Abstract
Glucocorticoids and other steroid hormones have been used as treatments against a number of diseases, especially inflammatory conditions in which the immune system is overactive. These treatments have varying degrees of responsiveness among individuals and in different tissues (including brain); therefore, it is important to determine what could account for these differences. In this study, we evaluated expression of steroid hormone receptors in immune cells from lymphoid and non-lymphoid tissues as a possible explanation for tissue-specific differences. We analyzed leukocytes (CD45+) in kidney, liver, spleen, and thymus tissues from healthy mice for expression of the receptor for stress hormone (glucocorticoid - GR) as well as other steroid hormones (androgen - AR, progesterone - PR) and found that all tissues expressed these steroid hormone receptors but with varying expression patterns. To determine whether tissue-specific differences were related to immune cell composition, we examined steroid hormone receptor expression in T lymphocytes from each of these tissues and found similar patterns of expression in these cells regardless of tissue source. Because glucocorticoids can also impact brain function, we further examined expression of the stress hormone receptor in brain tissue and found GR expressed in immune cells at this site. In order to investigate the potential impact in an area of neuropathology, we utilized a mouse model of West Nile Virus (WNV). We observed pathological changes in brains of WNV-infected animals and T lymphocytes in the areas of inflammation; however, these cells did not express GR. These data indicate that tissue-specific differences in steroid hormone receptor expression by immune cells could determine responsiveness with steroid hormone treatment.
Keywords: Steroid hormone receptors, T lymphocytes, neuroimmunology, viral infection
Introduction
Glucocorticoids (GCs) are powerful inhibitors of immune responses and serve a regulatory function by preventing the immune system from going awry and causing excessive damage within tissues (Adcock, 2003). Pharmacological doses of GCs and other steroid hormones limit immune responses by inhibiting pro-inflammatory cytokine/chemokine secretion, down-regulating activation/adhesion molecule expression on cell surfaces, and inducing apoptosis in immune cells (Evans-Storms and Cidlowski, 1995; Franchimont et al., 2000; Schleimer, 2004). They can also inhibit metabolism of arachidonic acid to interfere with production of immune-stimulating prostaglandins and leukotrienes (Pitzalis et al., 2002; Tuckermann et al., 2007). Glucocorticoids and other steroid hormones have also been shown to modify adaptive immune responses (Kirwan et al., 1999), including induction of T cell apoptosis and shifting cytokine responses from TH1 towards a TH2 pattern (Schaaf et al., 2005). Because of their strong impact on immune cells, glucocorticoids are commonly used for treatments of a number of autoimmune/inflammatory disorders (Kirwan et al., 1999); however under some conditions, resistance to glucocorticoids has been reported and related to several mechanisms - including reduced number of glucocorticoid receptors (GR) (Adcock and Barnes, 2008).
Glucocorticoids (GCs) are produced following activation of the hypothalamic-pituitary-adrenal (HPA) axis (Webster and Sternberg, 2004). Inflammatory and stress-related stimuli trigger the hypothalamus to release corticotropin-releasing hormone (CRH) that leads to adrenocorticotropin hormone (ACTH) secretion from the pituitary and subsequent GC release by adrenal glands. They can also limit their own production via a feedback mechanism on the brain (Noguchi et al., 2010). GCs primarily regulate cell function through binding of the glucocorticoid receptor (GR), a member of the steroid hormone receptor superfamily - intracellular receptors that predominantly act as transcription factors; and previous reports have shown glucocorticoid effects on lymphocyte development were dependent on expression of glucocorticoid receptors (Igarashi et al., 2001; Medina et al., 2001). Following ligand binding, the hormone-receptor complex translocates from the cytoplasm to the nucleus to initiate transcription of target genes (Webster et al., 2002). GR and other steroid hormone receptors also exhibit non-genomic functions by interacting directly with intracellular proteins to modify cellular activity (Bruscoli et al., 2006; Song and Buttgereit, 2006).
Because steroid hormones bind to their cytosolic receptors to mediate changes in cell activity, it is important to evaluate expression of steroid hormone receptors when assessing the use of steroid hormones as a treatment measure. Several studies have reported expression of steroid hormone receptors in a variety of tissues (including brain) (Paavonen, 1994) and immune cell types (Sternberg, 2006), such as dendritic cells (Wira and Fahey, 2004), monocyte/macrophage populations (Tuckermann et al., 2007), B lymphocytes (Igarashi et al., 2001), T lymphocytes (Cohen, 1993) and other cells (Gotovac et al., 2003; Miyaura and Iwata, 2002; Rai et al., 2004), demonstrating the ability of steroid hormones to act directly on these cells to modify immunity. Steroid hormone receptor expression has also been shown in microglia, which is the primary immune cell type in the brain and mediate inflammatory responses (Sierra et al., 2008). Therefore, glucocorticoids and other steroid hormones have the capacity to limit inflammation caused by pathogens that infiltrate brain tissue.
