s intracellular signaling pathway has been well studied. It has been shown that PKD1 mediates oxidative stress-induced NF-κB activation21 and BMP-2-induced JNK and p38 MAPK activation.22 However, PKD2 is a recently cloned, new member of the PKD family,23 and much less is understood about its role in intracellular signaling pathways. Recently, it was reported that overexpression of PKD2 potentiates MEK/ERK/RSK signaling,24 suggesting that endogenous PKD2 may mediate extracellular, stimulus-triggered MAPK activation. In the present study, our data show that PKD2 is the predominant isoform expressed in THP-1 cells and primary human monocytes and that transfection of PKD2 dominant-negative constructs markedly blocked lysoPC-induced phosphorylation of ERK and p38, indicating that endogenous PKD2 is an upstream regulator of MAPK activation. Knockdown of the endogenous PKD2 expression with the specific PKD2 siRNA also markedly inhibited lysoPC-induced phosphorylation of both ERK and p38, further confirming that lysoPC-induced endogenous PKD2 activity mediates the activation of both ERK and p38 in monocytes. Thus, the present study not only provides evidence that lysoPC induces PKD2 activation, but also provides evidence that PKD2 activation is essential for lysoPC-induced MAPK activation. To our knowledge, our results reveal for the first time that endogenous PKD2 is an upstream mediator of both ERK and p38 MAPK activation in living cells, in response to an extracellular stimulus.
PKC-dependent and independent activation of PKD has been reported (see reviews9, 10). Our previous studies have shown that PKCδ mediates both thrombin- and angiotensin II-induced PKD activation in smooth muscle cells.25, 26 PKCδ activation of PKD has also been found in other cell types in response to various stimuli.21, 27-29 In the present study, the activation of PKCδ, PKCε, PKCθ, PKCα and PKCζ was not detected in response to lysoPC stimulation in monocytic cells (data not shown), suggesting that none of these PKCs contributes to lysoPC-induced PKD activation in these cells. Furthermore, our data demonstrating that transfection of PKCδ siRNA has no effect on PKD2 activation support our conclusion that PKCδ has no role in lysoPC-induced PKD2 activation in monocytic cells.
Only in the last few years has the activation of this new kinase PKD been reported in vascular cells, including endothelial cells30 and smooth muscle cells,25, 26 and in platelets.31 Increasing evidence now points toward important roles for PKD-mediated signaling pathways in the regulation of myocardial contraction, hypertrophy and remodeling. However, the understanding of PKD function in the constituent cells of the heart and its role in circulation is still in its infancy (see review in 32). The present study provided new evidence of PKD activation in monocytes and explored the biological function of lysoPC-induced PKD activation. Our results identified that PKD2 is the upstream mediator of p38 and that the PKD2-p38 pathway modulates lysoPC-induced monocyte migration. These results provide evidence of the novel role of a PKD2-p38 pathway in cell migration. Therefore, our results suggest a possible new role of PKD2 in the development of atherosclerosis and possibly other inflammatory diseases.
Acknowledgements
The authors wish to thank Dr. Heather Williamson for her help in the experiments using human blood samples, Dr. Donald McGavin and Ms. Misty Bailey for critical reading of the manuscript.
Sources of Funding This work was supported by National Institutes of Health grants HL074341 (to M.-Z. Cui), AG026640 (to X. Xu) and HL29582 (to G. M. Chisolm). M.-Z. C. received a research award from the Philip Morris Foundation.
Footnotes
Disclosures None.
