Abstract
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase that is ubiquitous in the nervous system and interacts with a myriad of substrates. Its modulation of synaptic plasticity and associated mechanisms of learning and memory as well as neurodegeneration and cognitive disease highlights its importance in the human brain. Cdk5 is active throughout the neuron via its kinase activity, protein-protein interactions, and nuclear associations. It regulates functions thought vital to memory and plasticity, including synaptic vesicle recycling, dendritic spine formation, neurotransmitter receptor density, and neuronal excitability. Although conditional knockout of Cdk5 improves learning and plasticity, the associated deleterious effects of increased excitability cast doubts on the therapeutic efficacy of systemic inhibitors. However, through further work on the regulation of Cdk5 and its effectors, this important molecule promises to aid in elucidating key pathways involved in learning and memory and uncover innovative therapeutic targets to treat neurodegenerative and neuropsychiatric diseases.
Keywords: Cdk5, Learning and memory, Plasticity, Neurodegeneration, Cognition
1. Introduction
Learning and memory are vital to animal development and the acquisition of modifiable, plastic neuronal circuits allows for robust adaptation to dynamic surroundings. The ability to learn from experience and employ stored knowledge in the form of memories to modify behavior in response to selective environmental pressures is crucial in animal physiology and cognition.
Where and how memories are formed and stored is a founding question in neuroscience and remains a major topic of ongoing research. Neuroscience pioneer Ramon y Cajal proposed over a century ago that mature, communicating neurons modulate the efficiency of their associations to form memories [7]. Hebb later suggested the existence of dynamic neuronal metabolism and growth that described certain forms of learning [25]. Such synaptic plasticity is supported by the neurophysiological paradigm of long-term potentiation (LTP) [5] and growing direct evidence of synaptic remodeling. As a result, an accepted theory has emerged which states that memories are formed and stored within neuronal circuits capable of modifying the strength of the synapses that connect them [50].
Numerous neurodegenerative and neuropsychiatric diseases include deficits in cognition, underscoring the importance of memory formation and recall [2]. As maladies such as Alzheimer’s disease become more prevalent in the population [26], there is increasing need to unravel the pathophysiology behind them. By gaining better understanding of the mechanisms of learning and memory, we may shed light on how signaling mechanisms become dysregulated and contribute to these pathological conditions. The neuronal protein kinase Cdk5 has been implicated in regulating synaptic plasticity, learning, and memory [23]. Better understanding of the specific mechanisms by which Cdk5 mediates cognition has the potential to reveal targets for the development of new and more effective treatment strategies.
Cdk5 is a proline-directed serine/threonine kinase that modulates cortical lamination and morphology during development [21] through phosphorylating and interacting with many substrates in the mature nervous system [16]. Cdk5 may be considered to be a constitutively active kinase. Its activity is dependent upon its direct association with one of two noncyclin cofactors p35 and p39 [14, 34]. These cofactors are subject to cleavage by calpain. Thus, increases in intracellular calcium, which may occur during neuronal injury or neurotoxic insult, results in conversion of p35 or p39 to p25 or p27, respectively. The resulting Cdk5/p25 or Cdk5/p27 complexes engender aberrant activity that results in deleterious effects or neuronal death, and may lead to neurodegeneration and disease [22, 42].
While a number of deleterious effectors of Cdk5/p25 have been suggested to mediate neuronal injury, it is also possible that strong synaptic activity and calpain activation produces p25 locally during enhanced learning. Furthermore, the circumstances that cause Cdk5/p25 to exist in aberrant form are still unclear. For example, the kinetics of Cdk5-dependent phosphorylation of neurodegenerative targets such as tau or chromatin-associated histone H1 were shown to be unchanged in vitro, regardless of activation by p35 or p25 [43]. However, an earlier report [22] associated deviant Cdk5/p25 activity with pathological augmentation of its kinase actions. Either scenario does not eliminate the possibility of subcellular localization, posttranslational modifications, or protein-protein interactions playing a differentiating role in imparting aberrant activity upon Cdk5 in vivo.
