Abstract
Background
Women have approximately twice the risk of major depressive disorder (MDD) than men, yet this difference remains largely unexplained. Previous MDD research suggests high rates of endocrine dysfunction, which may be related to deficits in brain activity in stress response circuitry [hypothalamus, amygdala, hippocampus, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC)]. This functional magnetic resonance imaging (fMRI) study investigated the relationship between hypothalamic-pituitary-gonadal (HPG)-axis hormones and stress response circuitry dysfunction in MDD in women.
Methods
During the late follicular/midcycle phase of the menstrual cycle, female participants (10 with extensive histories of MDD, in remission, 10 healthy controls) were scanned while viewing negative and neutral arousal pictures. Group differences in blood oxygen-level dependent (BOLD) signal changes were analyzed using SPM2. Baseline gonadal hormones included estradiol, progesterone, and testosterone.
Results
fMRI results showed greater BOLD signal intensity changes in controls versus MDD in hypothalamus, amygdala, hippocampus, OFC, ACC, and subgenual ACC, findings unrelated to medication status. MDD women had a lower serum estradiol and higher serum progesterone compared to controls. Hypoactivations in hypothalamus, subgenual ACC, amygdala and OFC in MDD were associated with low estradiol and high progesterone.
Limitations
Generalizability of our findings is limited by small sample size and restriction to females, although this did not affect the internal validity of the results.
Conclusions
Hypoactivation of the stress response circuitry in MDD women is associated with dysregulation of the HPG-axis. Associations between brain activity deficits and hormonal disruption in MDD may ultimately contribute to understanding sex differences in MDD.
Keywords: Depression, stress, hormones, fMRI, HPG, women’s mental health, mood, HPA
INTRODUCTION
Major Depressive Disorder (MDD) is the fourth leading cause of morbidity and mortality worldwide (Murray and Lopez, 1997), and will become the leading cause of disease burden by 2030 (World Health Organization, 2009). Significant sex differences exist in MDD, with women demonstrating twice the prevalence than men (Kendler et al., 2006; Kessler et al., 2003). Despite the history of identification of sex differences in the prevalence of MDD, the neurobiological mechanisms to explain these differences remain unclear. One avenue of investigation to explain sex differences focuses on the disruption of hypothalamic-pituitary-adrenal and gonadal (HPA-HPG)-axes. HPG deficits in women with MDD include higher testosterone (Baischer et al., 1995), lower estrogen (Young et al., 2000) and higher progesterone (Hardoy et al., 2006) levels.
A substantial number of brain regions are shared between the HPG and HPA axes, stress response circuitry, and brain regions implicated in MDD. Our previous work demonstrated activation of the stress-response circuitry in healthy females that was modulated by gonadal hormones at different points in the menstrual cycle (Goldstein et al., 2005), and contributed to explaining sex differences in stress response circuitry in the brain in healthy individuals (Goldstein et al., 2010). The current study focuses on females with extensive histories of MDD, but currently in remission, to identify trait abnormalities in brain regions implicated in MDD, and whether gonadal hormonal disruptions are associated with brain activity deficits.
Functional neuroimaging has helped define the network of brain regions implicated in MDD. Individuals with MDD displayed reduced activation to paradigms using positive or rewarding stimuli in the hypothalamus (Yang et al., 2008), ventral striatum (Epstein et al., 2006; Forbes et al., 2009; Osuch et al., 2009; Pizzagalli et al., 2009; Smoski et al., 2009; Surguladze et al., 2005), parahippocampal gyrus (PHG) (Epstein et al., 2006; Yang et al., 2008), ACC (Lee et al., 2007; Yang et al., 2008), OFC (Osuch et al., 2009), insula (Lee et al., 2007), and dorsomedial prefrontal cortex (Epstein et al., 2006). In response to negatively-valenced stimuli, MDD patients compared to healthy controls showed increased activation in amygdala, caudate, ACC, OFC, middle and superior frontal gyri (Beauregard et al., 1998; Fahim et al., 2004; Irwin et al., 2004; Mitterschiffthaler et al., 2003; Sheline et al., 2001). In brief, the majority of functional imaging studies of MDD showed abnormally increased activity in response to negative emotional stimuli in the amygdala and ACC, and decreased activity in hippocampus, mPFC, and DLPFC in response to positive stimuli, in subjects who were acutely ill. In the few studies that consisted of subjects with MDD in remission, compared to healthy controls, they demonstrated hypoactivity in DLPFC and ACC in response to negative emotional and cognitive paradigms (Hooley et al., 2009; Hooley et al., 2005; Okada et al., 2009). Previous findings suggest that MDD is marked by dysfunction of prefrontal cortex that impairs the modulation of the amygdala (Drevets, 1999) in response to positive and negative stimuli.
The majority of investigations on MDD have focused on individuals with acute MDD, not those in full remission, limiting the ability to assess whether inconsistency in findings may be due to the clinical state versus trait characteristics of the patients. Further, although most studies include a majority of women, there is no separation of whether brain activity deficits are present in women only, men, or both. The current study tested the hypothesis that women with MDD in remission would show hypoactivity deficits in stress response circuitry in the brain which would be significantly associated with gonadal hormone (HPG-axis) dysregulation.
