Abstract
Objective
To determine if 5-HT1D receptors are located in the sphenopalatine ganglion.
Background
While the 5-HT1D receptor has been described in sensory and sympathetic ganglia in the head, it was not known whether they were also located in parasympathetic ganglia.
Methods
We used retrograde labeling combined with immunohistochemistry to examine 5-HT1D receptor immunoreactivity in rat sphenopalatine ganglion neurons that project to the lacrimal gland, nasal mucosa, cerebral vasculature and trigeminal ganglion.
Results
We found 5-HT1D receptor immunoreactivity in nerve terminals around postganglionic cell bodies within the sphenopalatine ganglion. All 5-HT1D immunoreactive terminals were also immunoreactive for calcitonin-gene related peptide but not vesicular acetylcholine transporter, suggesting that they were sensory and not preganglionic parasympathetic fibers. Our retrograde labeling studies showed that approximately 30% of sphenopalatine ganglion neurons innervating the lacrimal gland, 23% innervating the nasal mucosa and 39% innervating the trigeminal ganglion were in apparent contact with 5-HT1D receptor containing nerve terminals.
Conclusion
These data suggest that 5-HT1D receptors within primary afferent neurons that innervate the sphenopalatine ganglion are in a position to modulate the excitability of postganglionic parasympathetic neurons that innervate the lacrimal gland and nasal mucosa, as well as the trigeminal ganglion. This has implications for triptan (5-HT1D receptor agonist) actions on parasympathetic symptoms in cluster headache.
Keywords: parasympathetic, 5-HT1D, sumatriptan, trigeminal, axon reflex, calcitonin-gene related peptide
Introduction
Cluster headache is characterized by recurrent, short-lasting attacks of excruciating unilateral pain accompanied by ipsilateral autonomic signs1–3. The association of pain and autonomic signs, however, is common in several other distinct headache syndromes, including the other trigeminal-autonomic cephalalgias1, and is also present in those with migraine, albeit with less frequency (in approximately 30% of cases)2, 4.
A prevailing view of the primary headache disorders hypothesizes that pain arises from the activation of a functional complex of trigeminal primary afferent fibers, intracranial vasculature, and pain-regulatory centers of the brain5. Primary afferent activation causes the release of vasoactive peptides6 and could result in the further sensitization of trigeminovascular neurons and the propagation of pain7. Reflex activation of the brainstem parasympathetic nuclei and autonomic outflow through peripheral parasympathetic ganglia may also contribute to trigeminovascular activation and pain1. Importantly, the activation of parasympathetic outflow may explain the many autonomic signs associated with cluster headache (and migraine) during the attack1, 8.
Sumatriptan, and the other so-called triptans, have selective and profound clinical efficacy in the treatment of pain associated with migraine and cluster headache attacks, but also have significant efficacy in alleviating the accompanying autonomic signs in cluster headache9–13. Although the triptans are thought to exert their modulatory effects through the activation of a subset of serotonin receptors (5-HT1B, 5-HT1D, and 5-HT1F), the neuro-anatomy and physiology of these modulatory actions remain controversial and uncertain. The 5-HT1D receptors are of particular interest because they are expressed by trigeminal primary afferent neurons14–16, and are localized both in their central terminals14, 15 and in peripheral terminals innervating intracranial vasculature14, 17. The autonomic signs associated with cluster headache may be alleviated by triptans through a decrease in the reflex activation of brainstem parasympathetic outflow that results from reduced drive in trigeminovascular neurons following peripheral 5-HT1D receptor activation1.
We hypothesized that activation of 5-HT1D receptors at peripheral sites other than the primary afferent terminals in the intracranial vasculature may account for reduction in some of the autonomic signs associated with cluster headache. The aim of the present study was therefore to determine whether 5-HT1D receptors are located in cranial parasympathetic ganglia, and whether there is an association between 5-HT1D receptor immunoreactivity and postganglionic parasympathetic neurons that innervate the lacrimal gland and nasal mucosa. The sphenopalatine ganglion (SPG) was chosen for investigation because some of the cranial autonomic signs in cluster headache are most likely mediated by postganglionic parasympathetic neurons originating in the SPG.
Methods
Sprague-Dawley rats (n = 30) weighing between 200–250 g were used in this study. All experiments conformed to the Australian National Health and Medical Research Council code of practice for the use of animals in research, and were approved by the University of Melbourne Animal Experimentation Ethics Committee.