References
- 1.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Atherosclerosis: the new view. Sci Am. 2002;286:46–55. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- 3.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 4.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano T, Raines EW, Abraham JA, Klagsbrun M, Ross R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc Natl Acad Sci USA. 1994;91:1069–1073. doi: 10.1073/pnas.91.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rong JX, Berman JW, Taubman MB, Fisher EA. Lysophosphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1617–1623. doi: 10.1161/01.atv.0000035408.93749.71. [DOI] [PubMed] [Google Scholar]
- 7.Jing Q, Xin SM, Zhang WB, Wang P, Qin YW, Pei G. Lysophosphatidylcholine activates p38 and p42/44 mitogen-activated protein kinases in monocytic THP-1 cells, but only p38 activation is involved in its stimulated chemotaxis. Circ Res. 2000;87:52–59. doi: 10.1161/01.res.87.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Takahara N, Kashiwagi A, Maegawa H, Shigeta Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism. 1996;45:559–564. doi: 10.1016/s0026-0495(96)90024-4. [DOI] [PubMed] [Google Scholar]
- 9.Rykx A, De Kimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, Van Lint J. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 10.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 11.Cathcart MK, McNally AK, Morel DW, Chisolm GM., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989;142:1963–1969. [PubMed] [Google Scholar]
- 12.Auer A, von Blume J, Sturany S, von Wichert G, Van Lint J, Vandenheede J, Adler G, Seufferlein T. Role of the regulatory domain of protein kinase D2 in phorbol ester binding, catalytic activity, and nucleocytoplasmic shuttling. Mol Biol Cell. 2005;16:4375–4385. doi: 10.1091/mbc.E05-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui MZ, Zhao G, Winokur AL, Laag E, Bydash JR, Penn MS, Chisolm GM, Xu X. Lysophosphatidic acid induction of tissue factor expression in aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:224–230. doi: 10.1161/01.atv.0000054660.61191.7d. [DOI] [PubMed] [Google Scholar]
- 14.Oestvang J, Anthonsen MW, Johansen B. Role of secretory and cytosolic phospholipase A(2) enzymes in lysophosphatidylcholine-stimulated monocyte arachidonic acid release. FEBS Lett. 2003;555:257–262. doi: 10.1016/s0014-5793(03)01242-0. [DOI] [PubMed] [Google Scholar]
- 15.Yun MR, Okajima F, Im DS. The action mode of lysophosphatidylcholine in human monocytes. J Pharmacol Sci. 2004;94:45–50. doi: 10.1254/jphs.94.45. [DOI] [PubMed] [Google Scholar]
- 16.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 17.Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- 18.Zhu K, Baudhuin LM, Hong G, Williams FS, Cristina KL, Kabarowski JH, Witte ON, Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J Biol Chem. 2001;276:41325–41335. doi: 10.1074/jbc.M008057200. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Zhu K, Hong G, Wu W, Baudhuin LM, Xiao Y, Damron DS. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat Cell Biol. 2000;2:261–267. doi: 10.1038/35010529. [DOI] [PubMed] [Google Scholar]
- 20.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 21.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- 23.Sturany S, Van Lint J, Muller F, Wilda M, Hameister H, Hocker M, Brey A, Gern U, Vandenheede J, Gress T, Adler G, Seufferlein T. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J Biol Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 24.Sinnett-Smith J, Zhukova E, Rey O, Rozengurt E. Protein kinase D2 potentiates MEK/ERK/RSK signaling, c-Fos accumulation and DNA synthesis induced by bombesin in Swiss 3T3 cells. J Cell Physiol. 2007;211:781–790. doi: 10.1002/jcp.20984. [DOI] [PubMed] [Google Scholar]
- 25.Tan M, Xu X, Ohba M, Ogawa W, Cui MZ. Thrombin rapidly induces protein kinase D phosphorylation, and protein kinase C delta mediates the activation. J Biol Chem. 2003;278:2824–2828. doi: 10.1074/jbc.M211523200. [DOI] [PubMed] [Google Scholar]
- 26.Tan M, Xu X, Ohba M, Cui MZ. Angiotensin II-induced protein kinase D activation is regulated by protein kinase Cdelta and mediated via the angiotensin II type 1 receptor in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:2271–2276. doi: 10.1161/01.ATV.0000148449.92035.3a. [DOI] [PubMed] [Google Scholar]
- 27.Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta. 2007;1773:483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MS, Wang F, Puthanveetil P, Kewalramani G, Hosseini-Beheshti E, Ng N, Wang Y, Kumar U, Innis S, Proud CG, Abrahani A, Rodrigues B. Protein kinase D is a key regulator of cardiomyocyte lipoprotein lipase secretion after diabetes. Circ Res. 2008;103:252–260. doi: 10.1161/CIRCRESAHA.108.178681. [DOI] [PubMed] [Google Scholar]
- 29.Ozgen N, Obreztchikova M, Guo J, Elouardighi H, Dorn GW, 2nd, Wilson BA, Steinberg SF. Protein kinase D links Gq-coupled receptors to cAMP response element-binding protein (CREB)-Ser133 phosphorylation in the heart. J Biol Chem. 2008;283:17009–17019. doi: 10.1074/jbc.M709851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stafford MJ, Watson SP, Pears CJ. PKD: a new protein kinase C-dependent pathway in platelets. Blood. 2003;101:1392–1399. doi: 10.1182/blood-2002-08-2384. [DOI] [PubMed] [Google Scholar]
- 32.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: emerging roles in health and disease. Circ Res. 2008;102:157–163. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]