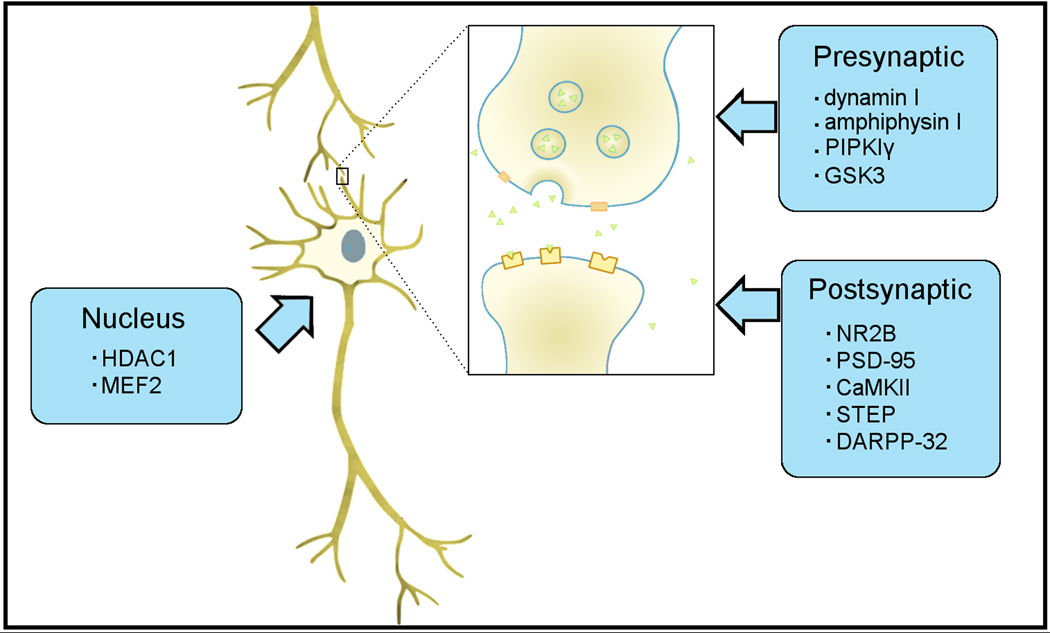
While Cdk5 occurs throughout neurons, here we will focus on three major regions within neurons where Cdk5 function appears critical in modulating synaptic plasticity, memory, and disease. Examples of functions through which it contributes to learning are discussed. These regions, the presynaptic and postsynaptic compartments, as well as the cell nucleus, host key functions of Cdk5 and its activators (Fig. 1).
Fig. 1.
Summary of several key molecules linked to synaptic plasticity with which Cdk5 interacts. In the presynaptic compartment, Cdk5 modulates the dephosphins dynamin I, amphiphysin I, and PIPKIγ as well as GSK3 to affect synaptic vesicle retrieval and priming. In the postsynaptic compartment, Cdk5 interacts with molecules controlling cytoskeletal architecture and receptor density, including NR2B, PSD-95, CaMKII, STEP, and DARPP-32. In the nucleus, Cdk5 interacts with HDAC1 to influence gene expression epigenetically and MEF2 to inhibit neuronal pruning.
2. Presynaptic signaling
The activity of the presynaptic terminal is crucial to interneuronal communication. As the source of synaptic vesicles carrying neurotransmitters and the site of vesicle recycling, the synaptic bouton is the final output of a neuron. Therefore, any regulator of vesicle fusion or recovery at the axon terminal is a potential modifier of synaptic strength for a given stimulus and may be involved in learning and memory.
Membrane depolarization at the axonal terminal results in synaptic vesicle fusion with the plasma membrane at the synaptic cleft, and neurotransmitter release [41]. A recent study suggests that Cdk5 functions as a key control point for the modulation of neurotransmitter release. Although the substrates involved remain unclear, this study indicates that Cdk5 activity is a major determinant in unmasking silent synapses [30].
Exocytosis at neuronal synapses is necessarily complemented by endocytosis. During gentle stimulation, vesicles are retrieved primarily via a well-described form of clathrin-dependent endocytosis (CDE), which is a relatively slow and low-capacity mechanism [20]. More robust stimulation which may occur during memory formation necessitates a distinct method of vesicle retrieval to ensure faithful signal transmission despite increased vesicle fusion and neurotransmitter release.