We previously showed that CD11c+ dendritic cells (DCs) and CD4+ T lymphocytes from bone marrow and spleen tissues express receptors for glucocorticoids (GR), androgens (AR), and progesterone (PR) and that dexamethasone- (a synthetic glucocorticoid) and progesterone induced changes in DC and T cell activity that could be reversed by treating cells with the GR and PR antagonist RU486 (Butts et al., 2008; Butts et al., 2007a; Butts et al., 2007b). This emphasizes the importance of assessing receptor expression when considering how steroid hormones might modify immune cell activity. While it is essential to understand the effects of glucocorticoids and other steroid hormones on immunity to determine their impact as a treatment agent to ameliorate disease, it is also important to determine their impact on different tissues when these agents are given systemically. The purpose of this study was to assess protein expression of the receptor for stress hormone and other steroid hormones in leukocytes from lymphoid and non-lymphoid tissues as a possible explanation for differential tissue effects. In addition, we used a model of West Nile Virus (WNV) infection and exposure to bacterial products (Clostridium sordellii lethal toxin - CsL) to examine leukocytes in the area of neuropathology and impact on peripheral tissues, respectively. Here, we show differences in steroid hormone expression patterns between lymphoid and non-lymphoid tissues, which were likely due to immune cell composition. In addition, T lymphocytes in brains of WNV-infected animals and associated with neuropathology did not express GR, and we observed lymphoid depletion and apoptosis in CsL-treated animals. This suggests that lack of GR expression in tissues could be a factor contributing to local severity and/or destructiveness of inflammation in the brain.
Materials and Methods
Animals
8- to 11-week-old female C57BL/6 mice were purchased from Taconic (Hudson, NY). Animals were housed under conditions of 12-hour day/night cycle and provided with food and water ad libitum. Animals were maintained in pathogen-free facilities, and all procedures were performed using approved protocols in accordance with the National Institute of Mental Health/NIH Animal Care and Use Committee.
Estrous cycle stage determination
Vaginal smears were acquired daily for 2-3 weeks to determine regularity of estrous cycles. Vaginal secretions were collected aseptically using a smooth polished glass dropper, which was sanitized using 70% EtOH and rinsed with normal saline before collection of each sample. Each dropper was filled with 10-15 μl of sterile normal saline (NaCl 0.9%) and the tip gently inserted into the vaginal opening, approximately 1mm. Following insertion, fluid was expelled into the vagina approximately two to three times prior to collection of sample.
Vaginal secretions collected from mice (approximately 75-100 μl) were placed on a microscope slide for subsequent evaluation. Vaginal smear cytology was used for detecting specific stage of estrous cycle. Proestrus typically occurs for approximately twelve hours; estrus happens between nine and twenty-seven hours; metaestrus occurs six to eight hours; and diestrus will take place between fifty-five and seventy hours. Unstained material was observed under a light microscope to determine estrus cycle stage based on proportion among three cell types: round, nucleated epithelial cells; irregular-shaped, anucleated cornified cells; and small, round leukocytes. Proportion of each cell type was used to determine estrous cycle stage as previously reported (Butts et al., 2010).
Antibodies
Purified antibodies that recognize the mouse glucocorticoid receptor (GR) (0.1 mg/ml; diluted 1:5000), androgen receptor (AR) (0.1 mg/ml; diluted 1:5000), and progesterone receptor (PR) (0.1 mg/ml; diluted 1:5000) were purchased from Affinity Bioreagents (Golden, CO). Fluorescein isothiocyanate (FITC)-conjugated goat antibodies (0.25 mg/ml) were used as a secondary antibody for steroid hormone receptors. Phycoerythrin (PE)-conjugated anti-mouse CD45 (0.1 mg/ml) and peridinin chlorophyll protein (PerCP)-conjugated CD3 (0.1 mg/ml) were purchased from BD Biosciences (San Diego, CA).
Isolation of cells from tissue
Animals were sacrificed to obtain kidney, liver, spleen, and thymus tissue collected in RPMI 1640 (Mediatech; Herndon, VA) containing 10% charcoal-stripped serum (CSS) (Biomeda; Foster City, CA), and 2% L-glutamine and 2% penicillin-streptomycin (both from Sigma). Charcoal-stripped serum was used as a replacement for fetal calf serum (FCS) as some components of serum have been shown to demonstrate hormone-mimicking properties. Red blood cells were removed by suspending cell pellets in ACK lysis buffer (BioWhittaker; Walkersville, MD) containing ammonium hydroxide for 10 minutes at 37°C. To obtain T lymphocytes, single cell suspensions of kidney, liver, spleen, and thymus tissue were labeled with magnetic bead-conjugated antibodies specific for mouse CD3+ T cells, incubated at 4°C for 15 minutes, and passed through magnetic columns using the Miltenyi Biotec magnetic bead-based method (approximately 85-90% purity).
