Epicardial Origin of VT: Mapping and Ablation
Idiopathic ventricular arrhythmias can originate from either the endocardial, mid-myocardial, or the epicardial regions of the heart. The outflow tracts and basal regions of the ventricle tend to be common locations of origin of these tachycardias. The anatomy of this region of the heart is both fascinating and at times challenging from a procedural standpoint. The orientation of the outflow tracts/basal regions of the heart allows mapping of VT's utilizing multiple anatomical approaches: (i) via the coronary vasculature, (ii) directly via a percutaneous pericardial approach (transverse sinus) and (iii) the atrial appendages.1 The intricate relationship of the outflow and basal regions of the ventricles to the coronary vasculature (arteries and veins) provides useful avenues for epicardial mapping and catheter ablation (figure 1). Coronary veins provide endovascular access to the epicardial regions of the ventricles, especially the crucial area between the junction of the great cardiac vein and the anterior interventricular vein (figure 1).
FIGURE 1. The Relationship Of The Ventricular Outflow Tracts to the Coronary Vasculature.
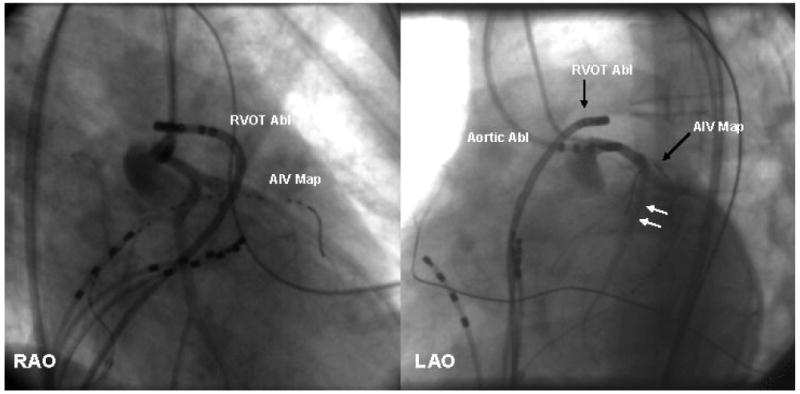
(left panel: left anterior oblique view and right panel: right anterior oblique view): Coronary arteriography, outflow, and coronary venous mapping from a patient with VT that was shown to originate from the region of the left main coronary artery. The left main coronary artery was mapped utilizing an ablation catheter via the aorta (Aortic Abl). These panels also show simultaneous mapping from the venous and the arterial systems. Note the close relationship between the anterior interventricular vein (location shown by the AIV map catheter) and the left anterior descending coronary artery adjacent to it (arrows). RVOT Abl= right ventricular outflow tract ablation catheter, Aortic Abl= aortic ablation catheter, AIV Map= anterior inter-ventricular vein mapping catheter
Coronary sinus venography was first described in the 1960's.2 Using occlusive venography, the middle cardiac vein, the great cardiac vein (GCV), and the anterior interventricular vein (AIV) can usually be visualized. The GCV runs in the posterior interventricular septum and the AIV runs parallel to the left anterior descending coronary artery and allow epicardial access to the anterior basal ventricular surface. Coronary venous mapping has been an attractive option to identify epicardial circuits in structural heart disease ever since surgical studies demonstrated that about 20% of VTs related to posterior/inferior myocardial infarctions had a critical reentrant circuit in the epicardium. In patients with anterior MI, access through the GCV and AIV allows mapping of late diastolic potentials over a wide area of the anterior wall without having to access the epicardium percutaneously.3 Coronary sinus/venous mapping was described by Arruda et al. in 19964 and radiofrequency ablation of idiopathic VT via this approach was described by Stellbrink et al. in 1997.5
The alternative (complementary) approach, percutaneous epicardial access, has certain limitations, especially pericardial bleeding and tamponade. Another limitation is the presence of epicardial fat which can mimic areas of scar due to the registration of low voltage. This can introduce an error in the delineation of scar and may limit lesion formation if the epicardial fat lies between the ablation catheter and targeted area. With the use of coronary venous mapping, these limitations can be avoided.
