Abstract
Nootropic agents or cognitive enhancers are purported to improve mental functions such as cognition, memory, or attention. The aim of our study was to determine the effects of two possible cognitive enhancers, huperzine A and IDRA 21, in normal young adult monkeys performing a visual memory task of varying degrees of difficulty. Huperzine A is a reversible acetylcholinesterase (AChE) inhibitor, its administration results in regionally specific increases in acetylcholine levels in the brain. In human clinical trials, Huperzine A resulted in cognitive improvement in patients with mild to moderate form of Alzheimer's disease (AD) showing its potential as a palliative agent in the treatment of AD. IDRA 21 is a positive allosteric modulator of glutamate AMPA receptors. It increases excitatory synaptic strength by attenuating rapid desensitization of AMPA receptors and may thus have beneficial therapeutic effects to ameliorate memory deficits in patients with cognitive impairments, including AD. The present study evaluated the effects of the two drugs in normal, intact, young adult monkeys to determine whether they can result in cognitive enhancement in a system that is presumably functioning optimally.
Six young pigtail macaques (Macaca nemestrina) were trained on delayed non-matching-to-sample task, a measure of visual recognition memory, up to criterion of 90% correct responses on each of the four delays (10s, 30s, 60s, and 90s). They were then tested on two versions of the task: Task 1 included the four delays intermixed within a session and the monkeys performed it with the accuracy of 90%. Task 2 included, in each of 24 trials, a list of six objects presented in succession. Two objects from the list were then presented for choice paired with novel objects and following two of the four delays intermixed within a session. This task with a higher mnemonic demand yielded an average performance of 64% correct. Oral administration of huperzine A did not significantly affect the monkeys' performance on either task. However, a significant negative correlation was found between the baseline performance on each delay and the change in performance under huperzine A, suggesting that under conditions in which the subjects were performing poorly (55 – 69%), the drug resulted in improved performance, whereas no improvement was obtained when the baseline was close to 90%. In fact, when the subjects were performing very well, huperzine A tended to reduce the performance accuracy, indicating that in a system that functions optimally, the increased availability of acetylcholine does not improve performance or memory, especially when the animals are close to the maximum performance. In contrast, oral administration of IDRA 21 significantly improved performance on Task 2, especially on the longest delay. This finding supports the potential use of this drug in treatment of cognitive and memory disorders.
1. Introduction
Agents that enhance cognitive function, or nootropic agents, improve mental performance through a variety of mechanisms, including amplification of excitatory neurotransmission mediated by acetylcholine or glutamate. In many cases, nootropic activity is evaluated in animal models, in which cognitive function has been compromised by drug treatment, aging, lesions, or genetic abnormalities. The type of enhancement is distinct from that in which normal function is augmented. A drug with a mechanism of action that may ameliorate deficits caused by loss of function of specific neural pathways would not necessarily have an effect under circumstances in which that pathway is intact and functioning normally. This distinction is important because there is considerable interest in identifying nootropic targets to augment normal function, but this cannot be accomplished only by extrapolating from drug actions in subjects with cognitive impairment.
The aim of our study was to determine the effects of two potential cognitive enhancers with distinct mechanisms of action, huperzine A and IDRA 21 (7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide) (Figure 1), in normal young adult monkeys performing a visual memory task of varying degrees of difficulty. Both of these agents have been shown to attenuate cognitive deficits caused by various experimental interventions in animal models. Huperzine A, a drug that augments cholinergic function by blocking acetylcholinesterase (AChE) activity, has been tested in clinical trials for dementia (Wang et al., 2009) and in animal models of cognitive impairment caused by blockade or loss of cholinergic neurons (Liang et al. 2008; Xiong, 1998), age (Ye et al., 2000) or ischemia (Wang et al., 2002; Wang et al., 2006; Wang et al., 2008; Zhou et al., 2001), but has not been shown to have effect in normal animals. In contrast, IDRA 21, a drug that prevents desensitization of the AMPA subtype of glutamate receptor, has been demonstrated to augment spatial memory in normal rats (Zivkovic et al., 1995), and to improve performance on a visual recognition memory task in young adult rhesus monkeys (Buccafusco et al., 2004). Therefore, we decided to examine whether huperzine A can enhance the performance of normal animals in the face of increasing mnemonic challenge in a visual recognition memory task with varying degrees of difficulty. As a comparison, we tested IDRA 21, with the expectation that this agent also would exert a nootropic action in normal monkeys. We compared the effects of the two drugs in normal, young adult monkeys and this allowed us to detect cognitive enhancement in a system that is presumably functioning optimally.
Figure 1.
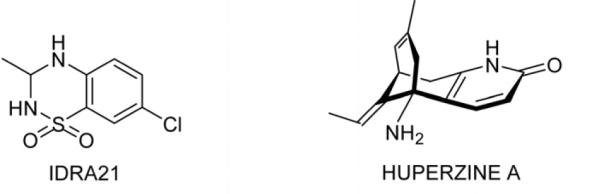
Chemical structure of IDRA 21 and Huperzine A.