Retrograde tracing
Retrograde tracing experiments were performed to identify SPG postganglionic parasympathetic neurons that innervate the lacrimal gland, nasal mucosa, the intracranial vasculature and the trigeminal ganglion. Each observation was made in at least three animals. Animals were anesthetized with ketamine (100 mg/kg; i.p.) and xylazine (10 mg/kg; i.p.). The depth of anesthesia was monitored by regularly checking for the corneal blink reflex and forelimb withdrawal reflex following noxious paw pinching. For lacrimal gland injections, a skin incision was made over the lateral aspect of jaw to reveal the lacrimal gland. Two injections of 0.5 µl of the retrograde tracer Fast Blue ((4'-Amino-2',5'-diethoxybenzanilide) 2% in distilled water; FB) were placed directly into the lacrimal gland with a Hamilton syringe. The injection site was washed extensively with 0.01M PBS and inspected for tracer leakage using a hand-held UV illumination device. No animals showed evidence of tracer leakage to surrounding tissues. Skin incisions were closed with stainless steel autoclips. Injections into the nasal mucosa (1 µl FB) were made with a Hamilton syringe, through the nostril and into the mucosa on the lower aspect of the lateral wall of the nasal cavity. For retrograde labeling from the intracranial vasculature, we targeted the middle meningeal artery in the dura and the middle cerebral artery. In the case of the middle meningeal artery, a skin incision was made over the calvarium and a small square piece of bone removed from the skull posterior to the orbital rim with a dental drill. A small piece of gel foam soaked in FB was placed directly onto the dura over the middle meningeal artery. In the case of the middle cerebral artery, a skin incision was made between the eye and ear, the temporalis muscle retracted, and then a small square piece of bone removed from the temporal bone to expose the underlying middle cerebral artery. The dura and arachnoid were cut and retracted from the middle cerebral artery and a small piece of gel foam soaked in FB was placed directly onto the artery. The square pieces of bone were placed back over the holes created to expose the intracranial vessels and were covered with parafilm to prevent leakage of the tracer to surrounding tissues. Skin incisions were closed with stainless steel autoclips.
In the case of the trigeminal ganglion injections, the animal was anaesthetized as above and placed in a stereotaxic headframe. The skin over the cranium was cut to expose the calvarium, and a small hole was drilled into the bone at a location defined by the stereotaxic co-ordinates noted below. The dura was then carefully retracted and a Hamilton syringe, loaded with 2 % FB, was lowered into the brain at stereotaxic coordinates: 3.0 mm posterior and 2.5 mm lateral to bregma18. The syringe was lowered 9.5–11.5mm until the sphenoid bone was encountered. As the needle passed through the TG, the animals blinked due to mechanical activation of trigeminal ganglion neurons associated with the blink reflex. This blink was used as a marker for correct placement of the syringe. 1 µl of 2 % FB was injected at this location into the trigeminal ganglion. The syringe was left in place for 5 minutes to minimize backtracking and then slowly withdrawn. The skin was closed with stainless steel autoclips and treated with Betadine. After surgery, each animal was placed under a heat lamp until they recovered from anesthesia, and then returned to their home cage.
All animals used for retrograde tracing were allowed to survive for 5 days to allow for transport of the tracer to neuronal somata in the SPG. Post-operative analgesia was not provided, but each animal was monitored carefully in the post-operative period and then daily throughout the survival period to ensure they were not showing signs of discomfort. We also tried longer survival times (up to 12 days) and a different tracer (2% True Blue) for retrograde tracing from the intracranial vasculature. True blue was used as previous studies reported retrograde labeling of the intracranial vasculature using True Blue19. Furthermore, we conducted a control experiment to determine whether retrograde labeling of SPG neurons could result from leakage of FB into the dura around the trigeminal ganglion when it was targeted with our stereotaxic technique. In this case, 1 µl of 2 % FB was injected onto the dura adjacent to the trigeminal ganglion (2 mm posterior and 2 mm lateral to the ganglion) using the same approach to that described above. There was no retrograde labeling in the SPG when the dura around the trigeminal ganglion was injected.
Tissue preparation
Each animal was given an overdose of sodium pentobarbitone (Lethobarb; 150 mg/kg; i.p.), and was perfused via the ascending aorta with 250 ml of heparinized 0.01M phosphate buffered saline (PBS) followed by 250 ml of 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). SPG (and in some cases trigeminal ganglia) were dissected, post-fixed in the above fixative for 1 hr, and left overnight in 30% sucrose in 0.01M PBS. The ganglia were sectioned at 20 µm using a cryostat (−19°C) the next day. Multiple series (1 in every 3 sections- 10 to 15 sections per ganglion) were collected on gelatinized glass slides (1% gelatin) and processed for immunoreactivity to the antisera described below.