METHODS
Subjects
A community-based sample of 10 women with an extensive history of MDD, in remission, and 10 healthy control women participated in this study. Healthy controls (HC) were made comparable on age, ethnicity, handedness, and verbal IQ. Exclusion criteria included: history of irregular menstrual cycle, neurologic disease, CNS damage, endocrine disorders, heart disease, or alcohol-related diseases; mental retardation; other medical illnesses that may significantly alter CNS function; current use of oral contraceptives or systemic, topical or inhaled steroids; and women who may have been pregnant.
Diagnostic information (for MDD and HC women) was systematically obtained by detailed structured clinical interview [Structured Clinical Interview for Diagnoses (SCID) DSM-IV version (First et al., 1996)] administered by a skilled Masters-level clinician. All diagnostic information was reviewed by a senior diagnostic expert (JMG) and final consensus diagnoses were assigned by JMG and consensus with another senior diagnostician. MDD subjects had a diagnosis of MDD, defined as either multiple major episodes (in 6 subjects) or single major episodes lasting several months to 2 years (in 4 subjects). Full remission was defined as the complete absence of any clinically significant DSM-IV MDD symptoms for the past one month; 7 of 10 MDD women met criteria for sustained full remission for the past 1 to 5 years. Comorbid diagnoses in the MDD group included alcohol dependence, in full remission for >3 years (1 subject) and generalized anxiety disorder, current (1 subject). Four MDD women were taking selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs): citalopram (1), sertraline (1), venlafaxine (2). HC women were excluded if they had a history of any DSM-IV axis I disorder or were taking any psychotropic medications.
Procedures
Approval for the following procedures was obtained through the Partners Human Research Committee. Subjects were recruited from the general community through flyers and online classified search engines. After diagnostic eligibility was determined, subjects tracked their cycles for three months and came to the imaging site during the late follicular/midcycle menstrual phase (days 10–15). Groups did not differ on mean cycle day (t=0.15; n.s.). Study procedures were explained, and written informed consent was obtained. MDD women completed the 17-item Hamilton Rating Scale for Depression [HAM-D (Hamilton, 1960)] with a trained study staff member to assess current mood state. All subjects scored <8, indicating they were not in the clinically symptomatic range. Subjects then underwent a baseline fasting blood draw followed by administration of a small standardized breakfast.
Neuroendocrine evaluation
Baseline blood samples were acquired fasting around 7:45 a.m. Two tubes of 7cc each were allowed to clot for 45–60 minutes. Blood was spun to separate serum from blood cells and stored frozen at −80° C in plastic tubes at the BWH Center for Clinical Investigation (CCI) Laboratory (Harvard Catalyst). Serum gonadal steroid hormones (estradiol, progesterone, and testosterone) were analyzed in duplicate with commercial radioimmunoassay (RIA) kits [estradiol (sensitivity 0.018 nmol/L, intra-assay variation 3.3%) and progesterone (sensitivity 0.095 nmol/L, intra-assay variation 1.4%): Ecelsys analyzer, Roche Diagnostics, Indianapolis, IN; testosterone (sensitivity 0.350 nmol/L, intra-assay variation 5.2%): Access Immunoassay System, Beckman Coulter, Miami, FL)] at the BWH Harvard Catalyst laboratory.
Prior to and after each scan, participants were administered the Profile of Mood States (POMS) to rate current mood, and the Spielberger State-Trait Anxiety Inventory (STAI) to rate current and “usual” or trait anxiety. In addition, the WAIS-III (Wechsler, 1997) vocabulary subtest was administered to obtain an approximate verbal IQ level.
fMRI procedures
In the MRI suite, participants were fitted with earplugs and lay prone on the scanner gurney. Foam padding was placed at the sides of the head to prevent head motion and the participant was given a response box with two buttons for the right hand. A Dell Latitude D820 Computer (Dell, Inc., Round Rock, TX) running Presentation experimental presentation program (Neurobehavioral Systems, Albany, CA) was used to produce the visual stimuli. Visual stimuli were presented via either a limited-view goggle system (Resonance Technology, Northridge, CA) or an LCD projector through a custom lens onto a screen situated in the magnet bore and viewed through a mirror attached to the head coil.
fMRI parameters
Scanning was performed with a quadrature full head coil and a 3T General Electric Signa MR scanner. Three functional scans, using a spin echo, T2*-weighted sequence (TR = 2000 ms; TE = 30 ms; FOV = 220×220 mm; matrix = 64×64; in-plane resolution = 3.44 mm; slice thickness = 5 mm; 31 contiguous slices aligned at 45° oblique to the AC-PC plane), were acquired for 185 time points per experimental run. Finally, a high-resolution 166-slice sagittal scan (T1-weighted MP-RAGE inversion recovery gradient echo sequence; TR = 6.6 ms; TE = 2.8 ms; FOV = 256×256 mm; matrix = 256×256; in-plane resolution = 1 mm; slice thickness = 1.2 mm) was acquired.
