Abstract
Epidemiologic studies of pancreatic cancer risk have reported null or non-significant positive associations for obesity, while associations for height have been null. Waist and hip circumference have been evaluated infrequently.
A pooled analysis of 14 cohort studies on 846,340 individuals was conducted; 2,135 individuals were diagnosed with pancreatic cancer during follow-up. Study-specific relative risks (RRs) and 95% confidence intervals (CIs) were calculated by Cox proportional hazards models, and then pooled using a random effects model.
Compared to individuals with a body mass index (BMI) at baseline between 21–22.9kg/m2, pancreatic cancer risk was 47% higher (95%CI:23–75%) among obese (BMI≥30kg/m2) individuals. A positive association was observed for BMI in early adulthood (pooled multivariate [MV]RR = 1.30, 95%CI=1.09–1.56 comparing BMI≥25kg/m2 to a BMI between 21–22.9kg/m2). Compared to individuals who were not overweight in early adulthood (BMI<25kg/m2) and not obese at baseline (BMI<30kg/m2), pancreatic cancer risk was 54% higher (95%CI=24–93%) for those who were overweight in early adulthood and obese at baseline. We observed a 40% higher risk among individuals who had gained BMI ≥10kg/m2 between BMI at baseline and younger ages compared to individuals whose BMI remained stable. Results were either similar or slightly stronger among never smokers. A positive association was observed between waist to hip ratio (WHR) and pancreatic cancer risk (pooled MVRR=1.35 comparing the highest versus lowest quartile, 95%CI=1.03–1.78).
BMI and WHR were positively associated with pancreatic cancer risk. Maintaining normal body weight may offer a feasible approach to reducing morbidity and mortality from pancreatic cancer.
Keywords: Pancreatic Cancer, Anthropometry, Pooled Analysis
INTRODUCTION
Worldwide, pancreatic tumors cause significant morbidity and mortality as the 7th and 9th most common cause of cancer death in males and females, respectively1. Pancreatic cancer has few early symptoms, is usually diagnosed at late stages, and has a median survival of only 6 months1. Thus, identifying modifiable factors may yield approaches to reducing the morbidity and mortality due to this disease.
Obesity (body mass index (BMI) ≥ 30 kg/m2) has been hypothesized to promote the development of pancreatic cancer2, 3. Based on 23 cohort (summary relative risk (RR)=1.14, 95%CI=1.07–1.22 per 5kg/m2 increase in BMI) and 15 case-control studies (summary odds ratio (OR)=1.00, 95%CI=0.87–1.15 per 5kg/m2 increase in BMI), a World Cancer Research Fund (WCRF) and American Institute of Cancer Research (AICR) panel determined that the evidence for a positive dose-response relationship for BMI was convincing for pancreatic cancer2. However, not all studies included adjusted for smoking habits, an important confounder for this association4 and moderate to high between-studies heterogeneity was present2. Few studies have examined associations between BMI in early adulthood or changes in BMI during adulthood and pancreatic cancer risk, results have generally been non-significant or null5–14. However, a recent large case-control study by Li et al 14 suggested that individuals who were obese (median = 59 yrs) had a younger age of diagnosis of pancreatic cancer compared to overweight (median = 61 yrs) and normal weight (median = 64 yrs) individuals (p<0.001).
Based on eight cohort studies (summary RR=1.11, CI=1.05–1.17 per 5cm increase in height with low heterogeneity) and ten case-control studies (summary OR=1.02, CI=0.96–1.07 per 5cm increase in height with moderate heterogeneity), the WCRF/AICR review concluded that adult height and factors that increase adult height are probable causes of pancreatic cancer, although the results were somewhat inconsistent. However, this working group noted that evidence exists for a biological mechanism through the modification of growth hormones, IGFs and sex hormone binding proteins2.
Although several studies assessed the association between BMI and pancreatic cancer risk, and height and pancreatic cancer risk, fewer studies have examined the independent effect of BMI at young ages, waist circumference, hip circumference or waist-to-hip ratio (WHR). Thus, we investigated the association between anthropometric factors and pancreatic cancer risk in a pooled analysis of 14 cohort studies9, 15–25. We also considered whether these associations differed by pancreatic cancer risk factors.
MATERIALS AND METHODS
Population
A pooled analysis of the primary data from fourteen cohort studies9, 15–25 was conducted in The Pooling Project of Prospective Studies of Diet and Cancer (Pooling Project), an international consortium. The current analysis used the same inclusion criteria and data set that have been used for analyses of dietary factors and pancreatic cancer risk in the Pooling Project26. To maximize the quality and comparability of the studies in the Pooling Project, each eligible study (Table 1) had to meet the following pre-specified inclusion criteria: a minimum of 50 incident pancreatic cancer cases, an assessment of usual diet, validation of the dietary assessment tool or a closely related instrument and publication of any diet and cancer association. Studies that met our inclusion criteria and agreed to participate sent their primary data for analysis. Because many cancers appear to have hormonal antecedents and because lifestyle factors may differ between women and men, studies including both women and men were split into two studies for our pooled analyses: a cohort of women and a cohort of men. This conservative approach, in which all estimates were calculated separately for women and men in those studies including both genders, allowed for potential effect modification by sex for every determinant of the outcome.
Table 1.
