Abstract
Our report describes RAFT copolymerization of multiple species of active peptide monomers with N-(2-hydroxypropyl(methacrylamide) (HPMA) under aqueous conditions. Resulting statistical copolymers are narrowly disperse and with highly controlled molecular weight and composition. Side chain peptide-copolymers were synthesized using a DNA condensing peptide (K12), and an endosomal escape peptide (K6H5) that had been modified with an aminohexanoic linker and capped with methacrylamide vinyl on the NH2-terminus. Copolymers of HMPA-co-K12 and HPMA-co-K12-co-K6H5 efficiently condensed DNA into small particles that maintain size stability even in 150 mM salt solutions. With increasing peptide content, the peptide-based polymers demonstrated gene delivery efficiencies to cultured human cells that were comparable to linear polyethylenimine.
Keywords: Nonviral gene delivery, peptide hybrid material, N-(2-hydroxypropyl)methacrylamide, RAFT polymerization, peptide-based gene delivery
Introduction
Biomimetic copolymers containing peptide motifs combine the highly specific functions of peptides with the scalable synthesis of synthetic polymers. Recently, these hybrid materials have received increased interest due to their applications in drug and gene delivery, tissue engineering, and biosensors, to name a few.1 The architectures of these materials include peptides incorporated as backbone segments in block copolymers or as pendant side chains. The latter offers a significantly higher concentration of peptides incorporated per polymer and also facile incorporation of multiple peptide sequences.
Side chain peptide-polymer bioconjugates can be synthesized by covalent coupling of peptides to polymer precursors or by direct polymerization of peptide monomers. Disadvantages of peptide coupling include unfunctionalized polymer side chains and generally lower peptide concentrations in the final polymer. Alternatively, synthetic copolymers with pendant peptides have been synthesized by free radical polymerization; however, resulting polymers were broadly disperse and poorly controlled.2–4 Living polymerization of peptide monomers have been demonstrated by atom transfer radical polymerization (ATRP), reverse addition-fragmentation polymerization (RAFT) and ring-opening metathesis polymerization (ROMP) techniques with peptides including elastin-based pentapeptides, integrin-binding sequences, and the antibiotic gramicidin.5–9 Limitations of these approaches included (a) restriction to short peptides (typically < 10 amino acids in length likely due to steric hindrance), (b) relatively low degrees of polymerization and (c) exclusion of lysine-containing peptides from RAFT syntheses due to potential aminolysis of the chain transfer agent.
Of particular relevance is the combination of a synthetic comonomer such as N-(2-hydroxypropyl)methacrylamide (HPMA) with peptide motifs that enable DNA condensation or elicit endosomal escape. Previous studies have combined a cationic material, poly-L-lysine (PLL) with HPMA. In one example, an HPMA copolymer containing active esters was covalently coupled in situ with available primary amines of PLL-DNA polyplexes. The modified polyplexes demonstrated enhanced serum stability in vitro and extended circulatory half-life in vivo10. Also, in an earlier report we explored the use of small oligomers of L-lysine copolymerized with HPMA through free radical polymerization.2 Although the incorporation efficiency of the peptides was poor and the copolymers disperse, the study demonstrated the potential for these types of peptide-based hybrid materials as nucleic acid carriers.
In our current study, we sought out a method for synthesis of side-chain peptide polymers that efficiently incorporate several components of varying molecular weight into a statistical copolymer while maintaining tight control of the resulting polymer’s molecular weight and composition. By doing so, down-stream purification steps could potentially be eliminated, conserving resources such as time and starting materials. Peptide compatibility with RAFT polymerization has been demonstrated by Börner and colleagues.11 More recently, Apostolovic and coworkers demonstrated RAFT copolymerization as a viable method to incorporate a functional and structurally constrained peptide into HPMA copolymers at quantitative yields.12 The success of their study bodes well for the development of novel materials based on multiple peptides copolymerized in a synthetic copolymer. We hypothesized that well-defined side chain peptide-polymers composed of multiple species of active peptides could be synthesized by RAFT polymerization and that these materials would have useful applications as gene transfer agents.
Material and Methods
Materials
N-(2-hydroxypropyl)methacrylamide (HPMA) was purchased from Polysciences (Warrington, PA). The initiator VA-044 was purchased from Wako Chemicals USA (Richmond, VA). All reagents used in solid phase chemical synthesis including NovaPEG Rink amide resin, HBTU, Fmoc-protected amino acids were purchased from EMD Biosciences (Darmstad, Germany). All other materials were reagent grade or better and were purchased from Sigma-Aldrich (St. Louis, Mo) unless otherwise stated.