fMRI stress response task paradigm
Stimulus materials were drawn from the International Affective Picture System (IAPS) (CSEA-NIMH, 1999). Pictures were drawn from the set according to affective valence (unpleasant, neutral) and arousal (high, low) based on normative ratings developed by Lang et al., 1993 (Lang et al., 1993) and adapted to the fMRI environment by our group (Goldstein et al., 2010; Goldstein et al., 2005). Two sets of pictures, each containing 72 images, were selected – one of unpleasant valence/high arousal and the other of neutral valence/low arousal. To create the 72 fixation slides, the neutral valence/low arousal slides were transformed using Fourier transforms to create a set of slides with the same physical properties of the original but without content that was readily recognizable. Validity of this task for activating the stress response circuitry was demonstrated previously (Goldstein et al., 2010; Goldstein et al., 2005). During the functional scans, participants were presented with three blocks of stimuli in a counterbalanced method. One block of stimuli consisted of six fixation images presented at the rate of one every five seconds for 30 seconds. The other blocks consisted of either six negative affect images or six neutral images, with blocks repeated four times during each six-minute functional scan. In all conditions, the participant was asked to press a button each time the picture being presented changed in order to ensure attention to the presented stimuli.
fMRI Data Analysis
fMRI data were preprocessed using Statistical Parametric Mapping (SPM2) (Wellcome Department of Cognitive Neurology, 2002) and using custom routines in MATLAB (Mathworks, Inc., 2000). Preprocessing commenced with correction for bulk-head motion. No individual runs exhibited head motion greater than 3.3mm across all runs. Images for each subject were spatially normalized using nonlinear volume-based spatial normalization techniques within SPM. The template used by SPM is the standard brain template developed at the Montreal Neurological Institute (MNI). Images were then spatial smoothed with a Gaussian filter (6mm at FWHM). Finally, well-established artifact detection tools (http://web.mit.edu.ezp-prod1.hul.harvard.edu/swg/software.htm) were used to identify and exclude outliers in the global mean image time series and movement parameters. Following preprocessing, statistical analysis was performed at the single-subject level using SPM. SPM treats each voxel’s BOLD time series according to a general linear model. Each epoch of trials was modeled using a boxcar function convolved with a canonical hemodynamic response function. Specific comparisons of interest (negative versus neutral) were tested using linear contrasts, and SPM maps were created based on these contrasts. These contrast values (estimates of the mean signal change at each voxel) were used in statistical analyses.
Voxel-wise analyses
Results from the individual subject level were submitted to a second level analysis in which subjects were treated as a random effect. Independent sample t-tests were used to compare the size of a particular effect between groups. Given our hypotheses about specific brain regions, we used an approach in SPM2 which limits voxel-wise analyses to voxels within our a priori ROIs. Anatomically-defined regions of interest included the hypothalamus, amygdala, hippocampus, OFC, ACC, and subgenual ACC (sgACC). False positives were controlled using a voxel-wise height threshold (p<0.05 uncorrected) and an extent threshold that jointly resulted in a cluster-level false-positive level of p<0.05, corrected for multiple comparisons within the search volume using family-wise error (FWE) correction. Anatomic borders of hypothesized regions were defined using a manually segmented MNI-152 brain. These borders were then implemented as overlays on the SPM2 canonical brain using the Wake Forest University (WFU) PickAtlas (Maldjian et al., 2003) toolbox for SPM.
Anatomical-ROI analyses
After identifying clusters within the ROIs, these anatomic overlays were used on the statistical maps of each individual to acquire signal change values across specific ROIs. Values indicated the degree of change in MR signal detected between the negative and neutral conditions and are expressed in terms of percent signal change (PSC). Average PSC values (beta weights averaged across all voxels within an anatomical region) were obtained for each ROI using the REX toolbox for SPM2 (Whitfield-Gabrieli, 2009). The PSC values were used to calculate effect sizes (ES) for the difference between groups. The formula for calculating these ES was: ES = [HC group mean (negative - neutral PSC) - MDD group mean (negative - neutral PSC)]/standard deviation of PSC value of the whole sample.
To assess the relationship between gonadal hormones and brain activation, serum estradiol and progesterone levels were entered as regressors at the second level of analysis, with our search area restricted to our a priori anatomical ROIs. We examined the degree to which addition of the hormone enhanced or attenuated the difference in PSC between groups. Finally, SPSS (SPSS, Chicago, IL) was used to calculate partial correlations between individual PSC values and a) estradiol (controlling for progesterone), and b) progesterone (controlling for estradiol) in order to quantify the effect of each of these hormones controlled for the other. These separate within-group partial correlations were compared using Z-value calculations to verify significant between-group differences in correlations.
