Abstract
Anxiety is characterized by cognitive biases, including attentional bias to emotional (especially threatening) stimuli. Accounts differ on the time course of attention to threat, but the literature generally confounds emotional valence and arousal and overlooks gender effects, both addressed in the present study. Nonpatients high in self-reported anxious apprehension, anxious arousal, or neither completed an emotion-word Stroop task during ERP recording. Hypotheses differentiated time course of preferential attention to emotional stimuli. Individuals high in anxious apprehension and anxious arousal showed distinct early ERP evidence of preferential processing of emotionally arousing stimuli along with some evidence for gender differences in processing. Healthy controls showed gender differences at both early and later processing stages. The conjunction of valence, arousal, and gender is critical in the time course of attentional bias.
Emotional disturbances are common in nearly all types of psychopathology, including mood and anxiety disorders (Berenbaum, Raghavan, Le, Vernon, & Gomez, 2003). What is sometimes called the cognitive approach to understanding and treating emotional disorders has focused on biased emotional information processing as an etiological and maintaining factor in various forms of psychopathology, including anxiety disorders (e.g., Beck, Emery, & Greenberg, 2005; Williams, Mathews, & MacLeod, 1996). A large body of research has demonstrated that anxiety is characterized by cognitive biases and impairments (for reviews, see Eysenck, & Calvo, 1992; Eysenck, Derakshan, Santos, & Calvo, 2007; McNally, 1998), particularly an attentional bias to threatening stimuli (Compton, Heller, Banich, Palmieri, & Miller, 2000; Nitschke & Heller, 2002). This phenomenon has been demonstrated in state and trait anxiety (Egloff & Hock, 2001) as well as in every anxiety disorder diagnostic category in DSM-IV-TR (American Psychiatric Association, 2000).
The broad finding of attentional bias to threat across various forms of anxiety is qualified by four important issues. First, pleasant stimuli are frequently not included in investigations of attentional bias to threat, so emotional valence may be a confound. That is, it may not be threat in particular that draws attention, but any emotionally intense stimulus. Second, when pleasant stimuli are included, they are not always matched to threatening stimuli on perceived arousal, nor are arousal levels always reported for emotional stimuli, so emotional arousal may be a confound. Failure to report arousal qualities of stimuli is problematic, because emotional arousal may be important in attracting attention (Keil, Bradley, Hauk, Rockstroh, Elbert, & Lang, 2002). Indeed, the prominent circumplex model of emotion decomposes emotion into two basic dimensions, valence and arousal (e.g., Barrett & Russell, 1999; Lang, Bradley, & Cuthbert, 1990). Further, research on neural processing in emotion has identified arousal as a critical factor in recruiting brain regions, such as right occipitotemporal regions (e.g., Compton et al., 2003; Deldin, Keller, Gergen, & Miller, 2000) and amygdala (e.g., Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000). It is therefore important to consider both dimensions as potential contributors to bias. Including pleasant stimuli that are matched to threatening stimuli on arousal value is necessary in order to demonstrate the specificity and generalizability of attentional bias to threat in anxiety. Third, a lack of attentional bias toward threat specifically (rather than toward both pleasant and threatening stimuli) has been found in some anxiety disorders, such as generalized anxiety disorder (e.g., Becker, Rinck, Margraf, & Roth, 2001; Martin, Williams, & Clark, 1991), but not consistently in other anxiety disorders, such as social phobia (e.g., Becker et al., 2001). Explaining this inconsistency and whether it is due to an emotional arousal confound may facilitate identifying mechanisms of attentional bias in anxiety.
Fourth, gender has not been systematically considered in the attentional bias literature. Lifetime prevalence rates of anxiety are estimated to be higher than any other class of psychological disorder (Kessler et. al., 2005), and women are estimated to be affected by anxiety disorders more than men, with some estimates as large as 2:1 (Craske, 2003). Despite these striking gender differences in rates of anxiety disorders, gender has not been consistently assessed in investigations of contributors to the development and maintenance of anxiety disorders, leaving open the possibility of gender differences in attentional bias in anxiety. In addition, gender has been shown to be important in neural processing of emotional stimuli. In research on amygdala, one of the most well established brain structures to play a role in emotion, gender has been shown to modulate its activation (Cahill, 2006). This brain region has also been shown to be activated by emotional arousal in general, not only by negative stimuli (e.g., Garavan, Pendergrass, Ross, Stein, & Risinger, 2001; Sabatinelli, Bradley, Fitzsimmons, and Lang, 2005), further highlighting the need to examine the conjunction of valence, arousal, and gender when examining neural processing of threat in anxiety.
Four prominent hypotheses have been explored regarding the timing of attentional bias to emotional stimuli in anxiety. Three of these hypotheses have focused on threat, and none has systematically considered gender. First, attention may be captured quickly and automatically by threatening stimuli in a variety of paradigms, including tasks in which masked emotional stimuli are conditioned without conscious awareness, or visual search tasks in which threat-relevant stimuli such as snakes and spiders are detected more rapidly than flower or mushroom stimuli (Öhman & Soares, 1998; Öhman, Flykt, & Esteves, 2001; Williams et al., 1996). Second, it may be difficult to disengage from threatening stimuli. This hypothesis has been investigated using spatial attention tasks in which a threatening cue stimulus is flashed to the left or right side of visual space, followed by a non-threatening target to which the participant is supposed to respond. When the target is flashed on the side of visual space opposite to a threatening cue, performance is slowed, suggesting prolonged engagement with threat (e.g., Fox, Russo, & Dutton, 2002). Third, there is evidence that threatening stimuli can be initially engaged, followed by avoidance, known as the vigilance-avoidance hypothesis (Mogg, Mathews, & Weinman, 1987). For example, trait-anxious individuals show attentional bias to threat in a visual probe task at a shorter stimulus duration (e.g., 500 ms) in the absence of evidence for prolonged engagement with the stimulus at longer stimulus durations (e.g., 1500 ms; see Mogg, Bradley, Miles, & Dixon, 2004). Finally, the emotionality hypothesis, although not cast in terms of emotional arousal, holds that emotional stimuli in general will draw more attention than neutral stimuli (e.g., Martin et al., 1991), although attentional biases to threatening versus pleasant stimuli may develop over different time frames (Bradley, Mogg, White, Groom, & de Bono, 1999).
Studying emotional processing in non-anxious participants is necessary to understand how attentional bias may operate uniquely in anxiety. A number of studies using pleasant and unpleasant stimuli with carefully matched levels of arousal have found that emotional arousal and not valence captures attention across pictures or words (e.g., Fischler & Bradley, 2006; Herbert, Junghöfer, & Kissler, 2008; Schupp, Junghöfer, Weike, & Hamm, 2004; Vuilleumier, 2005). Other evidence suggests that healthy controls bias attention preferentially to pleasant information when pleasant and unpleasant stimuli are carefully matched on levels of arousal (Engels et al., 2007; Herbert, Kissler, Junghöfer, Peyk, & Rockstroh, 2006; Herrington et al., 2005). Other studies that did not appear to match pleasant and unpleasant stimuli on levels of arousal suggest that non-anxious participants bias attention preferentially to threatening and/or unpleasant information. For example, attention is engaged by angry faces in a crowd (Hansen & Hansen, 1988) or unpleasant social information (Pratto & John, 1991). The diversity of task demands and emotional stimuli makes it difficult to assess whether processing differences are due to emotional valence and/or emotional arousal.
Gender may be another important factor in reconciling seemingly contradictory findings. In an fMRI study investigating processing of emotional pictures in control participants, women showed greater activity in primary and secondary visual areas to unpleasant than pleasant pictures (Lang et. al., 1998), whereas men tended to show greater visual activity to pleasant than unpleasant pictures, especially erotic pictures (Sabatinelli, Flaisch, Bradley, Fitzsimmons, Lang, 2004; Lang et. al., 1998). In addition, women rated unpleasant pictures as more unpleasant and more arousing than men in combination with showing larger changes in corrugator EMG (frown-associated muscle) activity while viewing unpleasant pictures (Bradley, Codispoti, Sabatinelli & Lang, 2001). Conversely, men rated pleasant (especially erotic) pictures as more pleasant and more arousing and responded with more skin conductance (sweat gland) activity (Bradley, Codispoti, Sabatinelli & Lang, 2001; Bradley & Lang, 2007). If women preferentially process threat while men preferentially process pleasant stimuli at higher levels of arousal, failing to examine gender would lead to unnecessary inconsistency across samples. A better understanding of the nature and time course of male and female non-anxious participants’ allocation of attention to emotional stimuli may have important treatment implications and may aid in understanding the higher prevalence rates of anxiety disorders in women (see also Narrow, First, Sirovatka, & Regier, 2007).