Idiopathic VT's and ventricular ectopy originate from the basal aspect of the ventricles which include the left and right ventricular outflow tracts, pulmonary artery, aortic cusps, aorto-mitral continuity,6 and mitral annulus. Recently, electrocardiographic analysis of the location of the tachycardia, particularly outflow tachycardias with transition in V3, and their ablation, has been subject of extensive study.7 In general, outflow tract tachycardias inscribe a sharp negative QRS complex in leads aVL and aVR with tall monophasic R waves in the inferior leads. Right ventricular outflow tract (RVOT) sites tend to have a LBBB (left bundle branch block) pattern in V1. RVOT tachycardias that are immediately anterior to the aorta have a precordial transition in V3 or later, while ‘free wall’ sites have a transition at V4 or later. Left ventricular outflow tract (LVOT) tachycardias frequently have a RBBB (right bundle branch block) morphology or early transition in the precordial leads. The right aortic cusp sites show a predominantly negative vector in lead V1 with a late transition and small broad R wave in V2, while left cusp sites can show a “M” or “W” configuration in V1 with early transition <V2.8, 9
The Present Study
Babam et al (journal editorial staff to insert) in their study have demonstrated that the transvenous approach is indeed safe and can be successful in 70% of idiopathic VT's when the earliest site of activation is in the coronary venous system. Of note, this success rate is similar to the transcutaneous epicardial approach. Further, 14% of idiopathic VT's (27 of 180 patients with idiopathic PVCs or VT's) were found to have their earliest site of activation epicardially. Epicardial arrhythmias in the proximal GCV had LBBB, while those in the distal GCV had RBBB. The majority of these VT's, 16/27, however, were of LBBB morphology and inferior axis, similar to aortic cusp and RVOT VT's, but could be distinguished from the latter due to their wide R wave > 75 ms in V1. This finding is in line with previous electrocardiographic criteria of epicardial VT/PVCs which have shown that these arrhythmias have a ‘pseudo-delta’ wave and a wider QRS, given the greater distance from the these epicardial sites of origin to the His-Purkinjie system. This study highlights several other important points:
Mapping and ablation within the great cardiac vein may be as successful as percutaneous epicardial ablation and can be carried out using open irrigated ablation catheters. The success rate in this study was 74%. However, it should be noted that mapping via the coronary veins does limit epicardial sampling as one is limited to areas of the myocardium that are subtended by a coronary vein. Therefore, percutaneous epicardial mapping should definitely be offered to those who fail transvenous mapping and ablation.
Coronary arterial anatomy needs to be defined. Surprisingly, proximity to a coronary artery leading to failure of ablation was seen in only one patient, though clearly, coronary angiography should be done routinely in every patient before ablation within the coronary venous system is attempted (See Figure 1).
As expected, sites of origin in the more “distal” GCV are more difficult to reach with standard ablation catheters and hence, carry a lower success rate.
Regardless of the ECG pattern (LBBB or RBBB), mapping in the GCV can be useful if initial mapping in the RVOT or LVOT, respectively, does not yield the earliest site of origin. 10 Of note, certain idiopathic tachycardias do not have a clear right or left bundle branch block morphology (equal R and S in V1) and therefore, may not follow a predetermined road map and require more detailed and extensive mapping.
Implications
This study adds to the literature supporting the efficacy of ablation via the coronary veins.11 Idiopathic VT's can be approached by stepwise mapping of the outflow tracts, aortic sinuses, coronary arteries and veins. Ablation can be performed safely from within the coronary venous system. Percutaneous epicardial mapping and ablation should be considered when coronary venous ablation fails. Both approaches need to take into account the proximity of coronary arteries, necessitating performance of coronary angiography pre and post ablation, and assessment of phrenic nerve capture testing prior to ablation. Epicardial techniques such as balloon protection can be effective in protecting the phrenic nerve.12-14 However, coronary artery injury is an ever present risk (especially near the proximal part of the anterior interventricular vein, see figure) and surgery is still necessary in some cases for VT's originating in this region.15 Cryoablation and potential experimental approaches such as cold saline irrigation are likely to aid these approaches to catheter ablation.11, 16, 17