RESULTS
Age, ethnicity, handedness, and verbal IQ did not differ between MDD and HC women (see Table 1). Within groups, HC women reported slightly higher state anxiety post-scan than pre-scan, but a decrease in their POMS vigor/activity rating (see Table 1). However, given that scores were in the low normative range, the magnitude of the changes was not clinically significant. MDD women did not demonstrate any significant changes in state anxiety or mood in response to the task. MDD and HC women did not differ in their current mood either pre-scan or post-scan. Similarly, there were no group differences on state anxiety pre-scan or post-scan (see Table 1). However, not surprisingly, MDD women demonstrated significantly higher trait anxiety than HC women. Taken together, this suggests that although MDD women have higher levels of anxiety as a trait of the disorder, on the day of the study visit, the scanning session and stress response paradigm did not evoke a higher level of state anxiety or mood changes in the MDD compared to HC groups.
Table 1.
Demographic and Clinical Characteristics and Mood and Anxiety Ratings in Depressed and Healthy Women
| Characteristic | HC mean (sd) |
MDD mean (sd) |
|---|---|---|
| Age (years) | 34.4 (4.5) | 34.2 (4.4) |
| Ethnicity (% Caucasian) | 100 | 100 |
| Handedness (% right-handed) | 100 | 100 |
| Verbal IQ1 | 14.3 (3.1) | 14.2 (1.3) |
| Age of symptom onset (years) | N/A | 20.9 (5.0) |
| Duration of illness (years) | N/A | 13.3 (8.5) |
| Number of episodes | N/A | 2.9 (1.9) |
| HAM-D score2 | N/A | 3.5 (2.5) |
| Mood Scale3 | ||
| Vigor4 | ||
| Pre-scan | 63.8 (8.3) | 56.5 (9.0) |
| Post-scan | 56.8 (14.0) | 55.8 (4.1) |
| Anxiety Scale5 | ||
| Trait Anxiety6 | 31.3 (8.8) | 39.3 (7.6) |
| State Anxiety7 | ||
| Pre-scan | 28.7 (5.9) | 32.5 (7.2) |
| Post-scan | 32.7 (7.0) | 33.7 (7.2) |
Scaled scores from WAIS Vocabulary subtest
Hamilton Depression Rating Scale (17-item)
Profile of Mood States (POMS) rates current mood (self-report); consists of 72 items, rated on a scale from 0 to 4 (0 = does not apply; 4 = the adjective describes emotional state extremely well). Table reflects a standardized mood state score for the Vigor subscale only; there were no significant within- or between-group differences on the other 5 subscales (Anxiety, Depression, Anger, Fatigue, Confusion); any score < 50 is in the low, normative range.). The POMS was not collected for one HC woman.
Significant within-group pre-scan to post-scan difference in the healthy control group, p<0.05, two-tailed
Spielberger State-Trait Anxiety Inventory (STAI) is a self-report of current and “usual” levels of anxiety. 40 statements are rated on a scale from 1 to 4 (1 = statement poorly reflects feelings of anxiety; 4 = statement accurately reflects feelings of anxiety). Statements reflect how the individual feels in general (reflecting trait-level anxiety) and current feelings of anxiety (reflecting state-level anxiety), out of which a standardized overall rating is also calculated. Any score < 50 is in the low normative range.
Significant between-group difference, p<0.05, two-tailed
Significant within-group pre-scan to post-scan difference in the healthy control group, p<0.05, two-tailed
MDD compared with HC women had lower serum estradiol at midcycle [HC: 153.9 pg/mL (108.2), MDD: 118.7 pg/mL (68.2); effect size (ES) = 0.26] and higher serum progesterone [HC: 0.59 ng/mL (0.59), MDD: 1.0 ng/mL (1.1); ES = 0.50]. One outlier HC subject had a serum progesterone level more than 3 SD above the group mean and thus was most likely beyond midcycle; this subject was not included in the analyses examining the effect of hormones on brain activity (see below). Although the effect size differences between MDD and HC women ranged from greater than a 0.33 to 0.50 standard deviation, given our small sample size, there were no statistically significant differences in average serum levels of gonadal hormones. Groups exhibited similar levels of serum testosterone [HC: 42.0 ng/dL (14.3), MDD: 41.9 ng/dL (13.2); ES = 0.01].
Compared to HC women, MDD women exhibited significantly lower activations in the negative-neutral contrast in the hypothalamus, amygdala, hippocampus, OFC, ACC, and sgACC (see Table 2; Figure 1), at a significance level of p<0.05, uncorrected for multiple comparisons. There were no regions of interest in which MDD women exhibited greater percent signal changes compared with HC women. Individual percent signal change values were extracted from each anatomical ROI and examined for group differences. These comparisons revealed significant hypoactivations (lower percent signal change) in MDD women in left hippocampus and OFC (at p<0.05), and at the trend level (p<0.10) in the hypothalamus, left amygdala, and ACC (see Table 3). Effect sizes of the differences in hypoactivations between MDD and HC women were substantial, primarily ranging from a half to almost full standard deviation difference in percent signal change in all ROIs, and significantly so in hippocampus and OFC (see Table 3).
Table 2.
