Abstract
Studies in children and adults with the reading disability developmental dyslexia have shown behavioral improvements after reading intervention. In another line of work, it has been shown that intensive training in a variety of cognitive and sensorimotor skills can result in changes in gray matter volume (GMV). This study examined changes in GMV following intensive reading intervention in children with dyslexia using voxel-based morphometry (VBM). Eleven dyslexic children underwent an eight week training focused on mental imagery, articulation and tracing of letters, groups of letters and words, which resulted in significant gains in reading skills. This was followed by an eight week null period (control) where no intervention was administered and no further significant gains in reading were observed. Structural scans were obtained before the intervention, after the intervention and after the null period. GMV increases between the first two time points were found in the left anterior fusiform gyrus/hippocampus, left precuneus, right hippocampus and right anterior cerebellum. However these areas did not change between time points two and three (control period), suggesting that the changes were specific to the intervention period. These results demonstrate for the first time that (1) training-induced changes in GMV can be observed in a pediatric sample and (2) reading improvements induced by intervention are accompanied by GMV changes.
Keywords: dyslexia, reading intervention, magnetic resonance imaging, language, gray matter volume
Introduction
Developmental dyslexia is a neurobiologically-based learning disability in which individuals have difficulty with word decoding, word recognition and spelling and these in turn may negatively impact other reading abilities such as reading comprehension and vocabulary growth (Lyon et al., 2003). These deficits exist even though the individual has the intelligence, educational opportunity and motivation to learn to read (Lyon et al., 2003; Eckert et al., 2004; Vellutino et al., 2004). Dyslexia is more commonly observed in males than females and estimated to affect between 5.3% and 11.8% of school aged children and (Katusic et al., 2001). Given this high incidence of dyslexia and the critical role of reading in the acquisition of knowledge and successful academic outcome, improving reading abilities in these children is an important priority for educators, policy makers and scientists. Over the past decade there has been increased interest amongst neuroscientists to quantify and characterize changes in brain structure, usually gray matter volume (GMV) following controlled learning experiences. These efforts, especially those focusing on the relationship between changes in brain structure and academic achievement in a formal learning environment (Draganski et al., 2006), have important implications for better understanding learning and skill acquisition in the classroom, especially in those children who encounter challenges in their efforts to acquire literacy. To date, no attempts have been made to measure changes in the brain’s gray matter in children with dyslexia following a formal, structured learning experience. Here we address this gap and make the connection between behavioral intervention for reading disabilities and measures of brain morphometry, to inquire about the nature of GMV changes following intensive tutoring of children with dyslexia. The results, in conjunction with current understanding of brain-behavioral relationships, will help inform both educators and researchers in an effort to better understand the neural basis for successful reading intervention and potentially to develop programs to best help children who have trouble reading.
There exists now a significant corpus of work characterizing the neuroanatomical profile of dyslexia (for a review see Eckert et al., 2004). This research includes post mortem studies (Galaburda et al., 1985) and in vivo magnetic resonance imaging (MRI) research comparing dyslexic with non-dyslexic populations. The initial MRI research involved manual tracing of a variety of brain regions implicated in language and reading, however more recent research has quantified the neuroanatomical differences in dyslexic children and adults by using a technique known as voxel-based morphometry (VBM) (Ashburner and Friston, 2000). Using this automated method, a variety of brain structures have been shown to have smaller gray matter volume (GMV) in dyslexics as compared to controls. VBM studies comparing adult dyslexic to age matched control groups shave shown less left temporal GMV (Brown et al., 2001; Vinckenbosch et al., 2005) and less bilateral temporal GMV for the dyslexic groups (Brambati et al., 2004; Steinbrink et al., 2008). Brambati et al. (2004) found less bilateral GMV for dyslexics in the cerebellar nuclei and Brown et al. (2001) also found less left inferior frontal and right cerebellar GMV in the dyslexics. The only two studies of children with dyslexia employing VBM have shown less GMV in bilateral inferior parietal lobule and temporal gyri and left inferior frontal gyrus (Hoeft et al., 2007) and less bilateral lingual gyrus GMV compared to controls as well as left supramarginal gyrus and left posterior cerebellar lobe (Eckert et al., 2005). These regions are consistent with those implicated in studies using other structural analysis methods as described in Eckert et al. (2004).
In parallel, functional brain imaging technologies (functional magnetic resonance imaging: fMRI; positron emission tomography: PET) have been used to investigate reading and language processing in the dyslexic brain. From these Pugh et al. (2001) has proposed a model describing the neural circuitry for reading in normal and disabled readers (2001). The model proposes that three left hemisphere regions are relied upon for typical reading: an inferior frontal region involved in phonological output, a temporo-parietal region involved in rule-based orthographic to phonological processing as well as semantic analysis, and an occipito-temporal region involved in single word identification. These areas are commonly found to be less activated in individuals with dyslexia during paradigms of reading or reading-related skills. Specifically, temporo-parietal and occipito-temporal regions consistently show hypoactivation for children and adults with dyslexia compared to normal readers in phonologically demanding (real and pseudoword reading) tasks; the inferior frontal cortex is sometimes hyperactive in dyslexics compared to controls on similar tasks (Shaywitz and Shaywitz, 2008). A recent activation likelihood estimate (ALE) meta-analysis of predominantly adult studies of functional brain imaging in dyslexics compared to controls found left hemisphere temporal and parietal areas were most likely to be less active in dyslexics than controls, although support for inferior frontal hyperactivation was not found (Maisog et al., 2008).
In a study of dyslexic children, different results were found for reading matched vs. age matched controls. When compared to both control groups the posterior network hypoactivation was found for dyslexics, however the hyperactivation in the frontal network was only found when compared to age matched controls, suggesting that the hypoactivation represents a functional deficit of dyslexia, while the hyperactivation is more representative of reading ability (Hoeft et al., 2007). Together these studies in children and adults point to a left hemisphere network that is impacted by an individual's reading disability. Notably these brain regions overlap with those that have demonstrated anatomical differences, as described above.
Most recently these functional brain imaging methodologies have been used to investigate whether the differences observed between dyslexic and normal readers change when the investigators intervene and improve reading ability in dyslexic individuals. Intervention studies in dyslexic children have shown changes in behavioral measures (i.e. increased performance in reading) and physiological changes measured using fMRI (Shaywitz et al., 2004; Aylward et al., 2003, Temple et al., 2003). While different types of interventions were given in these studies, similar patterns of increased activity were observed in bilateral frontal and temporo-parietal regions. An intervention study in adult dyslexics showed increases in activation in bilateral temporal and parietal areas as well as the right inferior frontal gyrus (Eden et al., 2004).