Anthropometric Factors by Cohort Study in the Pancreatic Analysis of the Pooling Project of Prospective Studies of Diet and Cancer
| Cohort1 | Gender | Follow-up Years |
Baseline Cohort Size2 |
Number of Cases |
Age Range (yrs) |
Median (Interquartile Range) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI at baseline (kg/m2) |
BMI in early adulthood(kg/m2) |
Height (m) | Waist Circumference (cm) |
Hip Circumference (cm) |
||||||
| ATBC | Male | 1984–1999 | 26,963 | 204 | 50–69 | 26.0(23.7–28.5) | 1.74(1.69–1.78) | |||
| BCDDP | Female | 1987–1999 | 39,864 | 93 | 40–93 | 24.2(22.0–27.4) | 1.63(1.57–1.68) | |||
| CNBSS3 | Female | 1980–2000 | 49,654 | 103 | 40–59 | 23.9(22.0–26.6) | 20.6(19.3–22.2) | 1.62(1.57–1.66) | ||
| CPS II | Female | 1992–2001 | 73,000 | 161 | 50–74 | 24.8(22.3–28.0) | 20.3(18.9–22.0) | 1.63(1.60–1.68) | 83.8(76.2–94.0) | |
| Male | 1992–2001 | 65,256 | 208 | 50–74 | 25.9(24.0–28.4) | 21.7(19.8–23.7) | 1.78(1.75–1.83) | 96.5(91.4–104.1) | ||
| CTS | Female | 1995–2003 | 96,380 | 103 | 22–104 | 23.6(21.3–27.2) | 20.8(19.4–22.5) | 1.65(1.60–1.68) | ||
| COSM | Male | 1998–2005 | 43,010 | 69 | 45–79 | 25.4(23.6–27.7) | 21.8(20.4–23.3) | 1.77(1.73–1.82) | 95.0(90.0–102.0) | 101.0(97.0–106.0) |
| HPFS | Male | 1986–2002 | 46,630 | 193 | 40–75 | 25.1(23.5–27.1) | 22.9(21.1–24.4) | 1.78(1.73–1.83) | 94.0(88.9–100.3) | 100.3(96.5–104.8) |
| IWHS | Female | 1986–2001 | 34,540 | 171 | 55–69 | 25.2(22.7–28.5) | 20.5(19.0–22.3) | 1.63(1.57–1.68) | 86.1(77.2–96.5) | 102.9(97.2–110.2) |
| MCCS | Female | 1990–2003 | 22,803 | 35 | 40–69 | 25.8(23.1–29.1) | 21.1(19.5–23.0) | 1.60(1.56–1.65) | 78.0(71.0–86.2) | 100.0(95.0–106.8) |
| Male | 1990–2003 | 14,895 | 28 | 40–69 | 26.8(24.7–29.0) | 22.4(20.8–24.2) | 1.73(1.68–1.78) | 92.5(86.7–99.0) | 100.5(96.5–105.0) | |
| NLCS3 | Female | 1986–1999 | 62,573 | 117 | 55–69 | 24.6(22.7–27.0) | 21.3(19.5–22.9) | 1.65(1.61–1.69) | ||
| Male | 1986–1999 | 58,279 | 140 | 55–69 | 24.8(23.4–26.5) | 21.7(20.3–23.2) | 1.76(1.72–1.81) | |||
| NYSC | Female | 1980–1987 | 22,083 | 48 | 15–107 | 24.0(21.0–27.0) | 1.63(1.57–1.68) | |||
| Male | 1980–1987 | 29,957 | 87 | 15–107 | 25.0(24.0–27.0) | 1.75(1.70–1.80) | ||||
| NHSb | Female | 1986–2002 | 66,895 | 173 | 40–65 | 24.2(22.0–27.5) | 20.9(19.5–22.7) | 1.63(1.60–1.68) | 76.2(71.1–84.5) | 99.1(94.0–106.7) |
| PLCO | Female | 1993–2004 | 28,019 | 60 | 55–74 | 25.9(23.2–29.8) | 20.8(19.5–22.4) | 1.63(1.57–1.68) | ||
| Male | 1993–2004 | 29,595 | 90 | 55–74 | 27.0(24.8–29.7) | 22.9(21.0–24.5) | 1.78(1.73–1.83) | |||
| SMC | Female | 1997–2004 | 35,944 | 52 | 49–83 | 24.5(22.3–27.1) | 20.3(18.8–22.0) | 1.64(1.60–1.68) | 82.0(76.0–90.0) | 102.0(97.0–108.0) |
| TOTAL | 846,340 | 2,135 | ||||||||
ATBC= Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, BCDDP=Breast Cancer Detection Demonstration Project Follow-up Cohort, CNBSS=Canadian National Breast Screening Study, CPS II=Cancer Prevention Study II Nutrition Cohort, CTS=California Teachers Study, COSM=Cohort of Swedish Men, HPFS=Health Professionals Follow-up Study, IWHS=Iowa Women’s Health Study, MCCS=Melbourne Collaborative Cohort Study, NLCS=Netherlands Cohort Study, NYSC=New York State Cohort, NHS=Nurses’ Health Study, PLCO=Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, SMC=Swedish Mammography Cohort
Baseline cohort size and number of cases determined after applying exclusion criteria.
CNBSS and NLCS are analyzed as case-cohort studies so the baseline cohort size does not reflect the exclusions.
For the Pooling Project, we divided the person-time of the Nurses’ Health Study into two segments corresponding to the 1980–1986 follow-up period (Part A) and follow-up beginning in 1986 (Part B) to take advantage of the increased comprehensiveness of the food-frequency questionnaire (FFQ) completed in 1986 compared to the FFQ completed in 1980. We excluded Part A because fewer than 50 pancreatic cancer cases were identified in the Nurses’ Health Study between 1980 and 1986. For the Swedish Mammography Cohort, we utilized 1997 as the baseline for the questionnaire data and the start of follow-up for the cohort members who had no history of cancer in 1997 because the 1997 questionnaire included information on smoking habits, an important pancreatic cancer risk factor4. The methods for the Pooling Project have been described in detail elsewhere27.
Exposure Assessment
Within each study, information on height and weight was collected by self-report on the baseline questionnaires in all cohorts except Melbourne Collaborative Cohort Study and Alpha-Tocopherol Beta-Carotene Cancer Prevention Study which measured height and weight. Weight during early adulthood (age 18 or 21 years) was also collected in 11 studies (Table 1). Seven studies measured waist and/or hip circumference. Smoking habits were ascertained in all studies. Thirteen studies ascertained physical activity; ten studies ascertained diabetes status.
Outcome Assessment
Invasive pancreatic cancer28 was ascertained by self-report with subsequent medical record review22, cancer registry linkage9, 15, 17, 18, 20, 21, 24, or both16, 19, 23, 25. Some studies additionally obtained information from death registries15–17, 19, 20, 22, 23.
Exclusions
In addition to predefined study-specific exclusions, we excluded individuals with loge-transformed energy intakes more than three standard deviations above or below the loge-transformed mean energy intake of their respective cohort population. We also excluded those with a prior cancer diagnosis other than non-melanoma skin cancer at baseline. In addition, individuals with a BMI ≤14 kg/m2 (n= 149 non-cases, n = 0 cases) or ≥50 kg/m2 (n=580 non-cases, n = 1 case) or with missing height or weight data (n=16,313 non-cases, n=61 cases) were excluded.