Synthesis of Peptide Monomers
Peptides, KKKKKKKKKKKK (K12), and KKKKKKHHHHH (K6H5) were synthesized on a solid support of NovaPEG Rink amide using standard Fmoc/tBu chemistry. Synthesis was carried out on an automated PS3 peptide synthesizer (Protein Technologies, Pheonix, AZ). Prior to peptide cleavage from resin, the amino terminal of the peptides was deprotected and modified with Fmoc-protected aminocaproic acid (Ahx) which was subsequently deprotected and capped with either methacryloy chloride or acryloyl chloride.2 These peptide monomers are respectively refered to as MaK12, AcK12, and MaK6H5. Synthesized peptides cleaved from resin by treating the solid support with a solution of TFA/H20/TIS (95:2.5:2.5, v/v/v) for 3 hrs with gentle mixing. Each peptide monomer was purified by RP-HPLC and products were confirmed by MALDI-TOF MS. MALDI-TOF MS calculated for MaK12 (MH+), 1736.26 found 1736.23. MALDI-TOF MS calculated for AcK12 (MH+) 1722.24, found 1722.22. MALDI-TOF MS calculated for MaK6H5 (MH+) 1652.99, found 1652.99.
Synthesis of ethyl cyanovaleric trithiocarbonate (ECT)
The synthesis of the RAFT chain transfer agent (CTA) ECT has been described previously by Convertine et al. 13 Briefly, over a 10 min period, ethanethiol (4.72 g, 76 mmol) was added to a stirred suspension of sodium hydride (60% in oil) (3.15 g, 79 mmol) in 150 mL of diethyl ether at 0 °C. The solution was stirred for a further 10 min before carbon disulfide (6.0 g, 79 mmol) was added. Crude sodium S-ethyl trithiocarbonate (7.85 g, 0.049 mol) was collected by filtration, suspended in 100 mL of diethyl ether, and reacted with iodine (6.3 g, 0.025 mol). After 1 h, the solution was filtered, washed with aqueous sodium thiosulfate, and thoroughly dried over sodium sulfate; the crude bis(ethylsulfanylthiocarbonyl) disulfide was then isolated by rotary evaporation. A solution of bis(ethylsulfanylthiocarbonyl) disulfide (1.37 g, 0.005 mol) and 4,4′-azobis(4-cyanopentanoic acid) (2.10 g, 0.0075 mol) in 50 mL of ethyl acetate was heated at reflux for 18 h. Following rotary evaporation of the solvent, the crude ECT was isolated by column chromatography using silica gel as the stationary phase and 50:50 ethyl acetate hexane as the eluent.
1H NMR(CDCl3): δ 1.36 t (SCH2CH3); δ 1.88 s (CCNCH3); δ 2.3–2.65 m (CH2CH2); δ 3.35 q (SCH2CH3).
Statistical RAFT copolymerization of HPMA with Peptide monomers
HPMA-co-K12 copolymers
Two sets of HPMA-co-K12 copolymers were synthesized. One set of copolymers varied the amount of K12 monomers in the feed for both MaK12, and AcK12 monomers from 1 mole % to 20 mole %. In these experiments the total moles of monomers was kept at 0.144 mmoles while the amount of K12 monomer was 1 mole %, 5 mole %, 10 mole %, and 20 mole % and HPMA amounts varied respectively. To carry out these polymerizations 1.43 × 10−3 mmoles, 7.18 × 10−3 mmoles, 14.36 × 10−3 mmoles, and 28.72 × 10−3 mmoles of either MaK12 or AcK12 were added to separate vials. To these vials 0.142 mmoles (20.35 mg), 0.136 mmoles (19.54 mg), 0.129 mmoles (18.51 mg), 0.115 mmoles (16.45 mg) of HPMA were added to respective vials making the total moles of monomer equal to 0.144 mmoles. The molar ratio of CTA:I used was 10, and the molar ratio of total monomers:CTA used was 190. Based on these ratios 0.0758 × 10−3 mmoles (0.201 mg) of ECT and 0.00758 × 10−3 mmoles (0.0247 mg) of VA-044 were added to monomers. The total volume of the RAFT copolymerization reaction was 206 μL in acetate buffer (1 M in H2O, pH 5.1). The solutions were then distributed into 5 mL round bottom flasks and sealed with a rubber septum. The aliquots were then purged with high purity N2 gas for 15 minutes prior to incubation in an oil bath (44 ºC) for 48 hours.