In evaluating when and how attention is deployed to emotional stimuli, research has relied heavily on dependent measures (such as reaction time) that do not allow continuous measurement of attentional processing across time. Many studies have used a modified Stroop task, called the emotion-word Stroop task, which has become a standard paradigm in the investigation of attentional biases in anxiety. The content of the distracter words is threatening (“die”), neutral (“sum”), or pleasant (“joy”). Participants must respond to the color of the word while ignoring the content or meaning of the word. Many studies (reviewed by Koven, Heller, Banich, & Miller, 2003; Nitschke & Heller, 2002; Williams et al., 1996) demonstrate that color naming is slowed in anxious and sometimes non-anxious participants when the distracter word is threatening. Reaction time to threatening words is typically much slower in clinically anxious individuals, suggesting that they have more difficulty than individuals without anxiety in filtering out threatening information, even when task-irrelevant. Unlike reaction time, event-related brain potentials (ERPs) offer millisecond-by-millisecond measurement of attentional processes. Surprisingly few studies have used ERP measures while investigating attentional bias to emotional stimuli in anxiety.
The present study employed ERPs to investigate the time course of attentional bias in the emotion-word Stroop task. P100 and N200 ERP components were examined to identify the onset of attentional bias. P100 for visual stimuli is a positive-going voltage fluctuation peaking approximately 100 ms after stimulus onset, likely originating from extrastriate areas of visual cortex and maximal over occipital regions. As more attention is allocated to a visual stimulus, more extrastriate neurons are recruited to process the stimulus, and P100 amplitude increases (e.g., Luck, Woodman, & Vogel, 2000). In a task where a neutral and an emotional face pair were flashed simultaneously in the left and right side of visual space, followed by a horizontal or vertical bar flashed to the left or right side replacing one of the face stimuli, P100 was larger when the bar replaced a fearful face than when it replaced a neutral face (Pourtois, Grandjean, Sander, & Vuillemier, 2004), suggesting that P100 can be sensitive to fear stimuli. N200 over posterior regions for visual stimuli peaks roughly between 150-250 ms post-stimulus and has been associated with involuntary stimulus discrimination and classification (Näätänen, 1990; Nobre, Allison, & McCarthy, 1998; Ritter, Simson, Vaughan, & Macht, 1982) and abstract linguistic processing (Grossi & Coch, 2005) and has been seen in studies involving processing of emotional content (Deldin et al., 2000; Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000; Kayser, Tenke, Nordby, Hammerborg, Hugdahl, & Erdmann, 1997; Schupp et al., 2004).
To assess later processing of emotional material, P300 and N400 components were measured. P300 (sometimes called P3b, late positive potential, LPP, or late positive complex, LPC) has a predominantly parietal distribution, peaking approximately 300-600 ms post-stimulus. P300 amplitude is associated with increased resource deployment (e.g., Yee & Miller, 1994) and is thought to reflect context updating and event categorization processes (e.g., Coles, Gratton, & Fabiani, 2000; Donchin & Coles, 1988). P300 is often larger for emotional than for neutral picture or word stimuli (e.g., Fischler & Bradley, 2006; Herbert, Junghöfer, & Kissler, 2008; Schupp, Junghöfer, Weike, & Hamm, 2004), reflecting prioritization of emotion processing. P300 latency is often independent of the timing of response-related motor processes and can serve as a more specific measure of stimulus evaluation duration (Donchin & Coles, 1988; Duncan-Johnson & Donchin, 1982). N400 is a negative-going waveform seen in response to words that is modulated by semantic meaning, with larger amplitude associated with improbable words and smaller amplitude associated with facilitated processing (e.g., for words of higher lexical frequency or words “primed” in a given sentential context, Kutas & Hillyard, 1980; van Petten and Kutas, 1990). N400 amplitude is reduced for emotional stimuli that are primed (e.g., emotional words congruent with prosody elicit smaller N400 than do those incongruent with prosody; Schirmer, Kotz, & Friederici, 2002; 2005) and for emotional words in a lexical decision task (judge whether the current stimulus is a word or non-word) where no explicit “priming” of emotion was conducted (Kanske & Kotz, 2007).
A small but growing number of studies are using ERPs to investigate the emotion-word Stroop task in non-clinical groups (e.g., Li, Zinbarg, & Paller, 2007; Pérez-Edgar & Fox, 2003; Thomas, Johnstone, & Gonsalvez, 2007; van Hooff, Dietz, Sharma & Bowman, 2008). Pérez-Edgar and Fox (2003) examined N100, N200, P300, and a late positive slow wave in children to pleasant, neutral, and unpleasant words. Unpleasant words produced smaller N100 (frontal sites) and N200 (at frontal, central, and occipital sites) than pleasant words. Unpleasant words were also associated with longer P300 latency (central sites) and pronounced late positive slow wave (600-1000 ms). These results suggest less early attentional bias coupled with longer evaluation for unpleasant words.
Other studies employed an emotion-word Stroop task with adults with unpleasant and neutral words (but not pleasant). Thomas et al. investigated several ERP components, N100 (at Cz), P200 (Pz), N200 (Fz), and P300 (Pz). Unpleasant words were associated with larger P200 over the right hemisphere as well as enhanced P300 amplitude, consistent with early and later preferential processing of unpleasant words with a posterior distribution. van Hooff et. al. investigated two ERP components in an unselected sample, P100 (maximal over occipital sensors) and “negative slow wave” (NSW; similar to N400, with maximum over frontal/frontocentral sensors). Unpleasant words were associated with larger P100, and there was no main effect of valence for NSW at 300-700 ms. Instead, this component became more negative for only a subset of unpleasant words that produced RT interference. In combination, unpleasant words were interpreted as being attended more than neutral words at an early stage (larger P100 for unpleasant words) coupled with larger NSW for a subset of unpleasant words at a later stage, perhaps reflecting suppression of conceptual representations (authors’ term), or lack of priming for a subset of unpleasant words producing RT interference.
More directly related to the present project, Li et al. (2007) included individuals high or low on trait anxiety (as measured by the Behavioral Inhibition Scale, Carver & White, 1994) in an emotion-word Stroop task with neutral and threat words. Enhanced occipital P100 to threat words was found for both rapid/“subliminal” and supraliminal presentation rates. The P100 effect was more pronounced as trait anxiety increased. P300 amplitude to threat words was moderated by trait anxiety only in the subliminal condition, with higher trait anxiety associated with larger P300 to threat. This study supports preferential processing of threat at both early and late stages, with late processing effects occurring only with rapid word presentation rates, presumably outside of conscious awareness. Taken together, ERP studies using emotion-word Stroop tasks suggest that preferential processing of unpleasant words can occur at both early and later stages. However, in the absence of pleasant words, these findings are not a test specifically of whether emotional valence or emotional arousal is preferentially associated with attentional bias.
In apparently the only ERP study to examine the emotion-word Stroop in adult patients (Metzger, Orr, Lasko, McNally, & Pitman, 1997), individuals with PTSD had small and late P300 across all word types (personal pleasant, neutral, and personal traumatic) compared to healthy controls. Nevertheless, within the PTSD group, P300 was larger to both pleasant and traumatic than neutral words, suggesting more resource deployment to arousing words generally, not just to trauma words, in line with P300 results reviewed above. A trend was also observed for longer P300 latency to trauma-related words in patients with PTSD, suggesting longer evaluation time for threat/trauma (this effect may have failed to reach significance due to a small sample size; PTSD N=9). These findings suggest that timing distinguishes both psychiatric status and emotional valence. To further capitalize on the potential of the emotional Stroop paradigm, the present study examines ERPs in carefully selected groups of anxious participants to elucidate the time course of attentional bias to emotional stimuli.
Anxiety is a broad, heterogeneous construct that is sometimes problematically treated as a unitary phenomenon (Lang, 1968). Anxiety can be analyzed in terms of at least two distinct dimensions, anxious apprehension and anxious arousal. Anxious apprehension is primarily characterized by worry and verbal rumination (Barlow, 1991; Heller, Nitschke, Etienne, & Miller, 1997), whereas anxious arousal is characterized by somatic tension and physiological arousal (Clark & Watson, 1991). Although these two types of anxiety are not mutually exclusive and may be present to varying degrees in different disorders, anxious apprehension is prominent in generalized anxiety disorder and obsessive compulsive disorder, and anxious arousal is prominent in panic attacks and high-stress situations (Nitschke, Heller, & Miller, 2000). These two dimensions of anxiety are also distinguished by different patterns of lateralized brain activity. EEG measures indicate that individuals scoring high on measures of anxious apprehension show greater activity (less alpha-band power) over the left than right hemisphere (Heller, Nitschke, Etienne, & Miller, 1997). The left hemisphere has been implicated in studies of obsessive-compulsive disorder (e.g., Baxter, Phelps, Mazziotta, Guze, Schwartz, & Selin, 1987; Swedo et al., 1989), generalized anxiety disorder (for a review see Nitschke & Heller, 2002; Wu, Buchsbaum, Hershey, Hazlett, Sicotte, & Johnson, 1991), and trait anxiety (Tucker, Antes, Stenslie, & Barnhardt, 1978), conditions marked by high levels of anxious apprehension. These findings linking the left hemisphere to anxiety disorders that feature worry and anxious apprehension are consistent with its specialization for language. Thus, anxiety-related impairments in various tasks might be accounted for by interference from iterative activity in left-hemisphere verbal processing circuits.