Statistical Analysis
Anthropometric measures were modeled continuously and categorically. For the categorical analysis, BMI at baseline was modeled using cutpoints proposed by the World Health Organization29. Due to lower average BMI values at younger ages, BMI in early adulthood was modeled using slightly different categories. Waist circumference, hip circumference and WHR were defined by sex-specific quartiles determined in an aggregated analysis, in which all studies with the factor of interest were combined into a single data set. Based on the height distribution among males and females, height was modeled categorically using separate absolute cutpoints for each sex.
RRs and 95% confidence intervals (CI) were calculated by fitting Cox proportional hazards models for each study. The models included stratification by age (years) at baseline and the calendar year at start of follow-up, and treated follow-up time (days) as the time scale. Multivariate relative risks (MVRR) were adjusted for smoking habits, personal history of diabetes, alcohol intake, and energy intake. As personal history of diabetes may be in the causal pathway between BMI and pancreatic cancer, we also conducted analyses removing personal history of diabetes as a covariate. We also conducted separate analyses in which we adjusted for smoking history using different categorizations of status, duration, and dose to replace the categorization we used for the main multivariate models.
To test for a linear trend in pancreatic cancer risk with each anthropometric factor, a continuous variable with values corresponding to the median value for each exposure category was included in the model; the statistical significance of the coefficient for that variable was evaluated using the Wald test.
Study-specific RRs, weighted by the inverse of the sum of their variance and the estimated between-studies variance component, were pooled using a random effects model. Between-studies heterogeneity was evaluated using the Q statistic and inconsistency was quantified by the I2 statistic. We also evaluated whether BMI and height were linearly associated with pancreatic cancer risk using a non-parametric regression analysis in an aggregated data set. To test for non-linearity, the model fit including the linear plus any cubic spline terms selected by a stepwise regression procedure was compared to the model fit with only the linear term using the likelihood ratio test. To avoid excess influence from extreme heights, these analyses were limited to individuals who were less than 2.0m in height (total number excluded = 441 individuals; 0 cases).
To examine variation in RRs by physical activity, we assessed the statistical significance of the cross-product term between the anthropometric factor and physical activity using a Wald test. We used a meta-regression model to evaluate whether associations with anthropometric factors varied by gender, smoking status, age at diagnosis and follow-up time as these are nominal variables or can only be assessed fully between-studies. We conducted sensitivity analyses excluding cases diagnosed during the first few years of follow-up to evaluate lag effects(5 years) and to address the concerns of reverse causation(2 years), as anthropometric factors(e.g., BMI) of cases that occurred close in time to the completion of the baseline questionnaire might have changed due to prediagnostic disease symptoms. Separate analyses were also conducted for adenocarcinomas, the most common pancreatic cancer subtype, in those studies that had information on histology and among those studies having more than 10 adenocarcinoma cases.
Finally, censored linear regression models30 were used to examine the mean age of diagnosis of pancreatic cancer continuously and by categories of BMI in early adulthood and BMI at age at enrollment (baseline) using an aggregated dataset, controlling for study-specific differences in age at diagnosis. In this model, we adjusted for the covariates presented in the main models. SAS software, version 9.1, was used.
RESULTS
The study population consisted of 314,585 men and 531,755 women among whom 1,019 men and 1,116 women developed pancreatic cancer (Table 1). Median BMI ranged from 23.6kg/m2 to 27.0kg/m2 across the studies.
Pancreatic cancer risk was increased by 47% among individuals with BMI≥30kg/m2 at baseline vs BMI between 21–22.9kg/m2 (Table 2, Figure 1a). Similar risk estimates were observed for females and males. Comparing BMI ≥30kg/m2 to BMI between 21–22.9kg/m2, the results were also similar when we limited our analyses to adenocarcinomas (Ncases = 1454; pooled MVRR=1.55, 95%CI=1.28–1.89), to individuals who were not diabetic (Ncases =1651; pooled MVRR= 1.47, 95%CI=1.16–1.85) and to never smokers (Ncases =748; pooled MVRR=1.52, 95%CI=1.13–2.05). The association did not change substantially when personal history of diabetes was excluded as a covariate. When we examined BMI ≥35kg/m2 compared to BMI between 21–22.9kg/m2, we observed modestly stronger risk estimates (pooled MVRR=1.55, 95%CI =1.19–2.03).