Activity-dependent bulk endocytosis (ADBE) monopolizes vesicle recycling during high frequency stimulation [11]. Compared to CDE, ADBE is a high-capacity process in which large portions of the membrane are pinched off, internalized, and formed into an endosome from which individual vesicles bud [48, 56]. Recently, Cheung et al. have discovered that the vesicles produced from ADBE and CDE replenish separate pools, with the former contributing to a reserve pool that becomes activated and utilized during intense stimulation [9]. These results suggest that the differential methods of vesicle retrieval may shape how a neuron responds to future stimuli.
ADBE is dependent on a class of molecules termed dephosphins [13]. Dephosphins, including dynamin I, synaptojanin, and phosphatidylinositol phosphate kinase Iγ (PIPKIγ), are necessarily dephosphorylated by calcineurin in a Ca2+-dependent fashion to trigger ADBE. Cdk5 rephosphorylates these dephosphins [32–33, 52] and is essential for vesicle recycling and continued neurotransmitter release. Ablation of Cdk5 activity in vivo halts vesicle endocytosis due to lack of dephosphin rephosphorylation [52]. Evidence that Cdk5 and its physiological activator p35 are enriched in synaptosomes [53] corroborates the role of Cdk5 in the presynaptic terminal.
Cdk5 has other important substrates that control ADBE. Clayton et al. describe glycogen synthase kinase 3 (GSK3) as a necessary component of the phosphorylation cascade of vesicle retrieval. Interestingly, GSK3 phosphorylation of dynamin I at Ser-774 is completely dependent upon previous dynamin I phosphorylation at Ser-778 by Cdk5 [12]. The subsequent dephosphorylation of these two sites by calcineurin initiates ADBE [10].
From these reports, it appears that high-intensity stimulation induces a cycle of activity-dependent phosphorylation/dephosphorylation of dephosphins. In this sequence Cdk5 functions as a master switch that primes various components in the machinery, while the phosphatase calcineurin initiates the ADBE vesicle retrieval mechanism. Such a scheme would allow the vesicle pool of the synaptic bouton to plastically change its response to stimuli based on experience.
3. Postsynaptic signaling
Numerous signaling pathways within dendritic spines that have been implicated in plasticity and learning are regulated by Cdk5. Neurotransmitter receptors are anchored in an insoluble intracellular matrix known as the postsynaptic density. A prominent component of this key structure is the scaffold protein PSD-95, which structurally links signaling complexes to receptors. Cdk5-dependent phosphorylation of this protein inhibits multimerization and clustering with ion channels [38], thus modulating the molecular architecture of the postsynaptic terminal.
Ca2+/calmodulin-dependent protein kinase (CaMKII) is another constituent of the postsynaptic density involved in the synaptic remodeling that underlies learning. Cdk5 and its cofactors interact with the α subunit of CaMKII within the postsynaptic density in a Ca2+-dependent manner [15] and may regulate the activity of this kinase [27].
Cdk5 also regulates excitatory ionotropic glutamate receptors that are essential to learning. For example, Cdk5 mediates interactions between calpain and the NR2B subunit of the NMDA receptor. Conditional Cdk5 knockout in adult mice improves hippocampal-dependent learning and plasticity due to higher levels of NR2B at the synapse caused by the disruption of the calpain/NR2B complex [23]. Interestingly, the enhanced cognition and plasticity are accompanied by elevations in basal excitability and contribute to the development of epileptiform activity and audiogenic seizures [24], supporting Cdk5’s role in tonal repression and baseline maintenance in neurons. Furthermore, NR2B receptor surface levels are regulated via phosphorylation of their C-terminal cytoplasmic domain by tyrosine kinases. The tyrosine phosphatase STEP hydrolyzes these phosphates and is regulated by Cdk5 [57]. Cdk5 has also been suggested to phosphorylate the NR2A subunit [35, 54]. Additionally, Cdk5 may regulate the anchoring of the GluR2 subunit of the AMPA receptor via phosphorylation of the synaptic adherens junction protein δ-catenin [45].