In contrast, anxious arousal shows more right than left lateral frontal activity (less alpha power) coupled with more right posterior activity (Heller & Nitschke, 1998; Nitschke, Heller, Palmieri, & Miller, 1999). Consistent with this observation, the right hemisphere is involved in vigilance and autonomic arousal (Compton et al., 2003; Heller, Nitschke, & Lindsay, 1997) and has been implicated in studies of patients with panic disorder or panic symptoms (Reiman, Raichle, Butler, Herscovitch, & Robins, 1984; Swedo et al., 1989) and in studies of non-patients in high-stress situations (Tucker, Roth, Arneson, & Buckingham, 1977). Taking into account lateralization of function in anxious apprehension and anxious arousal may therefore be useful in interpreting lateralized ERP data in these populations.
Much of the previous work examining attentional bias in anxious populations has relied on measures such as the State-Trait Anxiety Inventory (STAI; Spielberger, 1968), a measure of anxiety that is highly correlated with anxious apprehension and depression (less so with anxious arousal), indicating that the STAI is not specific to any one type of anxiety, or to anxiety at all (Nitschke, Heller, Imig, McDonald, & Miller, 2001). Thus, research relying solely on the STAI as a measure of anxiety conflates anxious apprehension and anxious arousal, in effect treating anxiety as a unitary construct, and conflates anxiety and depression.
The present study recruited participants carefully selected for high levels of either anxious apprehension or anxious arousal, both groups having low levels of co-occurring depression. A control group was low in all three. ERPs were used to examine the timing of attentional bias to emotional stimuli during an emotion-word Stroop task. This report focuses on behavioral and ERP data collected as part of a larger project including EEG and fMRI in separate sessions and using emotion- and color-word Stroop tasks. Portions of the fMRI data from about half of the participants in the present sample were published in Engels et al. (2007) and a smaller group in Mohanty et al. (2007).
A substantial effect on overt performance (reaction time; RT) was not anticipated. In nonclinical samples the RT impairment from emotional content is attenuated, and even with a very large sample statistical significance may depend on careful selection of anxiety measures (e.g., N=138 in Koven et al., 2003, in which anxiety sensitivity but not anxious apprehension or anxious arousal uniquely and significantly predicted Stroop interference for threat). However, both behavioral and brain data indicate robust effects of task (Koven et al., 2003; Engels et al., 2007; Mohanty et al., 2007). In the present study the focus was on ERP evidence of the timing of attentional bias to emotional stimuli in anxiety.
Critical differences in the timing of attention were explored in the two anxious groups and non-anxious controls.1 1) If attentional bias specifically to threat occurs early in anxiety, then components <300 ms (P100, N200) should show higher amplitude for threatening words in anxiety groups. Alternatively, if initial attentional bias is associated more broadly with emotional arousal, then components <300 ms should show greater amplitude for both high-arousing pleasant and threatening words in anxiety groups. 2) If extended resources are deployed to processing threatening words, or if threatening words are difficult to disengage from, then components >300 ms should be modulated, including larger P300 amplitude and/or longer P300 latency. N400 amplitude should be smallest for those words that are easiest to access (threat). Alternatively, if emotionally arousing words are associated with more extended processing more generally, then P300 amplitude should be larger, P300 latency longer, and N400 amplitude smaller for high-arousing pleasant and threatening words in anxiety groups. 3) If threatening or emotionally arousing words are preferentially processed initially prior to 300 ms, followed by subsequent avoidance, then early components (P100, N200) should show greater amplitude for threatening or arousing emotional words in anxiety groups coupled with smaller P300 amplitude and/or earlier P300, reflecting lack of continued engagement. N400 is expected to be modulated under “avoidance” conditions as well. Given a lack of guidance from the literature on N400 under such conditions, it is unclear whether it would be augmented or reduced. 4) Given the discrepancies in the literature regarding attentional deployment in non-anxious control participants, this group was included in order to investigate whether/when preferential attentional processing occurs in response to pleasant and/or threatening words matched on high levels of arousal, separate from effects for or moderation by anxiety. 5) Gender is included based on well established evidence of its importance in the neural processing of emotion.
Method
Of 4,457 college undergraduates screened for the study, participants were 832 (46 female) paid volunteers (mean age = 18.86, SD = .87) recruited via group questionnaire screening sessions. Participants were approximately 5% African American, 13% Asian/Pacific Islander, 1% Hispanic, and 81% European American. Participants were classified as high anxious apprehension (N = 21, 16 female), high anxious arousal (N = 26, 14 female), or low anxiety (N = 36, 16 female) on the basis of responses on the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990; Molina, & Borkovec, 1994) and the Mood and Anxiety Symptom Questionnaire (MASQ; Watson, Clark, et al., 1995; Watson, Weber, et al., 1995). Compared to the total participants screened for the study, the anxious apprehension group scored above the 80th percentile (>=63) on the PSWQ and below the 50th percentile on the MASQ Anxious Arousal scale. The anxious arousal group scored above the 80th percentile (>=33) on the MASQ Anxious Arousal scale and below the 50th percentile on the PSWQ. The control group scored below the 50th percentile on both scales (<=49; <=25, respectively). All three groups also scored below the 50th percentile (<=17) on a depressed-mood subscale (Nitschke et al., 2001) of the MASQ Anhedonic Depression scale. The PSWQ, MASQ-AA, and MASQ-AD were administered again when participants came to the lab individually. As expected, the three groups still differed significantly on the two anxiety scales (see Table 1 for means and standard deviations for each group). The groups did not differ in age. There was a trend for groups to differ on gender balance, χ2 (2, N = 83) = 5.45, p = .066. Only the anxious apprehension group had an uneven gender distribution with women outnumbering men, χ2 (1, N = 21) = 5.76, p = .016. All participants were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971) and were native speakers of English with self-reported normal color vision. Participants were given a laboratory tour, informed of the procedures of the study, and excluded if they endorsed: moderate to severe head injury, loss of consciousness for ten minutes or more, alcohol and/or drug abuse and/or dependence within the past three months (as defined by DSM-IV-TR, American Psychiatric Association, 2000), experience with electroshock therapy, multiple sclerosis, epilepsy, current pregnancy, claustrophobia, and/or contraindications for MRI participation (such as metal present in the body).
Table 1.
Questionnaire Scores Used in Group Selection
| PSWQ | MASQ-AA | MASQ-AD | ||||
|---|---|---|---|---|---|---|
| Group | Time 1 | Time 2 | Time 1 | Time 2 | Time 1 | Time 2 |
| Anxious Apprehension | ||||||
| 67 (3.6) | 63 (8.8) | 22 (2.4) | 24 (5.8) | 13 (2.6) | 15 (4.3) | |
| Anxious Arousal | ||||||
| 38 (8.1) | 35 (16.2) | 37 (3.6) | 32 (7.8) | 15 (1.9) | 14 (2.6) | |
| Control | ||||||
| 38 (8.5) | 36 (10.9) | 21 (2.2) | 22 (3.6) | 13 (2.5) | 13 (2.6) | |
Note. Questionnaire scores (mean (SD)) for each group at mass testing (Time 1) and laboratory session (Time 2). The anxious apprehension group scored higher than the other two groups on the Penn State Worry Questionnaire (PSWQ; p<.01) at Time 1 and Time 2, and the anxious arousal group and control group did not differ from each other. The anxious arousal group scored higher than the other two groups on the Mood and Anxiety Symptom Questionnaire Anxious Arousal scale (MASQ-AA; p< .01) at Time 1 and Time 2, and the anxious apprehension group and control group did not differ from each other. At Time 1, the anxious arousal group scored slightly but reliably higher than the anxious apprehension and control group on the MASQ Anhedonic Depression scale (MASQ-AD; p's<.05). The anxious apprehension and control group did not differ from each other. At Time 2, the anxious apprehension group scored higher than the control group (p<.05) but not the anxious arousal group. The anxious arousal and control group did not differ from each other.