Analysis of Hormone Receptor Expression
Cells from each tissue type (1.0 × 106/tube) were collected into polystyrene Falcon tubes (BD Biosciences, San Diego, CA) and washed with fluorescence-activated cell sorter (FACS) buffer containing PBS (Molecular Biologicals, Inc.; Columbia, MD), 2% CSS (Biomeda), and 0.2% sodium azide (Sigma). Cells were centrifuged for 5 minutes at 447.2 g followed by supernatant removal to prepare for cell labeling. Cells were incubated with 10μl anti-mouse CD45 (BD Biosciences) and CD3 (BD Biosciences) for approximately 20 minutes. Cells were washed with FACS buffer to remove excess antibody and centrifuged for 5 minutes at 447.2 g. Supernatant was removed by decanting, and cells were treated with Cytofix/Cytoperm solution (BD Biosciences, San Diego, CA) for 20 minutes to permeabilize cells, followed by a washing step with Cytofix/Cytoperm wash buffer and 5-minute centrifugation. Cells were incubated with 10μl mouse serum for 10 minutes to prevent non-specific binding of antibodies to intracellular proteins. 10μl of antibodies to mouse GR, AR, or PR (Affinity Bioreagents) or appropriate isotype control were added to tubes for 10 minutes. Fluorochrome-conjugated secondary antibody was added to each tube for an additional 10 minutes. All incubations were done at 4°C. Cells were collected using the FACSCalibur (BD Biosciences, San Diego, CA) and analyzed with FlowJo analysis software (Tree Star, Ashland, OR).
West Nile Virus (WNV) Infection
Mouse WNV infection studies were carried out in an animal biosafety level 3 facility under a protocol approved by the NIAID/NIH Animal Care and Use Committee using C57BL6/J mice purchased from the Jackson Laboratory (Bar Harbor, Maine). All experiments were initiated using female mice 8-12 weeks of age. WNV strain NY99-35262 was kindly provided by Dr. R. Lanciotti (CDC, Fort Collins, CO). Mice were injected subcutaneously in the scruff of the neck with 102 ffu (focus forming units) WNV-NY99 suspended in 50 μL HBSS and monitored daily for 18 days.
Clostridium Sordellii Lethal Toxin (CsL) Exposure
Prior to exposure, animals were synchronized to specific stages of the estrous cycle based on vaginal secretions. CsL exposure studies were carried out under a protocol approved by the NIMH/NIH Animal Care and Use Committee using C57BL/6 mice purchased from Taconic (Hudson, NY). Clostridium sordellii lethal Toxin (CsL) was administered intraperitoneally at doses of 250ng/kg and 500ng/kg. Mice given saline served as a control. Changes in animal behavior at one-hour intervals were observed, and tissue was collected at 12 hours after exposure. Tissues were immediately fixed in 4% paraformaldehyde for further processing. Pathological changes were assessed by PHL services (NCI-Frederick, Frederick, MD).
Histology
For immunohistochemistry experiments using healthy mice, animals were perfused intracardially with cold PBS containing penicillin and streptomycin followed by perfusion with 4% paraformaldehyde to fix tissues. Brains were collected and placed in 4% paraformaldehyde solution for 24 hours. After 24 hours, tissues were transferred into tubes containing a 70% ethanol solution and placed at 4 °C until used. Serial sagittal, horizontal, and coronal sections (5μm thick) were generated and placed onto slides by Histoserv, Inc. (Gaithersburg, MD). For WNV experiments, brain tissues were aseptically removed from mice and fixed in 10% normal buffered formalin for 24 hours followed by transfer to a 70% ethanol solution until ready for use. Hematoxylin and eosin (H&E) staining of control and CsL-exposed mice was performed by PHL to assess histopathology. Hematoxylin and eosin (H&E) staining of healthy mice and WNV-infected animals was performed by Histoserv, Inc.
Immunohistochemistry
Immunohistochemical staining was performed on paraffin embedded sections generated by Histoserv, Inc. After deparaffination and rehydration in 250 ml xylene solution, endogenous peroxidase activity was blocked with methanol containing 0.3% peroxide. Steam-induced antigen retrieval was performed using Target Retrieval Solution (Dako North America, Inc., Carpinteria, CA) for 20 min. Sections were then blocked for 20 min with Cyto Q Background Buster (Innovex Biosciences, Richmond, CA) at room temperature, followed by incubation for 15 minutes each with avidin and biotin solutions (Avidin/Biotin Blocking Kit, Vector Laboratories, Inc., Burlingame, CA) at room temperature. Sections were incubated overnight at 4° C with rabbit polyclonal anti-glucocorticoid receptor (Affinity BioReagents, Golden, CO) diluted (1:100) in Antibody Diluent with Background Reducing Components (Dako North America, Inc). Sections were subsequently incubated with polyclonal goat anti-rabbit biotinylated antibody (Dako North America, Inc.) for 30 minutes at room temperature. Immune complexes were detected using the Vectastain ABC Elite Kit (Vector Laboratories, Inc, Burlingame, CA) and Liquid DAB-Plus Substrate Kit (Invitrogen Corporation, Camarillo, CA) incubated at room temperature. Hematoxylin was used as a counterstain. Tissues were then dehydrated in xylene solution at room temperature and mounted in Permount (Fisher Scientific, Fair Lawn, NJ) at room temperature. Immunohistochemistry for receptors was performed using single staining of serial sections.