While these studies speak to physiological changes in brain function following intensive training regimens focused on reading, it is not yet known if there are parallel changes in cortical anatomy. Several longitudinal studies using VBM analysis have shown changes in subjects’ GMV after training. Draganski et al. (2004) followed a group of adults who were scanned before and after learning to juggle, and after not juggling for 3 months. An increase in GMV in area V5/MT (known to be integral to visual motion processing) was observed following the training, yet after the third scan, following a period of no training, there appeared to be a reversal of this pattern in the form of GMV decrease (although it was not significant over the time observed). Other longitudinal VBM studies have examined GMV change after a variety of tasks including more juggling tasks (Driemeyer et al., 2008; Boyke et al., 2008; Scholz et al., 2009), medical students studying for an exam (Draganski et al., 2006), mirror reading (Ilg et al., 2008), as well as repetitive transcranial magnetic stimulation (rTMS) on the left superior temporal gyrus (May et al., 2007), cognitive behavioral therapy (CBT) in a chronic fatigue syndrome population (de Lange et al., 2008) and pharmacological (quetiapine) treatment of a schizophrenic population (Stip et al., 2009).
Taken together, this literature has provides insight into the plasticity of the adult brain during learning. Increases in gray matter density seen early on (i.e. within one week after onset of training) (Driemeyer et al., 2008), suggest changes in spine/synapse density or cell body increases rather than neuronal or glial genesis. Longer term increases in hippocampal gray matter (Draganski et al., 2006) are more likely to reflect this slower process of neurogenesis. Anatomical changes after training have been observed in adults ranging from their early 20’s (Draganski et al., 2004) to early 60’s (Boyke et al., 2008), but has yet to be studied in a pediatric population.
To this point, changes in GMV after reading intervention have not been shown in children or adults with dyslexia. However, the above studies of training-induced changes in GMV and the fact that brain anatomy varies as a function of reading status (as shown for dyslexic versus non-dyslexic comparisons as well as in studies of illiterates; Castro-Caldas et al., 1998), suggest the possibility that such changes in the cortex might be measurable.
The current study was designed to investigate whether children with dyslexia who receive a reading intervention over an eight week period show changes in GMV. A longitudinal VBM analysis comparing GMV before the intervention, after the intervention and after an equal time period of non-intervention was performed to examine if any changes in gray matter could be observed as a result of the training. This three time point design follows the original Draganski et al. (2004) juggling studies. Based on the anatomical differences known to distinguish dyslexics from non-dyslexics (Eckert, 2004), the physiological changes previously reported following successful reading interventions (Aylward et al., 2003; Shaywitz et al., 2004; Eden et al., 2004) and the nature of the intervention used in the current study (visual imagery of words, multisensory integration and development of the sound representation of words) areas for which GMV changes were predicted included left hemisphere ventral visual, parietal and frontal cortices.
Materials and Methods
Subjects
The eleven dyslexic children (8 male, 3 female) whose data was submitted to this analysis were recruited as part of a larger study from a private school specializing in students with dyslexia. The school records were used to identify students with Woodcock-Johnson III Letter-Word Identification (W-J WID; Woodcock et al., 2001) scores less than 92. Average age of the eleven subjects was 9.1 years (Range 7 yrs 5 months-11 yrs 11 months). IQ scores were obtained prior to the intervention using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) which measures verbal (VIQ), performance (PIQ) and full scale (FSIQ) IQ. To be included in the study subjects had to score greater than 80 on these measures. Table 1 presents average scores and standard deviations for this group. Average IQ scores for this group all fell within the normal range (85–115), whilst reading of real words on the Letter-Word Identification fell well below the normal range. All subjects were free of any developmental disabilities, congenital or acquired neurological disorders and any injury or disease affecting brain function. Other exclusion criteria included diagnosed language or psychiatric disorders, hearing disorders, diagnosis of any major medical condition and any metallic implants, severe claustrophobia or any other contraindications to MRI scanning.
Table 1.
Behavioral profile, n=11 mean (sd)
| Age | 9.1 (1.3) years | |
| WASI | ||
| VIQ | 110 (3.5) | |
| PIQ | 102 (11.3) | |
| FSIQ | 107 (6.5) | |
| WJ Word ID | 77 (8.9) | |
Behavioral Tests
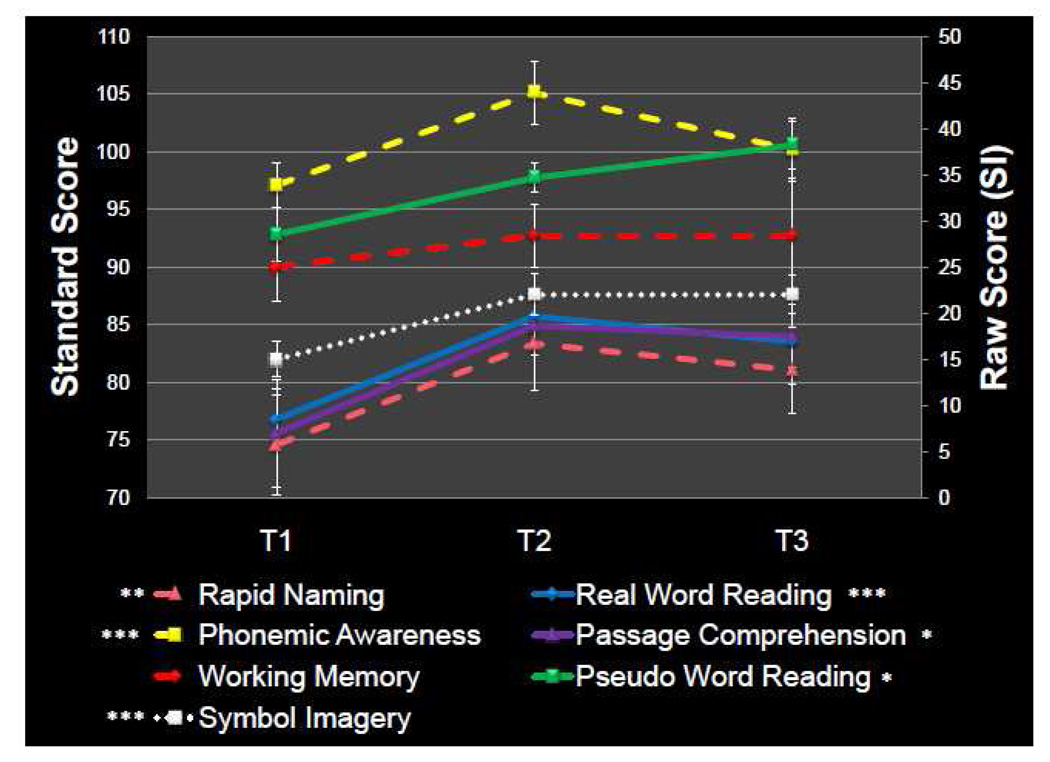
A battery of behavioral tests were administered prior to and after the intervention as well as after the period of no intervention. Researchers acquiring the behavioral data were blind to the child's status of intervention. Woodcock-Johnson Word Identification (W-J WID; single real word reading; Woodcock et al., 2001), Woodcock-Johnson Word Attack (W-J WA; single pseudo-word reading; Woodcock et al., 2001), and Woodcock-Johnson Passage Comprehension (W-J PC; Woodcock et al., 2001) were used as direct measures of reading ability. The Lindamood Auditory Conceptualization Test (LAC-3; Lindamood and Lindamood, 1971), which measures phonemic awareness, Rapid Automatized Naming (RAN L&N; Denckla and Rudel, 1976a; Denckla and Rudel, 1976b), which measures naming fluency for letters and numbers, and Digit Span (Wechsler, 1999), which measures working memory, all measure skills that support reading acquisition and were therefore of interest to this study (Wagner and Togersen, 1987). Symbol Imagery (Bell, 1997) which measures visual memory for letters was used because of the strong imagery component of the reading intervention. All test scores are reported as standard scores (Mean = 100, SD = 15) except Symbol Imagery where the raw score is reported. The measures of single word reading, pseudoword reading and passage comprehension used multiple test forms (in an alternating A/B schedule). While the other tests did not include multiple forms, these are highly reliable tests and should reflect actually changes in score as opposed to test/retest effects. Figure 1 presents the behavioral data acquired over the three time points.