Huperzine A is a plant-based alkaloid from a Chinese plant Huperzia serrata, that has nM potency as a reversible AChE inhibitor. Huperzine A is highly specific for AChE over BuChE, it crosses the blood-brain barrier and its potency is similar or superior to other AChE inhibitors (physostigmine, galanthamine, donepezil, and tacrine, for reviews, Wang and Tang, 2005; Zangara, 2003). Huperzine A has also been synthesized (Geib et al., 1991; Kozikowski et al., 1991; Tang et al., 1994) and both enantiomerically pure forms are being used in research and clinical trials. Huperzine A has been documented to cause regionally specific increases in ACh levels in rat brain (Cheng and Tang, 1998; Tang et al., 1989; Tang et al., 1994; Wang and Tang, 1998). A significant dose-dependent increase in norepinephrine (Zhu and Giacobini, 1995) and dopamine (Liang and Tang, 2006; Zhu and Giacobini, 1995) was also observed. In addition, huperzine A has been shown to have neuroprotective effects against neuronal damage caused by ischemia (Wang et al., 2006; Wang et al., 2008; Zhou et al., 2001), intracerebral infusion of beta-amyloid protein (Wang et al., 2001), NMDA toxicity (Gordon et al., 2001; Ved et al., 1997), organophosphate poisoning (Lallement et al., 2002a), and the nerve agent soman (Aracava et al., 2009; Eckert et al., 2007; Gordon et al., 2005; Haigh et al., 2008; Lallement et al., 1997; Lallement et al., 2002b; Tonduli et al., 2001). These observations have generated considerable interest in the potential clinical utility of huperzine A for the treatment of acute and chronic neurological disorders that affect cognitive function (Wang and Tang, 2005). It has also been shown to be a potential palliative agent in the treatment of Alzheimer's disease (AD) in human clinical trials. So far, Chinese clinical studies have shown an improvement in the memory of AD patients (Wang et al., 2009; Xu et al., 1995; Xu et al., 1999; Zhang et al., 2002). The phase IV clinical trials in China have demonstrated that huperzine A significantly improved memory deficits in elderly people with benign senescent forgetfulness, and patients with AD and vascular dementia, with minimal peripheral cholinergic side effects and no unexpected toxicity (Wang et al., 2009). In the U.S., a multicenter (29 centers in 17 states), double-blind, placebo controlled phase 2 clinical trial showed cognitive improvement in patients with mild to moderate AD (ClinicalTrials.gov; NCT00083590).
In order to understand the mechanisms by which huperzine A may enhance cognitive function, it is necessary to examine its effects in animal models. To date, all of the studies in rodents, in which huperzine A has been evaluated, have used models of spatial working memory impairment induced by a muscarinic antagonist scopolamine (Gao et al., 2000; Wang et al., 2007). These studies have all indicated that huperzine A ameliorated the induced memory impairment. In monkeys, huperzine A improved performance in spatial working memory in aged animals and young animals treated with scopolamine (Ye et al., 1999), reserpine or yohimbine (Ou et al., 2001). None of these studies examined the effects of huperzine A on intact animals in the absence of memory impairing treatments and conditions. Recently, huperzine A had no effect on attention, motivation and working memory in normal adult monkeys performing visual recognition task with short delays (Myers et al., 2010). The experimental data raise a question whether the effects of huperzine A are limited to conditions in which loss of function has occurred, or whether it is also effective in boosting normal cognitive function.
IDRA 21 is a positive allosteric modulator of glutamate AMPA receptors (Black, 2005; Yamada, 1998). It has been shown to increase excitatory synaptic strength by attenuating rapid desensitization of AMPA receptors. This property enhances long-term potentiation, especially within the hippocampus (Arai et al., 1996; Bertolino et al., 1993) and may thus have beneficial therapeutic effects. IDRA 21 and other AMPA modulators have been proposed as possible agents to ameliorate memory deficits observed in patients with cognitive impairments, including AD. In rats, it improved learning and memory in a spatial task (Uzunov et al., 1995; Zivkovic et al., 1995). Given orally to primates (patas monkeys), it blocked alprazolam-induced learning deficits in a complex behavioral task (Thompson et al., 1995). However, given to normal animals in the same experiment, it had no effect on the rate of learning, presumably because of a ceiling effect (Thompson et al., 1995).
In a more recent experiment in young adult and aged rhesus monkeys (Buccafusco et al., 2004), oral administration of IDRA 21 (dose range 0.15 -10 mg/kg) significantly improved performance on a computer-automated delayed matching-to-sample task. The improvement was especially robust in the young adult monkeys and affected mainly medium and longer delays (up to 180 sec). The performance in aged monkeys, which was significantly lower compared to young animals during baseline, also improved significantly but the effect was less robust.
The present study was designed to compare the effects of huperzine A (Experiment 1) or IDRA 21 (Experiment 2) on performance of normal subjects in the face of increasing mnemonic challenge in a visual recognition memory task with varying degrees of difficulty. By evaluating the effects of the two drugs in normal young adult monkeys we expected to determine if one or both drugs can promote cognitive enhancement in a system that is presumably functioning optimally.
Abstracts of this work were published previously (Flynn et al., 2000; Gale et al., 2001).
2. Material and Methods
2.1. Subjects
Six young pigtail macaques (Macaca nemestrina), four males (UY, ZK, KS, VB) and two females (KI, JV), were used. They were 1.5 – 4 years old and ranged in weight 3.5 – 5.5 kg at the beginning of this study. They were housed individually in rooms with a 12-h light/dark cycle and were maintained on primate chow (No. 5038, PMI Feeds, St. Louis, MO) supplemented with fresh fruit. Water was always available. Before participating in this experiment, all monkeys were subjects in previous behavioral studies. They all had been pre-trained in delayed nonmatching-to-sample (DNMS) with trial-unique objects, a measure of visual recognition memory (see below), and they all maintained a stable baseline performance on this task prior to this study. Their previous experimental history included intracerebral drug infusions intended to target perirhinal cortex in five subjects and the subthalamic nucleus in one subject (UY). All of these previous studies were finished at least one month before the initiation of this experiment. Post-mortem histological evaluation in four cases (KI, ZK, JV, UY) and MRI evaluation in two (KS, VB) showed no consistent damage within the infused brain regions.
All six monkeys were used in Experiment 1 and, at least one month after finishing testing in Experiment 1, four of the monkeys (KI, ZK, KS, UY) were used in Experiment 2. This study was conducted under a protocol approved by the Georgetown University Animal Care and Use Committee and in accordance with the Guide for Care and Use of Laboratory Animals adopted by the National Institutes of Health.
2.2. Apparatus and materials
Training was conducted in a Wisconsin General Testing Apparatus (WGTA) inside a darkened, sound-shielded room. The test tray, which was located at the level of the floor of the monkey's transport cage, contained three food wells spaced 18 cm apart and aligned 16 cm in front of the cage. The test compartment was illuminated with a 60-W incandescent bulb, but the monkey's compartment was always unlit. The two compartments were separated by an opaque vertical screen door, which was raised during stimuli presentations. The stimuli consisted of about 500 different junk objects that varied widely in color, shape, size, and texture. The food rewards were raisins or half peanuts.