Immunohistochemistry
Sections were processed to reveal immunoreactivity for the 5-HT1D receptor, the peptide neurotransmitter, calcitonin-gene related peptide (CGRP; located in trigeminal primary afferent fibers in the SPG)20 or vesicular acetylcholine transporter (VAChT; a marker for cholinergic nerve terminals21 which enables identification of pre-ganglionic parasympathetic terminals). Sections were washed 3 times in 0.01M PBS and incubated for one hour in a solution containing 10% normal horse serum and 0.5% Triton X-100 in 0.01M PBS, and then for two nights (at 4°C) in the primary antisera (for details see antibody specificity and characterization below). Primary antisera were diluted in 0.01M PBS containing 0.5% Triton X-100 and 0.1% sodium azide. Following 3 further washes in 0.01M PBS, sections were incubated in secondary antibody for 2 hrs, and washed again 3 times in 0.01M PBS. Secondary antisera (donkey anti-rabbit 594 or donkey anti-goat 488; AlexaFluor; Molecular Probes) were diluted in 0.01M PBS. The sections were cover-slipped using Dako (Carpinteria, CA) fluorescence mounting medium. Double labeling of the 5-HT1D receptor antibody and antisera raised against CGRP and VAChT was also performed and in these cases, both antisera were diluted in the same incubation solution.
Antibody specificity and characterization
The anti-5-HT1D receptor antibody used in this study is a polyclonal antibody raised in rabbit against an oligopeptide with sequence CLNPPSLYGKRFTTAQ, corresponding to amino acid residues 226–240 translated from the cDNA sequence of the rat, with an N-terminal cysteine added to the synthetic oligopeptide15. Specificity has been determined by the presence of 5-HT1D receptor immuno-reactivity in HEK cells expressing mouse 5-HT1D receptor, and absence of 5-HT1D receptor immuno-reactivity in HEK cells expressing mouse 5HT1B and -1F receptors15. Preabsorption of the primary antibody with the 5-HT1D receptor antigen peptide and omission of the primary antibody abolished immunoreactivity in rat sensory ganglia15. Absorption controls were also performed using substance P and peripherin15 and showed no reduction in 5-HT1D receptor immunoreactivity. In the current study, we extended this characterization by pre-incubating the 5-HT1D antibody with up to 900µg/ml CGRP (amino acids 1–37, Tocris Bioscience, Bristol, UK) for 24 hours prior to exposing the antibody to tissue, and showed no reduction in 5-HT1D immunoreactivity in the SPG or trigeminal ganglion. We have confirmed that the pattern of 5-HT1D receptor immunoreactivity observed in the trigeminal ganglion matches that of other studies using other 5-HT1D antibodies14, 17 (data not shown).
The anti-CGRP antibody (Biogenesis; #1720-9007) is raised in goat against synthetic rat Tyr-CGRP (23–37) and is thought to react with the whole CGRP molecule (1–37) and the C-terminal fragment (23–37) (manufacturer’s information). Western blot analysis has been used to verify the specificity of the anti-CGRP antibody in rat medulla extracts and pre-absorption with the manufacturer’s synthetic CGRP peptide abolishes immunostaining22.
The anti-VAChT antibody (Chemicon; #AB1578) is a polyclonal antibody raised in goat against a synthetic peptide corresponding to amino acids 511–530 predicted from the cloned rat VAChT (manufacturer’s information). It selectively labels CV-1 cells transfected with VAChT cDNA23, and pre-absorbion of the antibody with the manufacturer’s control peptide abolishes immunostaining24.
The specificity of secondary antibodies was tested in the present study by omission of the primary antibodies, which always resulted in no immunostaining.