Figure 1.
Behavioral Score Changes Over the Course of Reading Intervention: Test scores for each of the three time periods: before intervention (T1), after intervention (T2) and after the period of no intervention (T3). Solid lines represent direct measures of reading ability and dotted lines represent skills predictive of reading ability (as discussed in text). Symbol Imagery (in white) corresponds with the y-axis on the right because unlike the other measures the scores are raw and not standardized. Significant increases in score are noted (* p<0.05 ** p<0.01 ***p<0.001) as tested by post hoc t-tests run after a one way repeated measures ANOVA. No significant changes were seen between time points 2 and 3.
Reading Intervention
The reading intervention, Seeing Stars (Lindamood-Bell Learning Processes ©), focuses heavily on imaging/visualization starting with single letters and increasing in difficulty to imaging one syllable and up to two and three syllable words. In addition to visualization, the intervention also has a tactile/motor aspect in which the students finger trace letters, as well as a language production aspect in which they say the letter or sound name out loud while they are tracing the letter in the air. Thus, the intervention utilizes multiple sensory modalities in order to help improve internal visual and phonological representations. The use of imagery as a focus of the intervention is based on several studies relating the use of imagery in reading including a self report study of imagery during reading (Long et al., 1989), use of imagery in semantic retrieval (Kosslyn, 1976), and more direct measures of relating imagery during reading to improved processing and comprehension (Sadoski, 1983; Linden and Wittrock, 1981). The intervention was administered at the subjects’ school by employees of the Lindamood-Bell Corporation © who were specifically trained to administer the program. Subjects underwent eight weeks of this intervention followed by an eight week period of no intervention to serve as a control period. All behavioral testing prior to and following the intervention was conducted by research assistants who were members of the research team.
Imaging Procedures
Anatomical scans were obtained at the following three time points of the study: before the reading intervention (T1), after the reading intervention (T2) and after the period of no intervention (T3). At each of these three time points, three 3D T1-weighted MPRAGE images were obtained using a 3 Tesla Siemens Trio whole-body MRI system (TE= 4.38 ms, TR= 1600 ms, TI= 640 ms, FOV= 256mm, 160 slices, slice resolution 1mm, voxel size 1mm3). A blind image rating system using two raters was used to select the highest quality image from this set of three scans for each subject at each time point. This allowed for the selection of the image with the least amount of motion artifact, a problem that frequently occurs in this age group. An analysis of variance (ANOVA) was performed on the rating scores of the scans used for the three time points to ensure that there were no differences in rating scores across the three time points. As a precaution for avoiding head motion artifacts, children underwent training in a mock scanner prior to the acquisition of MRI data to help acclimate them to the MRI environment (i.e. confined space and noise). Additionally, to ensure fluctuations in the quality of the image was not a contributing factor in the results of this study, noise outside of the brain was measured in two spherical ROIs and shown to be stable across the three time points.
Analysis
To evaluate the efficacy of the reading intervention on standardized measures of reading and skills known to support reading acquisition, a one-way repeated measures ANOVA was conducted on all of the behavioral measures for the three time points, followed by post-hoc t-tests.
For the analysis of the MRI data, those images selected for analysis (one per subject at each time point) were processed according to the optimized VBM protocol (VBM2 toolbox pipeline) described by Good et al. (2001) in SPM2 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London). This analysis created a GMV template that is specific to this group using the first scan for each subject. Images were then segmented into gray matter, white matter and cerebral spinal fluid. The gray matter images were spatially normalized using the first time point as the source image for each subject. Although the VBM2 toolbox does not automatically modulate images, images were modulated here in order to make volume inferences from the results. The segmented/normalized/modulated images were smoothed using a default setting of 8mm FWHM. An absolute intensity threshold of 0.2 was used to remove voxels of low gray matter intensity from the analysis.
Statistical analysis for the VBM data was performed using the VBM2 toolbox. In order to determine clusters that significantly changed at any point during the study a one-way within-subjects ANOVA was performed at a height threshold of p<0.001 uncorrected, with an extent threshold of p<0.05. This height threshold has been used in previous VBM studies of dyslexia (Eckert, 2005) as well as other longitudinal VBM studies (Boyke et al., 2008; Driemeyer et al., 2008; Ilg et al., 2008). The cluster extent threshold here utilized the non-stationarity correction toolbox for SPM that allows for cluster level statistics on VBM data. Paired t-tests were then computed using the statistical analysis through the VBM2 toolbox to examine the direction of the effects (T2>T1, T3>T2 and T3>T1).
Average GMV signal (in arbitrary units) from these clusters was extracted using the MarsBaR toolbox (Brett et al., 2002). Average percent change in GMV for the clusters shown to increase significantly during the intervention was determined.
In addition to our main question about GMV changes brought about by reading intervention, we also wanted to explore whether the amount of reading improvement correlated with the amount of GMV change. To address this, a correlation matrix of GMV increases between T1 and T2 in regions identified by the above VBM analysis and behavioral test score changes between T1 and T2 was generated to obtain Pearson’s correlation coefficients. The behavioral tests included: single real word reading (W-J WID), pseudoword reading (W-J Word Attack), reading comprehension (W-J PC), phonemic awareness (LAC-3), rapid naming of letters and numbers (RAN) and Symbol Imagery (SI).