Ionotropic glutamate receptors may mediate synaptic remodeling via Ca2+-dependent signaling pathways such as those invoked via CaMKII. However, the second messenger cAMP is also essential to learning and G protein-coupled receptor (GPCR) signaling is modulated by Cdk5. For example, Cdk5 regulates the protein phosphatase-1 inhibitors DARPP-32 [4] and inhibitor-1[3, 39], both of which are activated by cAMP/PKA and have been implicated in postsynaptic plasticity mechanisms [8, 36]. Unpublished findings from our laboratory indicate that Cdk5 regulates cAMP-dependent signaling at multiple steps and may maintain a baseline tonus through these pathways in a manner that might be considered analogous with its presynaptic function.
In response to synaptic firing, localized conversion of p35 to p25 may redirect the kinase from its subcellular localization with membranous or cytoskeletal components, thereby removing its tonic regulatory influence on synaptic pathways. This remains to be determined and the functions of p25 in plasticity are presently unclear. Evidence indicates that Cdk5/p25 contributes to the loss of mitochondrial integrity [37]. Cdk5/p25 could influence mitochondrial translocation at active synapses, for example, through the phosphorylation of proteins such as the dynamin-related GTPase Drp1 that are targeted during mitosis by Cdk1 [51].
While the list of postsynaptic Cdk5 substrates is growing, understanding of its regulation remains incomplete. Although conditional Cdk5 knockout has proven to be a useful tool to study the function of Cdk5 in cognition, few selective inhibitors such as CP-681301 [55] and Indolinone A [18] have become readily available and appear ineffective systemically in our laboratory. The development of cell-specific Cdk5 conditional knockouts, systemic inhibitors with strong pharmacokinetic properties, and new strategies to selectively target specific Cdk5 signaling pathways will lead to important advances in understanding the regulation and function of Cdk5 in learning and memory mechanisms.
4. Nuclear signaling
More recently, Cdk5 has been connected to the regulation of neuronal gene transcription. This modulation of gene and protein expression provides a mechanism through which Cdk5 may produce long lasting changes in neuronal architecture, excitability, and viability. Because Cdk5, to our knowledge, has never been shown to be mutated in a pathological state, its effects in the nucleus could explain associations between wild-type Cdk5 and neurodegenerative insults such as Parkinson’s disease, amyotrophic lateral sclerosis, Alzheimer’s disease, and ischemia.
Alteration of histone acetylation is a critical part of chromatin remodeling and hence gene transcription [47]. Histone deacetylase 1 (HDAC1) action in gene promoter regions condenses chromatin, impedes access of transcription factors, and suppresses transcription [6]. Kim et al. have shown that p25-expressing transgenic mice undergo HDAC1 inactivation in a Cdk5-dependent manner [29]. How Cdk5 inactivates HDAC1 remains to be seen, but it is clear that this inactivation leads to abnormal cell cycle activity, DNA damage, and neuronal death [29]. Future studies may expand the association between HDACs and Cdk5 and the role of these pathways in the formation of memory.
Cdk5 maintains other links within the nucleus. The transcription factor myocyte enhancer factor 2 (MEF2) is a pro-survival signal in neurons which reduces interneuronal connections by decreasing the number of dendritic spines in an activity-dependent fashion [17]. This synaptic pruning which is paramount in the maturing nervous system is diminished in several conditions including fragile X syndrome, a common form of inherited mental retardation and autism [28]. Cdk5 inhibits MEF2 by phosphorylating residue Ser444 [19], such that under calpain-induced p25 production, dopaminergic striatal neurons are lost in a mouse model of Parkinson’s disease [49]. Loss of fragile x mental retardation protein in fragile X syndrome eliminates synaptic pruning via MEF2-activated targets [44]. Inhibition of MEF2 via Cdk5/p25 may create a condition of numerous inappropriate excitatory connections that would typically be eliminated by MEF2 pruning, leading to mental impairments and autistic phenotypes.