Stimuli and Experimental Design
Word presentation and response recording were controlled by STIM software (James Long Company, Caroga Lake, NY). Several pilot studies for this project as well as published work show that a blocked design is more effective in eliciting emotion-word Stroop interference than an intermixed design (e.g., Compton et al., 2003; Dalgleish, 1995). The emotion-word Stroop task consisted of blocks of pleasant or threat emotion words alternating with blocks of neutral words. Participants received 256 trials in 16 blocks (4 pleasant, 8 neutral, 4 threat) of 16 trials. A trial began with the presentation of a word for 1500 ms, followed by a fixation cross for 275 to 725 ms (onset to onset ITI 2000 +/- 225 ms). Each trial consisted of one word presented in 1 of 4 ink colors (red, yellow, green, blue) on a black background, with each color occurring equally often with each word type (pleasant, neutral, threat). In the EEG and the fMRI sessions, each participant was randomly assigned 1 of 8 possible orders designed specifically to control stimulus order effects. In 4 of the 8 presentation orders, the first and third blocks were neutral words, with pleasant and threat blocks second or fourth, with valence order counterbalanced across participants. The remaining 4 presentation orders complemented these, with the first and third blocks being either pleasant or threat emotion words and the neutral words second and fourth. These 8 orders of presentation were designed to ensure that the neutral and emotional words preceded each other equally often in order to avoid order effects. Stimulus familiarity was controlled by presenting each word just once per EEG session. The EEG sessions and fMRI sessions were counterbalanced so that each preceded the other equally often. Within a block, each color appeared 4 times, and trials were pseudo-randomized such that no more than 2 trials featuring the same color appeared in a row. After every fourth block, there was a brief rest period. In addition to the 16 word blocks, there were 4 fixation blocks, one at the beginning, one at the end, and two in the middle of the experiment: instead of a word, a brighter fixation cross was presented for 1500 ms, followed by the fixation cross that followed word stimuli.
The 256 word stimuli were selected from the Affective Norms for English Words set (ANEW: Bradley & Lang, 1999). Sixty-four pleasant (e.g., birthday, ecstasy, laughter), 64 threat (e.g., suicide, war, victim), and two sets of 64 neutral (e.g., hydrant, moment, carpet) words were carefully selected on the basis of established norms for valence, arousal, and frequency of usage in the English language (Bradley & Lang, 1999; Toglia & Battig, 1978). Specifically, pleasant and threat words were chosen to be particularly high in arousal. Words ranged from three to eight letters in length. Words were presented in capital letters using Tahoma 72-point font at a distance of 1.35 m from the participant's eyes, for a vertical span of 1.2 degrees and a horizontal span of 3-9 degrees. Instructions were read verbatim by experimenters to assure that participants understood task requirements. The participant performed 32 practice trials before the actual tasks began. No participants failed to understand the task instructions or the mapping between colors and buttons after completing practice trials.
Electrophysiological recordings
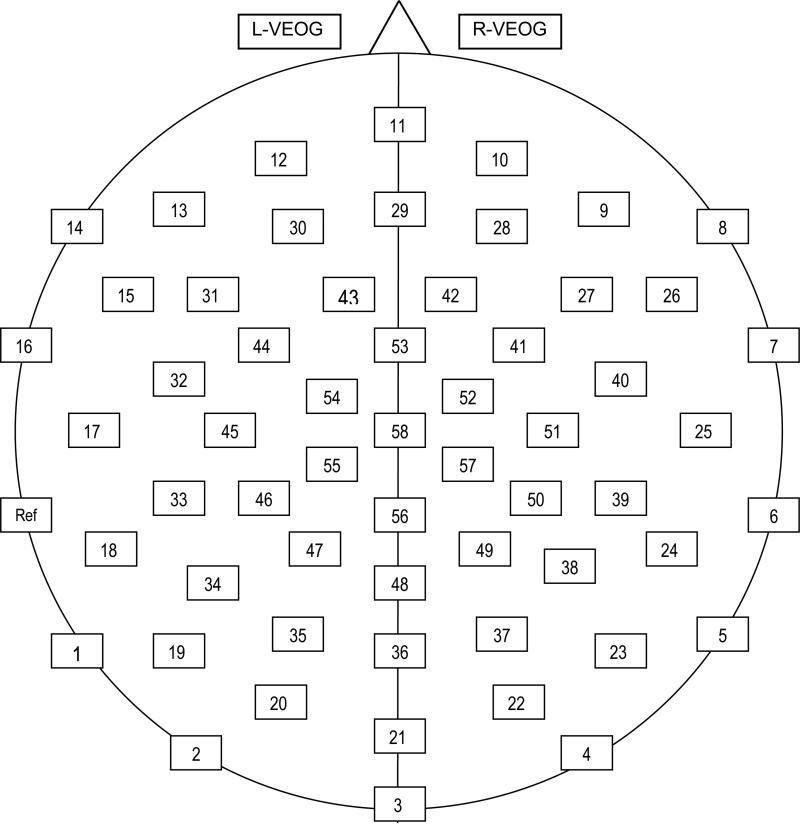
Participants were seated in a comfortable chair in a quiet room that was adjacent to a room where the experimenter controlled stimulus presentation and EEG data collection. The participant room was connected to the experimenter room by intercom. EEG was recorded with a custom-designed Falk Minow 64-channel cap with Ag/AgCl electrodes spaced equidistantly, extending inferiorly to the F9/F10 ring of the 10-10 System (see Figure 1). The left mastoid served as the online reference for all EEG and EOG sites. Electrodes placed above and below each eye and near the outer canthus of each eye recorded vertical and horizontal EOG for off-line eye-movement artifact correction of EEG. Electrode impedances were maintained below 20 Kohms. Half-power amplifier bandpass was .1 to 100 Hz, and data were digitized at 250 Hz. Electrode positions were recorded using a Zebris ELPOS digitizer (Zebris Medizintechnik, Tübingen, Germany).
Figure 1.
Custom-designed montage with electrodes spaced equidistantly. Montage extended to the more inferior F9/F10 ring of the 10-10 system. Electrode 58 is equivalent to Cz.
Data Reduction
Via Brain Electrical Source Analysis (BESA 5.1.8) software, muscle, movement, and other artifacts were removed manually, and eye blinks were corrected (Berg & Scherg, 1994). If a particular channel was off-scale for many trials (approximately 10%), all trials for that channel were removed from analyses; otherwise all channels for epochs in which a single channel was off-scale were discarded. Individual participants with more than 5% of channels discarded due to artifact (N=3) 2 were excluded from all analyses. The number of available pleasant, neutral, and threatening words3 was not differentially affected by artifact correction, Emotion F (2, 142) = 1.75, p = .181, and did not differ by group or gender. The number of neutral trials was double that for pleasant or threatening words. A random and even sampling of each of the 8 neutral blocks was taken to create a neutral average with 64 trials for each subject. Results based on these neutral averages are reported in footnote 5, referenced in the Results section. Trials accurately responded to were averaged for each emotion condition. ERP trials were rejected if reaction times were <350 ms or >1400 ms. The electrode configuration was then transformed to BESA's standard 81-channel montage using spherical spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989), reflecting the 10-10 system. An average reference was computed for each time point as the mean voltage over the 81 standard virtual scalp electrodes. Data were exported from BESA, and each channel baseline-adjusted by subtracting the average amplitude for the 200 ms before stimulus onset in custom Matlab software. Waveform averages were smoothed using a 101-weight, .1-20 Hz digital filter for P100 and N200 components and a 101-weight, .1-8 Hz digital filter for P300 and N400 (Cook & Miller, 1992; Edgar, Stewart, &: Miller, 2005; Nitschke, Miller, & Cook, 1998). To avoid spurious peaks driving amplitude measures, a combination peak/area measure was used. Voltage 48 ms around the peak was averaged for P100 and N200, and voltage 96 ms around the peak was averaged for P300 and N400. The difference in time averaged around the peak for early (<300 ms) and late (> 300 ms) components reflected faster versus slower resolution of the components. The latency associated with the peak was also recorded. All component scores were obtained for each of the 81 electrodes. Participants who displayed amplitude values more than 3 SD from the mean for a particular component at more than two electrode sites were excluded from all analyses (N=5, 3 female) 2.