2.3. Behavioral Procedure
2.3.1. Visual DNMS task
The monkeys were trained on visual DNMS with trial-unique objects, i.e., each object was used no more than once in an experimental session. In this task, each trial consisted of an acquisition phase, in which an unbaited sample object was presented over the central well. The monkey had to displace the sample object to advance on the trial. The screen door was lowered, blocking the view of the tray during the delay period. After a 10-sec delay, the sample object was presented concurrently with a novel object. In this phase (test), the novel object was baited with a food reward placed in the food well underneath it. The monkey had to displace the object to retrieve the food reward. This completed one trial. Following a 30-sec inter-trial interval, another trial began. The left/right position of the novel object in the test phase of each trial was determined according to a pseudorandom sequence. Testing continued in sessions of 25 trials per day until the animals reached a criterion of 90 correct choices in 100 consecutive trials (i.e. over 4 days). After reaching criterion at the 10 sec delay, the delay between the sample presentation and the choice test was gradually increased from to 30, 60, and 90 sec allowing the monkeys to reach criterion on each delay before introducing the longer delay. Once an animal reached criterion on the longest delay, it was ready to be evaluated on Task 1.
2.3.2. Task 1 - DNMS with mixed delays
All four delays, 10, 30, 60, and 90 sec, were intermixed within one session consisting of 24 trials, each delay was presented 6 times. The order of delays followed a pseudorandom sequence. The animals were given up to five sessions with mixed delays to ensure that they maintained the criterion performance.
2.3.3. Task 2 - DNMS with a list of sample objects
In this more difficult version of the task, the number of sample objects was increased from the original single object to 6 objects. The six sample objects were presented one after another, for 3 sec each, over the central well. In this version of the task, the monkey was not required to displace the object but only to observe the whole sequence of objects. According to a predetermined pseudorandom schedule, two of the six objects were then presented in each of two successive test trials: in each test trial, one sample object was presented concurrently with a novel object. The delay between the first appearance of the sample object and its presentation during the test phase was 15, 30, 60, or 90 sec. In addition, on some of the trials, only the delays of 90 sec were used to ensure that each delay was tested on approximately the same number of trials as first. In the test phase, the monkey was required to displace the novel object in order to receive the food reward. A daily session consisted of 25 trials, each of which employed either two delays out of the possible four or one delay of 90 sec.
2.4. Drug administration
(−)-Huperzine A (synthesized by A. Kozikowski) was given orally 1 hour prior to the behavioral testing at a dose of 100 μg (i.e., 20–30 μg/kg). This pretreatment time was based on previous reports in rats indicating that peak inhibition of AChE in brain is achieved by 30 – 60 minutes after oral administration and is maintained for 6 hours (Cheng and Tang, 1998; Tang et al., 1989). The dose for the monkeys was determined based on the dose given to human patients in clinical studies (i.e., clinical studies in AD patients is 200 or 400 μg twice a day; e.g., Wang et al., 2009). Two tablets (50 μg each) were crushed and mixed either with a small piece of banana or with a drop of honey. The experimenter gave the drug to the monkey in its home cage and waited until the monkey ingested the full dose.
In previous studies, AChE inhibition within the brain, tested in rats and mice, was dose dependent and comparable for various routes of administration, oral (Cheng & Tang, 1998; Wang & Tang, 1998), intraperitoneal (Tang et al., 1994), or intramuscular (i.m.) (Tang et al., 1989) at doses 100–800 μg/kg. In the aged monkeys, the optimal doses that improved performance were 1 and 10 μg/kg i.m., whereas the doses that significantly reversed the effects of scopolamine were 10 to 100 μg/kg (Ye et al., 1999). In normal young monkeys, i.m. doses of 5 – 40 μg/kg resulted in a dose dependent AChE inhibition, ranging from 30 – 75% with a peak 30 minutes after administration (Myers et al., 2010). Thus, our dose of 20 – 30 μg/kg is well within the range of the effective doses in the previous study in monkeys.
One hour after huperzine A administration, the monkey was transferred to the WGTA and given the behavioral task. The test session lasted on average 30 – 45 minutes. One or two baseline sessions alternated with drug sessions, which were separated by at least three days to allow complete clearance of the drug. Three drug sessions were administered using Task 1 and six drug sessions using Task 2.
IDRA 21 (provided by A. Kozikowski) was first suspended in a few drops of Tween 80 (Sigma-Aldrich) and then mixed in a flavored drink Kool-Aid (4mg/ml; Kraft Foods, Inc.). It was given to the monkeys orally via a hand-held syringe at a dose 2.5 mg/kg 1 hour before testing. Three or four drug sessions alternated with baseline sessions. Drug sessions were separated by at least three days in order to allow for complete drug clearance between treatments.
In previous studies, Thompson et al. (1995) used a dose of 3 or 5.6 mg/kg to antagonise the effects of alprazolam in monkeys. In another study in monkeys (Buccafusco et al., 2004), the optimal dose of IDRA 21 for obtaining maximal performance improvement ranged between 0.3 and 10 mg/kg across animals, with an average of 1.9 mg/kg; this dose is comparable to the dose (2.5 mg/kg) used in our study.
3. Experimental Design
3.1. Experiment 1
Two monkeys (JV and VB) were tested on Task 1 only and the remaining 4 monkeys were tested on both Task 1 and 2.
3.1.1. Data analysis
Each monkey's performance was scored as percent correct responses at each delay in each session. At first, scores of the 6 monkeys tested on Task 1 were analyzed by 2 × 3 × 4 ANOVA with treatment (drug, baseline), session (1,2, and 3) and delay (10, 30, 60, and 90 sec) as repeated measures. No significant effect of session or interaction of session with any of the other factors was found (see Results), indicating that performance did not change across sessions. Thus data from the three sessions were combined within treatments and within delays and were further analyzed by 2 × 4 ANOVA with treatment and delay as repeated measures. The scores of the 4 monkeys that were tested on Task 2 were analyzed by 2 × 6 × 4 ANOVA with treatment (drug, baseline), session (1–6) and delay (15, 30, 60, and 90 sec) as repeated measures. Again, no significant effect of session or interaction of session with any of the other factors was found (see Results), indicating that performance did not change across sessions. Thus data from the six sessions were combined within treatments and within delays and were further analyzed by 2 × 4 ANOVA with treatment and delay as repeated measures. Finally, the scores of the 4 monkeys that were tested on both tasks were analyzed by 2 × 2 × 4 ANOVA with treatment (drug, baseline), task (Task 1 versus Task 2) and delay as repeated measures. For the purpose of comparison between tasks, the delay of 10s in Task 1 and 15s in Task 2 were considered equal. Hyunh-Feldt correction for repeated measures was applied when required.