Nuclear and epigenetic mechanisms controlling learning and memory may also contribute to the etiology of neuropsychiatric disorders such as addiction which have strong cognitive components. For example, increases in Cdk5-dependent inhibitory phosphorylation of MEF2 may contribute to increases in synaptic spine density in response to chronic cocaine exposure [46]. This neuroadaptive morphological effect is dependent upon Cdk5 activity [40] and Cdk5 itself serves as an epigenetic target of cocaine via the invocation of the ΔFosB transcription factor [1, 31].
5. Conclusions
Cdk5 is essential to learning and memory in the adult brain and has the ability to affect many neuronal divisions, including the pre- and postsynaptic compartments and the nucleus. For example, as a governor and primer of ADBE, Cdk5 augments plasticity presynaptically. By facilitating the proteolysis of NMDA receptor subunits postsynaptically, it functions to modulate excitability and synapse strengthening. Epigenetically, deregulated Cdk5 can lead to transcription of genes that affect dendritic spine number and induce memory deficits. Unlike other protein kinases involved in plasticity, Cdk5 is constitutively active and the dephosphorylation of its substrate sites in response to Ca2+ conductance may take precedence over its directed holoenzyme activity in mediating synaptic remodeling.
Regulatory mechanisms controlling Cdk5 activity remain unclear, but the relationship between the physiological function of Cdk5 in cognition and its contribution to neuronal injury and death is palpable. A reasonable hypothesis linking Cdk5 and synaptic plasticity is that the machinery of the cell cycle was adopted to serve synaptic remodeling, allowing the conversion of naïve synapses to form strong connections, and thereby recording experiences within neuronal circuits. Cdk5 may be the adaptive product of CNS evolution, but its neurotoxic potential may lie within its vestigial cell cycle-like nature.
Cdk5 reduction enhances cognition and systemic drugs that inhibit its activity have been considered as a therapeutic strategy for neurodegenerative diseases. However, the consequence of its essential role in cognition may be that targeting its enzymatic activity produces unacceptable side effects. While this may reduce the enthusiasm for Cdk5 drug discovery, it is important to note that there may be very high therapeutic value in inhibiting Cdk5 acutely during neuronal injury such as that associated with stroke or traumatic brain injury, or when risk is high such as during protracted labor or during cardiovascular or neurovascular repair surgeries. For other neurological or neuropsychiatric diseases, selectively targeting specific regulatory pathways or blocking excessive aberrant activity may be an effective alternative to explore.
Since its discovery, Cdk5 has been found to be essential to CNS function and its role in peripheral tissue and disease is growing. As our understanding of its role in cognition continues to expand, Cdk5 will likely prove to be a powerful and focused tool in profiling behavioral and disease states. The knowledge gained from the study of Cdk5 will lead to new perspectives and deeper comprehension of the key pathways involved in memory and novel therapeutic approaches for neurological and neuropsychiatric disorders.
Acknowledgements
Support for this work was provided by U.S. National Institutes of Health Grants to J.A.B. (MH79710, MH083711, and DA016672). We wish to thank the Medical Scientist Training Program, University of Texas Southwestern Medical Center.
Abbreviations
- ADBE
activity-dependent bulk endocytosis
- CaMKII
Ca2+/calmodulin-dependent protein kinase
- CDE
clathrin-dependent endocytosis
- Cdk5
cyclin-dependent kinase 5
- GSK3
glycogen synthase kinase 3
- HDAC1
histone deacetylase 1
- LTP
long-term potentiation
- MEF2
myocyte enhancer factor 2
- PIPKIγ
phosphatidylinositol phosphate kinase Iγ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare they hold no conflicts of interest.