Results
Behavioral Results
One-way repeated measures ANOVAs showed significant within-subjects effects over the three time points for all behavioral measures with the exception of working memory (Digit Span). Specifically, there were significant increases in the scores for single real word reading (W-J WID) F(2,20)=10.77, p=0.001; pseudoword reading (W-J Word Attack) F(2,20)=6.321, p=0.007; reading comprehension (W-J PC), F(2,20)=5.420, p=0.013; phonemic awareness (LAC-3) F(2,20)=5.150, p=0.016; rapid naming (RAN), F(2,20)=7.655, p=0.003; and Symbol Imagery (SI), F(2,20)=30.723, p<0.001. Working memory as measured by the Digit Span tests did not show significant changes, F(2,20)=0.444, p=0.648.
Post hoc t-tests were run on all behavioral measures (except Digit Span) to compare scores between T1 and T2, T1 and T3 as well as between T2 and T3. For the comparisons of scores between T1 and T2, single real word reading (W-J WID), phonemic awareness (LAC-3), and Symbol Imagery (SI) were each significant at p<0.001. Rapid Naming of letters and numbers (RAN) was significant at p<0.01. Pseudoword reading (W-J Word Attack) and reading comprehension (W-J PC) were both significant at p<0.05. Each of these measures was still significant when comparing T3 with T1 except for phonemic awareness (LAC-3). However, there were no significant changes in performance when comparing the scores between T2 and T3. A graphic representation of these behavioral score changes over the three Time Points are shown in Figure 1.
Anatomical Results: ANOVA
The F test employed in theVBM2 toolbox identified seven regions with significant changes in GMV during the course of the study F(2,20). In the left hemisphere, the anterior fusiform gyrus extending into the hippocampus (BA 20; x= −36, y= −11, z= −24; F=55.58), the superior frontal gyrus (BA 10; x= −11, y= 58, z= −12; F=22.92) and the precuneus (BA 7; x= −17, y= −60, z= 31; F=18.32) were identified. In the right hemisphere, the hippocampus (x= 32, y= −12, z= −16; F=20.54), the anterior cerebellum (x= 8, y= −45, z= −10; F=16.18), the precuneus (BA 7; x= 4, y= −60, z= 30; F=15.95) and the caudate (x= 9, y= 16, z= 9; F=14.96) were significant. Details for these clusters can be found in Table 2.
Table 2.
VBM Results
| Significant Clusters Identified by One Way ANOVA (within subjects) | ||||||
|---|---|---|---|---|---|---|
| Hemisphere | Talairach Coordinates | Cluster Size | F statistic | Z Score | BA | Anatomical Description |
| L | x = −36 y = −11 z = −24 | 369 | 55.58 | 5.68 | 20 | Anterior Fusiform Gyrus |
| L | x = −11 y = 58 z = −12 | 344 | 22.92 | 4.58 | 10 | Superior Frontal Gyrus |
| R | x = 32 y = −12 z = − 16 | 182 | 20.54 | 4.19 | - | Hippocampus |
| L | x = −17 y = −60 z = 31 | 289 | 18.32 | 4.01 | 7 | Precuneus |
| R | x = 8 y = −45 z = −10 | 56 | 16.18 | 3.82 | - | Anterior Cerebellum |
| R | x = 4 y = −60 z = 30 | 85 | 15.95 | 3.80 | 7 | Precuneus |
| R | x = 9 y = 16 z = 9 | 239 | 14.96 | 3.70 | - | Caudate |
| Significant Clusters Identified by T2-T1 Contrast | ||||||
| Hemisphere | Talairach Coordinates | Cluster Size | t statistic | Z Score | BA | Anatomical Description |
| L | x = −36 y = −11 z = −24 | 372 | 7.83 | 5.24 | 20 | Anterior Fusiform Gyrus |
| R | x = 31 y = −14 z = −15 | 281 | 5.72 | 4.35 | - | Hippocampus |
| L | x = −17 y = −60 z = 31 | 656 | 5.37 | 4.17 | 7 | Precuneus |
| R | x = 7 y = −46 z = −11 | 114 | 5.34 | 4.16 | - | Anterior Cerebellum |
| Significant Clusters Identified by T3-T1 Contrast | ||||||
| L | x = −36 y = −11 z = −24 | 618 | 10.47 | 6.05 | 20 | Anterior Fusiform Gyrus |
| R | x = 32 y = −12 z = − 16 | 460 | 6.21 | 4.58 | - | Hippocampus |
| L | x = −17 y = −60 z = 31 | 679 | 5.64 | 4.32 | 7 | Precuneus |
| R | x = 9 y = 16 z = 9 | 748 | 5.46 | 4.22 | - | Caudate |
| R | x = 8 y = −45 z = −10 | 249 | 5.23 | 4.11 | - | Anterior Cerebellum |
Anatomical Results: Paired t-tests
Post hoc t-tests performed using the VBM2 toolbox showed that each of the clusters identified by the ANOVA represented a significant increase in GMV over the course of the study. Specifically, the clusters which increased significantly between T1 and T2 (during the reading intervention) were: the left anterior fusiform gyrus extending into the hippocampus (BA 20; x= −36, y= −11, z= −24), left precuneus (BA 7; x= −17, y= −60, z= 31) right hippocampus (x= 31, y= −14, z= −15), and right anterior cerebellum (x= 7, y= −46, z= −11). Clusters from the ANOVA that demonstrated a significant increase between T1 and T3 were: the left anterior fusiform gyrus (BA 20; x= −36, y= −11, z= −24), right hippocampus (x= 32, y= −12, z= −16), left precuneus (BA 7; x= −17, y= −60, z= 31), right caudate (x= 9, y= 16, z= 9) and right anterior cerebellum (x= 8, y= −45, z= −10). Details for these clusters can be found in Table 2. The only regions shown to increase significantly during the null period (between T2 and T3) were the left superior frontal gyrus and right precuneus.