Kinetic studies of HPMA-co-K12 copolymerization were done using total mole amount of 0.653 mmoles. This included 0.620 mmoles (88.8 mg) of HPMA and 0.0326 mmoles (58.1 mg) of MaK12. The molar ratio of CTA:I used was 10, and the molar ratio of total monomers:CTA used was 190. Based on these ratios 3.43 ×10−3 mmoles (0.904 mg) of ECT and 0.343 × 10−3 mmoles (0.111 mg) of VA-044 were added to monomers. The total volume of the RAFT copolymerization reaction was 936 μL in deuterated acetate buffer (1 M in D2O, pH 5.1). The solution was aliquotted into equivalent volumes into nine 5 mL round bottom flasks and sealed with a rubber septum. The aliquots were then purged with N2 gas for 15 minutes prior to placing in an oil bath set at 44 ºC. At designated time points, aliquots were removed from the oil bath and immediately quenched by submersing in liquid nitrogen. Frozen aliquots were then thawed and analyzed by NMR.
HPMA-co-K6H5
The mole % of HPMA and MaK6H5 in feed was 95% and 5% respectively. The total moles of monomers were 0.144 mmoles, including 0.136 mmoles (19.54g) of HPMA and 7.20 × 10−3 mmoles of MaK6H5. The molar ratio of CTA:I used was 10, and the molar ratio of total monomers:CTA used was 190. Based on these ratios 0.0758 × 10−3 mmoles (0.201 mg) of ECT and 0.00758 × 10−3 mmoles (0.0247 mg) of VA-044 were added to monomers. The total volume of the RAFT copolymerization reaction was 206 μL in deuterated acetate buffer (1 M in H2O, pH 5.1). The solution was then distributed into 5 mL round bottom flasks and sealed with a rubber septum. The solution was then purged with high purity N2 gas for 15 minutes prior to incubation in an oil bath (44 ºC) for 48 hours.
HPMA-co-MaK12-co-MaK6H5
Kinetic studies were done using total mole amount of 0.653 mmoles. This included 0.587 mmoles (84.2 mg) of HPMA, 0.0326 mmoles (58.1 mg) of MaK12 and 0.0326 mmoles (54.0 mg) of MaK6H5. The molar ratio of CTA:I used was 10, and the molar ratio of total monomers:CTA used was 190. Based on these ratios 3.43 ×10−3 mmoles (0.904 mg) of ECT and 0.343 × 10−3 mmoles (0.111 mg) of VA-044 were added to monomers. The total volume of the RAFT copolymerization reaction was 936 μL in deuterated acetate buffer (1 M in deuterated H2O, pH 5.1). The solution was aliquotted into equivalent volumes into nine 5 mL round bottom flasks and sealed with a rubber septum. The aliquots were purged with high-purity N2 gas for 15 minutes prior to incubation in an oil bath (44 ºC). At designated time points, aliquots were removed from the oil bath and immediately quenched by submersing in liquid nitrogen. Frozen aliquots were then thawed and analyzed by NMR.
1H Nuclear Magnetic Resonance
Polymerization kinetic for copolymers of HPMA-co-MaK12, and HPMA-co-MaK12-co-MaK6H5 were assessed by 1H NMR to determine the conversion of vinyl-coupled hydrogens. As described above, copolymerizations were carried out in acetate buffered deuterated water. Each time point of polymerization was quenched by immersion in liquid nitrogen. After the samples had been thawed 0.5 mL of deuterated water was added to the solution (0.144 mmoles of monomer) making the final volume 0.604 ml (final concentration of Monomer: 0.238 mol/L). The samples were then applied to NMR using a Bruker AV300 running at 300 Hz. Conversion of monomers was determined based on the ration of vinyl protons (δ 5.4 and 5.6) relative to 3-methyl protons (δ 1.1) of HPMA. These peaks on the NMR spectrum were well separated from peaks generated from the addition of MaK12. 1H NMR (300 Hz, deuterated H20): δ 1.1 (d, 3H), 1.9 (d, 3H), 2.6 (s, 1H), 2.9–3.2(m, 2H), 3.9 (m, 1H), 5.4 (s, 1H), 5.6(s, 1H), 8.03(s, 1H).