Image Acquisition and Data Analysis
Sections were examined and photographed using a Zeiss Axioskop fluorescence microscope (Zeiss, Oberkocken, Germany) fitted with a Zeiss 62 HE filter set and an AxioCam MRm camera. Examination of 5-HT1D receptor immunoreactivity and double labeling with CGRP or VAChT was performed through the 63× or 100× objective. Networks of 5-HT1D receptor immunoreactive fibers and varicosities that surrounded postganglionic neurons were given an “innervation density score” on a scale ranging from 0 to 3, to indicate the density of pericellular 5-HT1D receptor nerve terminals surrounding each soma. We made the assumption that the varicosities noted were terminals on the basis of electron microscopic observations that varicosities in the SPG contain synaptic densities, small clear vesicles and large dense core vesicles25, 26. Only terminals in direct apposition to the soma were considered. A score of 3 was given to cell profiles that were surrounded by a dense network of 5-HT1D receptor immunoreactive terminals that made apparent contact with more than half the circumference of the cell profile; a score of 2 given to cell profiles associated with 5-HT1D receptor immunoreactive terminals that made apparent contact with less than half, but more than one quarter of the circumference of the cell profile; and a score of 1 given to cell profiles if less than one quarter of the circumference was surrounded by 5-HT1D receptor immunoreactive terminals. Neuronal profiles that were not contacted by any 5-HT1D receptor immunoreactive terminals were scored as 0. To prevent double counting, only cells with a nucleus visible under the microscope were examined. Innervation scores were determined for all of the SPG somata in every section, and also for retrogradely labeled postganglionic neurons from the lacrimal gland, nasal mucosa and trigeminal ganglion.
To generate figures that best represented what we observed down the microscope, we imaged representative sections using confocal microscopy (Zeiss 510 META confocal microscope), and have presented Z-projections to show the arrangement of the fiber labeling through the entire thickness of the section. Figures were prepared using Image J (NIH) and Corel Draw software. Individual images were contrast and brightness adjusted. No other manipulations were made to the images.
Results
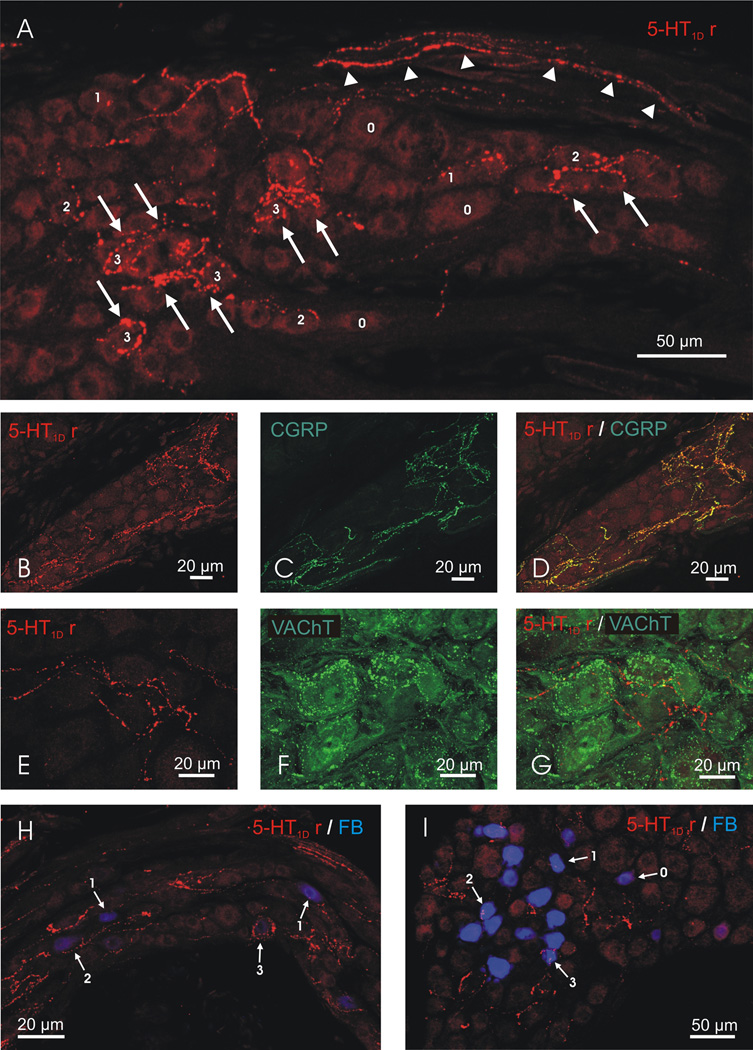
Immunohistochemical labeling of 5-HT1D receptors revealed that 5-HT1D receptor immunoreactivity was confined to fibers traversing the SPG and terminals that are in close apposition to neuronal cell bodies in the SPG (n = 5 animals, Figure 1A). No cell bodies in the SPG were 5-HT1D receptor immunoreactive. In many cases, SPG neurons were completely surrounded by complex, pericellular “basket-like” arrangements of 5-HT1D receptor immunoreactive terminals. We quantified the extent of innervation in a total of 6146 cell profiles in 3 animals and found that 1.5 ± 0.5% (mean ± SEM) of SPG cell profiles were almost completely surrounded by 5-HT1D receptor immunoreactive terminals (score = 3), 4.2 ± 0.6% had terminals in apparent contact with less than half but more than one quarter of the profile (score = 2), and 20.9 ± 3% had terminals in contact with less than one quarter of the profile (score = 1). Overall, we found 5-HT1D receptor immunoreactive terminals in close apposition to almost 27% of the SPG neurons. All of these 5-HT1D receptor immunoreactive fibers and terminals in the SPG were double labeled with antisera directed against CGRP (n = 3 animals, Figure 1B, C and D) and no 5-HT1D receptor immunoreactive fibers and terminals in the SPG were double labeled with antisera directed against VAChT (n = 3 animals, Figure 1E, F and G), suggesting that they were of sensory, not preganglionic parasympathetic origin.