Anatomical Results: % GMV Change
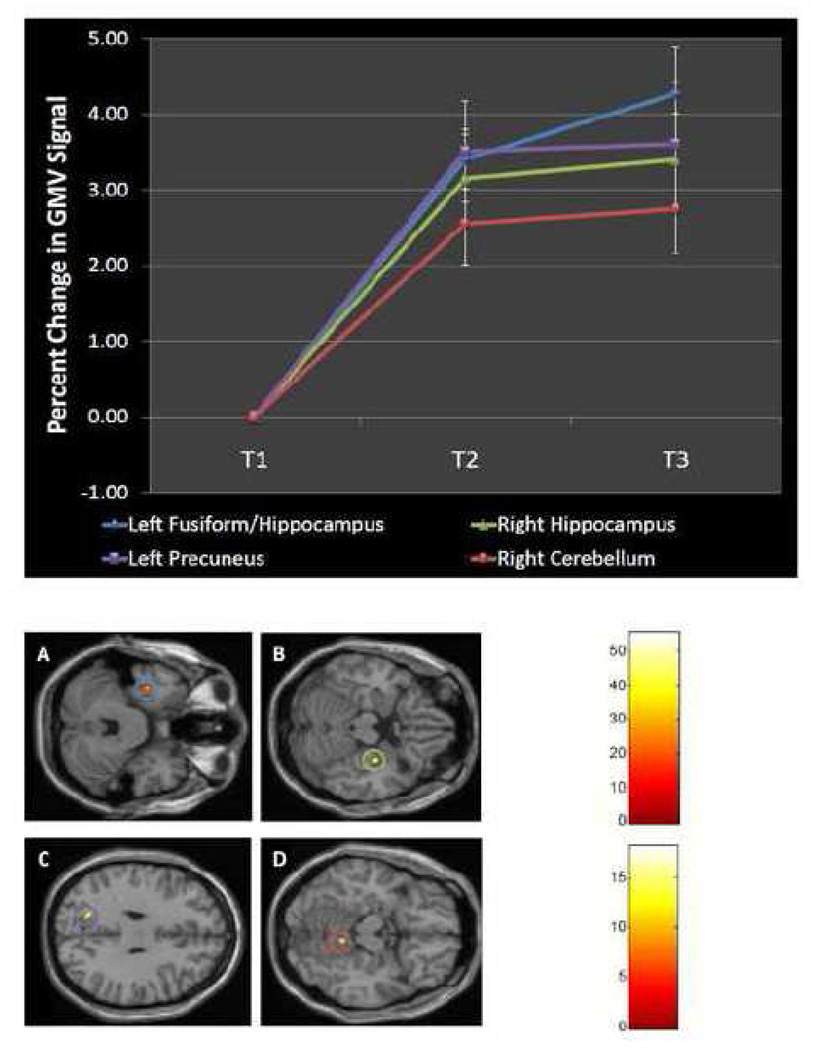
For those clusters that were identified as showing an increase in GMV during the intervention (between T1 and T2), the percent change in GMV signal across the three time points was determined using the GMV data extracted using the MarsBaR toolbox (Brett et al., 2002). For these four clusters the average percent GMV signal increases were: 3.40% in the left anterior fusiform, 3.15% in the right hippocampus, 3.51% in the left precuneus and 2.55% in the right cerebellum. Figure 2 pictures these four clusters and graphs the increase in GMV percentage over the three time points.
Figure 2.
Gray Matter Volume Increases Over the Course of Reading Intervention: Top) Percent change in GMV signal for the four clusters identified in the VBM2 toolbox pipeline. Bottom) Statistical parametric maps showing the four clusters. Ovals around clusters correspond to the color scheme in the top of the figure. A=left fusiform/hippocampus B=right hippocampus C=left precuneus D=right cerebellum. Scales represent the F score. Top scale corresponds to the left fusiform/hippocampal cluster, bottom scale corresponds with the right hippocampus, left precuneus and right cerebellum.
Anatomical-Behavioral Correlations
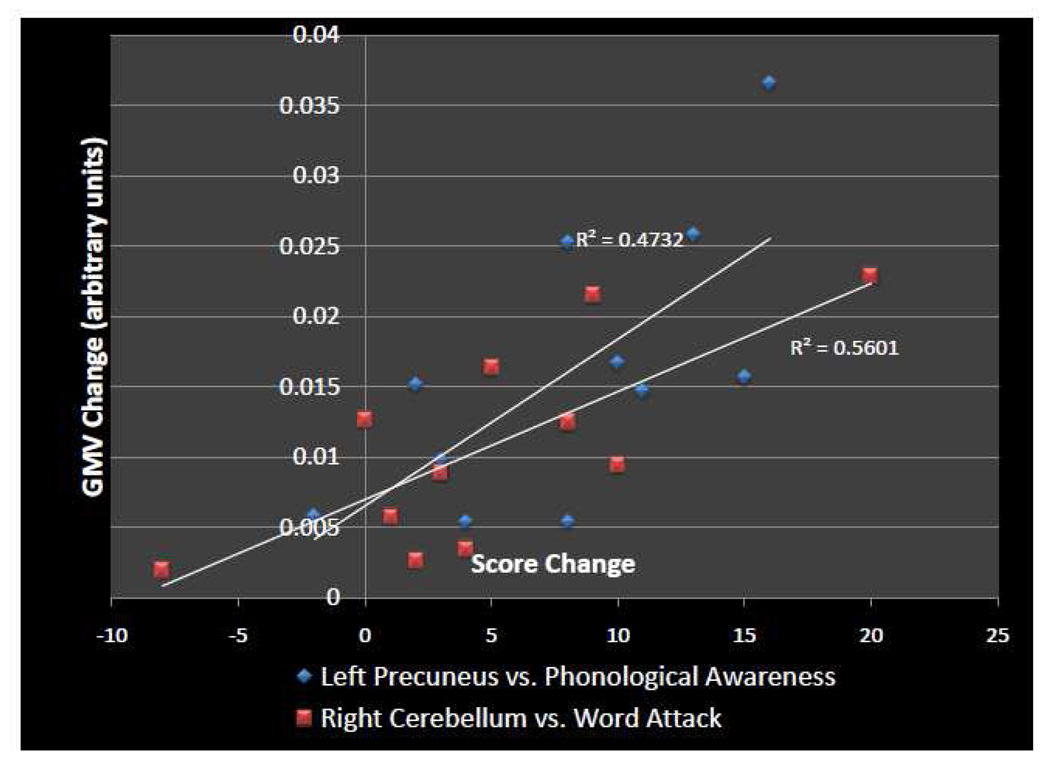
Correlation analysis between the change in behavioral scores and the GMV increase in the four clusters identified by the VBM analysis revealed two significant correlations. The amount of change in phonemic awareness (LAC-3) correlated positively with GMV change in the left precuneus (R=0.688, p<0.05) and the changes in pseudoword reading (Word Attack) correlated positively with GMV change in the right cerebellum (R= 0.748, p<0.01) (see Figure 3 for the scatter plots of these correlations). However, these correlations do not survive correction for multiple comparisons and were not significant comparing T1 to T3.
Figure 3.
Correlations of Behavioral Score Change with GMV Change: Scatter plot of GMV change in arbitrary units vs. score change. Blue diamonds represent GMV change in the left precuneus cluster vs. score change on the phonological awareness test (LAC-3). The Pearson’s correlation coefficient for this association is R=0.688 (R2=0.4732) and was significant at p<0.05. Red squares represent GMV change in the right cerebellum cluster vs. score change on the pseudo word reading (Word Attack) test. The Pearson’s correlation coefficient for this association is R=.7484 (R2=0.5601) and was significant at p<0.01.