Table 2.
Pooled Multivariate-Adjusted Relative Risks1 and 95% Confidence Intervals for Pancreatic Cancer According to Body Mass Index at Baseline and in Early Adulthood
| Categories of BMI (kg/m2) | I2 | PH2 | PH by sex3 | Ptrend4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI at Baseline | Range | <21 | 21–22.9 | 23–24.9 | 25–29.9 | ≥30 | ||||
| All | Cases | 196 | 290 | 457 | 847 | 345 | ||||
| MVRR | 1.16(0.96–1.40) | 1 (Ref) | 1.07(0.92–1.25) | 1.18(1.03–1.36) | 1.47(1.23–1.75) | 9% | 0.35 | 0.99 | <0.001 | |
| Females | Cases | 148 | 177 | 221 | 378 | 192 | ||||
| MVRR | 1.15(0.92–1.44) | 1 (Ref) | 1.08(0.88–1.32) | 1.29(1.04–1.61) | 1.46(1.17–1.80) | 0% | 0.56 | 0.002 | ||
| Males | Cases | 48 | 113 | 236 | 469 | 153 | ||||
| MVRR | 1.19(0.85–1.68) | 1 (Ref) | 1.07(0.85–1.34) | 1.09(0.88–1.34) | 1.50(1.07–2.11) | 38% | 0.14 | 0.06 | ||
| BMI in Early Adulthood | Range | <18.5 | 18.5–20.9 | 21–22.9 | 23–24.9 | ≥25 | ||||
| All | Cases | 163 | 519 | 426 | 276 | 214 | ||||
| MVRR | 0.90(0.75–1.09) | 0.96(0.84–1.10) | 1 (Ref) | 1.12(0.93–1.33) | 1.30(1.09–1.56) | 6% | 0.39 | 0.74 | <0.0001 | |
| MVRR5 | 0.95(0.79–1.15) | 0.99(0.87–1.13) | 1 (Ref) | 1.09(0.92–1.29) | 1.21(1.01–1.45) | 0% | 0.55 | 0.62 | 0.03 | |
| Females | Cases | 121 | 351 | 239 | 113 | 94 | ||||
| MVRR | 0.88(0.67–1.14) | 0.93(0.79–1.10) | 1 (Ref) | 1.01(0.81–1.27) | 1.27(0.99–1.62) | 0% | 0.43 | 0.02 | ||
| MVRR5 | 0.92(0.70–1.21) | 0.96(0.81–1.14) | 1 (Ref) | 0.98(0.78–1.24) | 1.16(0.90–1.50) | 0% | 0.7 | 0.18 | ||
| Males | Cases | 42 | 168 | 187 | 163 | 120 | ||||
| MVRR | 0.97(0.68–1.37) | 1.00(0.75–1.32) | 1 (Ref) | 1.21(0.88–1.67) | 1.31(0.97–1.76) | 27% | 0.25 | 0.002 | ||
| MVRR5 | 1.02(0.72–1.45) | 1.03(0.78–1.35) | 1 (Ref) | 1.19(0.87–1.62) | 1.21(0.88–1.68) | 29% | 0.23 | 0.06 | ||
| BMI Change | <25kg/m2 in early adulthood, <30kg/m2 at baseline | ≥25kg/m2 in early adulthood, <30kg/m2 at baseline6 | <25kg/m2 in early adulthood, ≥30kg/m2 at baseline | ≥25kg/m2 in early adulthood and ≥30kg/m2 at baseline6 | ||||||
| All | Cases | 1203 | 125 | 181 | 89 | |||||
| MVRR | 1 (Ref) | 1.30(1.08–1.57) | 1.38(1.14–1.66) | 1.54(1.24–1.93) | 0% | 0.97 | 0.79 | |||
| Females | Cases | 703 | 51 | 121 | 43 | |||||
| MVRR | 1 (Ref) | 1.43(1.07–1.91) | 1.29(1.06–1.58) | 1.50(1.09–2.06) | 0% | 0.95 | ||||
| Males | Cases | 500 | 74 | 60 | 46 | |||||
| MVRR | 1 (Ref) | 1.22(0.95–1.56) | 1.42(0.90–2.21) | 1.59(1.16–2.17) | 0% | 0.73 | ||||
| Absolute BMI Change | Loss > 2 kg/m2 | BMI +/− 2kg/m2 | 2-≥5kg/m2 | 5-≥10kg/m2 | >10kg/m2 | |||||
| All | Cases | 79 | 391 | 493 | 491 | 144 | ||||
| MVRR | 1.44 (1.13–1.85) | 1 (Ref) | 0.98 (0.85–1.12) | 1.13 (0.98–1.30) | 1.40 (1.13–1.72) | 0% | 0.63 | 0.83 | 0.04 | |
| Females | Cases | 52 | 218 | 252 | 281 | 115 | ||||
| MVRR | 1.31 (0.95–1.81) | 1 (Ref) | 0.91 (0.75–1.11) | 1.06 (0.88–1.28) | 1.43 (1.10–1.85) | 7% | 0.38 | 0.10 | ||
| Males | Cases | 27 | 173 | 241 | 210 | 29 | ||||
| MVRR | 1.58 (1.05–2.39) | 1 (Ref) | 1.04 (0.85–1.27) | 1.22 (0.96–1.56) | 1.34 (0.88–2.05) | 0% | 0.51 | 0.27 | ||
Multivariate relative risks (MVRR) were adjusted for smoking status (never smokers; past smokers, pack-years <15yrs; past smokers, pack-years ≥15yrs; current smokers, pack-years <40yrs, current smokers, pack-years ≥ 40yrs), history of diabetes (no, yes), alcohol intake (0,0.1–14.9,15–29.9,≥30g/day) and energy intake (continuously); age in years and year of questionnaire return were included as stratification variables.
P-value, test for between-studies heterogeneity is based on the highest category
P-value, test for between-studies heterogeneity due to sex is based on highest category
P-value, test for trend
Multivariate relative risks were additionally adjusted for BMI at baseline
PLCO(Female) and SMC were excluded from this category due to small number of cases in this category
Figure.
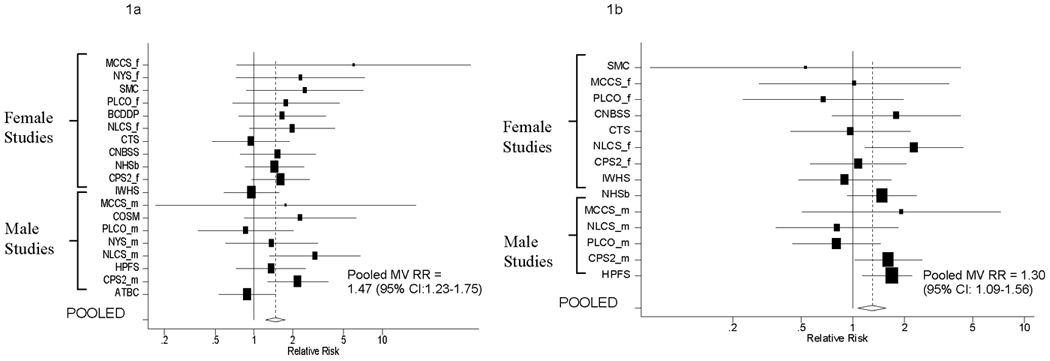
Multivariate Adjusted Relative Risks and 95% Confidence Intervals for Pancreatic Cancer According to BMI at Baseline (Figure a; BMI≥30kg/m2) and BMI at Younger Ages (Figure b; BMI ≥25kg/m2) compared to BMI between 21–22.9 kg/m2 by Study
The black squares and horizontal lines correspond to the study-specific relative risks and 95% confidence intervals. The area of the black squares is proportional to the inverse of the sum of the between-studies variance and the study-specific variance. The studies are ordered within each sex strata according to their weight in calculating the pooled estimate. The diamond represents the pooled multivariate relative risk and the 95% confidence interval. The vertical dashed line represents the pooled multivariate relative risk.
As suggested by the categorical analyses, the non-parametric regression analyses showed a linear association between BMI at baseline and pancreatic cancer risk (p-value, test for non-linearity > 0.10). The pooled MVRR for a 5kg/m2 increment in BMI was 1.14 (95%CI=1.07–1.21).