Molecular Weight and Compositional Characterization
Size Exclusion Chromatography (SEC)
Molecular weight analysis was carried out by size exclusion chromatography. To prepare materials for analysis, copolymers were extensively dialyzed against distilled H2O removing any unreacted monomers and remove residual buffer salts and then lyophilized to recover them as a fluffy while solid. Then the copolymers were dissolved at 5 mg/mL in running buffer (0.3 M sodium acetate buffered to pH 4.4 with acetic acid) for analysis by SEC as described by Hennink and coworkers.14 This was done by applying samples to a OHpak SB-804 HQ column (Shodex) in line with a miniDAWN TREOS light scattering detector (Wyatt) and a Optilab rEX refractive index detector (Wyatt). Absolute molecular weight averages (Mn and Mw), and dn/dc were calculated using ASTRA software (Wyatt).
Ninhydrin Assay
Content of K12 and K6H5 peptides within HPMA copolymers were determined through ninhydrin assay of primary amines. Briefly, a stock solution of reagent was made by dissolving 0.1340 g of ninhydrin and 0.0234 g hydrindantin in 5 mL of 2-methoxyethanol, and then 3.325 ml of 4N acetate buffer (pH 5.5) was added. Standards of glycine at a concentration of 200 μM to 0 μM were then made through serial dilutions. Samples were then prepared at an approximate concentration of 40 μM of primary amines in double distilled and deionized water. To 0.5 ml of sample 0.25 ml of ninhydrin reagent solution was added and then mixed well. The reaction mixture was boiled for 15 min and allowed to cool. After the solution had reached room temperature, 0.75 mL of ethanol was added. Then, 100 μl of the final solution was transferred to wells on a UV-transparent 96-well plate, and the absorbance was measure at 440 nm on a Tecan Safire2 96-well plate reader.
Amino Acid Analysis
To confirm K12 and/or K6H5 content with in HPMA copolymers, amino acid analysis was carried out at UC Davis’s proteomics core facility with an L-8800 Hitachi analyzer that utilizes a sodium citrate buffer system. HPMA content was calculated by subtracting amino acid content in mg from the total weight of the copolymers used for analysis.
Polyplex formulation and characterization
The gWiz-Luc plasmid (endotoxin free, purchased from Aldevron, Fargo, ND) was diluted in double distilled H2O to a concentration of 0.1 mg/mL and mixed with an equal volume of polymer (also diluted in double distilled H2O) at a lysine to DNA phosphate ratio (N/P) of 5 as previously described.2 Branched polyethyleneimine (PEI, MW 25,000) and Poly-L-lysine hydrobromide (PLL, MW15,000 – 30,000) were used as positive controls. After mixing, the polyplexes were allowed to form for 5 minutes at room temperature. Twenty μL of each polyplex solution (containing 1 μg DNA) was mixed with either 80 μL of double distilled H20 or PBS (where PBS was used to dilute polyplex solutions, the final ionic strength of the solution was 150 mM) and then used to determine the particle size of the polyplexes by dynamic light scattering (DLS) measured on a Brookhaven Instruments Corp ZetaPALS instrument. Particle sizing measurements were performed at a wavelength of 659.0 nm with a detection angle of 90º at RT.
In vitro transfection efficiency
Human cervix epithelial adenocarcinoma cells (HeLa, passage 25, ATCC # CCL-2) were seeded in complete cell culture medium (MEM + 10% FBS + 1% antibiotic/antimicrobial) at a density of 5 × 104 cells/well in each well of a 24-well plate. The 24-well plates were placed in a 37ºC incubator with 5% CO2 for 24 hours to allow the HeLa cells to attach. Polyplexes were formed as described above at N/P = 5 using 1 μg of gWiz-Luc plasmid DNA in 20 μL total volume. Each sample was brought up to 200 μL with OptiMEM I (Invitrogen). The cells were washed twice with PBS and the transfection solutions were added. The 24-well plates were returned to the incubator for four hours, at which time the polyplex solution was replaced with complete cell culture media after washing the cells with PBS. The cells were returned to the incubator for a further 44 hours. After a total incubation of 48 hours, the luciferase expression was quantified with a luciferase assay kit (Promega Corp.) according to the manufacturer’s instructions, except that a freeze-thaw cycle at −20ºC was included after the addition of the lysis buffer to ensure complete cell lysis. Luminescence intensity was measured on a Tecan Safire2 plate reader with integration for 1 s. The total protein content in each well was measured by a BCA Protein Assay Kit (Pierce) according to the manufacturer’s instructions so that the luciferase activity measured in each well could be normalized by the total protein content in each well. Furthermore, the BCA protein assay was also used to asses cytotoxicity of the materials. Each sample was tested in replicates with n = 6. The statistical significance of differences in the samples was determined using Student’s t-test, where a p-value of less than 0.05 was considered significant.