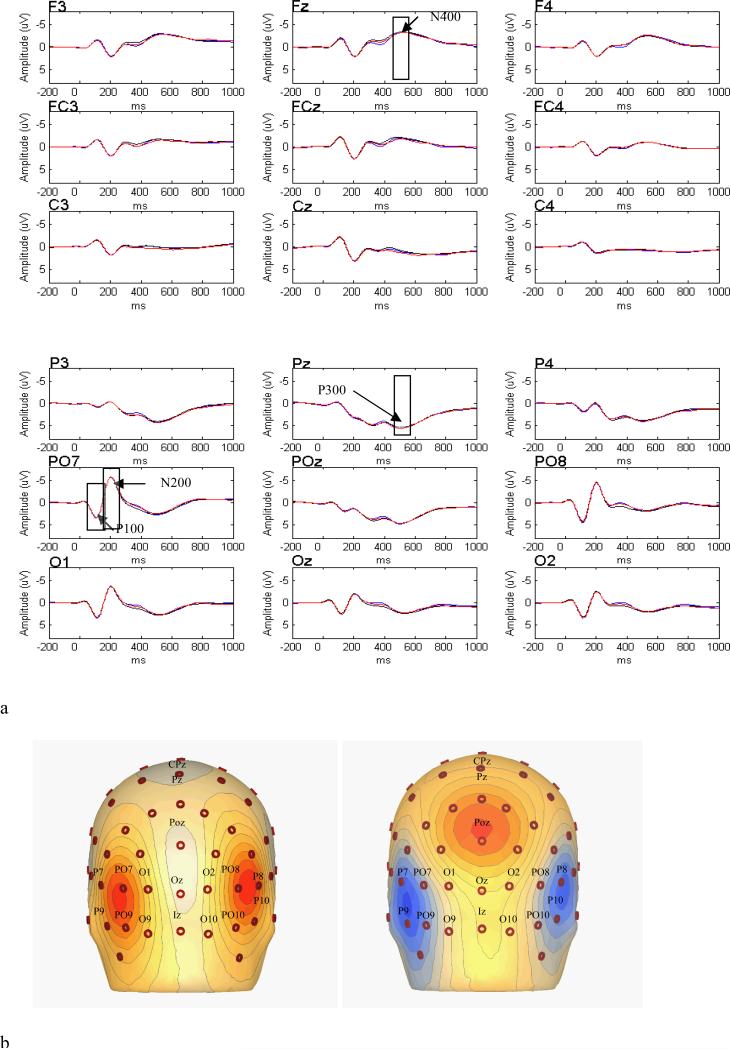
Four ERP components were scored: P100 (88-128 ms), N200 (160-240 ms), P300 (448-580 ms) and N400 (448-580 ms). For P100 and N200, sites for analysis were chosen based upon examination of current source density (CSD) estimates across conditions and across groups. Current source density is a transformation of the EEG to its second spatial derivative, essentially a spatial high-pass filter that reduces the spread of focal brain activity on the scalp surface and enhances the contribution of the underlying cortical surface to the recorded electrode signal (Hoechstetter et al., 2004; Nunez et al., 1999). Amplitude values at sites where CSD activity was maximal for P100 (P7, P8, PO7, PO8, O1, O2) and N200 (P7, P8, P9, P10, PO7, PO8, PO9, PO10) were averaged together by hemisphere for each component separately (see Figure 2b). Note that voltage associated with these sensors, and not CSD activity was subsequently analyzed). Amplitude values at sites for P300 (P1, P2, P3, P4) and N400 (FC1, FC2, FC3, FC4) were also averaged by hemisphere for each component and were chosen by integrating previous literature (e.g., Pérez-Edgar & Fox, 2003; Schirmer, Kotz, & Friederici, 2005) with inspection of the grand-average waveforms where effects were maximal.
Figure 2.
a) Grand-average event-related potential waveforms for representative frontal and posterior sensors, highlighting P100, N200, P300, and N400 (blue, black, and red tracings represent pleasant, neutral, and threatening words, respectively). Stimulus onset was at time = 0 ms. b) Current source density plots illustrating areas of maximal voltage at 100 ms (P100; left graph) and 200 ms (N200; right graph) after stimulus onset. Values range from red = + 0.63 uV/cm^2 to blue = - 0.63 uV/cm^2. The typical bilateral polarity reversal is apparent from P100 to N200.
Results
Behavioral Performance
RT for pleasant, neutral, and threat word trials was analyzed for correct trial responses between 350 and 1400 ms (mean 671 ms, SD 102 ms). Performance accuracy was relatively high (mean error rate 4.9%, SD = 3.4). A Group (anxious apprehension, anxious arousal, control) × Gender (female, male) × Emotion (pleasant, neutral, threat) MANOVA was conducted exploring linear (valence: comparing pleasant with threat) and quadratic (arousal: comparing pleasant and threat with neutral) orthogonal univariate trends on the emotion factor. P-values reflect the Huynh-Feldt correction for sphericity where appropriate. An alpha level of .05 was used. A main effect of Gender, F (1,77) = 4.12, p = .046, indicated that women were slower to respond than men by approximately 50 ms, in line with other choice-RT studies (e.g., Conroy & Polich, 2007). This main effect was qualified by a Group × Gender × linear Emotion interaction, F (2,77) = 3.58, p = .034, ε = .968. Group × Gender ANOVAs were conducted for pleasant and threat words separately, and Gender × Emotion ANOVAs were conducted for each group separately. Neither set of analyses produced a clear dissection of the 3-way interaction.
Early Visual Sensory Processing of Emotional Words
A Group (anxious apprehension, anxious arousal, control) × Gender (female, male) × Emotion (pleasant, neutral, threat) × Hemisphere4 (left, right) MANOVA including linear and quadratic trends was conducted separately for P100, N200, P300, and N400 amplitude and also for P300 latency (see Figure 2 for grand-average waveforms for representative channels).
P100
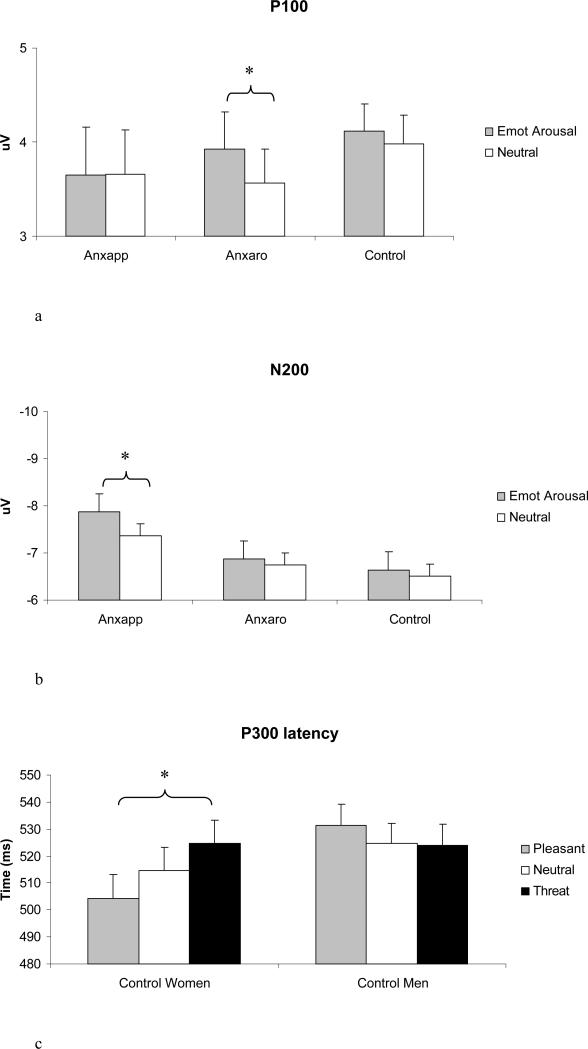
P100 amplitude was larger in women, F (1,77) = 4.48, p = .038, and was larger over the right than left hemisphere, F (1,77) = 16.19, p < .001. The gender main effect was qualified by a Group × Gender × linear Emotion interaction, F (2,77) = 5.59, p = .005 (see Figure 3). This effect was explored with Gender × Emotion ANOVAs conducted separately for each group, examining both linear and quadratic trends in light of hypotheses 1 and 3. No main effect of emotion was found in the anxious apprehension group. Anxious arousal women had larger P100 amplitude than anxious arousal men, F (1,24) = 5.63, p = .0265. The anxious arousal group showed larger P100 to emotionally arousing words, quadratic Emotion F (1,24) = 4.56, p = .043. A Gender × linear Emotion interaction, F (1,34) = 6.40, p = .016, emerged in the control group in the absence of main effects of Gender or Emotion. Separate ANOVAs were conducted for control women and control men. Control women did not show main effects or interactions with emotion. Control men showed larger P100 amplitude to threat than to pleasant words, linear Emotion F (1,19) = 4.68, p = .043
Figure 3.
Posterior components P100 and N200 amplitude and P300 latency. Error bars represent 1 SE. a) P100 emotional arousal effect in anxious arousal group. b) N200 emotional arousal effect in anxious apprehension group. c) P300 linear emotion effect in control women.
N200
Emotionally arousing stimuli enhanced N200 amplitude, quadratic Emotion F (1,77) = 5.02, p = .028. N200 was larger over the left than the right hemisphere, F (1,77) = 9.76, p = .003. Figure 3 illustrates two effects, Group × linear Emotion, F (2,77) = 3.90, p = .024, and Group × Gender × linear Emotion, F (2,77) = 4.39, p = .016. In light of hypotheses 1 and 3, Gender × Emotion ANOVAs were conducted separately for each group exploring both linear and quadratic trends. The anxious apprehension group had larger N200 for emotional arousal, quadratic Emotion F (1,19) = 4.57, p = 0.465, in the absence of a main effect or interaction with gender. The control group showed effects for linear Emotion, F (1,34) = 4.33, p = .045, and Gender × linear Emotion, F (1,34) = 9.42, p = .004, which was investigated with separate ANOVAs for control women and men. Only the control men showed a main effect of Emotion, linear F (1,19) = 12.58, p = .002, with larger N200 amplitude to pleasant than to threat words.