3.2. Experiment 2
Because the monkeys were performing close to the maximum accuracy on Task 1, this task was not used in Experiment 2 to avoid a possible ceiling effect. Four subjects (UY, KI, ZK, KS) from Experiment 1 were tested here on Task 2. A new baseline performance was determined before initiation of the drug administration.
3.2.1. Data analysis
Each monkey's performance was scored as percent correct responses at each delay in each session. The data were collapsed across sessions and analyzed by 2 × 4 ANOVA with treatment (drug, baseline) and delay (15, 30, 60, and 90 sec) as repeated measures.
4. Results
4.1. Experiment 1: huperzine A treatment
4.1.1. Task 1 - DNMS with mixed delays
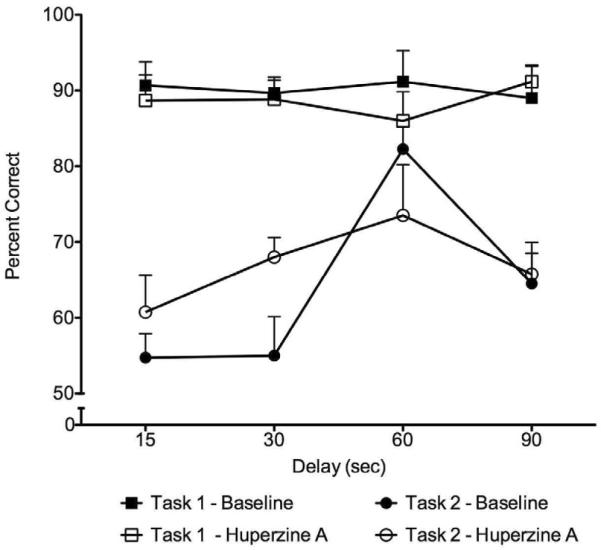
On Task 1, there were no significant effects of session (F=3.35, df=2,10, p=0.10) or interaction between session and treatment (F=2.14, df=2,10, p=0.14), indicating that performance (with or without drug) did not change across sessions. The data for each monkey, combined across the three sessions, are presented in Table 1 and Figure 2. As shown in Table 1, average baseline performance on Task 1 was 90% correct; with huperzine A treatment performance was at 89% correct. There were no significant effects of treatment (F1,5 = 0.24, p = 0.64), delay (F3,15 = 0.13, p = 0.92), or interactions between these two factors (F3,15=0.51, p=0.54), based on ANOVA.
Table 1.
The scores are percent correct responses at each delay interval during baseline and after huperzine A administration on Task 1 and Task 2. X represents mean values averaged either across subjects within a delay or across delays for each subject. Within-subject analysis of variance with repeated measures indicated no significant effects of treatment, delay, or interaction between these two factors for either Task 1 or Task 2 (all ps>0.05).
| TASK1 | BASELINE | HUPERZINE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | 10 s | 30 s | 60 s | 90 s | X | 10 s | 30 s | 60 s | 90 s | X |
| UY | 80 | 94 | 72 | 78 | 81 | 78 | 80 | 94 | 95 | 87 |
| KI | 84 | 84 | 95 | 78 | 85 | 89 | 95 | 95 | 89 | 92 |
| ZK | 100 | 89 | 89 | 100 | 95 | 89 | 95 | 89 | 100 | 93 |
| KS | 95 | 89 | 100 | 94 | 95 | 100 | 95 | 89 | 89 | 93 |
| JV | 96 | 95 | 97 | 100 | 97 | 95 | 81 | 72 | 89 | 84 |
| VB | 89 | 87 | 94 | 84 | 89 | 81 | 87 | 77 | 85 | 83 |
| X | 91 | 90 | 91 | 89 | 90 | 89 | 89 | 86 | 91 | 89 |
| TASK2 | BASELINE | HUPERZINE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | 15 s | 30 s | 60 s | 90 s | X | 15 s | 30 s | 60 s | 90 s | X |
| UY | 64 | 53 | 50 | 52 | 55 | 72 | 65 | 50 | 56 | 61 |
| KI | 50 | 70 | 53 | 47 | 55 | 66 | 70 | 74 | 62 | 68 |
| ZK | 74 | 88 | 84 | 83 | 82 | 56 | 85 | 83 | 70 | 74 |
| KS | 58 | 76 | 60 | 64 | 65 | 73 | 73 | 59 | 58 | 66 |
| X | 62 | 72 | 62 | 62 | 64 | 67 | 73 | 67 | 62 | 67 |
Figure 2.
Percent correct responses on each delay in Task 1 (squares) and Task 2 (circles) during baseline (filled symbols) and after huperzine A administration (open symbols). Within-subject analysis of variance with repeated measures indicated no significant effects of treatment, delay, or interaction between these two factors for either Task 1 or Task 2 (all ps>0.05).
4.1.2. Task 2 - DNMS with a list of sample objects
On Task 2, as in Task 1, there was no significant effect of session (F5,20 =1.40, p=0.30) or interaction between session and treatment (F5,20=0.21, p=0.90). Thus the scores were combined within treatment and within delay across the six sessions and are presented in Table 1 and Figure 2. The average baseline performance on Task 2 was 64%; after huperzine A treatment performance was at 67%. There were no significant effects of treatment (F1,3 = 0.40, p = 0.57), delay (F3,9 = 1.82, p = 0.25), or interaction between these two factors (F3,9 = 0.33, p = 0.81), based on ANOVA.
4.1.3. Comparison across tasks
As expected, the 4 monkeys that were tested on both tasks had significantly lower scores on Task 2 than Task 1 (F1,3 = 72.14, p = 0.003). Using a repeated measures ANOVA, there were no significant effects of treatment (F1,3 = 0.68, p = 0.47) or delay (F3,9 = 1.52, p = 0.28); interactions were also not significant. Thus, no effect of the drug treatment was found when the monkeys performed either an easy task (Task 1) or a more difficult one (Task 2).