For WNV experiments, 4-5 μm sections of paraffin-embedded slides were pre-treated using Diva Decloaking solution (Biocare Medical Inc) and blocked with 10% normal goat serum (Vector Laboratories) at room temperature. Slides were then incubated with rabbit polyclonal anti-glucocorticoid receptor (1:100 dilution; Affinity BioReagents, Golden, CO) or anti-CD3 antibody (1:400 dilution; Dako North America, Inc.) in Antibody Diluent with Background Reducing Components solution (Dako North America, Inc.). After incubation with anti-mouse polymer-horse radish peroxidase (Dako North America, Inc, Carpinteria, CA), sections were developed with streptavidin ABC system utilizing biotinylated goat anti-rabbit IgG (mouse adsorbed; Biocare Medical, Inc) and a diaminobenzidine chromogen (Dako North America, Inc.) followed with counterstaining in a hematoxylin solution.
Statistical analysis
For all statistical analyses, the level of significance was set at a probability of no more than 0.05. Data are presented as mean values ± standard deviation. Statistical analysis was performed using Student's t test or one-way analysis of variance (ANOVA). Post-hoc pair-wise comparison was performed using a Bonferroni procedure. Values were calculated using SigmaStat 3.0 software.
Results
Lymphoid and non-lymphoid tissues express steroid hormone receptors
We previously identified steroid hormone receptor expression at the RNA and protein levels in dendritic cells and CD4+ T cells harvested from bone marrow and spleen tissue (Butts et al., 2008; Butts et al., 2007b) and wanted to compare expression of these receptors in leukocytes from other lymphoid and non-lymphoid tissues. We measured protein expression levels of glucocorticoid receptor (GR), androgen receptor (AR), and progesterone receptor (PR) in CD45+ cells from kidney, liver, spleen, and thymus tissue of healthy mice using flow cytometry to assess steroid hormone receptor expression patterns (Figure 1). Fewer CD45+ cells were isolated from kidney and liver tissues compared to spleen and thymus, and there were differences in immune cell composition in each tissue (kidney - 10±5.6% CD45+CD3+ cells, 0.2±0.1% CD45+CD11c+ cells; liver - 15±8.7% CD45+CD3+ cells, 0.9±0.6% CD45+CD11c+ cells; spleen - 30±7.2% CD45+CD3+ cells, 1.2±0.3% CD45+CD11c+ cells; thymus - 80±14.6% CD45+CD3+ cells, 1.1±0.5% CD45+CD11c+ cells). GR was expressed in approximately 20-80% of CD45+ cells; AR was expressed in approximately 10-40% of CD45+ cells; and PR was expressed in approximately 20-70% of cells, depending on tissue source (Table 1). Since percentages do not provide information regarding the number of receptors expressed by individual cells, we also assessed mean fluorescent intensity (MFI), which provides information on relative receptor numbers expressed by individual cells. We observed that intensity of GR expression was greater than AR or PR in CD45+ cells, and MFI values for GR were higher in lymphoid (spleen, thymus) compared to non-lymphoid (kidney, liver) tissues (Table 2). These data indicate that immune cells from lymphoid and non-lymphoid tissues express GR, AR, and PR protein but with variable expression patterns, which could account for differences in responsiveness.
Figure 1. Steroid hormone receptor protein expression in lymphoid and non-lymphoid tissues.
Expression of steroid hormone receptors by leukocytes was measured in freshly isolated cells from kidney, liver, spleen, and thymus using flow cytometry. Dot plots show glucocorticoid receptor (GR), androgen receptor (AR), and progesterone receptor (PR) are expressed by CD45+ cells from each tissue. Cross-hatch lines were determined by comparing with isotype controls. Data shown is representative of 9 independent experiments.
Table 1. Steroid hormone receptor expression in leukocytes from lymphoid and non-lymphoid tissues.
Flow cytometry was used to evaluate steroid hormone receptors expressed in CD45+ leukocytes from kidney, liver, spleen, and thymus. Table shows percentage of total cells expressing the specified steroid hormone receptor. Data are presented as mean values ± standard deviation. Statistical analysis evaluated each tissue type with the indicated steroid hormone receptor and was performed using one-way analysis of variance (ANOVA). Statistical significance was reached for each steroid hormone receptor when all tissues were compared. (n=9).
| Tissue | P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor | Kidneya | Liverb | Spleenc | Thymusd | a vs. b | a vs. c | a vs. d | b vs. c | b vs. d | c vs. d | all groups |
| Glucocorticoid | 55.2 ± 14.1 | 40.2 ± 16.2 | 28.4 ± 3.7 | 56.5 ± 22.9 | 0.315 | 0.006 | 1.00 | 0.739 | 0.217 | 0.004 | 0.002 |
| Androgen | 21.8 ± 5.4 | 10.6 ± 2.6 | 26.9 ± 4.3 | 30.1 ± 4.8 | <0.001 | 0.117 | 0.002 | <0.001 | <0.001 | 0.796 | <0.001 |
| Progesterone | 67.1 ± 25.1 | 35.8 ± 23.6 | 57.6 ± 26.2 | 42.6 ± 23.3 | 0.066 | 1.00 | 0.254 | 0.414 | 1.00 | 1.00 | 0.045 |
Table 2. Mean fluorescence intensity of steroid hormone receptor expression in lymphoid and non-lymphoid tissues.