The BMI-pancreatic association was similar among the different models that adjusted for smoking habits as: 1) smoking status (never, past, current), 2) smoking status and smoking duration, 3) smoking status and amount smoked, 4) smoking status, smoking duration among past smokers, and amount smoked among current smokers, or 5) smoking status and smoking pack-years (data not shown).
BMI in early adulthood was also positively (Table 2, Figure 1b) and linearly (pooled MVRR=1.20, 95%CI=1.10–1.30 for a BMI increment of 5kg/m2, p-value, test for non-linearity > 0.10) associated with pancreatic cancer risk. The association between BMI in early adulthood and pancreatic cancer risk was similar when the study population was limited to non-diabetics (pooled MVRR=1.28, 95%CI=1.06–1.55), or when the case definition was limited to adenocarcinomas (pooled MVRR=1.18, 95%CI=0.95–1.47; p-value, test for trend <0.01) comparing BMI≥25 kg/m2 to BMI between 21–22.9kg/m2. The association between BMI in early adulthood and pancreatic cancer risk was stronger when the study population was limited to never smokers (pooled MVRR=1.51, 95%CI=1.13–2.01) for the same contrast. In analyses that mutually adjusted for BMI at baseline and BMI in early adulthood, we found similar risk estimates for BMI in early adulthood (pooled MVRR=1.21, 95%CI:1.04–1.45 comparing BMI≥25kg/m2 to BMI between 21–22.9kg/m2) and BMI at baseline (pooled MVRR=1.46, 95%CI:1.17–1.81 comparing BMI≥30kg/m2 to BMI between 21–22.9kg/m2).
Pancreatic cancer risk was stronger among individuals who were overweight in early adulthood (BMI≥25kg/m2) and obese at baseline (BMI≥30kg/m2) compared to individuals with a BMI<25kg/m2 in early adulthood and BMI<30kg/m2 at baseline (pooled MVRR=1.54, 95%CI=1.24–1.93) (Table 2). Results were stronger when the analysis was limited to never smokers (pooled MVRR=1.93, 95%CI=1.31–2.85). When we categorized the absolute difference of BMI at baseline and BMI at younger ages, we observed positive associations among individuals who had lost 2kg/m2 (MVRR=1.44, 95% CI=1.13–1.85), and gained ≥10kg/m2 (MVRR=1.40, 95%CI=1.13–1.72), but no statistically significant associations for those who gained 2-≤;5kg/m2 or 5-≤10kg/m2, compared to individuals whose BMI remained stable (BMI +/− 2kg/m2). To examine whether preclinical disease was related to the higher pancreatic cancer risk in those individuals who had lost 2kg/m2 from early adulthood to baseline, we excluded the first two years and the first five years from follow-up; the estimates were similar to the overall results (pooled multivariate RR removing 1st 2 years of follow-up = 1.47, 95% CI=1.14–1.89; pooled multivariate RR removing 1st 5 years of follow-up = 1.56, 95% CI=0.94–2.58).
No statistically significant associations with pancreatic cancer risk were observed for waist or hip circumference comparing the highest versus lowest quartile (Table 3). However, a 35% greater risk was observed among individuals in the highest versus lowest quartile of WHR (n = 6 studies; p-value, test for heterogeneity due to sex = 0.90). As we only have 3 studies contributing to the male and 4 studies contributing to the female specific estimates, we did not present the results for males and females, separately. However, the risk estimates, although not statistically significant, were similar to the overall (combined) results. Results were similar when we additionally adjusted for BMI at baseline.
Table 3.
Pooled Multivariate-Adjusted Relative Risks1 and 95% Confidence Intervals for Pancreatic Cancer According to Waist Circumference, Hip Circumference, and WHR
| Quartiles | I2 | PH2 | PH by sex3 | Ptrend4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| Waist Circumference5–6 | |||||||||
| Cases | 167 | 141 | 210 | 225 | |||||
| MVRR | 1 (Ref) | 0.89(0.71–1.12) | 0.95(0.73–1.25) | 1.16(0.92–1.46) | 10% | 0.36 | 0.48 | 0.07 | |
| MVRR7 | 1 (Ref) | 0.86(0.66–1.13) | 0.90(0.67–1.20) | 1.04(0.73–1.47) | 26% | 0.22 | 0.56 | 0.7 | |
| Hip Circumference5–6 | |||||||||
| Cases | 144 | 127 | 123 | 173 | |||||
| MVRR | 1 (Ref) | 0.93(0.73–1.19) | 0.85(0.66–1.09) | 1.07(0.85–1.35) | 0% | 0.65 | 0.53 | 0.59 | |
| MVRR7 | 1 (Ref) | 0.90(0.70–1.16) | 0.78(0.59–1.02) | 0.95(0.69–1.30) | 0% | 0.89 | 0.31 | 0.53 | |
| WHR5–6 | |||||||||
| Cases | 87 | 143 | 137 | 185 | |||||
| MVRR | 1 (Ref) | 1.24(0.94–1.63) | 1.12(0.85–1.48) | 1.35(1.03–1.78) | 0% | 0.99 | 0.90 | 0.06 | |
| MVRR7 | 1 (Ref) | 1.23(0.94–1.63) | 1.12(0.84–1.49) | 1.34(1.00–1.79) | 0% | 0.93 | 0.91 | 0.09 | |
See Table 2 for adjustment factors
P-value, test for between-studies heterogeneity is based on the highest category
P-value, test for between-studies heterogeneity due to sex is based on highest category
P-value, test for trend
Due to smaller case numbers and fewer number of studies that collected information on waist circumference, hip circumference and WHR, we are unable to present analyses separately by sex.
The top and bottom 1% of the population was excluded from the analysis.
Multivariate relative risks were additionally adjusted for BMI at baseline
Height was not associated with pancreatic cancer risk overall (Table 4) or among non-diabetics or never smokers. The nonparametric regression analyses showed that the association between height and pancreatic cancer risk was consistent with a linear (p-value, test for non-linearity>0.10), but null, association for men (pooled MVRR=1.02, 95%CI=0.97–1.06) and women (pooled MVRR=1.00, 95%CI=0.95–1.06) for an increment of 0.05m of height. Further, no association was observed when the outcome was limited to adenocarcinomas of the pancreas (data not shown).
Table 4.