4.1.4. Inverse relationship between baseline performance and performance after huperzine A treatment
In Task 1, when the monkeys' baseline performance was close to 90% correct, four out of six subjects achieved lower scores than baseline when they were pretreated with huperzine A. In contrast, on Task 2, when the monkeys' performance was below 70% correct, three out of four subjects Task 2 achieved higher scores when pretreated with huperzine A. We further analyzed the relationship between the individual baseline scores and the effect of the drug across animals in both tasks. For each monkey, the performance on each delay under baseline was compared with its performance under the drug treatment and the difference between the two performance scores was expressed as a difference score, with a positive value for increases and a negative value for decreases. A significant negative correlation was found between the baseline performance and the difference score (Pearson r = −0.32, p = 0.033), indicating an inverse relationship. This result suggested that under conditions in which the subjects were performing poorly (55 – 69%), the drug resulted in improved performance, whereas no improvement was obtained when the baseline was close to 90%. In fact, when the subjects were performing very well, huperzine A tended to reduce the performance accuracy.
4.2. Experiment 2: IDRA 21 treatment
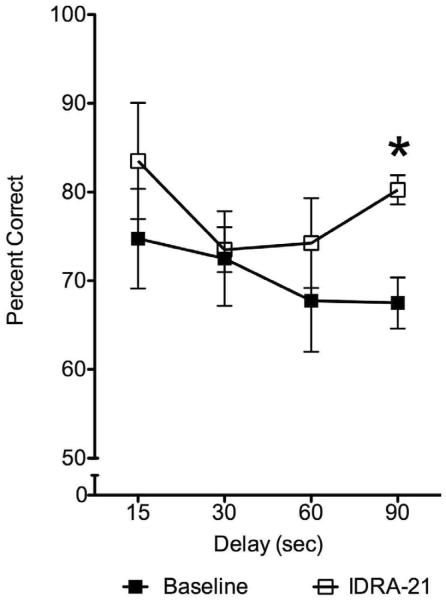
The scores combined across sessions within treatment and within delay are presented in Table 2 and Figure 3. ANOVA showed a significant effect of drug (F=72.11, df= 1, p<0.003) but no significant effect of delay (F=2.94, df=3, p=0.09) or interaction between these two factors (F=0.92, df=3, p=0.47). Although the drug treatment improved performance on each delay, the largest improvement (from 68 to 80%) was on the 90s delay (paired t-test, p<0.002). The average performance across subjects increased from 71% to 78%. Thus, in normal young subjects pretreatment with IDRA 21 significantly improved memory on a difficult version of a recognition memory task. These results demonstrated that the memory enhancing action of IDRA 21 is not limited to models of drug-induced memory impairments but is also manifest in normal subjects.
Table 2.
The scores are percent correct responses at each delay interval during baseline and after IDRA 21 administration on Task 2. X represents mean values averaged either across subjects within a delay or across delays for each subject. Within-subject analysis of variance with repeated measures resulted in a significant effect of drug (p<0.003) but no significant effect of delay, or interaction between drug and delay. Although the drug treatment improved performance on each delay, the largest improvement was observed on the 90s delay (paired t-test, p<0.002).
| BASELINE | IDRA 21 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | 15 s | 30 s | 60 s | 90 s | X | 15 s | 30 s | 60 s | 90 s | X |
| UY | 74 | 63 | 64 | 64 | 66 | 79 | 72 | 66 | 78 | 74 |
| KI | 63 | 65 | 54 | 66 | 62 | 67 | 71 | 71 | 78 | 72 |
| ZK | 90 | 86 | 72 | 76 | 81 | 93 | 81 | 89 | 85 | 87 |
| KS | 72 | 76 | 81 | 64 | 73 | 95 | 70 | 71 | 80 | 79 |
| X | 75 | 73 | 68 | 68 | 71 | 84 | 73 | 74 | 80 | 78 |
Figure 3.
Percent correct responses on each delay in Task 2 during baseline (filled symbols) and after IDRA 21 administration (open symbols). Within-subject analysis of variance with repeated measures resulted in a significant effect of drug (p<0.003) but no significant effect of delay, or interaction between drug and delay. IDRA 21 treatment improved performance on each delay with the largest improvement observed on the 90s delay (paired t-test, p<0.002); * denotes significant difference between baseline and IDRA 21 treatment in this delay.
5. Discussion
We found that, overall, huperzine A did not significantly affect the performance of normal young adult monkeys on a visual recognition memory task. This lack of effect was independent of task difficulty. However, across both task conditions, a significant inverse relationship was detected between the subjects' baseline performance and the effect of the drug: huperzine A adversely affected performance when the baseline was at relatively high accuracy (90% or better), while it enhanced performance when the baseline was at relatively low accuracy (70% or less).
Our results suggest that huperzine A may exert a nootropic action in normal animals only under conditions where performance is close to chance, and that this action disappears as performance improves. The improvement of performance under conditions when the baseline accuracy is low may be due to enhancement of attention and/or motivation in the face of low rates of reinforcement. The fact that the drug treatment tended to impair performance when baseline accuracy was high, suggests that the drug may exert multiple actions, some of which are deleterious to optimal performance in normal animals. This result is consistent with previous findings showing that whereas cholinergic agonists may improve learning in subjects with deficient brain acetylcholine system, there may be only a little gain from a cholinergic therapy in normal subjects. Cholinergic drugs typically improve learning and memory in normal subjects only within a limited dose range and doses above the optimum may either eliminate facilitation (e.g. Ogura and Aigner, 1993; Sweeney et al., 1990) or even result in an impairment (Dumery et al., 1988; Ennaceur and Melliani, 1992; Miyamoto et al., 1988). Similarly, an inverted U-shaped dose-response curve was found following the treatment with AChE inhibitors in Alzheimer's patients (e.g. Braida and Sala, 2001; Chatterjee et al., 1993) or lesioned animals (Sweeney et al., 1990). The effect of huperzine A on memory impairments in monkeys treated with reserpine or yohimbine also exhibited an inverted U-shaped dose-response curves (Ou et al, 2001).