Results and Discussion
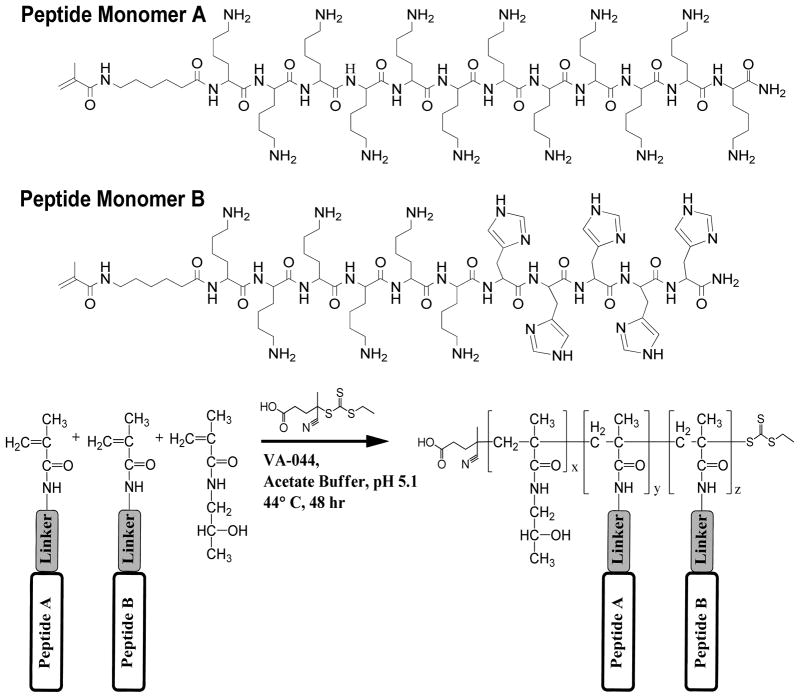
To test our main hypothesis, peptide monomers composed of either twelve L-lysine residues (MaK12) or six L-lysine residues and five L-histadine residues (MaK6H5) were copolymerized with N-(2-hydroxypropyl)methacrylamide (Mw of comonomers: HPMA 143.2 Da, MaK12 1732.2 Da, and MaK6H5 1653.0 Da). Peptides were chosen for their ability to either efficiently condense nucleic acids (K12),15 or elicit endosomal escape (K6H5).16 HPMA was chosen as a model comonomer because of its commercial availability, its use as a comonomer in clinically-tested drug delivery materials and its extensive use in peptide-polymer conjugates.17 Peptides were synthesized by solid phase peptide synthesis using standard Fmoc/tBu chemistries. Prior to cleavage from resin the amine terminus of the peptide was modified with a linker (6-aminocaproic acid) and then capped with either a methacrylamido- (MaK12 and MaK6H5) or acrylamido-functional group (AcK12) for polymerization (Scheme 1).
Scheme 1.
RAFT Polymerization of Peptide-HPMA copolymers
Copolymerization of peptide monomers with HPMA was carried out under aqueous conditions at 44 ºC using the RAFT agent ethyl cyanovaleric trithiocarbonate (ECT) and VA-044 as the initiator. ECT has previously demonstrated excellent properties as a RAFT agent for HPMA and other methacrylamido- and acrylamido-based monomers.18 An acetic acid buffer at pH 5.1 with a molar strength of 1 M was used to ensure ε-amines of L-lysine were fully protonated, thereby protecting the trithiocarbonate from nucleophilic attack.