Discussion
This study followed eleven children with dyslexia who underwent reading intervention and examined if there were increases in GMV along with any intervention-induced gains in reading performance. From an educational standpoint, the intervention was successful, as it resulted in behavioral gains for measures of reading ability as well as for skills that are associated with good reading acquisition. These gains may be due to the multi-sensory approach used in the intervention (phonological training and visual imagery), but future studies will need to determine which components of programs such as the one employed here are critical in driving these increases in reading performance. Following the experimental design used in previous training-induced plasticity studies examining changes in GMV (Draganksi et al., 2004; Draganski et al., 2006) MRI scans were obtained before and after the intervention as well as after a null period where no intervention was given. We predicted increases in GMV in left hemisphere ventral visual, parietal and frontal cortices as these are brain areas that (1) subserve the skills targeted by the intervention program, (2) are known to be involved in the process of reading, (3) have been shown to be under-activated in dyslexic readers, and (4) have been shown to increase in activity following a successful reading intervention. The students showed significant gains in reading (as well as reading-related measures) following the intervention and GMV increases specific to the intervention period (T2 compared to T1) were observed in four areas: left anterior fusiform (extending into the hippocampus), left precuneus, right cerebellum and right hippocampus. Importantly, all of the behavioral gains (except for phonemic awareness) and the changes in these four regions were still significant when comparing T3 with T1, but were not significant when comparing T3 with T2, demonstrating that both behavioral and GMV changes observed on the second scan were associated with the period of intervention. The maintenance of these gains through the null period is encouraging in terms of the in classroom benefit for these children. The left fusiform and precuneus findings support our original predictions based on the nature of the intervention and what is known about the neural signature for reading and reading disability, while the increases in GMV in bilateral hippocampus and the right cerebellum may suggest that a more general learning network was engaged during the intervention period. Importantly, the GMV increases reported here provide evidence that the learning associated with the intervention has structural brain correlates. Understanding how specific changes in behavior relate to specific structural changes in the brain after intensive intervention may be useful in understanding which regions of the brain are targeted by specific interventions and, if the focus of the intervention is further tailored with this knowledge, it might eventually provide a better understanding of how children successfully learn in the general classroom and in special education settings.
Increased GMV in Left Anterior Fusiform/Hippocampus and Right Hippocampus
The peak of the cluster in the left anterior fusiform gyrus falls within the predicted changes for the ventral visual pathway. However, a significant portion of this cluster extended into the left hippocampus. This along with the cluster identified in the right hippocampus suggest a bilateral increase in hippocampal GMV. These changes may reflect general learning that is occurring during the intervention period as has been demonstrated in the right hippocampus for students preparing for a medical exam (Draganski et al., 2006). Each of these regions will be discussed in turn.
The cluster with the peak in the left anterior fusiform is part of the ventral visual stream. However, it is more anterior than regions known to be involved in the processing of single words (the so called “visual word form area”; Cohen et al., 2002) and closer to regions that have been implicated in object naming/processing rather than word processing. Renvall et al. (2003) found this region to be more active for naming objects in a complex scene compared to naming colored circles. In a study looking at both naming and viewing of words and objects, multiple regions of the anterior fusiform were identified (Moore and Price, 1999). One peak in BA 20/37 was found to be more active for words and objects (over meaningless stimuli) irrespective of the task, and another peak (slightly anterior and superior in BA 20) was found to be more active specifically during object naming but not word naming (Moore and Price, 1999). The students in the current study were required to make connections between a letter or groups of letters and the sound they make. A possible interpretation is that students with dyslexia are relying on anterior located regions traditionally associated with object processing in order to compensate for regions in the posterior ventral stream that are not supporting word processing in ways that is typical for normal readers. Additionally, Anderson et al. (2000) showed a region in the anterior fusiform that was more active during encoding than retrieval during a word pair association task. Hence another possibility is that the intervention placed increased demand on this region in encoding the connections between letters/groups of letters and sounds, resulting in an increase in GMV. Future studies using functional imaging may be able to address both of these possibilities more directly.
Turning to the hippocampus, it is notable that a GMV study of learning in medical students found hippocampal gray matter to increase over all time points of the study (Draganski et al., 2006). The authors suggest that this could be due to the fact that neurogenesis occurs in the hippocampus, while it does not in other areas where GMV increases have been observed. However, in the current study both the left and right hippocampi showed a significant increase only during the intervention period while increases during the null period did not meet statistical significance. Hence we cannot reinforce the interpretation offered by Draganski and colleagues, although we do not rule out the possibility that future studies could show a more robust increase during the control period.
Increase in GMV in the Left Precuneus
An increase in GMV in the left precuneus is consistent with predictions made for the study, based on the fact that the intervention has a strong emphasis on visual imagery. The precuneus has been implicated in various functions including visuo-spatial imagery and memory retrieval (for a review see Cavanna and Trimble, 2006). The left precuneus has been implicated in visual imagery of letters, specifically the visuo-spatial aspects of the imagery (Raij, 1999). Thompson et al. (2009) found bilateral precuneus activation during a spatial location task compared to a spatial-transformation task. In their study subjects viewed an arrangement of letter stimuli and later were primed with a letter and trisected circle; subjects had to decide which third of the trisected circle would be facing the middle of the screen if it was in the position of the primed letter (Thompson et al., 2009). In addition to these studies in typical populations linking letter imagery to the left precuneus, there is also evidence of differences the precuneus in dyslexic readers. A meta-analysis by Maisog et al. (2008) found the left precuneus to be less likely to be active in dyslexic adults compared to controls. In another study, the right precuneus was found to have less GMV in dyslexic adults compared to controls (Menghini et al., 2008).
Further, the amount of GMV increase in the left precuneus in our study showed a positive correlation with score change for the phonemic awareness test (LAC-3). As this is one of the skills targeted by the intervention (in addition to visual imagery), it is encouraging to find a direct relationship between the amount of GMV increase and the amount of improvement in this skill. However, a correlation between visual imagery, another integral part of the intervention, and precuneus GMV increases was not found and as mentioned in the results section, the correlations reported for this study did not survive a correction for multiple comparisons, suggesting these results are somewhat tentative.