Figure 1.
(A) Confocal Z-projection of a section of rat SPG showing 5-HT1D receptor immunoreactivity confined to fibers traversing the SPG (arrowheads) and to terminals that were in close apposition to neuronal cell bodies (numbered 0–3 according to the innervation score they were given). (B–D) Confocal Z-projection of a section of rat SPG showing double labeling for 5-HT1D receptor (B) and CGRP (C) immunoreactivity. All 5-HT1D receptor immunoreactive fibers and terminals were CGRP immunoreactive (D; yellow). (E–G) Confocal Z-projection of a section of rat SPG showing double labeling for 5-HT1D receptor (E) and VAChT (F) immunoreactivity. No 5-HT1D receptor immunoreactive fibers or terminals were VAChT immunoreactive (G). Confocal Z-projection of a section of rat SPG showing 5-HT1D receptor immunoreactivity in fibers and terminals (red) in close apposition to retrogradely labeled cell bodies (blue) of postganglionic neurons that innervate the lacrimal gland (H) and the trigeminal ganglion (I). In H and I, the numbers 0–3 indicate the innervations score each of the retrograde labeled cells were given.
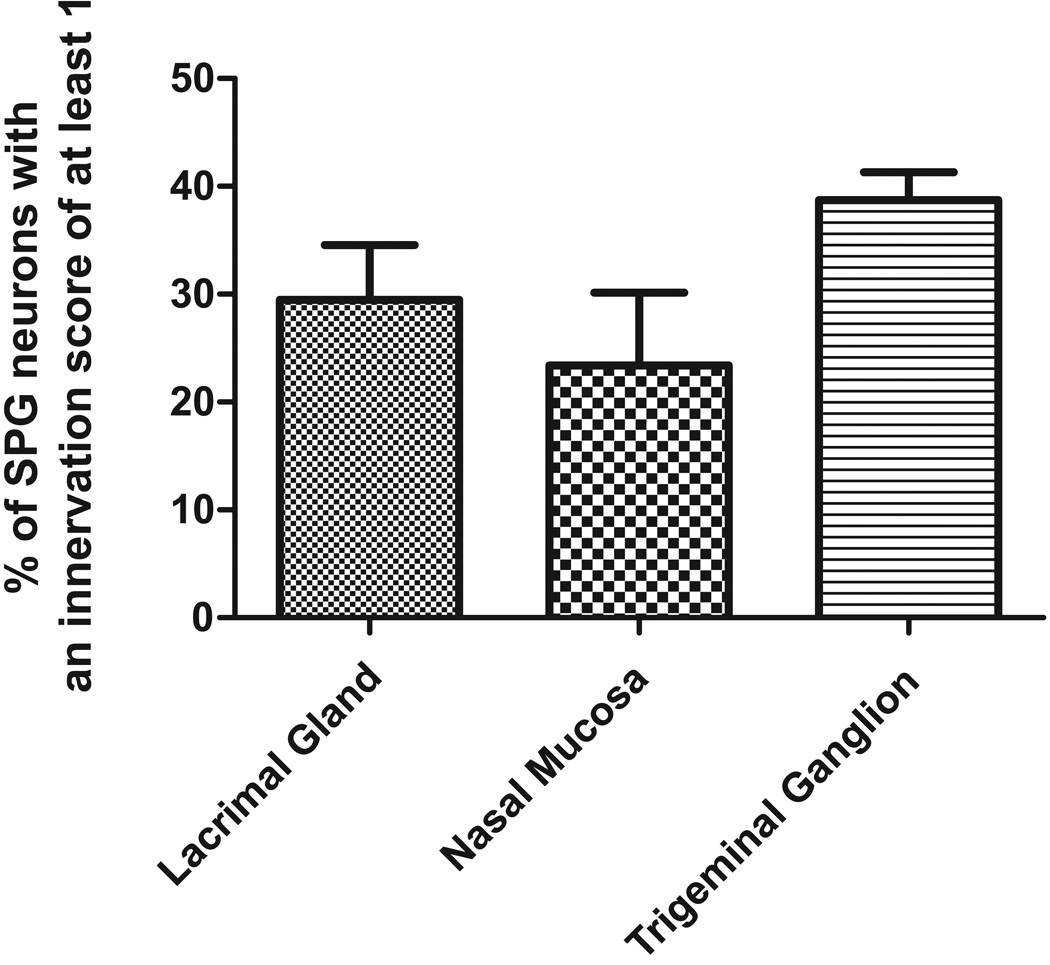
We identified postganglionic parasympathetic neurons that innervate the lacrimal gland (n = 3 animals) and nasal mucosa (n = 3 animals) by retrograde labeling with Fast Blue. There were a total of 299 cells retrogradely labeled from injections into the lacrimal gland and 291 cells retrogradely labeled following injections into the nasal mucosa. Neurons that innervate the lacrimal gland were located predominantly in the caudal part of the ganglion with relatively less labeling in the rostral part, whereas those that innervate the nasal mucosa were distributed equally in both the rostral part and caudal tail of the ganglion. Innervation scores of retrograde labeled cells showed that 29.5 ± 5% of the cells retrogradely labeled from the lacrimal gland had 5-HT1D receptor immunoreactive terminals around them (e.g. Figure 1H, Figure 2). A similar relationship was observed between 5-HT1D receptor immunoreactive terminals and cells that innervated the nasal mucosa. A total of 23.4 ± 7% of the cells retrogradely labeled from the nasal mucosa had 5-HT1D receptor terminals around them (Figure 2).
Figure 2.
Summary histogram of the percentage of retrogradely labeled cells from each target that were in close apposition to 5-HT1D receptor immunoreactive nerve terminals.