Initial studies focused on the copolymerization of MaK12 relative to AcK12 peptides. In these experiments amount of peptide monomers in feed were varied from 1 mole % to 20 mole % while the remainder of feed was HPMA. Copolymerization of both methacrylamido- and acrylamido- modified peptides with HPMA were carried out in parallel and demonstrated near quantitative yields of peptide monomer incorporation into the final copolymer for the full range of peptide monomer percentages tested. Interestingly, the MaK12 peptide monomer was copolymerized more efficiently at 20 mole% of peptide in feed than its acrylamido-counterpart (Figure 1). As shown in Table 1, the molecular weights of the resulting polymers were near the predicted value with low polydispersities (Mw/Mn < 1.2). We then studied the copolymerization kinetics of HPMA-co-MaK12 copolymers with peptide feed amount of 5 mole %. Conversion of monomers was followed by 1H NMR. Kinetics closely followed a pseudo-first order model characteristic of living radical polymerization (Figure 2). Elution profiles from size exclusion chromatography analysis demonstrated an initial bimodal size distribution that quickly became unimodal as seen in Figure 3. Additionally, studies were included to determine lysine content at each time point through both ninhydrin assay and amino acid analysis. As demonstrated in Figure 4c, lysine content of copolymers remained constant at near quantitative yields throughout polymerization. Also, as the polymerization experiment proceeded the number molecular weight average, Mn, remained close to predicted values. The polydispersity fell from 1.8 to 1.1 through the course of the experiment as can be seen in Figure 4.
Figure 1.

Correlation between MaK12 monomer feed concentration in mole % and concentration of peptides in the HPMA copolymers.
Table 1.
Physical and Chemical Characterization of HPMA-Peptide Copolymers Copolymerized via RAFT
| Copolymera | CTAo/Mo | Predicted | Actual | Mw/Mn | K12 (%)c | K6H5 (%)c |
|---|---|---|---|---|---|---|
| Mn (kDa) | Mn (kDa)b | |||||
| P-01-00 | 240 | 38.18 | 31.31 | 1.16 | 0.58 | NA |
| P-05-00 | 240 | 53.44 | 55.17 | 1.05 | 4.43 | NA |
| P-10-00 | 240 | 72.51 | 89.00 | 1.02 | 9.66 | NA |
| P-20-00 | 240 | 110.64 | 110.50 | 1.28 | 19.41 | NA |
| P-00-05 | 190 | 41.55 | 44.83 | 1.15 | NA | 4.15 |
| P-05-05 | 190 | 56.70 | 45.80 | 1.08 | 3.94 | 4.41 |
HPMA-peptide samples are named by using a P-xx-yy notation where P- indicates that the sample is a copolymer of HPMA with –xx- mole % of MaK12 peptide monomer and –yy- mole % of MaK6H5 peptide monomer in the polymerization reaction.
Number average molecular weight was determined using SEC couled with light scattering and refractive index detectors.
Mole % of K12 were determined by both ninhydrin assay and amino acid analysis, reported values are from amino acid analysis.
Figure 2.

Pseudo first-order rate plot of the bulk copolymerization of HPMA with 5 mole % of MaK12 in the feed.
Figure 3.
Time-point SEC of HPMA-MaK12 statistical copolymers. Initial distributions demonstrate a bimodal molecular weight distribution that quickly resolves to single distribution peak with narrow polydispersity.
Figure 4.

RAFT Statistical copolymerization of HPMA-co-MaK12 copolymers using a feed of 5 mole % of MaK12 peptide monomer. (a) Polydispersity vs. conversion, (b) Mn growth vs. conversion, (c) and incorporation of K12 and copolymer’s number average (Mn) vs. time.
To demonstrate RAFT polymerization’s usefulness in a synthesis of copolymer that included multiple peptides, HPMA copolymers were synthesized with 5 mole % of MaK12 and 5 mole % MaK6H5 peptide monomers in the feed with the remainder of monomers being HPMA. Copolymerization of the 2-peptide HPMA copolymer was monitored by 1H NMR, amino acid analysis, and SEC. Conversion of monomers to polymers appeared to follow pseudo-first order kinetics (see Figure 5), and the final K12 and K6H5 contents were 3.9 and 4.4 mole %, respectively. Again, copolymers with molecular weights near predicted values and low polydispersities were obtained as shown in Table 1 and Figures 6a and b. Both HPMA-MaK12, and HPMA-MaK12-MaK6H5 copolymers displayed multi-modal distributions of molecular weight in early time points that quickly resolved into a single distribution of copolymers (Figures 3 and 7). Also, the Mn of HPMA-co-MaK12-co-MaK6H5 initially deviated from predicted values with very broad polydispersity produced by the multi-modal molecular weight distributions. This had a rather unique effect of maintaining Mn values of approximately 19 kDa while conversion increased from 15% to 50 %. During this same period the copolymer’s Mw/Mn ratio diminished from 3.1 to 1.5 and the SEC elution of the copolymer resolved to a single molecular weight distribution which indicates that the growing copolymer chains were reaching a steady equilibrium. The final copolymer reached a conversion of 86% of monomers and had an Mn near the predicted value with low polydispersity (final Mw/Mn = 1.08). A possible explanation for bimodal molecular weight distributions in early time points is the formation of macroCTA’s by the initial addition of peptide monomers to ECT. This would cause heterogeneous RAFT efficiency as has been described by Huang et al.19 This phenomenon could possibly result in some heterogeneity in the copolymers during early stages of RAFT polymerization; however, the inclusion of peptide monomers remained consistently close to the feed peptide monomer concentration. As polymerization proceeded RAFT efficiencies became homogeneous as is indicated by the copolymer presenting a single distribution of molecular weights with a narrow polydispersity (Mw/Mn).