Increase in GMV in the Right Cerebellum
The right anterior cerebellum also contained a cluster of increased GMV after the reading intervention. While we did not predict a change here, the findings are notable given the theoretical model linking the cerebellum and dyslexia (Nicolson et al., 2001; Stein and Walsh, 1997; Fawcett and Nicolson, 2007; Laycock et al., 2008), specifically in ways that account for the sensorimotor problems that have been reported in individuals with reading disability. For example, using PET, Nicolson et al. (1999) showed that dyslexic adults had lower right cerebellum activation compared to controls while learning a motor sequence and also when later performing that learned motor sequence. The cerebellum has also been included in a number of reports investigating GMV differences in dyslexic subjects using VBM (Eckert et al., 2005; Brown et al., 2001; Brambati et al., 2004) and other methods used to evaluate anatomical aberrations (reviewed by Eckert et al., 2004). For example, the right anterior lobe of the cerebellum has been shown to have less overall volume in dyslexic children compared to controls (Eckert et al., 2003). Further, a magnetic resonance spectroscopy (MRS) study showed male dyslexic adults to have biochemical asymmetry in the cerebellum that was suggestive of differences in the cellular density of dyslexics compared to controls (Rae et al., 1998). In a later study by these investigators using anatomical measures, dyslexic adults were found to have abnormally symmetric cerebellar gray matter compared to controls; controls had less left hemisphere gray matter than the dyslexic group (Rae et al., 2002).
It is notable that the amount of increase in GMV observed in the right anterior cerebellum following the intervention showed a positive correlation with the change in score on the pseudoword reading (Word Attack) measure. There is previous evidence for a relationship between phonological decoding skills (e.g. pseudoword reading) and the anterior cerebellum. Subjects characterized as phonological dyslexics (pseudoword decoding scores <90) were shown to have a leftward asymmetry in the anterior cerebellum (Leonard et al., 2001). It is possible that an increase in the right anterior cerebellum reflects a shift to a less asymmetrical anterior cerebellum. However, the subjects in our study were not as weak in their pseudoword reading abilities as the subjects reported by Leonard et al. (2001) (our subjects’ weakness was most prominent for real word reading) and asymmetry of the anterior cerebellum prior to the intervention was not investigated. Also, as previously mentioned, the evidence for a relationship between GMV increases in the right cerebellum and pseudoword reading advancement is tentative (the correlation did not survive a correction for multiple comparisons).
Learning and Structural Plasticity
While our study design followed that employed by Draganski et al. (2004) who measured GMV prior to and following a training period (during which subjects learned to juggle) and again following by a period where no practice occurred, there is an important difference in our study, in that reading interventions should provide a lasting improvement (and it did) and subjects do not cease to read. In other words, the skill learned by the participants in the juggling studies (Draganski et al., 2004; Boyke et al., 2008; Driemeyer et al., 2008) was entirely novel to the subjects and the importance of maintaining long-term improvements was not of the same value as reading gains are to a dyslexic student. However, in this regard our study bears some resemblance to another study by Draganski and colleagues (Draganski et al. 2006) in which they followed medical students while they studied for an exam before the semester break. The type of learning the medical students did for their exam and the learning the children did in this study represent skills that are useful and more likely to be used regularly than those used in the juggling studies.
The pattern of GMV change (except for the hippocampus) for both the jugglers and the medical students showed an initial increase during the learning phase followed by a small but non-significant decrease during the null period (Draganski et al., 2004; Draganski et al., 2006; Boyke et al., 2008; Driemeyer et al., 2008). This trend suggests that practice may be necessary to maintain the structural changes achieved while learning. The GMV change in the current study shows significant increases during the intervention and non-significant increases in the period after the intervention. This pattern is consistent with the behavioral data, where the scores showed significant improvement during the intervention, followed by non-significant changes in the eight weeks afterward. This is an important finding for educational purposes as it suggests these children are maintaining their behavioral gains without the intervention, but it raises an interesting question as to what cortical mechanisms support these sustained gains. May and Gaser (2006) offer a thorough review of the morphometry and plasticity literature including the possible neuronal correlates of the GMV changes. Importantly, while it is possible to speculate on the nature of these GMV changes after various interventions, it is not possible to determine from the current study or the previous longitudinal VBM literature whether these changes are due to learning or practice effects. Additional experimental groups including those varying in length of interventions and control groups including but not limited to those matched for cognitive effort and baseline behavior would be necessary in order to make more definitive conclusions.
Another important distinction from previous longitudinal VBM studies (Draganski et al., 2004; Draganski et al., 2006; May et al., 2007; Boyke et al., 2008; Driemeyer et al., 2008; Ilg et al., 2008; de Lange et al., 2008; Scholz et al., 2009) is that the subjects here are children. The participants in the previous studies range from young adults through elderly subjects, but no subjects below the age of 20 have been studied as of yet. One might predict that changes in GMV in pediatric populations may be especially pronounced, since GMV is already undergoing dramatic changes as part of typical development (Sowell et al., 1999a; Sowell et al., 1999b). Even though GMV has not yet been investigated in the context of intervention in children, white matter integrity has been studied. Keller and Just (2009) showed that increases in phonological decoding ability correlated with increased fractional anisotropy (FA) in the left anterior centrum semiovale. While these changes do not correspond with the GMV changes reported in this study, this is not unexpected as both the tissue type analyzed and interventions used are different. Specifically, the duration, intensity and approach of the intervention may modulate which brain regions are impacted. Future studies examining a variety of anatomical measures and addressing different types of interventions will be able to assess the more integral relationships between anatomical changes and reading intervention. Further, because gray matter undergoes significant changes during development from childhood through adulthood (Sowell et al., 1999a; Sowell et al., 1999b) these studies will also need to include a wider age range.
Limitations
There are a few important considerations to take into account while interpreting the results of this study. The group was made up of eleven dyslexic children, and while this is similar to group sizes used in previous studies examining GMV changes following training (Draganski et al., 2004;) and studies comparing dyslexic subjects to controls (Brambati et al., 2004; Vickenbosh et al., 2005; Steinbrink et al., 2008), it should be noted that the sample size is small. It is also important to appreciate that we did not have a dyslexic control group that did not receive the intervention to compare with the dyslexic sample receiving the intervention. Instead, the null period following the reading intervention was used as a within subject developmental control period, which is typical in studies in the field of education, where it is difficult to withhold intervention from students who have significantly fallen behind on their academic skills. Further research into the nature of these changes and their relation to reading skills will help translate what is learned in the research environment to helping children directly in the classroom.
Conclusions
This study showed gains in reading skills and increased GMV in dyslexic children after an eight week reading intervention. GMV increases were observed in the left hemisphere in anterior fusiform/hippocampus and precuneus. The left anterior fusiform region is commonly engaged in tasks involving object processing and object naming and may suggest that the dyslexic students are relying on this region to help improve their processing of words. The left precuneus has been implicated in visual imagery and specifically in tasks involving imagery of individual letters. Right hemisphere GMV changes following the intervention were found in the cerebellum and hippocampus. There is a theoretical framework implicating the cerebellum in dyslexia and this study adds a novel contribution to this theory. Finally, the GMV increases in the left hippocampus (extending from the cluster reported for the anterior fusiform gyrus) and right hippocampus may reflect more general learning that is occurring during the intervention. The increases in GMV were restricted to the intervention period and were not observed after the intervention ended, suggesting that these increases in GMV are related to the intervention. This is the first longitudinal VBM analysis in children and demonstrates that changes in brain structure are brought about by intervention. These findings provide encouragement that learning can result in both lasting behavioral and structural changes in children who struggle in learning to read. Further investigation will improve understanding not only for how the brain responds to learning, but in how these findings may be translated into refining interventions and improve the learning experience.