Pooled Multivariate-Adjusted Relative Risks1–2 and 95% Confidence Intervals for Pancreatic Cancer According to Height
| Categories of Height (m) | I2 | PHeterogeneity3 | Ptrend4 | |||||
|---|---|---|---|---|---|---|---|---|
| Range | <1.60 | 1.60-<1.65 | 1.65-<1.70 | ≥1.70 | ||||
| Females | Cases | 299 | 339 | 287 | 190 | |||
| MVRR1 | 1 (Ref) | 1.04(0.89–1.22) | 0.96(0.77–1.19) | 1.03(0.84–1.25) | 0% | 0.51 | 0.72 | |
| MVRR2 | 1 (Ref) | 1.05(0.89–1.23) | 0.97(0.78–1.21) | 1.06(0.87–1.29) | 1% | 0.43 | 0.88 | |
| Range | <1.70 | 1.70-<1.75 | 1.75-<1.80 | ≥1.80 | ||||
| Males | Cases | 142 | 235 | 283 | 359 | |||
| MVRR1 | 1 (Ref) | 1.02(0.77–1.36) | 1.05(0.85–1.30) | 1.18(0.93–1.49) | 11% | 0.35 | 0.20 | |
| MVRR2 | 1 (Ref) | 1.03(0.77–1.38) | 1.06(0.86–1.32) | 1.20(0.96–1.51) | 9% | 0.37 | 0.13 | |
See Table 2 for adjustment factors
Multivariate relative risks were additionally adjusted for BMI at baseline (continuous).
P-value, test for between-studies heterogeneity is based on the highest category
P-value, test for trend
Overall, the associations of BMI at baseline(Table 5), BMI in early adulthood(Table 5), and height (data not shown) with pancreatic cancer risk were not modified by lifestyle and cohort characteristics. Although the interactions were not statistically significant, the positive associations for BMI at baseline and in early adulthood appeared stronger in never and past smokers than among current smokers. The association between BMI at baseline and pancreatic cancer risk varied by physical activity level; a slightly stronger positive association was observed in the high physical activity group (p-value, test for interaction=0.02). In addition, results for BMI at baseline, BMI in early adulthood and height were similar when we compared results from analyses limited to the first five years of follow-up with those of five or more years of follow-up, excluded cases diagnosed during the first two years of follow-up (data not shown), or stratified by the median age at diagnosis of the cases (Table 5).
Table 5.
Pooled Multivariate Relative Risks1 and 95% Confidence Intervals for Body Mass Index by Levels of Other Pancreatic Cancer Risk Factors
| Factor | BMI at Baseline (5kg/m2) |
P-value,heterogeneity2 | P-value,interaction3 | BMI in Early Adulthood (5kg/m2) |
P-value,heterogeneity2 | P-value,interaction3 | ||
|---|---|---|---|---|---|---|---|---|
| Cases | Continuous RR(95% CI) |
Cases | Continuous RR (95% CI) |
|||||
| Overall | 2135 | 1.14(1.07–1.21) | 0.24 | 1598 | 1.20(1.10–1.30) | 0.62 | ||
| Sex | ||||||||
| Females | 1116 | 1.13(1.06–1.21) | 0.39 | 0.90 | 918 | 1.14(1.02–1.28) | 0.37 | 0.23 |
| Males | 1019 | 1.14(1.01–1.29) | 0.13 | 680 | 1.27(1.12–1.44) | 0.88 | ||
| Smoking Status | ||||||||
| Never4–5 | 748 | 1.19(1.08–1.31) | 0.20 | 0.12 | 628 | 1.28(1.12–1.46) | 0.52 | 0.11 |
| Past4,6 | 642 | 1.22(1.10–1.34) | 0.73 | 548 | 1.30(1.13–1.50) | 0.58 | ||
| Current7 | 628 | 1.07(0.95–1.21) | 0.25 | 338 | 1.02(0.84–1.23) | 0.50 | ||
| Physical Activity8–9 | ||||||||
| Low | 825 | 1.13(1.00–1.28) | 0.002 | 0.02 | 652 | 1.14(1.00–1.30) | 0.75 | 0.31 |
| Medium | 587 | 1.15(1.04–1.28) | 0.57 | 458 | 1.16(0.99–1.36) | 0.78 | ||
| High | 473 | 1.29(1.15–1.45) | 0.80 | 397 | 1.31(1.11–1.55) | 0.60 | ||
| Median Age at Diagnosis (years) | ||||||||
| <69 | 1042 | 1.15(1.07–1.25) | 0.35 | 0.82 | 749 | 1.19(1.05–1.33) | 0.58 | 0.91 |
| ≥69 | 1093 | 1.14(1.05–1.22) | 0.78 | 849 | 1.20(1.07–1.35) | 0.57 | ||
| Follow-up Time (years) | ||||||||
| < 5 10–11 | 726 | 1.20(1.10–1.31) | 0.50 | 0.24 | 511 | 1.12(0.96–1.31) | 0.38 | 0.26 |
| ≥5 | 1394 | 1.12(1.05–1.20) | 0.47 | 992 | 1.23(1.12–1.36) | 0.95 | ||
See Table 2 for adjustment factors. The stratification variable was excluded from the model.
P-value, test for between-studies heterogeneity
P-value, test for interaction
ATBC was excluded from the never and past smoking analyses because this study only included current smokers.
NLCS(Males) was excluded from this strata due to small case numbers (n<10)
MCCS(Females) was excluded from this strata due to small case numbers (n<10)
MCCS(Males and Females) were excluded from this strata due to small case numbers (n<10)
NYSC(Female and Males) was excluded from the physical activity analysis since they did not measure physical activity.
CNBSS was excluded from the physical activity analysis for BMI in early adulthood due to small case numbers (n<10).
MCCS(Females) were excluded from this strata due to small case numbers (n<10)
MCCS(Males) were excluded from this strata due to small case numbers(n<10)
Using an aggregated dataset and controlling for study-specific differences in age at diagnosis, we explored differences in mean age at diagnosis of pancreatic cancer continuously and by categories of BMI in early adulthood and BMI at age of enrollment (baseline). When examining BMI at age of enrollment as a continuous variable, mean age of diagnosis of pancreatic cancer was significantly decreased by 0.11 years for each 1kg/m2 unit increase in BMI. In the categorical analysis, the mean age of diagnosis of pancreatic cancer was decreased by 3.38 years when comparing ≥30kg/m2 to <21kg/m2. Similarly, after adjusting for BMI at age at enrollment, mean age of diagnosis of pancreatic cancer was decreased significantly by 0.09 years for each 1kg/m2 unit increase in BMI at early adulthood, and 2.44 years when comparing ≥25 kg/m2 to <18.5kg/m2.