Figure 5.

Pseudo first-order rate plot of the bulk copolymerization of HPMA with 5 mole % of MaK12 and 5 mole % of MaK6H5 in the feed.
Figure 6.
RAFT Statistical copolymerization of HPMA-co-MaK12-co-MaK6H5 copolymers using a feed of 5 mole % MaK12 and 5 mole % of MaK6H5 peptide monomers. (a) Polydispersity vs. conversion, (b) Mn growth vs. conversion, (c) and incorporation of K12, K6H5, and the copolymer’s number average molecular weight (Mn) vs. time.
Figure 7.
SEC analysis of statistical copolymers of MaK12-MaK5H6-HPMA copolymers during various time points of compolymerization. Initial samples displayed bimodal distributions that were broadly disperse. However, as polymerization proceeded, copolymers became more uniform in molecular weight with a single molecular weight distribution and a narrow polydispersity (Mw/Mn 1.08)
In order to determine the benefit of including multiple peptide components into the copolymers, materials were tested for their ability to function as nucleic acid carriers. This analysis included the ability of the HPMA-peptide copolymers to form complexes with plasmid DNA (termed “polyplexes”) and the copolymers’ ability to deliver plasmid to logarithmically-growing HeLa cells in culture. HPMA-peptide copolymers displayed the ability to effectively condense the plasmid into small particles (<200 nm) as displayed in figure 8. Also, the particles formed by the HPMA-peptide copolymers demonstrated salt stability when in PBS buffers, whereas the size of polyplexes formed by cationic peptides and homopolymers of linear poly(ethylenimine) (PEI), and poly(L-lysine) (PLL) grew considerably in the presence of salt. The salt stability of HPMA-peptide copolymers were consistent with polyplexes formed by PEG-modified cationic polymers.20 Additionally, the transfection efficiency of HPMA-K12 copolymers approached that of PEI at high K12 content (20 mole % of K12) reaching efficiencies well beyond that of PLL (figure 9), contrasting sharply to the diminished transfection efficiency caused by PEGylating PEI.21 Although HPMA copolymers with increased amounts of oligolysine transfected HeLa cells more readily, the increased cationic character also increased the cytotoxicity of the copolymers (Figure 10). However, the HPMA-peptide copolymers demonstrated much less toxicity its PLL counterpart.
Figure 8.

Particle sizing of polyplexes formed with gWiz-Luc luciferase plasmid with either cationic peptides or HPMA-peptide copolymers. In all formulations an N/P of 5 was used. HPMA-peptide samples are named by using a P-xx-yy notation where P- indicates that the sample is a copolymer of HPMA with –xx- mole % of MaK12 peptide monomer and –yy- mole % of MaK6H5 peptide monomer in the polymerization reaction.
Figure 9.

Transfection efficiency of PEI, PLL, cationic peptides, and HPMA-peptide copolymers. Gene delivery efficiency of selected carriers (formulated at N/P = 5) to HeLa cells as measured by luciferase activity 48 h after transfection. Results are reported as the mean RLU/mg protein ± SD for replicate samples, with all samples having n = 6. Nomenclature follows the same convention as described in figure 8. *P-20-00 demonstrated statistical significance with all other materials except PEI (p < 0.05). **P-10-00 and P-05-05 demonstrated statistical significance to all materials except PEI and P-20-00 (p < 0.01). ***P-05-00 demonstrated statistical significance to K6H5, K12, P-01-00, and DNA (P<0.05).
Figure 10.

Toxicity of the PLL, PEI, cationic peptides and HPMA-peptide copolymers on HeLa cells. Protein content was determined through a BCA protein assay. The protein content was normalized to untreated cells.