Later Processing of Emotional Words
P300
The only effect for P300 amplitude was a trend for enhancement of emotionally arousing words, F (1,77) = 2.95, p = .090. There was a main effect of Group for P300 latency, F (2,77) = 4.33, p = .017. P300 latency was longer in controls than for anxious apprehension subjects (mean 520 ms, SD, 36 for controls; mean 494 ms, SD 31 for anxious apprehension, Dunnett p = .015). A quadratic Emotion × Hemisphere interaction, F (1,77) = 6.68, p = .012, ε = .971, followed up with simple-effects tests showed that emotionally arousing words were evaluated more quickly than neutral only in left-hemisphere channels, quadratic Emotion F (1,82) = 4.51, p = .037. A Group × quadratic Emotion, F (2,77) = 3.37, p = .039, qualified by a Group × Gender × linear Emotion interaction, F (2,77) = 4.36, p = .016, was investigated with separate Gender × Emotion ANOVAs for each group. The anxious apprehension and anxious arousal groups showed no significant effects. A Gender × linear Emotion interaction, F (1,34) = 10.33, p = .003, emerged, and simple-effects tests pointed to longer-latency P300 in control women for threat than for pleasant words, linear Emotion F (1,15) = 13.45, p = .002, ε = .929 (see Figure 3).
N400
Emotionally arousing words prompted smaller N400 amplitude than did neutral words (pleasant, neutral, and threat means were -1.63, -1.80, and – 1.61 uV, respectively), quadratic F (1,77) = 3.98, p = .050. N400 was also larger for left than right frontocentral sensors, F(1,77) = 7.23, p = .009.
Discussion
Attentional bias to threatening stimuli has been a common finding in clinical anxiety. Gender has not been consistently investigated in this literature, despite greater prevalence rates of anxiety disorders in females and well established differences in neural processing of emotional stimuli (Cahill, 2006). Inconsistent inclusion of pleasant stimuli or failure to match pleasant with threatening stimuli on emotional arousal are also widespread practices that do not allow identification of a specific attentional bias to threat. The present study investigated gender and matched pleasant and threat words on emotional arousal. Individuals reporting high levels of either of two kinds of anxiety, anxious apprehension and anxious arousal, were investigated. A substantial effect on overt performance (RT) as a function of group was not anticipated nor found, consistent with previous behavioral studies using nonclinical samples. The important behavioral finding was very good performance, avoiding possible interpretation problems from task-difficulty confounds.
Primary hypotheses concerned ERP evidence for the timing of processes related to attentional bias to emotional stimuli in anxious participants. Non-anxious participants were included as a comparison group. The first hypothesis was that early visual ERP components < 300 ms would be modulated by either threat or emotionally arousing stimuli in general, reflecting a processing bias for emotional words. This hypothesis received support. The anxious apprehension group showed an enhanced N200 to emotionally arousing words. The anxious arousal group provided evidence of an even earlier processing bias, with larger P100 to such stimuli. The control group did not show evidence of prioritization of emotionally arousing stimuli at any latency. These findings are in line with the findings of Li et al. (2007) of larger P100 to threat than to neutral stimuli in trait-anxious participants. Anxious arousal individuals in the present study showed this enhanced P100 to pleasant stimuli, indicating preferential attention to emotionally arousing and not threatening stimuli alone, showing the importance of including pleasant stimuli in such studies.
Anxious arousal women showed evidence of greater early visual processing (larger P100 amplitude) than did anxious arousal men, irrespective of emotional content. It is possible that this reflects a gender difference in generalized tonic arousal and/or vigilance for stimuli that were cued by a fixation cross that appeared for approximately 500 ms prior to the onset of a word stimulus. Whereas most of the available literature has emphasized attentional bias specifically to threatening stimuli, present results indicate (a) that emotional arousal (not only negative valence) affects early visual processing in anxiety and (b) that anxiety subtypes differ in the time course of this effect: anxious apprehension at 200 ms vs. anxious arousal as early as 100 ms.
The second hypothesis, that threatening or emotionally arousing words would be preferentially processed as manifested by later (> 300 ms) ERPs in anxious groups, was not supported, in that P300 and N400 amplitude did not differentiate groups.
The third hypothesis, that threatening or emotionally arousing words would be preferentially processed early in combination with later avoidance, was also not supported. Neither anxiety group showed smaller P300 amplitude, shorter P300 latency, or modulation of N400 amplitude by threat or arousal, as would be consistent with avoidance of emotional stimuli.
The groups demonstrated equivalent processing of emotional words as indexed by P300 amplitude, with enhanced P300 associated with emotional arousal. The marginal significance level of this finding can be considered sufficient, as it actually warrants a one-tailed test in the context of extensive past reports (e.g., Schupp et al., 2004). Li et al. (2007) found that P300 amplitude was larger for threat than for neutral words but was larger in trait anxious vs. control participants only in a subliminal condition, not in a supraliminal condition as in the present study.
Groups demonstrated equivalent processing of emotional words as indexed by N400 amplitude, with smaller N400 amplitude to emotionally arousing than to neutral words. This pattern of findings is consistent with reports involving emotional stimuli that indicate facilitated processing of emotional words (e.g., Kanske & Kotz, 2007; Schirmer, Kotz, & Friederici, 2002; 2005). It is worth noting that what has been called an N450 component peaking approximately 400-500 ms with a frontocentral distribution has been reported in color-word Stroop studies, with greater negativity on incongruent than neutral and/or congruent trials (e.g., Curtin & Fairchild, 2003; Liotti, Woldorff, Perez, & Mayberg, 2000; Rebai, Bernard, & Lannou, 1997; West & Alain, 1999). This component is presumably associated with conflict detection or selection of competing responses, with N450 amplitude perhaps reflecting the amount of cognitive resources devoted to cognitive control. Given the pattern of effects in the present study, it seems unlikely that neutral words were more difficult to process (thus associated with larger N450), given no effect on P300 amplitude, performance accuracy, or RT. Instead, it seems more likely that emotionally arousing words were easier to process, consistent with other studies involving emotional word stimuli (in particular, Kanske & Kotz, 2007). Future research could readily address the issue of whether negativity occurring approximately 400-500 ms is associated with greater cognitive control or facilitated processing of emotion by manipulating task demands that require different levels of cognitive control in the context of emotional words.
A question could be raised as to whether the effects observed in the present research could be attributed to the competing task demands inherent in the emotion-word Stroop task, or whether they reflect early processing of emotional words independent of the need for increased cognitive control. Present findings argue that the early emotional arousal effects are driven by early attentional processing, rather than being specific to demands of the emotion-word Stroop task. First, in the emotion and color-word Stroop tasks, word reading is more automatic than color naming. Since color is the dimension to which the participant is to respond (task-relevant), cognitive control is needed to override word meaning (task-irrelevant) and execute a response. Prefrontal regions such as dorsolatoral prefrontal cortex (DLPFC) and anterior cingulate (ACC) are critical in implementing this control. A recent paper exploring source analysis of ERPs collected during the color-word Stroop task showed that a frontocentral negativity around 400 ms (N400/N450) was larger for incongruent than for congruent words and was explained by an ACC source (Hanslmayr, Pastötter, Bäuml, Gruber, Wimber, & Klimesch, 2008). Other studies concur with the timing of interference effects occurring ~400 ms in both emotion-word and color-word Stroop tasks (e.g., Hanslmayer et. al., 2008; van Hooff et. al., 2008). Thus, present early ERP effects around 100-200 ms are entirely consistent with early attentional prioritization of emotionally arousing words via a process distinct from the demands/interference effects engendered by the Stroop task.
Second, several ERP studies demonstrate that emotional arousal is prioritized over neutral information early in information processing, at least in individuals with elevated anxiety, in tasks other than the emotion-word Stroop. For example, in a study involving schematic flower and spider stimuli in two conditions, (a) emotion Stroop (name the color of the spider or flower) and (b) categorization (identify whether object is a spider or a flower), both spider phobic and socially phobic participants showed larger P100 amplitude than did control participants, across condition and across stimulus type (Kolassa, Musial, Kolassa, & Miltner, 2006). This pattern is consistent with generalized vigilance for emotionally arousing stimuli (threatening and mildly pleasant) in anxiety. Similarly, in a study where participants named emotional words, individuals with panic disorder but not healthy control participants showed larger ERP amplitudes at approximately 100-200 ms (what the authors called “the P2/N2 time window”) for panic-related than for neutral words (Pauli, Amrhein, Muhlberger, Dengler, & Wiedemann, 2005). Pleasant words were not included in this study. Results support prioritization of emotional arousal at an early time point in individuals with anxiety. Thus, across several experimental task demands, early ERP effects ~100 and 200 ms were observed, with preferential processing of emotionally arousing stimuli in anxious participants.