Our data are consistent with a recent study in young adult monkeys performing at about 85% accuracy in a visual recognition memory task with short delays (Myers et al., 2010). This study reported that huperzine A did not affect attention, motivation or working memory in a task that resembled our Task 2, except for the fact that it was limited to shorter delays. The effect of huperzine A on low accuracy performance may explain the beneficial effects of this drug in aged monkeys, as reported by Ye et al. (1999). The monkeys were tested on a visuospatial delayed response task with delays that were selected for each monkey to generate a baseline of 67% accuracy (Ye et al., 1999). Under these conditions, huperzine A, in doses of 1 and 10 μg/kg i.m., improved performance. Because the accuracy level of the baseline performance was comparable to that observed in most subjects on Task 2, it is likely that the mechanism by which huperzine A enhanced performance of poorly performing subjects may be similar for both the young and aged animals.
We included IDRA 21 in our study as a comparison with huperzine A because it has been previously shown that IDRA 21 enhances learning and memory in normal animals, both in rodent and primate studies. Moreover, this compound has been shown to be considerably more potent than the well-known nootropic agent aniracetam, in reversing scopolamine-induced cognitive deficits. The results we obtained were consistent with these previous findings and they contrasted with the relative ineffectiveness of huperzine A. In Experiment 2 we found that IDRA 21 significantly improved performance across delays, with more pronounced improvement on the longest delay. These data are consistent with the results reported by Buccafusco et al. (2004) who obtained improvement in performance on a visual recognition task. Our results extend this previous finding by showing an improvement not only with long delays but also with an increased memory load (6 objects). In the Buccafusco et al. study, the optimal dose of IDRA 21 ranged between 0.3 and 10 mg/kg, with an average of 1.9 mg/kg; this dose is comparable to the doses (2.5 mg/kg) used in our study. In view of the fact that some animals in the Buccafusco et al. study required higher doses to achieve an optimal effect, it is possible that the relatively low dose used in our study may have underestimated the full potential of the IDRA-21 effect.
IDRA 21, a member of the class of drugs known as ampakines, positively modulates AMPA receptors. It increases excitatory synaptic strength by attenuating rapid desensitization of AMPA receptors and by this action it enhances long-term potentiation. Our findings are consistent with observations using several ampakines other than IDRA 21, some of which enhance AMPA receptor-mediated function via different mechanisms. These ampakines have been shown to augment learning and memory in a variety of tasks in both animals and humans (Arai and Kessler, 2007; Ingvar et al., 1997; Lynch, 2004; O'Neill et al., 2004). These ampakines, which belong to several different chemical classes (O'Neill et al., 2004), enhance AMPA receptor currents by attenuating desensitization and/or slowing deactivation of the receptors (Arai and Kessler, 2007).
The contrast between the effects of huperzine A and IDRA 21 may reflect the distinct manner with which these drugs alter synaptic transmission. Blockade of AChE activity, as accomplished by huperzine A, represents an intervention targeted at the sole mechanism by which ACh transmission is actively terminated in the synapse. In contrast, the attenuation of desensitization of AMPA receptors, as accomplished by IDRA 21, represents an intervention, which modulates the response of a subset of AMPA receptors in an activity-dependent fashion. Thus, the latter intervention is less likely to override normal homeostatic control mechanisms allowing for an amplification of normal function without deleterious effects.
The lack of a clear effect of huperzine A to improve cognitive function in normal young monkeys in our study is consistent with previous findings in animals from other laboratories. These findings are in contrast with the positive effect of huperzine A in patients with cognitive impairment and aged animals suggesting that huperzine A, as other AChE inhibitors, exerts its function in a system which does not function normally, because it has been compromised by the disease of aging, or by the experimental depletion of acetylcholine. Thus, huperzine A can be used to restore or ameliorate a compromised system back to a normal level of functioning, however, it may not be effective in enhancing cognitive function beyond normal levels. In contrast, IDRA 21 has shown a consistent effect in improving performance on memory tasks in animals, even when the animals were not compromised by drugs, lesions, or age. Along with similar results using other ampakines, this suggests that this class of compounds is especially promising for augmenting memory beyond the normal level of functioning. In fact, the observation that IDRA 21 improved the performance of normal young animals more than that of aged subjects (Buccafusco et al., 2004) suggests that this type of cognitive enhancement may be best suited for use in normal subjects.
Huperzine A did not significantly affect performance of young macaques on visual recognition task.
Negative correlation was found between baseline and performance under huperzine A.
Increased availability of acetylcholine did not improve performance in optimally working system.
IDRA 21 improved performance on long delays of the task with high mnemonic demand.
Potential use of this drug in treatment of cognitive and memory disorders supported.