Regions of Activation in Comparisons of Negative Valence to Neutral Valence Conditions: Healthy Control Women (HC) Compared with Women with MDD (MDD)
| Region | Hemisphere | x | y | z1 | Z | Voxels | Uncorrected p-value2 |
Voxel-level FWE-corrected p-value3 |
Cluster-level FWE-corrected p- value4 |
|---|---|---|---|---|---|---|---|---|---|
| Hypothalamus | −6 | −8 | −4 | 2.17 | 13 | 0.015 | n.s. | n.s. | |
| Amygdala | L | −18 | −5 | −9 | 2.67 | 41 | 0.004 | n.s. | 0.081 |
| Hippocampus | R | 12 | −36 | 4 | 2.75 | 11 | 0.003 | 0.064 | n.s. |
| L | −11 | −39 | 6 | 2.30 | 24 | 0.011 | n.s. | n.s. | |
| OFC5 | R | 37 | 16 | −11 | 2.78 | 119 | 0.003 | n.s. | n.s. |
| L | −33 | 13 | −18 | 3.32 | 129 | 0.000 | 0.037 | n.s. | |
| ACC5 | 0 | 51 | 18 | 2.90 | 273 | 0.002 | n.s. | 0.036 | |
| sgACC5 | −9 | 5 | −13 | 2.29 | 8 | 0.011 | n.s. | n.s. |
Coordinates are presented in Talairach space
Voxel-wise Z-score significance level p<0.05 uncorrected for multiple comparisons within a hypothesized ROI; ROIs listed represent regions of significantly activated clusters within the apriori hypothesized ROI
FWE rate (family-wise error rate) used for SVC (small volume correction): Voxel-level significance level (FWE-corrected within the search volume of interest)
FWE rate (family-wise error rate) used for SVC (small volume correction): Cluster-level significance level (FWE-corrected within the search volume of interest)
OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; sgACC = subgenual anterior cingulate cortex
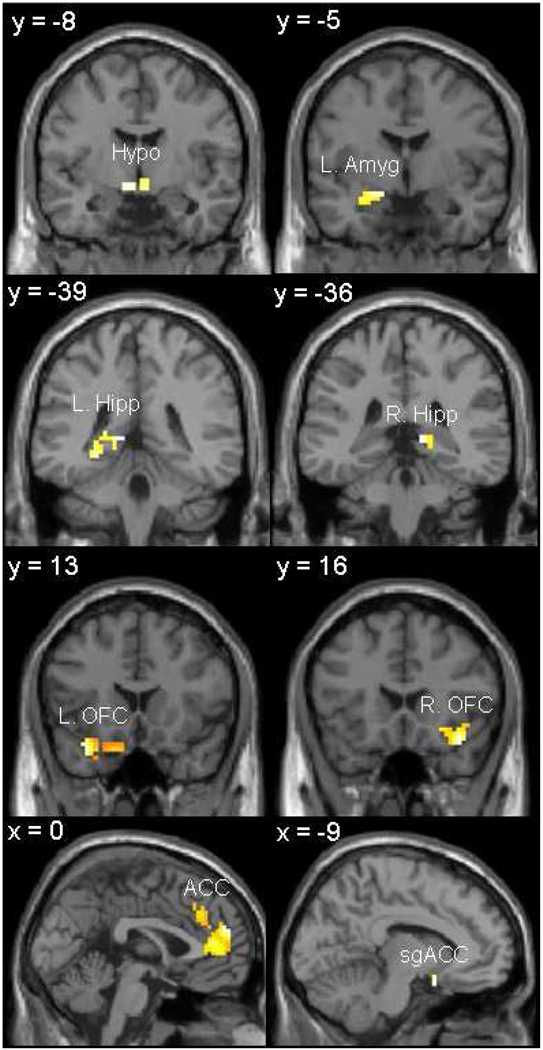
Figure 1. Significant Hypoactivation of Stress Response Circuitry Regions in Women with Depression.
Activations of hypothesized regions of interest were derived using restriction to within anatomical borders (defined by a manually segmented MNI brain) with the small volume correction tool in SPM2. Activations in Figure 1 are selected from Table 3, centered on the peak voxel of activation with a p<0.05 (uncorrected).
Hypo = anterior hypothalamus; Amyg = Amygdala; Hipp = hippocampus; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; sgACC = subgenual anterior cingulate cortex.
Table 3.