Footnotes
Conflict of Interest Disclosures: Dr. Shivkumar is supported by the NHLBI (R01HL084261) The University of California, Los Angeles has intellectual property relating to epicardial mapping and ablation
References
- 1.Sosa E, Scanavacca M, d'Avila A. Catheter ablation of the left ventricular outflow tract tachycardia from the left atrium. J Interv Card Electrophysiol. 2002;7:61–65. doi: 10.1023/a:1020872216950. [DOI] [PubMed] [Google Scholar]
- 2.Gensini GG, Digiorgi S, Coskun O, Palacio A, Kelly AE. Anatomy of the Coronary Circulation in Living Man; Coronary Venography. Circulation. 1965;31:778–784. doi: 10.1161/01.cir.31.5.778. [DOI] [PubMed] [Google Scholar]
- 3.Della Bella P, Tondo C, Carbucicchio C, Riva S, Fassini G, Galimberti P. Transcoronary venous mapping of ventricular tachycardia. Elmsford, NY: Blackwell Publishing, Inc.; 2003. [Google Scholar]
- 4.Arruda M, Chandrasekaran K, Reynolds D. Idiopathic epicardial outlfow tract ventricular tachycardia: implications for radiofrequency catheter ablation. Pacing Clin Electrophsyiol. 1996;19:611. [Google Scholar]
- 5.Stellbrink C, Diem B, Schauerte P, Ziegert K, Hanrath P. Transcoronary venous radiofrequency catheter ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8:916–921. doi: 10.1111/j.1540-8167.1997.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 6.Steven D, Roberts-Thomson KC, Seiler J, Inada K, Tedrow UB, Mitchell RN, Sobieszczyk PS, Eisenhauer AC, Couper GS, Stevenson WG. Ventricular tachycardia arising from the aortomitral continuity in structural heart disease: characteristics and therapeutic considerations for an anatomically challenging area of origin. Circ Arrhythm Electrophysiol. 2009;2:660–666. doi: 10.1161/CIRCEP.109.853531. [DOI] [PubMed] [Google Scholar]
- 7.Tanner H, Hindricks G, Schirdewahn P, Kobza R, Dorszewski A, Piorkowski C, Gerds-Li JH, Kottkamp H. Outflow tract tachycardia with R/S transition in lead V3: six different anatomic approaches for successful ablation. J Am Coll Cardiol. 2005;45:418–423. doi: 10.1016/j.jacc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Dixit S, Gerstenfeld EP, Lin D, Callans DJ, Hsia HH, Nayak HM, Zado E, Marchlinski FE. Identification of distinct electrocardiographic patterns from the basal left ventricle: distinguishing medial and lateral sites of origin in patients with idiopathic ventricular tachycardia. Heart Rhythm. 2005;2:485–491. doi: 10.1016/j.hrthm.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Lin D, Ilkhanoff L, Gerstenfeld E, Dixit S, Beldner S, Bala R, Garcia F, Callans D, Marchlinski FE. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5:663–669. doi: 10.1016/j.hrthm.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 11.Obel OA, d'Avila A, Neuzil P, Saad EB, Ruskin JN, Reddy VY. Ablation of left ventricular epicardial outflow tract tachycardia from the distal great cardiac vein. J Am Coll Cardiol. 2006;48:1813–1817. doi: 10.1016/j.jacc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4:95–98. doi: 10.1016/j.hrthm.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Biase L, Burkhardt JD, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Horton R, Sanchez J, Gallinghouse JG, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Natale A. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957–961. doi: 10.1016/j.hrthm.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Fan R, Cano O, Ho SY, Bala R, Callans DJ, Dixit S, Garcia F, Gerstenfeld EP, Hutchinson M, Lin D, Riley M, Marchlinski FE. Characterization of the phrenic nerve course within the epicardial substrate of patients with nonischemic cardiomyopathy and ventricular tachycardia. Heart Rhythm. 2009;6:59–64. doi: 10.1016/j.hrthm.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Atienza F, Arenal A, Ormaetxe J, Almendral J. Epicardial idiopathic ventricular tachycardia originating within the left main coronary artery ostium area: identification using the LocaLisa nonfluoroscopic catheter navigation system. J Cardiovasc Electrophysiol. 2005;16:1239–1242. doi: 10.1111/j.1540-8167.2005.40773.x. [DOI] [PubMed] [Google Scholar]
- 16.Thyer IA, Kovoor P, Barry MA, Pouliopoulos J, Ross DL, Thiagalingam A. Protection of the coronary arteries during epicardial radiofrequency ablation with intracoronary chilled saline irrigation: assessment in an in vitro model. J Cardiovasc Electrophysiol. 2006;17:544–549. doi: 10.1111/j.1540-8167.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 17.Hammill SC. Epicardial ablation: reducing the risks. J Cardiovasc Electrophysiol. 2006;17:550–552. doi: 10.1111/j.1540-8167.2006.00459.x. [DOI] [PubMed] [Google Scholar]