DISCUSSION
In this pooled prospective analysis, a positive association was observed between BMI at baseline, BMI in early adulthood, weight loss > 2kg/m2 and weight gain ≥ 10kg/m2 during adulthood, and WHR and pancreatic cancer risk. Further, being obese and overweight at baseline and early adulthood was associated with a slightly younger age of diagnosis of pancreatic cancer. However, waist circumference, hip circumference, and height were unrelated to pancreatic cancer risk.
Our results support the conclusion by a recent WCRF/AICR panel of a convincing positive dose-response relationship between BMI and pancreatic cancer2; however, moderate to high between-studies heterogeneity was present in that review. Some of the heterogeneity may have been due to variation in how BMI was modeled across studies and the small number of cases in some studies. In general, epidemiologic studies with smaller number of cases were more likely to report null findings31. In two recent large cohort studies and one large case-control not included in the review, positive associations, similar to those reported here, were observed for BMI and pancreatic cancer risk14, 32, 33.
In contrast to our results, BMI in early adulthood was not associated with pancreatic cancer risk in four case-control studies5, 10, 11, 12. When we examined change in BMI from young adulthood to baseline, we observed a positive association for loss of >2kg/m2 or gain of ≥10kg/m2. Other studies that have examined change in adult weight or BMI, have reported on % weight change or absolute weight or BMI change; results have been inconsistent6, 8, 12, 34. The results of these studies may have also differed from ours due to the different characterization of the exposure, choice of covariates, small sample size, recall bias, selection bias and use of proxy information.
Studies have shown that greater central adiposity compared to peripheral adiposity is more strongly related to insulin resistance and diabetes35, two potential pancreatic cancer risk factors4. Our analysis suggests that in addition to obesity, abdominal adiposity may be independently associated with higher pancreatic cancer risk. Besides the studies in our pooled analysis, three other pancreatic cancer studies have examined central adiposity33, 36, 37. In NIH-AARP Diet and Health Study33, waist circumference was positively associated with pancreatic cancer risk in women, but not men, whereas no statistically significant association was found for WHR33. In EPIC37 and the Asian Pacific Cohort Consortium36, higher risk of pancreatic cancer was observed for higher waist circumference36, 37 and WHR37.
We observed no association between height and pancreatic cancer risk, which is similar to the majority of previously conducted studies2, 32, 38, 39. Only EPIC, not included in our analysis, observed a positive association between height and pancreatic cancer risk37.
Similar to many previous studies conducted, the majority of participants in each of the component studies were Caucasian. Thus, we did not have enough power to examine differences by race and ethnicity. However, the studies included in our analysis comprise populations from different geographic regions with different age ranges and education levels which may be considered a strength, particularly if the results are consistent across studies. One advantage of our study was that we were able to classify the main exposure and covariates uniformly, thereby lessening potential sources of heterogeneity across studies. However, height and weight were collected differently across studies; height and weight were self-reported in 12 studies and measured in 2 studies. Even though studies have shown that the under-stating of weight and over-stating of height can occur when comparing self-reported to measured factors40, 41, most studies have shown that the correlation is high between self-reported and measured height and weight42, 43. Thus, rankings of height and weight will be expected to be accurate even if there is systematic under- or over- reporting. In addition, all studies collected information on important pancreatic cancer risk factors including age, alcohol intake and smoking habits, and the majority of studies collected diabetes history.
Our pancreatic cancer case definition may also represent different subtypes of pancreatic cancer, and individual subtypes may be associated with different etiologies. When we limited the case definition for pancreatic cancer to adenocarcinomas, we observed similar estimates for all anthropometric factors as those reported for all pancreatic cancers. Thus, our conclusions are applicable at least to the largest group of pancreatic cancers.
In each component study, data on anthropometric factors were collected prior to cancer diagnosis; thus, a cancer diagnosis would not have influenced the reporting of anthropometry as may occur in a case-control study. However, individuals who were diagnosed close in time to baseline may have already experienced changes in anthropometry due to prediagnostic symptoms; in analyses where we excluded the first two and five years of cases, the results were similar to the overall results. Due to the inclusion of 14 cohort studies we had greater statistical power than individual studies to examine the association between anthropometry and pancreatic cancer risk and to assess whether or not these associations were modified by other pancreatic cancer risk factors.
Identifying modifiable risk factors for pancreatic cancer may help lessen the morbidity and mortality from this highly fatal disease that has few known potentially modifiable risk factors. We found positive associations between BMI at baseline, BMI in early adulthood, and WHR and pancreatic cancer risk in this pooled analysis of 846,340 individuals. Waist circumference, hip circumference, and height were not associated with pancreatic cancer risk. These results are in accordance with the WCRF/AICR recommendation to maintain body weight within the normal range2, as obesity – a potentially modifiable characteristic - is linked to a number of cancers, as well as diabetes and cardiovascular disease44.
Appropriate Article Category. Epidemiology
Novelty
Although several studies assessed the association between BMI and pancreatic cancer risk, and height and pancreatic cancer risk, fewer studies have examined BMI at young ages, changes in adult BMI, waist and hip circumference, or waist-to-hip ratio in a prospective design with high statistical power.
Impact of the paper
We prospectively assessed the association of anthropometric factors and pancreatic cancer risk in The Pooling Project of Prospective Studies of Diet and Cancer. Our analyses included 14 prospective cohort studies in which 2,135 incident pancreatic cancer cases were identified. Overall, our findings suggest a positive association between BMI at baseline, BMI at younger ages, waist to hip ratio and risk of pancreatic cancer.
Acknowledgment
This work was supported by National Institutes of Health grants #CA098566 and #CA55075.