References
- 1.Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 2.Bibb JA, Mayford MR, Tsien JZ, Alberini CM. Cognition enhancement strategies. J Neurosci. 2010;30:14987–14992. doi: 10.1523/JNEUROSCI.4419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibb JA, Nishi A, O'Callaghan JP, Ule J, Lan M, Snyder GL, Horiuchi A, Saito T, Hisanaga S, Czernik AJ, Nairn AC, Greengard P. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J Biol Chem. 2001;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- 4.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 7.Cajal SRY. The Croonian Lecture: La Fine Structure des Centres Nerveux. Proceedings of the Royal Society of London. 1894;55:444–468. [Google Scholar]
- 8.Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung G, Jupp OJ, Cousin MA. Activity-dependent bulk endocytosis and clathrin-dependent endocytosis replenish specific synaptic vesicle pools in central nerve terminals. J Neurosci. 2010;30:8151–8161. doi: 10.1523/JNEUROSCI.0293-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clayton EL, Anggono V, Smillie KJ, Chau N, Robinson PJ, Cousin MA. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J Neurosci. 2009;29:7706–7717. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28:6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton EL, Sue N, Smillie KJ, O'Leary T, Bache N, Cheung G, Cole AR, Wyllie DJ, Sutherland C, Robinson PJ, Cousin MA. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat Neurosci. 2010;13:845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 14.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 15.Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner. J Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 17.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 18.Gillardon F, Steinlein P, Burger E, Hildebrandt T, Gerner C. Phosphoproteome and transcriptome analysis of the neuronal response to a CDK5 inhibitor. Proteomics. 2005;5:1299–1307. doi: 10.1002/pmic.200400992. [DOI] [PubMed] [Google Scholar]
- 19.Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 20.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- 22.Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T. Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem. 2002;277:44525–44530. doi: 10.1074/jbc.M207426200. [DOI] [PubMed] [Google Scholar]
- 23.Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawasli AH, Koovakkattu D, Hayashi K, Anderson AE, Powell CM, Sinton CM, Bibb JA, Cooper DC. Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5. PLoS One. 2009;4:e5808. doi: 10.1371/journal.pone.0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebb DO. The Organization of Behavior Wiley. New York: 1949. [DOI] [PubMed] [Google Scholar]
- 26.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 27.Hosokawa T, Saito T, Asada A, Ohshima T, Itakura M, Takahashi M, Fukunaga K, Hisanaga S. Enhanced activation of Ca2+/calmodulin-dependent protein kinase II upon downregulation of cyclin-dependent kinase 5-p35. J Neurosci Res. 2006;84:747–754. doi: 10.1002/jnr.20975. [DOI] [PubMed] [Google Scholar]
- 28.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, Guan JS, Lee BH, Moy LY, Giusti P, Broodie N, Mazitschek R, Delalle I, Haggarty SJ, Neve RL, Lu Y, Tsai LH. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Voronov S, Letinic K, Nairn AC, Di Paolo G, De Camilli P. Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases. J Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 35.Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L, Braus GH, Dietz GP, Jakobs S, Bahr M, Weishaupt JH. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- 38.Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen C, Nishi A, Kansy JW, Fernandez J, Hayashi K, Gillardon F, Hemmings HC, Jr, Nairn AC, Bibb JA. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem. 2007;282:16511–16520. doi: 10.1074/jbc.M701046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 43.Peterson DW, Ando DM, Taketa DA, Zhou H, Dahlquist FW, Lew J. No difference in kinetics of tau or histone phosphorylation by CDK5/p25 versus CDK5/p35 in vitro. Proc Natl Acad Sci U S A. 2010;107:2884–2889. doi: 10.1073/pnas.0912718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poore CP, Sundaram JR, Pareek TK, Fu A, Amin N, Mohamed NE, Zheng YL, Goh AX, Lai MK, Ip NY, Pant HC, Kesavapany S. Cdk5-mediated phosphorylation of delta-catenin regulates its localization and GluR2-mediated synaptic activity. J Neurosci. 2010;30:8457–8467. doi: 10.1523/JNEUROSCI.6062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 48.Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J Physiol. 2003;553:345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweatt JD. Mechanisms of Memory. San Diego: Elsevier; 2003. [Google Scholar]
- 51.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 52.Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- 53.Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 55.Wen Y, Yu WH, Maloney B, Bailey J, Ma J, Marie I, Maurin T, Wang L, Figueroa H, Herman M, Krishnamurthy P, Liu L, Planel E, Lau LF, Lahiri DK, Duff K. Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron. 2008;57:680–690. doi: 10.1016/j.neuron.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]