Mean fluorescent intensity (MFI), generated by the flow cytometer software package, was evaluated to estimate number of steroid hormone receptors expressed by individual CD45+ cells from kidney, liver, spleen, and thymus tissue. MFI shown is based on data collected in experiment from Figure 1.
| Tissue | Receptor | MFI |
|---|---|---|
| Kidney | GR | 446 |
| AR | 363 | |
| PR | 397 | |
| Liver | GR | 444 |
| AR | 327 | |
| PR | 420 | |
| Spleen | GR | 582 |
| AR | 368 | |
| PR | 424 | |
| Thymus | GR | 661 |
| AR | 375 | |
| PR | 422 |
Steroid hormone receptor expression is similar in T lymphocytes from different tissues
It is possible that discrepancies in steroid hormone receptor expression by leukocytes between lymphoid and non-lymphoid tissues are related to the composition of immune cells in each tissue. We wanted to investigate this possibility and assessed GR, AR, and PR in a specific immune cell population (CD3+ cells, T lymphocytes) known to express steroid hormone receptors. Although the total numbers of T lymphocytes in kidney, liver, spleen, and thymus tissue varied (kidney - 10±5.6% CD45+CD3+ cells; liver - 15±8.7% CD45+CD3+ cells; spleen - 30±7.2% CD45+CD3+ cells; thymus - 80±14.6% CD45+CD3+ cells), proportions of T cells expressing these steroid hormone receptors were similar and not statistically significant (Figure 2). These data provide evidence that the steroid hormone receptor expression pattern of T lymphocytes is similar in lymphoid and non-lymphoid tissues, regardless of tissue type.
Figure 2. Steroid hormone receptor expression in T lymphocytes from lymphoid and non-lymphoid tissues.
Expression of steroid hormone receptors by freshly-isolated CD3+ T lymphocytes from kidney, liver, spleen, and thymus tissues was measured using flow cytometry. Graph shows no statistically significant difference in T cells expressing GR, AR, and PR between tissues. Mean ± SEM. (n=7)
Glucocorticoid receptor protein expression by immune cells in brain tissue
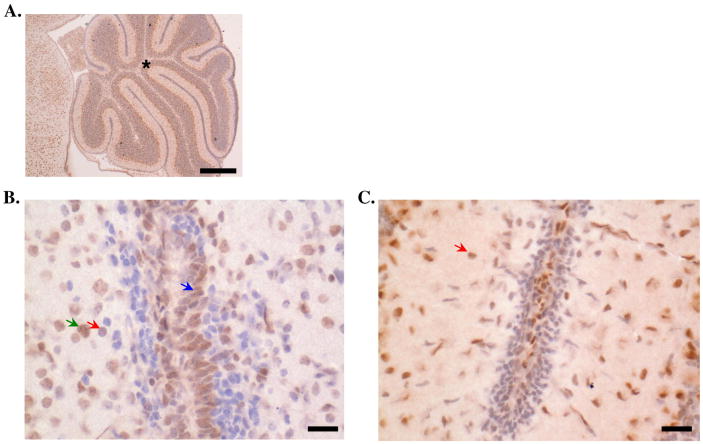
Elevated concentrations of glucocorticoids (produced during chronic stress and under inflammatory conditions) can generate a negative feedback response by binding receptors in the brain and limiting corticotropin-releasing hormone (CRH) production by the hypothalamus (Bakker et al., 2000; Kovacs et al., 2000; Tait et al., 2008). Brain tissue is also susceptible to infection and often results in infiltration of peripheral immune cells that can effectively clear invading pathogens but also lead to destruction of inflamed areas. Brain tissue contains immune cells, of which the most common type are microglia cells (Kuhlmann et al., 2009; Poluektova et al., 2005). To investigate whether the machinery for immune cells to respond to glucocorticoids was present in brain tissue, we used immunohistochemistry to examine GR expression by microglia in brains of healthy mice. We detected GR protein in brains of mice and found it expressed in cells suggestive of “resting” microglia (round, hyperchromatic cells) (Figure 3). There were several other cell types expressing GR in brain tissue of these animals, including astrocytes and neurons in the granular and Purkinje layers of the cerebellum. These data show that microglia do indeed contain the molecular machinery to respond to glucocorticoids.
Figure 3. Glucocorticoid receptor expression in brain tissue.
Expression of the stress hormone receptor, GR, was evaluated in mouse brain tissue via immunohistochemistry. Asterisk is located in the granular layer of the cerebellum. GR expression (indicated by positive cytoplasmic staining) was seen in several cell types identified based on morphology, including microglia (red arrows; approximately 8-10 μm in diameter, round, hyperchromatic cells with no appreciable cytoplasm), astrocytes (green arrows; approximately 10-20 μm in diameter, large, vesiculate nuclei with distinct nucleoli and indistinct cytoplasm) and granule cells of the external granular layer (blue arrows; located within the inner and outer granular layers of the cerebellum, approximately 8-10 μm in diameter, round, hyperchromatic cells with scant cytoplasm). Micrographs of cerebellum at 20× (scale bar=300 μm, (A) and 400× (scale bar=50 μm, (B, C) magnification are shown. In Panel C, several microglia cells were identified with cytoplasmic GR staining. Micrographs shown are representative of 5 experiments.