Percent Signal Change (PSC) Values in Hypothesized Stress Response Circuitry Regions Comparing Negative to Neutral Stimuli: Healthy Control Women (HC) Compared with Women with MDD (MDD)
| Hypothesized Regions1 | HC PSC |
MDD PSC |
Difference in PSC: HC vs. MDD |
Effect size of between-group difference2 |
|
|---|---|---|---|---|---|
| Region | Hemisphere | ||||
| Hypothalamus | 0.61 | 0.35 | 0.26 | 0.73 † | |
| Amygdala | R | 0.64 | 0.52 | 0.12 | 0.48 |
| L | 0.68 | 0.37 | 0.31 | 0.78 † | |
| Hippocampus | R | 0.60 | 0.42 | 0.18 | 0.59 |
| L | 0.66 | 0.31 | 0.35 | 0.86 * | |
| OFC3 | R | 0.36 | 0.22 | 0.14 | 0.50 |
| L | 0.35 | 0.03 | 0.32 | 0.91 * | |
| ACC3 | 0.42 | 0.00 | 0.42 | 0.78 † | |
| sgACC3 | 0.26 | 0.11 | 0.15 | 0.29 | |
Significance levels:
p<0.05;
p<0.10; two-tailed
Hypothesized regions of interest activations were anatomically-defined
ES (Effect sizes) = standard deviations calculated as: differences between negative versus neutral percent signal changes in HC vs. MDD; differences are divided by standard deviation of percent signal change value of the whole sample
OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; sgACC = subgenual anterior cingulate cortex
Results are underscored by further analysis of the effect of medication status in the MDD women on fMRI results. Percent signal change values in each group (HC vs. unmedicated MDD) were compared excluding the four MDD women taking antidepressants. Removal of these subjects resulted in persistent hypoactivation in all ROIs in the MDD compared with HC women, demonstrating that hypoactivity in the stress response circuitry in MDD was not due to medications.
To examine the effect of circulating gonadal hormones on group differences in percent signal change, estradiol and progesterone were entered as covariates at the second-level analysis in SPM. Findings showed that estradiol and progesterone levels attenuated group differences in the hypothalamus, left amygdala, sgACC, and left OFC, and enhanced group differences in the remaining ROIs (right amygdala, bilateral hippocampus, right OFC, ACC), suggesting that in the former regions, group differences were in part driven by abnormalities in levels of estradiol and progesterone.
In order to quantify these hormonal effects on brain activity, we examined the partial correlations of estradiol and progesterone (i.e., estradiol unopposed by progesterone and vice versa) on hypoactivations in our regions of interest. As shown in Table 4, there was a significant difference in the association of serum progesterone, unopposed by estradiol, to brain activity in HC vs. MDD women in hypothalamus, hippocampus, and OFC. In HC women, these brain regions were positively correlated with progesterone, while in MDD women they were negatively correlated (see Table 4). Group differences in estradiol’s effect on activity in the amygdala and hippocampus were at a trend level, with estradiol (unopposed by progesterone) having a positive correlation in MDD and a negative correlation in HC women. However, the strongest effects (i.e., largest effect sizes) were in progesterone’s effect comparing HC and MDD women. Findings suggest an association between hormonal dysfunction in MDD and hypoactivation of critical regions in stress response circuitry in the MDD group, with estradiol and progesterone having, in large part, opposing effects.
Table 4.
Partial Correlations between Percent Signal Change (PSC) Values in Hypothesized Stress Response Circuitry Regions and Serum Estradiol (Unopposed by Progesterone) and Progesterone (Unopposed by Estradiol): Healthy Control Women (HC) Compared with Women with MDD (MDD)
| Estradiol (controlling for Progesterone) |
Progesterone (controlling for Estradiol) |
||||||
|---|---|---|---|---|---|---|---|
| Hypothesized Regions | HC r |
MDD r |
Test of difference between partial correlations (z) |
HC r |
MDD r |
Test of difference between partial correlations (z) |
|
| Region | Hemisphere | ||||||
| Hypothalamus | −0.11 | −0.17 | 0.11 | 0.57† | −0.48 | 2.10* | |
| Amygdala | R | −0.31 | 0.42 | −1.38† | 0.11 | −0.43 | 1.02 |
| L | −0.26 | −0.13 | −0.24 | −0.47 | −0.46 | −0.02 | |
| Hippocampus | R | −0.16 | 0.48 | −1.23† | 0.64† | −0.26 | 1.84* |
| L | −0.20 | −0.17 | −0.06 | 0.51 | −0.32 | 1.61* | |
| OFC1 | R | −0.28 | −0.30 | 0.04 | 0.45 | 0.43 | 0.04 |
| L | −0.34 | −0.08 | −0.49 | 0.33 | −0.62† | 1.91* | |
| ACC1 | −0.21 | −0.14 | −0.13 | 0.43 | −0.08 | 0.97 | |
| sgACC1 | −0.01 | −0.18 | 0.31 | 0.05 | −0.20 | 0.45 | |
Significance levels:
p < 0.05;
p < 0.11; two-tailed
OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; sgACC = subgenual anterior cingulate cortex
DISCUSSION
In this preliminary study, women with a history of severe major depression (in remission) demonstrated significant hypoactivations in stress response circuitry that were, in part, associated with gonadal hormone dysregulation, as evidenced during the late follicular/midcycle peak of the menstrual cycle. These findings provide initial evidence that hormonal dysregulation and brain activity deficits in response to stress contribute to trait characteristics in women with MDD. Results demonstrated hypoactivation in MDD women across the circuitry (anterior hypothalamus, amygdala, hippocampus, ACC, OFC, and sgACC), with significant hypoactivations in the OFC, ACC, and hippocampus.