We also attempted to identify postganglionic parasympathetic neurons that innervate the middle cerebral artery or middle meningeal artery by retrograde labeling with Fast Blue or True Blue. No retrogradely labeled SPG neurons were observed following injections of FB around the middle cerebral artery (n = 3 animals) and only 3 SPG neurons were labeled following injections of FB around the middle meningeal artery (n = 3 animals). Attempts to modify our technique for labeling neurons that innervate the intracranial vasculature were not successful, as we were only able to label 3 cells that innervated the middle cerebral artery (n = 1 animal) and 1 cell that innervated the middle meningeal artery (n = 1 animal) using a survival time of 12 days. Furthermore, we could only label 4 cells that innervate the middle meningeal artery (n = 1 animal) and no cells that innervate the middle cerebral artery (n = 1 animal) when using True Blue with a 12-day survival time. We are confident that this lack of labeling is not a limitation of our technique because we noted retrograde labeling of significant numbers of sensory neurons in the trigeminal ganglion in each of these animals (data not shown). Innervation scores were not determined for the intracranial vasculature groups because we considered these numbers to reflect a lack of significant innervation of the intracranial vasculature by the SPG.
In a further experimental group, we identified postganglionic parasympathetic neurons that innervate the trigeminal ganglion (n = 3 animals). There were a total of 329 SPG cells retrogradely labeled with injections of FB into the trigeminal ganglion (Figure 1I) and these were predominantly in the rostral part of the SPG. 38.7 ± 3 % of these had 5-HT1D receptor terminals around them (Figure 2).
Discussion
In this study, we report that 5-HT1D receptor immunoreactivity is present in the parasympathetic sphenopalatine ganglion. The 5-HT1D receptor immunoreactivity was within fibers traversing the ganglia and terminals making apparent contact with postganglionic parasympathetic neurons within the ganglia. No somata were 5-HT1D receptor immunoreactive. We were able to show that the 5-HT1D receptor immunoreactive terminals were in close apposition to a significant number of SPG postganglionic neurons (up to 27%). This close anatomical association of pericellular nerve terminals and postganglionic neurons argues strongly that activation of 5-HT1D receptor immunoreactive terminals in the ganglia is likely to result in modulation of parasympathetic postganglionic neurons in the SPG.