References
- 1.GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwideed. Lyon: IARCPress; 2004. [Google Scholar]
- 2.World Cancer Research Fund, Panel AIfCRE, Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. American Institute for Cancer Research; 2007. [Google Scholar]
- 3.Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002;132:3451S–3455S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]
- 4.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–282. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Fryzek JP, Schenk M, Kinnard M, Greenson JK, Garabrant DH. The association of body mass index and pancreatic cancer in residents of southeastern Michigan, 1996–1999. Am J Epidemiol. 2005;162:222–228. doi: 10.1093/aje/kwi183. [DOI] [PubMed] [Google Scholar]
- 6.Ogren M, Hedberg M, Berglund G, Borgstrom A, Janzon L. Risk of pancreatic carcinoma in smokers enhanced by weight gain. Results from 10-year follow-up of the Malmo preventive Project Cohort Study. Int J Pancreatol. 1996;20:95–101. [PubMed] [Google Scholar]
- 7.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 8.Friedman GD, van den Eeden SK. Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol. 1993;22:30–37. doi: 10.1093/ije/22.1.30. [DOI] [PubMed] [Google Scholar]
- 9.Verhage BA, Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry and pancreatic cancer risk: an illustration of the importance of microscopic verification. Cancer Epidemiol Biomarkers Prev. 2007;16:1449–1454. doi: 10.1158/1055-9965.EPI-07-0201. [DOI] [PubMed] [Google Scholar]
- 10.Ji BT, Hatch MC, Chow WH, McLaughlin JK, Dai Q, Howe GR, Gao YT, Fraumeni JF., Jr Anthropometric and reproductive factors and the risk of pancreatic cancer: a case-control study in Shanghai, China. Int J Cancer. 1996;66:432–437. doi: 10.1002/(SICI)1097-0215(19960516)66:4<432::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Zatonski W, Przewozniak K, Howe GR, Maisonneuve P, Walker AM, Boyle P. Nutritional factors and pancreatic cancer: a case-control study from South-West Poland. Interactional Journal of Cancer. 1991;48:390–394. doi: 10.1002/ijc.2910480314. [DOI] [PubMed] [Google Scholar]
- 12.Eberle CA, Bracci PM, Holly EA. Anthropometric factors and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control. 2005;16:1235–1244. doi: 10.1007/s10552-005-0354-y. [DOI] [PubMed] [Google Scholar]
- 13.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandera EV, Freudenheim JL, Marshall JR, Zielezny M, Priore RL, Brasure J, Baptiste M, Graham S. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States) Cancer Causes Control. 1997;8:828–840. doi: 10.1023/a:1018456127018. [DOI] [PubMed] [Google Scholar]
- 16.Calton BA, Stolzenberg-Solomon RZ, Moore SC, Schatzkin A, Schairer C, Albanes D, Leitzmann MF. A prospective study of physical activity and the risk of pancreatic cancer among women (United States) BMC Cancer. 2008;8:63. doi: 10.1186/1471-2407-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang ET, Canchola AJ, Lee VS, Clarke CA, Purdie DM, Reynolds P, Bernstein L, Stram DO, Anton-Culver H, Deapen D, Mohrenweiser H, Peel D, et al. Wine and other alcohol consumption and risk of ovarian cancer in the California Teachers Study cohort. Cancer Causes Control. 2007;18:91–103. doi: 10.1007/s10552-006-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvera SA, Rohan TE, Jain M, Terry PD, Howe GR, Miller AB. Glycemic index, glycemic load, and pancreatic cancer risk (Canada) Cancer Causes Control. 2005;16:431–436. doi: 10.1007/s10552-004-5028-7. [DOI] [PubMed] [Google Scholar]
- 19.Patel AV, McCullough ML, Pavluck AL, Jacobs EJ, Thun MJ, Calle EE. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control. 2007;18:287–294. doi: 10.1007/s10552-006-0081-z. [DOI] [PubMed] [Google Scholar]
- 20.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomarkers Prev. 2005;14:1571–1573. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 21.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 22.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev. 2001;10:429–437. [PubMed] [Google Scholar]
- 23.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006;98:407–413. doi: 10.1093/jnci/djj094. [DOI] [PubMed] [Google Scholar]
- 25.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783–792. doi: 10.1093/aje/155.9.783. [DOI] [PubMed] [Google Scholar]
- 26.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, Hankinson SE, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–776. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith-Warner S, Spiegelman D, Ritz J, Albanes D, Beeson W, Bernstein L, Berrino F, van den Brandt P, Buring J, Cho E, Colditz G, Folsom A, et al. Methods for pooling results of epidemiologic studies: the pooling project of prospective studies of diet and cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization; International statistical classification of diseases and related health problems, 10th revision. Vol. 1
- 29.Physical status: The Use and Interpretation of Anthropometry. World Health Organization; Report of a WHO Expert Committee. 1995 [PubMed]
- 30.Lawless JF. Statistical models and methods for lifetime data.ed. New York: John Wiley and Sons; 1982. [Google Scholar]
- 31.Michaud DS, Fuchs CS. Obesity and pancreatic cancer: overall evidence and latency period. Cancer Epidemiol Biomarkers Prev. 2005;14:2678. doi: 10.1158/1055-9965.EPI-05-0428. author reply -9. [DOI] [PubMed] [Google Scholar]
- 32.Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, Beral V. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer. 2009;124:2400–2405. doi: 10.1002/ijc.24196. [DOI] [PubMed] [Google Scholar]
- 33.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A, Silverman DT. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 34.Hanley AJ, Johnson KC, Villeneuve PJ, Mao Y. Physical activity, anthropometric factors and risk of pancreatic cancer: results from the Canadian enhanced cancer surveillance system. Int J Cancer. 2001;94:140–147. doi: 10.1002/ijc.1446. [DOI] [PubMed] [Google Scholar]
- 35.Abate N. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care. 1996;19:292–294. doi: 10.2337/diacare.19.3.292. [DOI] [PubMed] [Google Scholar]
- 36.Ansary-Moghaddam A, Huxley R, Barzi F, Lawes C, Ohkubo T, Fang X, Jee SH, Woodward M. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol Biomarkers Prev. 2006;15:2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 37.Berrington de Gonzalez A, Spencer EA, Bueno-de-Mesquita HB, Roddam A, Stolzenberg-Solomon R, Halkjaer J, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Boeing H, Pischon T, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:879–885. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- 38.Friedman GD, van den Eeden SK. Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol. 1993;22:30–37. doi: 10.1093/ije/22.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr Physical activity, body weight, and pancreatic cancer mortality. Br J Cancer. 2003;88:679–683. doi: 10.1038/sj.bjc.6600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 41.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115:223–230. doi: 10.1093/oxfordjournals.aje.a113294. [DOI] [PubMed] [Google Scholar]
- 42.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 43.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]