Acknowledgements
This work was supported by NICHD (P50 HD40095). We would like to thank Ashley Wall, Emma Cole, Corinna Moore, Jenni Rosenberg, Iain DeWitt and Alison Merikangas for aiding with the data acquisition. We also thank our participants and their families for volunteering their time, the Jemicy School for allowing us to conduct the intervention at their school and the staff from Lindamood Bell Learning Processes for providing the intervention.
Abbreviations
- VBM
voxel based morphometry
- GMV
gray matter volume
- fMRI
functional MRI
- PET
positron emission tomography
- ALE
activation likelihood estimate
- FA
fractional anisotropy
- MRS
magnetic resonance spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony J. Krafnick, Email: Krafnick-ajk53@georgetown.edu.
D. Lynn Flowers, Email: Flowers-lflowers@triad.rr.com.
Eileen M. Napoliello, Email: Napoliello-emn5@georgetown.edu.
Guinevere F. Eden, Email: Eden-edeng@georgetown.edu.
References
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FIM. The effects of divided attention on encoding-and retrieval-related brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12(5):775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thomson JB, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Bell N. Symbol Imagery Test. San Luis Obispo, CA: Gander Publishing; 1997. [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. The Journal of Neuroscience. 2008;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]; Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage. [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain: Learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121:1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohen L, Léhericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JWM, Toni I. Increase in prefrontal cortical volume following cognitive behavioral therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Naming of object-drawings by dyslexic and other learning disabled children. Brain and Language. 1976a;3:1–15. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Rapid “automatized” naming (RAN): dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976b;14(4):471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May MayA. Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning—revisited. Public Library of Science One. 2008;3:1–5. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. The Neuroscientist. 2004;10(3):1–10. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Fawcett AJ, Nicolson RI. Dyslexia, learning, and pedagogical neuroscience. Developmental Medicine & Child Neurology. 2007;49:306–311. doi: 10.1111/j.1469-8749.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. PNAS. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. The Journal of Neuroscience. 2008;28(16):4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clinic Proceedings. 2001;76(11):1081–1092. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:634–641. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM. Using imagery to retrieve semantic information: A developmental study. Child Development. 1976;47:434–444. [Google Scholar]
- Laycock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, Griffiths PD, Nicolson RI. Cerebellar volume and cerebellar metabolic characteristics in adults with dyslexia. Annals of the New York Academy of Sciences. 2008;1145:222–236. doi: 10.1196/annals.1416.002. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, King WM, Freeman A. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Lindamood C, Lindamood P. Lindamood Auditory Conceptualization (LAC) Test. Austin, TX: Pro-Ed.; 1971. [Google Scholar]
- Linden M, Wittrock MC. The teaching of reading comprehension according to the model of generative learning. Reading Research Quarterly. 1981;17(1):44–57. [Google Scholar]
- Long SA, Winograd PN, Bridge CA. The effects of reader and text characteristics on imagery reported during and after reading. Reading Research Quarterly. 1989;25(3):353–372. [Google Scholar]
- Lyon GR. Defining dyslexia, comorbidity, teachers’ knowledge language and reading: A definition of dyslexia. Annals of Dyslexia. 2003;53:1–14. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A Meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Current Opinion in Neurology. 2006;19:407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Gänßbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cerebral Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1:1–10. [Google Scholar]
- Menghini D, Hagberg GE, Petrosini L, Bozzali M, Macaluso E, Caltagirone C, Vicari S. Structural correlates of implicit learning deficits in subjects with developmental dyslexia. Annals of the New York Academy of Sciences. 2008;1145:212–221. doi: 10.1196/annals.1416.010. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Raberger T, Wimmer H. On the automaticity/cerebellar deficit hypothesis of dyslexia: balancing and continuous rapid naming in dyslexic and ADHD children. Neuropsychologia. 2003;41:1493–1497. doi: 10.1016/s0028-3932(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Talcott J, Richardson AJ, Stein JF. Metabolic abnormalities in developmental dyslexia detected by H magnetic resonance spectroscopy. The Lancet. 1998;351:1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, Dixon RM, Lee MA, Thompson CH, Styles P, Richardson AJ, Stein JF. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Raij T. Patterns of brain activity during visual imagery of letters. Journal of Cognitive Neuroscience. 1999;11(3):282–299. doi: 10.1162/089892999563391. [DOI] [PubMed] [Google Scholar]
- Renvall K, Laine M, Hiltunen J, Rinne JO, Kaasinen V, Sipilä H, Cornelissen K, Martin N. Naming multiple objects: Neural correlates as measured by positron emission tomography. Applied Neuropsychology. 2003;10(4):224–233. doi: 10.1207/s15324826an1004_4. [DOI] [PubMed] [Google Scholar]
- Sadoski M. An exploratory study of the relationship between reported imagery and the comprehension and recall of a story. Reading Research Quarterly. 1983;19(1):110–123. [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1367–1368. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: The neurobiology of reading and dyslexia. Development and Psychopathology. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999a;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999b;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. TRENDS in Neuroscience. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller H-P, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stip E, Mancini-Marïe A, Letourneau G, Fahim C, Mensour B, Crivello F, Dollfus S. Increased grey matter densities in schizophrenia patients with negative symptoms after treatment with quetiapine: A voxel-based morphometry study. International Clinical Psychopharmacology. 2009;24:34–41. doi: 10.1097/YIC.0b013e32831daf6c. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli DE. Neural Deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. PNAS. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Marrett S, Saad ZS, Ruff DA, Martin A, Bandettini PA. Functional but not structural changes associated with learning: An exploration of longitudinal Voxel-Based Morphometry (VBM) Neuroimage. 2009;48:117–125. doi: 10.1016/j.neuroimage.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WL, Slotnick SD, Burrage MS, Kosslyn SM. Two forms of spatial imagery. Psychological Science. 2009;20(10):1245–1253. doi: 10.1111/j.1467-9280.2009.02440.x. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S. Gray matter alteration in dyslexia: Converging evidence from volumetric and voxel-by-voxel MRI analyses. Neuropsychologia. 2005;43:324–331. doi: 10.1016/j.neuropsychologia.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101(2):192–212. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Woodcock RW, McGrew K, Mather N. The Woodcock-Johnson Tests of Achievement. Third Edition. Riverside: Itasca, IL; 2001. [Google Scholar]