Our findings indicate that 5-HT1D receptor /CGRP immunoreactive fibers and terminals observed in this study are sensory in origin. The lack of 5-HT1D receptor immunoreactive fibers and terminals double labeled with antisera directed against VAChT excludes the possibility that the 5-HT1D receptor immunoreactive fibers in this ganglia are of preganglionic parasympathetic origin. This is consistent with the report by Suzuki and colleagues20, who showed that no CGRP immunoreactive nerve terminal “baskets” were seen in the ipsilateral SPG two weeks after sectioning the nerves connecting the maxillary branch of the trigeminal nerve to the SPG. Indeed, in the same study, Suzuki and colleagues showed that transection of the sympathetic nerves entering the SPG caused no change to the peptide staining of the terminal baskets, suggesting that they were not of sympathetic origin. Furthermore, Harriot and Gold (2008) did not observe CGRP in the rat superior cervical ganglia (SCG), which is the origin of sympathetic postganglionic fibers in cranial tissues. The 5-HT1D receptor immunoreactive fibers we observed in the SPG in our study all contained CGRP and therefore could not be of sympathetic origin.
Suzuki, Hardebo et al.20 argued that these peptidergic sensory fibers linked the sensory and autonomic systems in the periphery, and also suggested that peptide release in these sensory terminals in the SPG might occur by a mechanism similar to axon reflex in the periphery. This provides an alternative route and mechanism for activation of parasympathetic neurons by the trigeminovascular system to that involving reflex activation of brainstem parasympathetic outflow1, 27. We propose that this peripheral route may function in addition to the brainstem connections to modulate parasympathetic activity in cluster headache and migraine (Figure 3), and that triptan activation of the 5-HT1D receptor at this location could act to modulate parasympathetic drive through the SPG.
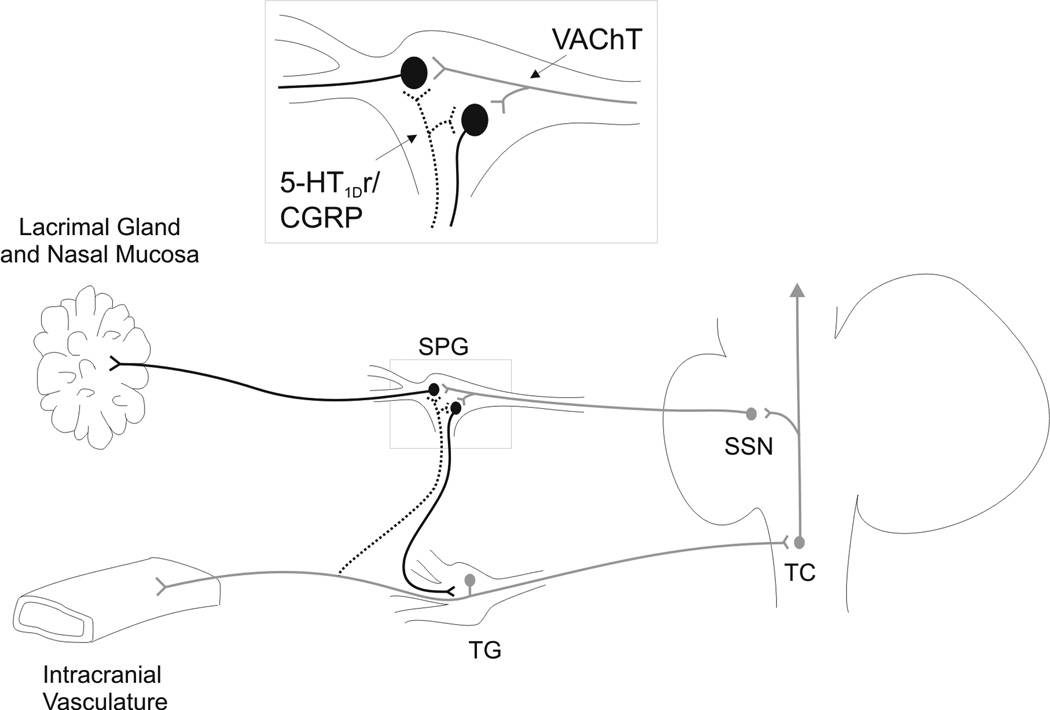
Figure 3.