Acknowledgements
This study was supported by NIH/AG14580. The authors would like to thank Kenita Barrow, Laura Williams, Cristina Giampaolo, and Anthony Flynn for help with behavioral training and testing of the monkeys and Marco Pieroni for providing material for Figure 1 and valuable comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aracava Y, Pereira EF, Akkerman M, Adler M, Albuquerque EX. Effectiveness of donepezil, rivastigmine, and (+/−)huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J. Pharmacol. Exp. Ther. 2009;331:1014–1024. doi: 10.1124/jpet.109.160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Guidotti A, Costa E, Lynch G. Effect of the AMPA receptor modulator IDRA 21 on LTP in hippocampal slices. Neuroreport. 1996;7:2211–2215. doi: 10.1097/00001756-199609020-00031. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: From AMPA receptors to synapses and behavior. Current Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Vicini S, Costa E. Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus. Receptors Channels. 1993;1:267–278. [PubMed] [Google Scholar]
- Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179:154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- Braida D, Sala M. Eptastigmine: ten years of pharmacology, toxicology, pharmacokinetic, and clinical studies. 2001;7:369–386. doi: 10.1111/j.1527-3458.2001.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV. The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology. 2004;46:10–22. doi: 10.1016/j.neuropharm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Morris MK, Bowers D, Williamson DJ, Doty L, Heilman KM. Cholinergic treatment of an amnestic man with a basal forebrain lesion: theoretical implications. J. Neurol. Neurosurg. Psychiatry. 1993;56:1282–1289. doi: 10.1136/jnnp.56.12.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DH, Tang XC. Comparative studies of huperzine A, E2020, and tacrine on behavior and cholinesterase activities. Pharmacol. Biochem. Behav. 1998;60:377–386. doi: 10.1016/s0091-3057(97)00601-1. [DOI] [PubMed] [Google Scholar]
- Dumery V, Derer P, Blozovski D. Enhancement of passive avoidance learning through small doses of intra-amygdaloid physostigmine in the young rat. Its relation to the development of acetylcholinesterase. Dev Psychobiol. 1988;21:553–65. doi: 10.1002/dev.420210606. [DOI] [PubMed] [Google Scholar]
- Eckert S, Eyer P, Worek F. Reversible inhibition of acetylcholinesterase by carbamates or huperzine A increases residual activity of the enzyme upon soman challenge. Toxicology. 2007;233:180–186. doi: 10.1016/j.tox.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- Flynn AM, Malkova L, Giampaolo CM, Williams LD, Kozikowski AP, Gale K. Program No 75212 Neuroscience Meeting Planner. Society for Neuroscience; New Orleans, LA: 2000. Effects of huperzine on recognition memory in monkeys. Online. [Google Scholar]
- Gale K, Barrow KV, Kozikowski AP, Malkova L. Program No 9511 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2001. IDRA 21 improves recognition memory in normal monkeys. Online. [Google Scholar]
- Gao Y, Tang XC, Guan LC, Kuang PZ. Huperzine A reverses scopolamine-and muscimol-induced memory deficits in chick. Acta Pharmacol. Sin. 2000;21:1169–1173. [PubMed] [Google Scholar]
- Geib SJ, Tuckmantel W, Kozikowski AP. Huperzine A--a potent acetylcholinesterase inhibitor of use in the treatment of Alzheimer's disease. Acta Crystallogr. C. 1991;47(Pt 4):824–827. doi: 10.1107/s0108270190008952. [DOI] [PubMed] [Google Scholar]
- Gordon RK, Nigam SV, Weitz JA, Dave JR, Doctor BP, Ved HS. The NMDA receptor ion channel: a site for binding of Huperzine A. J. Appl. Toxicol. 2001;21(Suppl. 1):S47–51. doi: 10.1002/jat.805. [DOI] [PubMed] [Google Scholar]
- Gordon RK, Haigh JR, Garcia GE, Feaster SR, Riel MA, Lenz DE, Aisen PS, Doctor BP. Oral administration of pyridostigmine bromide and huperzine A protects human whole blood cholinesterases from ex vivo exposure to soman. Chem. Biol. Interact. 2005;157–158:239–246. doi: 10.1016/j.cbi.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Haigh JR, Johnston SR, Peppernay A, Mattern PJ, Garcia GE, Doctor BP, Gordon RK, Aisen PS. Protection of red blood cell acetylcholinesterase by oral huperzine A against ex vivo soman exposure: next generation prophylaxis and sequestering of acetylcholinesterase over butyrylcholinesterase. Chem. Biol. Interact. 2008;175:380–386. doi: 10.1016/j.cbi.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an Ampakine of Memory Encoding in Humans. Experiment. Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Miller CP, Yamada F, Pang YP, Miller JH, McKinney M, Ball RG. Delineating the pharmacophoric elements of huperzine A: importance of the unsaturated three-carbon bridge to its AChE inhibitory activity. J. Med. Chem. 1991;34:3399–3402. doi: 10.1021/jm00116a010. [DOI] [PubMed] [Google Scholar]
- Lallement G, Baille V, Baubichon D, Carpentier P, Collombet JM, Filliat P, Foquin A, Four E, Masqueliez C, Testylier G, Tonduli L, Dorandeu F. Review of the value of huperzine as pretreatment of organophosphate poisoning. Neurotoxicol. 2002a;23:1–5. doi: 10.1016/s0161-813x(02)00015-3. [DOI] [PubMed] [Google Scholar]
- Lallement G, Demoncheaux JP, Foquin A, Baubichon D, Galonnier M, Clarencon D, Dorandeu F. Subchronic administration of pyridostigmine or huperzine to primates: compared efficacy against soman toxicity. Drug. Chem. Toxicol. 2002b;25:309–320. doi: 10.1081/dct-120005893. [DOI] [PubMed] [Google Scholar]
- Lallement G, Veyret J, Masqueliez C, Aubriot S, Burckhart MF, Baubichon D. Efficacy of huperzine in preventing soman-induced seizures, neuropathological changes and lethality. Fundam. Clin. Pharmacol. 1997;11:387–394. doi: 10.1111/j.1472-8206.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Tang XC. Comparative studies of huperzine A, donepezil, and rivastigmine on brain acetylcholine, dopamine, norepinephrine, and 5-hydroxytryptamine levels in freely-moving rats. Acta Pharmacol. Sin. 2006;27:1127–1136. doi: 10.1111/j.1745-7254.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Huang XT, XC T. Huperzine A reverses cholinergic and monoaminergic dysfunction induced by bilateral nucleus basalis magnocellularis injection of beta-amyloid peptide (1–40) in rats. Cell. Mol. Neurobiol. 2008;28:87–101. doi: 10.1007/s10571-007-9158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. AMPA receptor modulators as cognitive enhancers. Curr. Opin. Pharm. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Myers TM, Sun W, Saxena A, Doctor BP, Bonvillain AJ, Clark MG. Systemic administration of the potential countermeasure huperzine reversibly inhibits central and peripheral acetylcholinesterase activity without adverse cognitive-behavioral effects. Pharmacol. Biochem. Behav. 2010;94:477–481. doi: 10.1016/j.pbb.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Narumi S, Nagaoka A, Coyle JT. Effects of continuous infusion of cholinergic drugs on memory impairment in rats with basal forebrain lesions. J Pharmacol Exp Ther. 1989;248:825–835. [PubMed] [Google Scholar]