Integration of these results with the broader neuroimaging literature on MDD is limited by the dearth of studies examining response to emotional stimuli in MDD women in remission. However, hypoactivation in the dorsal ACC in response to negative stimuli was previously reported in MDD individuals in the remitted state (Hooley et al., 2009). In contrast to Hooley and colleagues, who reported an elevated amygdala response to negative stimuli, our analyses showed hypoactivation in the amygdala in MDD women. However, this inconsistency is likely related to the impact of state anxiety and mood on brain activity in the amygdala. That is, Hooley et al. (2009) reported a significant increase in negative mood compared to baseline. In our study, despite an elevated trait anxiety reported by MDD women, they did not differ from HC women on current state of mood or anxiety before or after scanning. This implies that hypoactivation in our ROIs cannot be explained by variation in transient anxiety levels and may reflect a trait characteristic of the illness.
MDD women in our study showed decreased levels of estradiol and higher progesterone compared to HC women during the midcycle phase. Abnormal gonadal functioning has been previously demonstrated in women in an acute episode of MDD (Young et al., 2000). Similar to Young et al. (2000) who reported 30% lower estradiol levels in the follicular phase in MDD women, serum estradiol in our MDD group was approximately 25% lower than the HCs. Importantly, our subjects were in remission, and thus this implies that decreased estradiol is likely a trait characteristic in women with MDD. Further, MDD women had elevated serum progesterone, even during midcycle when progesterone levels are relatively low. This is consistent with previous reports demonstrating elevated progesterone in the luteal phase in MDD women in remission (Hardoy et al., 2006), and previous findings that effective ECT treatment did not alter progesterone levels in MDD women (Baghai et al., 2005).
Extending previous neuroendocrine work, we showed that gonadal hormone dysfunction, in part, accounted for variation in brain activity differences in anterior hypothalamus, left amygdala, left hippocampus, and subgenual ACC, in response to stress comparing MDD and HC women. This is not wholly surprising given that in healthy individuals, stress response circuitry regions, such as anterior hypothalamus, amygdala, and hippocampus, are governed by the coordinated action of HPA and HPG-axis hormones. They are regions dense in estrogen α and/or β receptors and progesterone receptors (Donahue et al., 2000; Guerra-Araiza et al., 2002; Kato et al., 1994; Osterlund et al., 2000a; Osterlund et al., 2000b). In fact, in previous imaging studies, estradiol and progesterone levels have been significantly associated with brain activity in response to reward (Dreher et al., 2007), emotional expressions (van Wingen et al., 2008), and fear extinction and learning (Milad et al., 2009; Milad et al., 2010).
Our results extend these findings to women with MDD and underlying hormonal dysfunction. For example, when unopposed by progesterone, estradiol was negatively associated with activation across the stress response circuitry in healthy control and MDD women, extending our previous findings (Goldstein et al., 2010; Goldstein et al., 2005) demonstrating in healthy women that higher estradiol at ovulation was associated with lower activation. Exceptions to this trend were seen in the right amygdala and hippocampus, in which activations were positively associated (at the trend level) with estradiol unopposed by progesterone in the MDD women. In contrast, progesterone, unopposed by estradiol, had stimulatory effects on almost all regions of interest in healthy control women, but inhibitory effects in MDD, with significant effect sizes when comparing the correlations between MDD and HC women. Further work (currently underway) that includes the assessment of the adrenal response to stress in MDD is necessary in order to fully interpret these results, given that high levels of adrenal response, previously found in MDD, can inhibit gonadal hormone levels in women. In addition, progesterone can be released by the adrenal cortex and thus a full understanding of the affected hormonal pathways in MDD must include pituitary, adrenal and gonadal hormone responses to stress.
In the remitted state, medication status serves as a potential confound in interpretation of differences in the brain’s response to stress and the impact of hormones. However, although four of the MDD women were taking antidepressant medication, exclusion of these subjects did not change the findings, demonstrating that hypoactivations in MDD were not driven by medication status.
The generalizability of our findings is limited by small sample size and restriction to female subjects. However, MDD women were carefully made comparable to healthy women on a number of potential confounds. They were in remission so we were investigating trait effects, and the majority was unmedicated. Further, although these women had gonadal hormone abnormalities, they were all cycling. Thus, we in fact sampled against our hypothesis investigating hormonal dysregulation and the brain in MDD, underscoring our results. Further, although generalizability may be limited, this would not negate the internal validity of the results. In addition, even though we had a small sample size, we still demonstrated FWE-corrected results significant in two regions. Further, using analyses to obtain signal intensity changes in our regions of interest based on anatomy, we demonstrated substantial effect size differences in hypoactivations (half to almost one standard deviation) comparing MDD and HC women (see Table 3).