West Nile Virus infection leads to brain tissue degradation and T cell infiltration in areas of inflammation
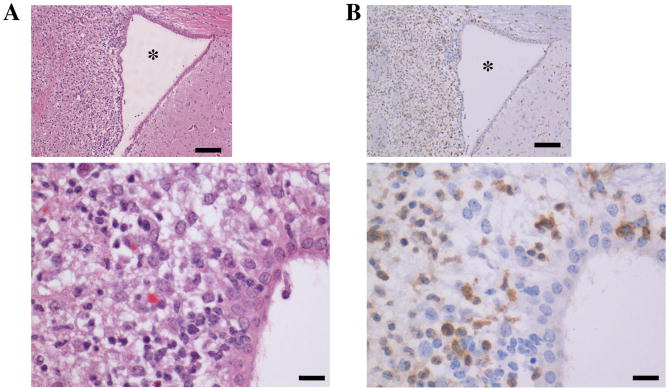
Although microglia are the most common type of leukocyte in the brain, immune cells from peripheral tissues can travel to the brain during infection. West Nile Virus (WNV) is usually transmitted through mosquito bite and in some cases leads to neuroinvasive disease (meningitis, encephalitis) and immunopathology (Lim et al.; Murphy et al., 2005). WNV infection in a mouse model leads to leukocyte infiltration (including T cells, monocytes, and NK cells), encephalitis, and death within approximately 12 days (Lim et al., 2006). Since T lymphocytes express GR in lymphoid and non-lymphoid tissues in healthy mice, we wanted to determine whether GR+ T lymphocytes were recruited to sites of inflammation in brain tissue of WNV-infected animals and found that these animals showed T lymphocyte accumulation in areas of inflammation (Figure 4); however, these cells did not express GR protein.
Figure 4. Neuropathology following West Nile Virus (WNV) infection.
Mice were infected with WNV, and brain tissue was collected 12 days after infection for immunohistochemistry. Asterisk is located in the lumen of the lateral ventricle. Evaluation of H&E sections (A) of brains of infected mice at 100× (scale bar=100 μm) and 400× (scale bar=50 μm) magnification revealed pronounced vacuolation of the neutrophil adjacent to the lateral ventricle, with infiltration of mixed inflammation (primarily lymphocytes and neutrophils). Serial sections were evaluated for CD3 expression (B) at 100× (scale bar=100 μm) and 400× (scale bar=50 μm) magnification and revealed T cells (positive membrane staining) as the predominant leukocytic infiltrate in affected areas. There was also mild to moderate multifocal, nonsuppurative meningitis present in the cerebellum and cerebrum of infected mice. Micrographs shown are representative of 3 experiments.
Inflammatory conditions result in loss and/or redistribution of immune cells from tissues
We and others have previously shown that exposure to infectious agents or their products, induces glucocorticoid release (Castagliuolo et al., 2001; Jamieson et al., 2010; Moayeri et al., 2005; Sternberg et al., 1989). To explore the impact on immune cells, we exposed mice to lethal toxin from Clostridium sordellii (CsL) (Geny et al., 2007) and examined histopathological changes in different tissues. We observed changes in a number of tissues following CsL exposure (Table 3), including kidney, liver, spleen, and thymus. As shown in Figure 5, there was extensive lymphoid depletion in spleen tissue and apoptosis in thymus tissue in the majority of infected mice that was not observed in control animals. This pattern is consistent with the effects of increased glucocorticoid release that is known to occur during inflammatory conditions and exposure to bacterial products.
Table 3. Histopathological assessment of Clostridium sordellii exposure.
Histological changes in lymphoid and non-lymphoid tissues were assessed in C57BL/6 mice administered Clostridium sordellii (CsL) intraperitoneally. Pathological conditions listed in the table were similar in animals given low and high doses of CsL and not observed in saline-treated animals. (n=5 mice/group).
| C. sordelli Study | |
|---|---|
| Behavior | Clinical signs of illness and symptomology of disease |
| Adrenal Glands | Zona reticularis apoptosis; medullary melanosis; cortex angiectasis |
| Colon | Small intestine hyperplasia; surface epithelium vacuolation; gut-associated lymphoid tissue hyperplasia/apoptosis |
| Kidney | Medullary angiectasis; chronic nephropathy |
| Liver | Hepatocellular vacuolation; subacute inflammation |
| Lungs | Hemorrhage |
| Pancreas | Acinar necrosis; acute inflammation; |
| Skin | Acute inflammation; subcutaneous hemorrhage |
| Spleen | Extramedullary hematopoiesis; lymphoid depletion, white pulp apoptosis; melanosis |
| Stomach | Gland dilation |
| Thymus | Cortical and medullary apoptosis |
| Uterus | Dilation; endometrial stroma angiectasis |
Figure 5. Tissue Pathology following Clostridium sordellii exposure.