- O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA Receptor Potentiators for the Treatment of CNS Disorders. Current Drug Targets - CNS & Neurological Disorders. 2004;3:181–194. 181. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Ogura H, Aigner TG. MK-801 impairs recognition memory in rhesus monkeys: comparison with cholinergic drugs. J Pharmacol Exp Ther. 1993 Jul;266(1):60–4. [PubMed] [Google Scholar]
- Ou LY, Tang XC, Cai JX. Effect of huperzine A on working memory in reserpine- or yohimbine-treated monkeys. Eur. J. Pharmacol. 2001;433:151–156. doi: 10.1016/s0014-2999(01)01500-x. [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Bachman ES, Coyle JT. Effects of different doses of galanthamine, a long-acting acetylcholinesterase inhibitor, on memory in mice. Psychopharmacol. (Berl) 1990;102:191–200. doi: 10.1007/BF02245921. [DOI] [PubMed] [Google Scholar]
- Tang XC, De Sarno P, Sugaya K, Giacobini E. Effect of huperzine A, a new cholinesterase inhibitor, on the central cholinergic system of the rat. J. Neurosci. Res. 1989;24:276–285. doi: 10.1002/jnr.490240220. [DOI] [PubMed] [Google Scholar]
- Tang XC, Kindel GH, Kozikowski AP, Hanin I. Comparison of the effects of natural and synthetic huperzine-A on rat brain cholinergic function in vitro and in vivo. J. Ethnopharmacol. 1994;44:147–155. doi: 10.1016/0378-8741(94)01182-6. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Guidotti A, DiBella M, Costa E. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proc. Natl. Acad. Sci. U S A. 1995;92:7667–7671. doi: 10.1073/pnas.92.17.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonduli LS, Testylier G, Masqueliez C, Lallement G, Monmaur P. Effects of Huperzine used as pre-treatment against soman-induced seizures. Neurotoxicol. 2001;22:29–37. doi: 10.1016/s0161-813x(00)00015-2. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Zivkovich I, Pirkle WH, Costa E, Guidotti A. Enantiomeric resolution with a new chiral stationary phase of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide, a cognition-enhancing benzothiadiazine derivative. J. Pharm. Sci. 1995;84:937–42. doi: 10.1002/jps.2600840807. [DOI] [PubMed] [Google Scholar]
- Ved HS, Koenig ML, Dave JR, Doctor BP. Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. Neuroreport. 1997;8:963–968. doi: 10.1097/00001756-199703030-00029. [DOI] [PubMed] [Google Scholar]
- Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis. J. Neural. Transm. 2009;116:457–465. doi: 10.1007/s00702-009-0189-x. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang T, Ma H, Liu J, Fu F, Liu K. Prolonged effects of poly(lactic-co-glycolic acid) microsphere-containing huperzine A on mouse memory dysfunction induced by scopolamine. Basic Clin. Pharmacol. Toxicol. 2007;100:190–195. doi: 10.1111/j.1742-7843.2007.00041.x. [DOI] [PubMed] [Google Scholar]
- Wang LS, Zhou J, Shao XM, Tang XC. Huperzine A attenuates cognitive deficits and brain injury in neonatal rats after hypoxia-ischemia. Brain Res. 2002;949:162–170. doi: 10.1016/s0006-8993(02)02977-3. [DOI] [PubMed] [Google Scholar]
- Wang R, Tang XC. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer's disease. Neurosignals. 2005;14:71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang HY, Tang XC. Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by beta-amyloid protein-(1–40) in rat. Eur. J. Pharmacol. 2001;421:149–156. doi: 10.1016/s0014-2999(01)01030-5. [DOI] [PubMed] [Google Scholar]
- Wang T, Tang XC. Reversal of scopolamine-induced deficits in radial maze performance by (−)-huperzine A: comparison with E2020 and tacrine. Eur. J. Pharmacol. 1998;349:137–142. doi: 10.1016/s0014-2999(98)00199-x. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Wang J, Zhang HY, Tang XC. Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J. Neurochem. 2008;106:1594–1603. doi: 10.1111/j.1471-4159.2008.05504.x. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Tang LL, Yan H, Wang YJ, Tang XC. Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol. Biochem. Behav. 2006;83:603–611. doi: 10.1016/j.pbb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Cheng DH, Tang XC. Effects of huperzine A on nucleus basalis magnocellularis lesion-induced spatial working memory deficit. Zhongguo Yao Li Xue Bao. 1998;19:128–132. [PubMed] [Google Scholar]
- Xu SS, Gao ZX, Weng Z, Du ZM, Xu WA, Yang JS, Zhang ML, Tong ZH, Fang YS, Chai XS, et al. Efficacy of tablet huperzine-A on memory, cognition, and behavior in Alzheimer's disease. Zhongguo Yao Li Xue Bao. 1995;16:391–395. [PubMed] [Google Scholar]
- Xu SS, Cai ZY, Qu ZW, Yang RM, Cai YL, Wang GQ, Su XQ, Zhong XS, Cheng RY, Xu WA, Li JX, Feng B. Huperzine-A in capsules and tablets for treating patients with Alzheimer disease. Zhongguo Yao Li Xue Bao. 1999;20:486–490. [PubMed] [Google Scholar]
- Yamada KA. Modulating excitatory neurotransmission: potential treatment for neurological disease. Neurobiol. Dis. 1998;5:67–80. doi: 10.1006/nbdi.1998.0190. [DOI] [PubMed] [Google Scholar]
- Ye JW, Cai JX, Wang LM, Tang XC. Improving effects of huperzine A on spatial working memory in aged monkeys and young adult monkeys with experimental cognitive impairment. J. Pharmacol. Exp. Ther. 1999;288:814–819. [PubMed] [Google Scholar]
- Ye JW, Shang YZ, Wang ZM, Tang XC. Huperzine A ameliorates the impaired memory of aged rat in the Morris water maze performance. Acta Pharmacol. Sin. 2000;21:65–69. [PubMed] [Google Scholar]
- Zangara A. The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease. Pharmacol. Biochem. Behav. 2003;75:675–686. doi: 10.1016/s0091-3057(03)00111-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang X, Chen Q, Shu L, Wang J, Shan G. Clinical efficacy and safety of huperzine Alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Zhonghua Yi Xue Za Zhi. 2002;82:941–944. [PubMed] [Google Scholar]
- Zhou J, Zhang HY, Tang XC. Huperzine A attenuates cognitive deficits and hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci. Lett. 2001;313:137–140. doi: 10.1016/s0304-3940(01)02265-0. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Giacobini E. Second generation cholinesterase inhibitors: effect of (L)-huperzine-A on cortical biogenic amines. J. Neurosci. Res. 1995;41:828–835. doi: 10.1002/jnr.490410613. [DOI] [PubMed] [Google Scholar]
- Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. J. Pharmacol. Exp. Ther. 1995;272:300–309. [PubMed] [Google Scholar]