Interestingly, early effects ~100 ms (e.g., P100) are not typically found for emotionally arousing stimuli in non-anxious participants (e.g., Junghöfer, Bradley, Elbert & Lang, 2001; Keil, Bradley, Hauk, Rockstroh, Elbert, & Lang, 2002). Although early effects ~100 ms are not consistently found in non-anxious controls, other early effects have been demonstrated. For example, larger P200 ~200 ms for emotionally arousing than for neutral adjectives was observed in a task where participants encoded these adjectives for a later recall task (Herbert, Junghöfer, & Kissler, 2008). An “early posterior negativity” peaking ~260 ms has consistently differentiated arousing and neutral stimuli when participants simply watch pictures, including pictures that are presented very rapidly (e.g., Junghöfer, Bradley, Elbert, & Lang, 2001; Schupp, Junghöfer, Weike, & Hamm, 2003; Schupp, Stockburger, Codispoti, Junghöfer, Weike, & Hamm, 2007). Thus, across a variety of experimental paradigms, early ERP effects consistent with preferential processing of emotionally arousing stimulus features have been demonstrated in both anxious and non-anxious participants, with earlier effects more consistently found in anxious participants.
Present data suggest that investigating threat to the exclusion of pleasant stimuli may be misleading. Both anxiety groups showed evidence of preferential processing of emotionally arousing stimuli in general, rather than of threat alone. Interventions for anxiety disorders may benefit from a focus on emotional arousal rather than threat alone. Anxiety disorders are thought to be associated with specific fear structures (Foa & Kozak, 1986; Lang, 1977, 1979) that become activated when elements of the fear structure are encountered. Furthermore, the fear structure may enhance resource allocation and attentional processing of stimuli represented in the structure (Foa, Feske, Murdock, Kozak, & McCarthy, 1991). If the fear structure primarily contains information associated with high levels of emotional arousal (both pleasant and threat), therapists conducting exposure therapy with anxiety clients could highlight the role of emotional arousal in contributing to fear experiences. For example, a client with panic disorder with agoraphobia and high levels of anxious arousal, who has learned to fear interoceptive cues, could be exposed to pleasurable and highly arousing situations (e.g., a brisk walk with a trusted friend) as well as threatening and highly arousing situations (e.g., a crowded train in which escape is difficult) to elicit interoceptive cues and associated fear structures involved in panic attacks. Exposure to emotionally arousing situations may more completely elicit one's fear structure and result in faster extinction of associations between arousal cues and panic attack responses (Lang, Melamed, & Hart, 1970), including gender in investigations of emotional processing in anxiety is also important.
Anxious arousal women showed evidence of greater processing of stimuli than anxious arousal men at an early stage. Non-anxious men showed evidence of preferential processing of threat at an early stage (100 ms), whereas non-anxious women showed evidence of preferential processing of threat at a later stage (300 ms). Taken together, these results points to variance that (a) would be missed if gender were not included as part of the investigation and (b) could have crucial implications for understanding the greater prevalence rates of anxiety disorders in women. Interventions for women with high levels of anxious arousal may benefit from an appreciation of a tendency for early tonic arousal or vigilance for all stimuli, irrespective of emotional content. Conversely, men high in anxious arousal may under-prioritize information at initial sensory processing stages. These processing tendencies may have implications for the kind of information that becomes relevant to one's fear structure and is an area inviting further research.
The present study indicates that emotional valence, emotional arousal, and gender are important in attentional bias. Inconsistent inclusion of pleasant stimuli in investigations of attentional bias has limited the generalizability of results claiming special importance for threat in attentional capture and/or maintenance in anxiety. Failing to examine gender in investigations of attentional bias masks emotional processing differences that may be relevant to understanding greater prevalence rates and risk for anxiety disorders in women. Systematically investigating the role of emotional valence, emotional arousal, and gender in attentional bias in anxious and non-anxious individuals may foster understanding of the etiology and treatment of anxiety disorders and appears to be a fruitful avenue for continued investigation.
Acknowledgments
This research was supported by National Institute of Drug Abuse (R21 DA14111), the National Institute of Mental Health (R01 MH61358, T32 MH19554), and the University of Illinois Beckman Institute and Intercampus Research Initiative in Biotechnology. The authors thank Emily Cahill, Laura Crocker, and Christina Murdock for their contributions to this project.
Footnotes
Differential predictions for individuals high in anxious apprehension or anxious arousal were not made, as most studies of anxious participants do not differentiate anxious apprehension and anxious arousal. These groups were distinguished in the present study to determine whether the time course of processing emotional stimuli differs as a function of anxiety type, which could have implications for understanding and treating anxiety disorders characterized by these dimensions of anxiety.
Total number of participants was 83 after excluding participants for the following reasons: a) more than 5% of channels discarded due to artifact (N=3, 1 female) and b) amplitude values more than 3 SD from the mean for a given component at more than two electrode sites (N=5, 3 female).
Total number of neutral trials (low arousal, N = 128) was double the number of each emotion condition (pleasant and unpleasant N = 64 each). To examine possible condition effects on the number of trials available after artifact removal, the number of neutral trials remaining was divided by 2 for this analysis.
Previous studies finding lateralization differences in anxious apprehension and anxious arousal have relied on regional EEG power spectrum or fMRI and not ERP time-course data. Hemisphere was included in the present investigation for exploratory reasons.
In order to explore whether quadratic effects of emotion for P100 amplitude in the anxious arousal group and N200 amplitude in the anxious apprehension group could have been driven by differing trial numbers per condition, 64 trials from the neutral condition were randomly selected from the total 128 trials. Analyses were then run using the subsampled neutral condition. The main findings remained in terms of the pattern of the means, but in two instances the p-values became weaker. Specifically, the gender effect in the anxious arousal group using the 64 neutral trial average became a trend, F (1,24) = 3.12, p = .090. Similarly, the emotional arousal effect in the anxious apprehension group became weaker, F (1,19) = 3.63, p = .072.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Fourth edition. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2:58–71. [Google Scholar]
- Barrett LF, Russell JA. The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science. 1999;8:10–14. [Google Scholar]
- Baxter LR, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Archives of General Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- Beck A, Emery G, Greenberg RL. Anxiety disorders and phobias: A cognitive perspective. Basic Books; New York: 2005. [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional stroop effect in anxiety disorders: General emotionality or disorder specificity? Journal of Anxiety Disorders. 2001;15:147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Raghavan C, Le H-N, Vernon LL, Gomez JJ. A taxonomy of emotional disturbances. Clinical Psychology Science and Practice. 2003;10:206–226. [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–318. [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Stimuli, instruction manual, and affective ratings. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. Cambridge University Press; New York, NY: 2007. pp. 581–607. [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. The Journal of Neuroscience. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality & Social Psychology. 1994;104:532–536. [Google Scholar]
- Clark L, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Gratton G, Fabiani M. Event related brain potentials. In: Cacioppo J, Tassinary L, editors. Principles of psychophysiology: Physical, social, and inferential elements. Cambridge University Press; New York: 2000. pp. 413–455. [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, et al. Paying attention to emotion: An fMRI investigation of cognitive and emotional stroop tasks. Cognitive, Affective & Behavioral Neuroscience. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Heller W, Banich MT, Palmieri PA, Miller GA. Responding to threat: Hemispheric asymmetries and interhemispheric division of input. Neuropsychology. 2000;14:254–264. doi: 10.1037//0894-4105.14.2.254. [DOI] [PubMed] [Google Scholar]
- Conroy MA, Polich JP. Normative variation of P3a and P3b from a large sample: Gender, topography, and response time. Journal of Psychophysiology. 2007;21:22–32. [Google Scholar]
- Cook EW, 3rd, Miller GA. Digital filtering: Background and tutorial for psychophysiologists. Psychophysiology. 1992;29:350–367. doi: 10.1111/j.1469-8986.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Craske MG. Origins of phobias and anxiety disorders: Why more women than men? Elsevier Ltd; Kidlington, Oxford, UK: 2003. [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional Stroop task in groups of anxious, expert, and control subjects: A comparison of computer and card presentation formats. Cognition & Emotion. 1995;9:341–362. [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Right-posterior face processing anomaly in depression. Journal of Abnormal Psychology. 2000;109:116–121. doi: 10.1037//0021-843x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biological Psychology. 1982;14:1–52. doi: 10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Stewart JL, Miller GA. Digital filtering in EEG/ERP research. In: Handy TC, editor. Event-related potentials: A handbook. MIT Press; Cambridge, MA: 2005. pp. 85–113. [Google Scholar]