Histopathological analysis of C57BL/6 mice administered C. sordellii lethal toxin (CsL) intraperitoneally. was assessed 12 hours following exposure. Micrographs (600× magnification, inset - 100× magnification) show lymphoid depletion in spleen tissue (A) and apoptosis in thymus tissue (B) that was not identified in control (saline-treated) animals. (C) Graphs show percentage of control and infected mice exhibiting alterations in lymphoid architecture. Mean ± SEM * = p<0.05 (n=5 mice/group)
Discussion
Steroid hormones, especially glucocorticoids, play an important role in regulating immune responses and limiting over-activation of the immune system (Webster et al., 2002). We previously identified steroid hormone receptor expression in immune cells from lymphoid tissues and showed that steroid hormone effects on these cells could be blocked by treatment with steroid hormone receptor antagonists (Butts et al., 2008; Butts et al., 2007a; Butts et al., 2007b). In the present study, we sought to further explore steroid hormone receptor protein expression in immune cells to compare lymphoid and non-lymphoid tissues, including brain, in healthy animals and under inflammatory conditions. Our findings indicate that steroid hormone receptors are differentially expressed in immune cells from lymphoid and non-lymphoid tissues and that this is likely related to the type of immune cells comprised in each tissue since proportions of CD3+ T lymphocytes expressing steroid hormone receptors was similar in each of the tissues examined. We have also studied the impact of steroid hormones on differentiation of a subset of T lymphocytes (CD4+) following activation by agents that promote TH1 responses and observed a shift from TH1 toward TH17 (unpublished results), which is similar to recently reported effects of aldosterone on T cell activation (Herrada et al., 2010).
In this study, we evaluated GR expression in the brain and found that GR was not only expressed in microglia but also in other cell types (including astrocytes and neurons – granule cells). Furthermore although microglia expressed GR in healthy mice, T lymphocytes surrounding inflammatory regions in brains of WNV-infected animals did not. This suggests that a subset of GR-insensitive T lymphocytes could have been recruited into brain tissue following WNV infection and raises the possibility that brain inflammation in response to WNV may be destructive because the infiltrating leukocytes that cause the tissue damage lack the molecular machinery to respond to endogenous glucocorticoids that dampen inflammation. These results are similar to a previous report showing down-regulation of GR, MR, and ERα gene expression in microglia following inflammatory challenge in the brain (Sierra et al., 2008) but differ from a study showing an increase in GR expression and decrease in glucocorticoid sensitivity in splenic T cells of mice following thermal injury (D'Elia et al., 2010). Indeed, we also found that mice exposed to the lethal toxin of Clostridium sordellii exhibited lymphoid depletion in splenic tissue and apoptosis in thymic tissue, which is consistent with patterns of glucocorticoid-induced immune cell apoptosis.
Some individuals are more susceptible to excessive inflammation and tissue pathology following immune system activation. This has been associated with blunted glucocorticoid responses or glucocorticoid resistance due to impaired glucocorticoid receptors or inappropriately low levels of GR (Boldizsar et al., 2006; DeRijk et al., 2002; Sternberg, 2006). Our findings suggest that differential cell and tissue expression of steroid hormone receptors could correlate with the presence of inflammation during infection. Thus, the degree of expression of GR and other steroid hormone receptors should be taken into consideration when evaluating risk for inflammatory tissue pathology during infection.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH)/NIH and by a biodefense grant from the National Institute of Allergy & Infectious Diseases (NIAID)/NIH Intramural Research Program. We also thank Dr. Cecilia Tami for careful manuscript review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM. Glucocorticoids: new mechanisms and future agents. Curr Allergy Asthma Rep. 2003;3:249–257. doi: 10.1007/s11882-003-0047-0. [DOI] [PubMed] [Google Scholar]
- Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- Bakker JM, Kavelaars A, Kamphuis PJ, Cobelens PM, van Vugt HH, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment increases susceptibility to experimental autoimmune disease in adult rats. J Immunol. 2000;165:5932–5937. doi: 10.4049/jimmunol.165.10.5932. [DOI] [PubMed] [Google Scholar]
- Boldizsar F, Palinkas L, Czompoly T, Bartis D, Nemeth P, Berki T. Low glucocorticoid receptor (GR), high Dig2 and low Bcl-2 expression in double positive thymocytes of BALB/c mice indicates their endogenous glucocorticoid hormone exposure. Immunobiology. 2006;211:785–796. doi: 10.1016/j.imbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bruscoli S, Di Virgilio R, Donato V, Velardi E, Baldoni M, Marchetti C, Migliorati G, Riccardi C. Genomic and non-genomic effects of different glucocorticoids on mouse thymocyte apoptosis. Eur J Pharmacol. 2006;529:63–70. doi: 10.1016/j.ejphar.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Butts CL, Bowers E, Horn JC, Shukair SA, Belyavskaya E, Tonelli L, Sternberg EM. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts CL, Candando KM, Warfel J, Belyavskaya E, D'Agnillo F, Sternberg EM. Progesterone regulation of uterine dendritic cell function in rodents is dependent on the stage of estrous cycle. Mucosal Immunol. 2010;3:496–505. doi: 10.1038/mi.2010.28. [DOI] [PubMed] [Google Scholar]
- Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, Tonelli L, Sternberg EM. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007a;19:287–296. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- Butts CL, Shukair SA, Duncan KM, Harris CW, Belyavskaya E, Sternberg EM. Effects of dexamethasone on rat dendritic cell function. Horm Metab Res. 2007b;39:404–412. doi: 10.1055/s-2007-980195. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Karalis K, Valenick L, Pasha A, Nikulasson S, Wlk M, Pothoulakis C. Endogenous corticosteroids modulate Clostridium difficile toxin A-induced enteritis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G539–545. doi: 10.1152/ajpgi.2001.280.4.G539. [DOI] [PubMed] [Google Scholar]
- Cohen JJ. Programmed cell death and apoptosis in lymphocyte development and function. Chest. 1993;103:99S–101S. doi: 10.1378/chest.103.2_supplement.99s. [DOI] [PubMed] [Google Scholar]
- D'Elia M, Patenaude J, Dupras C, Bernier J. T cells from burn-injured mice demonstrate a loss of sensitivity to glucocorticoids. Am J Physiol Endocrinol Metab. 2010;299:E299–307. doi: 10.1152/ajpendo.00084.2010. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol. 2002;81:103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- Evans-Storms RB, Cidlowski JA. Regulation of apoptosis by steroid hormones. J Steroid Biochem Mol Biol. 1995;53:1–8. doi: 10.1016/0960-0760(95)00034-w. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- Geny B, Khun H, Fitting C, Zarantonelli L, Mazuet C, Cayet N, Szatanik M, Prevost MC, Cavaillon JM, Huerre M, Popoff MR. Clostridium sordellii lethal toxin kills mice by inducing a major increase in lung vascular permeability. Am J Pathol. 2007;170:1003–1017. doi: 10.2353/ajpath.2007.060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotovac K, Sabioncello A, Rabatic S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: lower quantity of GCR in patients with post-traumatic stress disorder (PTSD) Clin Exp Immunol. 2003;131:335–339. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF, Fardella CE, Kalergis AM. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson AM, Yu S, Annicelli CH, Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan JR, Balint G, Szebenyi B. Anniversary: 50 years of glucocorticoid treatment in rheumatoid arthritis. Rheumatology (Oxford) 1999;38:100–102. doi: 10.1093/rheumatology/38.2.100. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Goldschmidt T, Antel J, Wegner C, Konig F, Metz I, Bruck W. Gender differences in the histopathology of MS? J Neurol Sci. 2009;286:86–91. doi: 10.1016/j.jns.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Lim JK, Glass WG, McDermott DH, Murphy PM. CCR5: no longer a “good for nothing” gene--chemokine control of West Nile virus infection. Trends Immunol. 2006;27:308–312. doi: 10.1016/j.it.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Webster JI, Wiggins JF, Leppla SH, Sternberg EM. Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect Immun. 2005;73:4238–4244. doi: 10.1128/IAI.73.7.4238-4244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TD, Grandpre J, Novick SL, Seys SA, Harris RW, Musgrave K. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246–251. doi: 10.1089/vbz.2005.5.246. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Makino S, Matsumoto R, Nakayama S, Nishiyama M, Terada Y, Hashimoto K. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology. 151:4344–4355. doi: 10.1210/en.2010-0266. [DOI] [PubMed] [Google Scholar]
- Paavonen T. Hormonal regulation of immune responses. Ann Med. 1994;26:255–258. doi: 10.3109/07853899409147900. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Pipitone N, Perretti M. Regulation of leukocyte-endothelial interactions by glucocorticoids. Ann N Y Acad Sci. 2002;966:108–118. doi: 10.1111/j.1749-6632.2002.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Rai T, Ohira H, Tojo J, Abe K, Yokokawa J, Takiguchi J, Shishido S, Sato Y. Expression of human glucocorticoid receptor in lymphocytes of patients with autoimmune hepatitis. Hepatol Res. 2004;29:148–152. doi: 10.1016/j.hepres.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Lewis-Tuffin LJ, Cidlowski JA. Ligand-selective targeting of the glucocorticoid receptor to nuclear subdomains is associated with decreased receptor mobility. Mol Endocrinol. 2005;19:1501–1515. doi: 10.1210/me.2005-0050. [DOI] [PubMed] [Google Scholar]
- Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc. 2004;1:222–230. doi: 10.1513/pats.200402-018MS. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait AS, Butts CL, Sternberg EM. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J Leukoc Biol. 2008;84:924–931. doi: 10.1189/jlb.0208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Forster I, Habenicht AJ, Reichardt HM, Tronche F, Schmid W, Schutz G. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117:1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol. 2004;181:207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]