In conclusion, results of our study revealed significant associations between hormonal dysregulation and brain activity deficits in response to stress in women with a history of severe major depression in remission. We demonstrated hypoactivations in MDD women across the stress response circuitry, unrelated to medication status. Further, gonadal hormone abnormalities were evidenced by lower estradiol and higher progesterone levels in MDD which were significantly associated with decreased activations in MDD in the anterior hypothalamus, amygdala, sgACC, and OFC. Findings indicate that hormonal dysregulation and stress response circuitry dysfunction in MDD may be trait characteristics, given that only subjects who were in remission of MDD symptoms were included in the sample. We would argue further that these findings have important implications for understanding the pathophysiology of sex differences in MDD (given sex differences in gonadal hormones), a hypothesis currently under investigation. Moreover, our approach has critical implications for the design of studies of MDD, underscoring the importance of attending to the gender of subjects, the women’s hormonal status, and clinical status of state versus trait characteristics of the illness.
Acknowledgements
This work was supported by grants from the National Institute of Health to J.M.G. ORWH-NIMH P50 MH082679 and pilot funds for fMRI scans from NIH NCRR-GCRC M01 RR02635 at Brigham and Women's Hospital's General Clinical Research Center. We thank Drs. Tamara Gersh, Seung-Schik Yoo, and Matthew Jerram for help in earlier phases of the study, Harlyn Aizley, M.Ed. for clinical interviewing of the subjects, and Jo-Ann Donatelli, Ph.D. for her contributions to diagnostic review. We also appreciate the input of Stuart Tobet, Ph.D. and Robert Handa, Ph.D. (Co-PIs on ORWH-NIMH P50 MH082679) regarding their comments on earlier drafts of the manuscript.
Role of Funding Source
Funding for this work was provided by ORWH-NIMH Grant P50 MH082679 and some pilot funds for fMRI scans from NIH NCRR-GCRC M01 RR02635 at Brigham and Women's Hospital's General Clinical Research Center. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
Dr. Goldstein designed the study, wrote the protocol, and oversaw the final manuscript. Drs. Goldstein and Holsen worked on the background literature and interpretation of results. Drs. Holsen, Whitfield-Gabrieli, and Lee, and Sarah Spaeth and Lauren Ogden conducted the statistical analyses. Dr. Klibanski oversaw the neuroendocrine assessments and interpretation of endocrine results. Drs. Holsen and Goldstein wrote the first draft of the manuscript. All authors and acknowledged consultants contributed to and have approved the final manuscript.
REFERENCES
- Baghai TC, di Michele F, Schule C, Eser D, Zwanzger P, Pasini A, Romeo E, Rupprecht R. Plasma concentrations of neuroactive steroids before and after electroconvulsive therapy in major depression. Neuropsychopharmacology. 2005;30:1181–1186. doi: 10.1038/sj.npp.1300684. [DOI] [PubMed] [Google Scholar]
- Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–559. doi: 10.1016/0306-4530(94)00081-k. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- CSEA-NIMH. Center for Research in Psychophysiology. Gainesville, Florida: NIMH Center for Emotion & Attention, University of Florida; 1999. International Affective Picture System [digitized photographs] [Google Scholar]
- Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, Blaustein JD, Reichlin S. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res. 2000;856:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Fahim C, Stip E, Mancini-Marie A, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, Beauregard M. Abnormal prefrontal and anterior cingulate activation in major depressive disorder during episodic memory encoding of sad stimuli. Brain Cogn. 2004;54:161–163. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. Washington, D.C: Am Psych Press; 1996. [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. 2006;26:379–384. doi: 10.1097/01.jcp.0000229483.52955.ec. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun-Todd DA. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res. 2009;172:83–91. doi: 10.1016/j.pscychresns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Scott LA, Hiller JB, Yurgelun-Todd DA. Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol Psychiatry. 2005;57:809–812. doi: 10.1016/j.biopsych.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Irwin W, Anderle MJ, Abercrombie HC, Schaefer SM, Kalin NH, Davidson RJ. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. Neuroimage. 2004;21:674–686. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28:454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychopathology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lee BT, Seong Whi C, Hyung Soo K, Lee BC, Choi IG, Lyoo IK, Ham BJ. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitecture atlas-based interrogation of fmri data sets. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. 1053–8119. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.04.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Suckling J, Poon L, Simmons A, Andrew C, Sharma T. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Yamashita H, Ueda K, Takami H, Yamawaki S. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci. 2009;63:423–425. doi: 10.1111/j.1440-1819.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000a;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000b;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RW. Brain activation to favorite music in healthy controls and depressed patients. Neuroreport. 2009;20:1204–1208. doi: 10.1097/WNR.0b013e32832f4da3. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale - third edition: Administration and scoring manual. San Antonio: The Psychological Corporation and Harcourt Brace; 1997. [Google Scholar]
- Whitfield-Gabrieli S. Region of Interest Extraction (REX) Toolbox. Boston, MA: 2009. [Google Scholar]
- World Health Organization, G. Projections of mortality and burden of disease to 2030. World Health Organization; 2009
- Yang JC, Park K, Eun SJ, Lee MS, Yoon JS, Shin IS, Kim YK, Chung TW, Kang HK, Jeong GW. Assessment of cerebrocortical areas associated with sexual arousal in depressive women using functional MR imaging. J Sex Med. 2008;5:602–609. doi: 10.1111/j.1743-6109.2007.00737.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]