Conclusion
In conclusion, our study presented the synthesis of well-defined statistical side-chain peptide-HPMA copolymers with lysine- and histadine-based, long (>10 amino acids) peptides by RAFT polymerization. Copolymerization reactions demonstrated pseudo-first order kinetics characteristic of living radical polymerization. Controlled growth of copolymers, inclusion of multiple peptides and the narrow polydispersities of resulting copolymers suggests the robustness of this technique for polymerization and synthesis of graft copolymers. These materials proved to be effective nucleic acid delivery vehicles that can potentially be used directly in vivo without further modification due to their salt stability. Although our study focused on two peptides with functionally distinct roles, this technique can possibly be extended to other peptides with diverse functional and structural characteristics. This may facilitate the development of novel and multi-functional materials for self-assembly, drug-delivery, and biotechnology.
Acknowledgments
This work was supported by an NSF CAREER Award (CBET-0448547) to S.H.P. and NIH/NINDS 1R01NS064404. We also appreciated helpful discussions with Pavla Kopečková.
References
- 1.Lutz JF, Börner HG. Prog Polym Sci. 2008;33:1–39. [Google Scholar]
- 2.Burke RS, Pun SH. Bioconjugate Chem. 2010;21:140–150. doi: 10.1021/bc9003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obrien-Simpson NM, Ede NJ, Brown LE, Swan J, Jackson DC. J Am Chem Soc. 1997;119:1183–1188. [Google Scholar]
- 4.Murata H, Sanda F, Endo T. Macromolecules. 1997;30:2902–2906. [Google Scholar]
- 5.Ayres L, Adams P, Lowik D, van Hest JCM. Biomacromolecules. 2005;6:825–831. doi: 10.1021/bm049421p. [DOI] [PubMed] [Google Scholar]
- 6.Ayres L, Vos MRJ, Adams P, Shklyarevskiy IO, van Hest JCM. Macromolecules. 2003;36:5967–5973. [Google Scholar]
- 7.Maynard HD, Okada SY, Grubbs RH. J Am Chem Soc. 2001;123:1275–1279. doi: 10.1021/ja003305m. [DOI] [PubMed] [Google Scholar]
- 8.Godwin A, Hartenstein M, Muller AHE, Brocchini S. Angew Chem, Int Ed. 2001;40:594–597. doi: 10.1002/1521-3773(20010202)40:3<594::AID-ANIE594>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Trillo F, Dureault A, Bayley JPM, van Hest JCM, Thies JC, Michon T, Weberskirch R, Cameron NR. Macromolecules. 2007;40:6094–6099. [Google Scholar]
- 10.Oupický D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Mol Ther. 2002;5:463–472. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 11.ten Cate MGJ, Rettig H, Bernhardt K, Börner HG. Macromolecules. 2005;38:10643–10649. [Google Scholar]
- 12.Apostolovic B, Deacon SPE, Duncan R, Klok H. Biomacromolecules. 2010;11:1187–1195. doi: 10.1021/bm901313c. [DOI] [PubMed] [Google Scholar]
- 13.Convertine AJ, Benoit DSW, Duvall CL, Hoffman AS, Stayton PS. J Controlled Release. 2009;133:221–229. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang XL, van der Horst A, van Steenbergen MJ, Akeroyd N, van Nostrum CF, Schoenmakers PJ, Hennink WE. Pharm Res. 2006;23:595–603. doi: 10.1007/s11095-006-9574-4. [DOI] [PubMed] [Google Scholar]
- 15.Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Bioconjugate Chem. 1997;8:81–8. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 16.Midoux P, Monsigny M. Bioconjugate Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 17.Ríhová B, Bilej M, Větvička V, Ulbrich K, Strohalm J, Kopecek J, Duncan R. Biomaterials. 1989;10:335–342. doi: 10.1016/0142-9612(89)90075-6. [DOI] [PubMed] [Google Scholar]
- 18.Duvall CL, Convertine AJ, Benoit DSW, Hoffman AS, Stayton PS. Mol Pharmaceutics. 2010;7:468–476. doi: 10.1021/mp9002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CF, Nicolay R, Kwak Y, Chang FC, Matyjaszewski K. Macromolecules. 2009;42:8198–8210. [Google Scholar]
- 20.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. Gene Therapy. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 21.Mishra S, Webster P, Davis ME. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]