- Egloff B, Hock M. Interactive effects of state anxiety and trait anxiety on emotional Stroop interference. Personality and Individual Differences. 2001;31:875–882. [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fischler IS, Bradley MM. Event-related potential studies of language and emotion: Words, phrases and task effects. Progress in Brain Research. 2006;156:185–204. doi: 10.1016/S0079-6123(06)56009-1. [DOI] [PubMed] [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. Processing of threat-related information in rape victims. Journal of Abnormal Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition & Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pendergrass PC, Ross TJ, Stein EA, Risinger RC. Amygdala responses to both positively and negatively valenced stimuli. NeuroReport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Grossi G, Coch D. Automatic word form processing in masked priming: An ERP study. Psychophysiology. 2005;42:343–355. doi: 10.1111/j.1469-8986.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Hansen RD. Finding the face in the crowd: An anger superiority effect. Journal of Personality and Social Psychology. 1988;54:917–924. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml K-H, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience. 2008;20:215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition & Emotion. 1998;12:421–447. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Lindsay DL. Neuropsychological correlates of arousal in self-reported emotion. Cognition and Emotion. 1997;11:383–402. [Google Scholar]
- Herbert C, Junghöfer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45:487–498. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Herbert C, Kissler J, Junghöfer M, Peyk P, Rockstroh B. Processing of emotional adjectives: Evidence from startle EMG and ERPs. Psychophysiology. 2006;43:197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: A new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Research. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JE, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke C, Nordby H, Hammerborg D, Hugdahl K, Erdmann G. Event-related potential (ERP) asymmetries to emotional stimuli in a visual half-field paradigm. Psychophysiology. 1997;34:414–426. doi: 10.1111/j.1469-8986.1997.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rochstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture-processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kolassa I-T, Musial F, Kolassa S, Miltner W. Event-related potentials when identifying or color-naming threatening schematic stimuli in spider phobic and non-phobic individuals. BMC Psychiatry. 2006;6:38. doi: 10.1186/1471-244X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven NS, Heller W, Banich MT, Miller GA. Relationships of distinct affective dimensions to performance on an Emotional Stroop Task. Cognitive Therapy and Research. 2003;27:671–680. [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Fear reduction and fear behavior: Problems in treating a construct. In: Shlien JM, editor. Research in psychotherapy. Volume 3. American Psychological Association; Washington, D.C.: 1968. pp. 90–102. [Google Scholar]
- Lang PJ. Imagery in therapy: An information processing analysis of fear. Behavior Therapy. 1977;8:862–886. doi: 10.1016/j.beth.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lang PJ, Melamed BG, Hart J. A psychophysiological analysis of fear modification using an automated desensitization procedure. Journal of Abnormal Psychology. 1970;76:220–234. doi: 10.1037/h0029875. [DOI] [PubMed] [Google Scholar]
- Li W, Zinbarg RE, Paller KA. Trait anxiety modulates supraliminal and subliminal threat: Brain potential evidence for early and late processing influences. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:25–36. doi: 10.3758/cabn.7.1.25. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38:701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Martin M, Williams RM, Clark DM. Does anxiety lead to selective processing of threat-related information? Behaviour Research and Therapy. 1991;29:147–160. doi: 10.1016/0005-7967(91)90043-3. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Information-processing abnormalities in anxiety disorders: Implications for cognitive neuroscience. Cognition & Emotion. 1998;12:479–495. [Google Scholar]
- Metzger LJ, Orr ST, Lasko NB, McNally RJ, Pitman RK. Seeking the source of emotional Stroop interference effects in PTSD: A study of P3s to traumatic words. Integrative Physiological and Behavioral Science. 1997;32:43–51. doi: 10.1007/BF02688612. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18:689–700. [Google Scholar]
- Mogg K, Mathews A, Weinman J. Memory bias in clinical anxiety. Journal of Abnormal Psychology. 1987;96:94–98. doi: 10.1037//0021-843x.96.2.94. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho M-H, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment and treatment. Wiley; Chichester, UK: 1994. pp. 265–283. [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Narrow WE, First MB, Sirovatka MS, Regier DA, editors. Age and gender considerations in psychiatric diagnosis: A research agenda for DSM-V. American Psychiatric Publishing Inc; Washington, DC: 2007. [Google Scholar]
- Nitschke JB, Heller W. The neuropsychology of anxiety disorders: Affect, cognition, and neural circuitry. In: D'haenen H, den Boer JA, Westenberg H, Willner P, editors. Textbook of biological psychiatry. Wiley; Chichester: 2002. pp. 975–988. [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The neuropsychology of emotion. Oxford University Press; New York: 2000. pp. 298–319. [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Nitschke JB, Miller GA, Cook EW. Digital filtering in EEG/ERP analysis: Some technical and empirical comparisons. Behavior Research Methods, Instruments & Computers. 1998;30:54–67. [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Modulation of human extrastriate visual processing by selective attention to colours and words. Brain. 1998;121:1357–1368. doi: 10.1093/brain/121.7.1357. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB, Shi Z, Carpenter MR, Srinivasan R, Tucker DM, et al. EEG coherency II: Experimental comparisons of multiple measures. Clinical Neurophysiology. 1999;110:469–486. doi: 10.1016/s1388-2457(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology. General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJF. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of Experimental Psychology. General. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pauli P, Amrhein C, Mühlberger A, Dengler W, Wiedemann G. Electrocortical evidence for an early abnormal processing of panic-related words in panic disorder patients. International Journal of Psychophysiology. 2005;57:33–41. doi: 10.1016/j.ijpsycho.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Individual differences in children's performance during an emotional Stroop task: A behavioral and electrophysiological study. Brain and Cognition. 2003;52:33–51. doi: 10.1016/s0278-2626(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuillemier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pratto F, John OP. Automatic vigilance: The attention-grabbing power of negative social information. Journal of Personality and Social Psychology. 1991;61:380–391. doi: 10.1037//0022-3514.61.3.380. [DOI] [PubMed] [Google Scholar]
- Rebai M, Bernard C, Lannou J. The Stroop's test evokes a negative brain potential, the N400. International Journal of Neuroscience. 1997;91:85–94. doi: 10.3109/00207459708986367. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310:683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG, Macht M. Manipulation of event-related potential manifestations of information processing stages. Science. NeuroReport. 1982;21815:909–911. 1109–1112. doi: 10.1126/science.7134983. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Flaisch T, Bradley MM, Fitzsimmons JR, Lang PJ. Affective picture perception: gender differences in visual cortex? 2004 doi: 10.1097/00001756-200405190-00005. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. Sex differentiates the role of emotional prosody during word processing. Cognitive Brain Research. 2002;14:228–233. doi: 10.1016/s0926-6410(02)00108-8. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. On the role of attention for the processing of emotions in speech: Sex differences revisited. Cognitive Brain Research. 2005;24:442–452. doi: 10.1016/j.cogbrainres.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Selective visual attention to emotion. The Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Self-evaluation questionnaire. STAI Form X-2. Consulting Psychologists Press; Palo Alto, CA: 1968. [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Archives of General Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- Thomas SJ, Johnstone SJ, Gonsalvez CJ. Event-related potentials during an emotional Stroop task. International Journal of Psychophysiology. 2007;63:221–231. doi: 10.1016/j.ijpsycho.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Toglia MP, Battig WF. Handbook of semantic word norms. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- Tucker DM, Antes JR, Stenslie CE, Barnhardt TM. Anxiety and lateral cerebral function. Journal of Abnormal Psychology. 1978;87:380–383. [PubMed] [Google Scholar]
- Tucker DM, Roth RS, Arneson BA, Buckingham V. Right hemisphere activation during stress. Neuropsychologia. 1977;15:697–700. doi: 10.1016/0028-3932(77)90076-8. [DOI] [PubMed] [Google Scholar]
- van Hooff JC, Dietz KC, Sharma D, Bowman H. Neural correlates of intrusion of emotion words in a modified Stroop task. International Journal of Psychophysiology. 2008;67:23–34. doi: 10.1016/j.ijpsycho.2007.09.002. [DOI] [PubMed] [Google Scholar]
- van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Memory & Cognition. 1990;18:380–393. doi: 10.3758/bf03197127. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assesnheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Event-related neural activity associated with the Stroop task. Cognitive Brain Research. 1999;8:157–164. doi: 10.1016/s0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Wu JC, Buchsbaum MS, Hershey TG, Hazlett E, Sicotte N, Johnson JC. PET in generalized anxiety disorder. Biological Psychiatry. 1991;29:1181–1199. doi: 10.1016/0006-3223(91)90326-h. [DOI] [PubMed] [Google Scholar]
- Yee CM, Miller GA. A dual-task analysis of resource allocation in dysthymia and anhedonia. Journal of Abnormal Psychology. 1994;103:625–636. doi: 10.1037//0021-843x.103.4.625. [DOI] [PubMed] [Google Scholar]