Schematic of connections between cranial sensory and parasympathetic pathways putatively involved in pain and autonomic symptoms associated with cluster headache and migraine. Trigeminal primary afferent neurons with somata in the trigeminal ganglion (TG) relay information from intracranial vasculature to the brainstem trigeminal complex (TC), which passes this information to higher pain regulatory centers in the brain. Reflex connections with brainstem parasympathetic outflow nuclei, such as the superior salivatory nucleus (SSN), can influence postganglionic parasympathetic neurons in the SPG that project to the lacrimal gland and nasal mucosa and are likely responsible for some of the autonomic symptoms of cluster headache and migraine. Postganglionic parasympathetic neurons that innervate the lacrimal gland and nasal mucosa can also receive direct inputs from collateral fibers of 5-HT1D receptor immunoreactive trigeminal primary afferent neurons (dotted line). These trigeminal fibers have previously been shown to be from the maxillary (V2) division (see Suzuki et al. 1989). This peripheral connection (in the SPG) may also be responsible, at least in part, for some of the autonomic signs associated with cluster headache and migraine. Furthermore, triptans could act on 5-HT1D receptor immunoreactive terminals at this site to reduce the autonomic signs associated with these conditions. A reciprocal connection between the two ganglia may provide a mechanism for feedback to modulate the activity of trigeminal primary afferent neurons in cluster headache and migraine.
Ultra-structural examinations have revealed that CGRP immunoreactive terminals in the SPG make synaptic contacts with postganglionic neurons25, and that the CGRP immunoreactivity is in large dense core vesicles in these terminals26. Substance P labeled “basket-like” terminals have also been reported in the SPG20, 28, 29. Activation of 5-HT1D receptors has been shown to inhibit release of substance P and CGRP from peripheral terminals30, 31 and glutamate from central terminals32 of primary afferent neurons. Our finding that CGRP immunoreactive terminals in the SPG also express 5-HT1D indicates that activation of these receptors in the SPG may inhibit CGRP release and decrease the probability of activation of postganglionic parasympathetic neurons in the SPG.
Our retrograde labeling studies show that many of the postganglionic parasympathetic SPG neurons that innervate the lacrimal gland and nasal mucosa have 5-HT1D receptor immunoreactive terminals around them. Given that these terminals are peptidergic and likely to be of sensory origin (see above), we suggest that trigeminal afferent activity associated with cluster headache and migraine could modulate excitability of parasympathetic neurons innervating the lacrimal gland and nasal mucosa, resulting in some of the autonomic signs observed in these conditions. Further to this, activation of the 5-HT1D receptor in the SPG by triptans is likely to influence the activity of these same parasympathetic neurons. Thus triptans may (at least in part) reduce secretions of the lacrimal gland and nasal mucosa by acting at this site, and this could account for some of the efficacy of triptans in the treatment of autonomic signs associated with cluster headache and migraine. It would be useful in future studies to test whether triptan injections into the SPG decrease the parasympathetic outflow to the lacrimal gland and nasal mucosa, but at present there is a distinct lack of an available experimental model to test this hypothesis.
Interestingly, we were unable to retrogradely label a significant number of neurons that innervate the cerebral vasculature in the present study despite reports that the SPG innervates the middle cerebral artery and the middle meningeal artery in rats19, 20, 33–37. The reason for this is unclear as we have used similar approaches to these previous studies, including the same retrograde tracers (Fast Blue and True Blue) and extended survival times (up to 12 days). Of note however, is that retrograde labeling in most of these previous studies was only qualitatively reported and thus it is not clear if there was significant retrograde labeling of SPG cells following injections around the vessels. The single study that did quantify retrograde labeling, following injections of True Blue onto the surface of the middle cerebral artery of rats, counted every section throughout the entire SPG and reported only 16 retrograde labeled cells36. In addition, one of the studies used anterograde tracing from the SPG35 and therefore it is possible that the reported labeling of terminals in the vasculature might have resulted from uptake of tracer by axons of passage that originate from outside of the SPG, because axons of sympathetic and sensory neurons are known to pass through the SPG8. Thus there appears to be a lack of definitive evidence that a significant number of postganglionic parasympathetic neurons, originating in the SPG, innervate the cerebral vasculature or vasculature of the dura. We suggest that triptan activation of 5-HT1D receptors in the SPG is unlikely to have a significant effect on the intracranial vasculature because there is such minimal innervation. It follows that triptan action on 5-HT1D receptors in the SPG is unlikely to attenuate trigeminovascular pain by reducing parasympathetic drive through the SPG.
Our observation that postganglionic parasympathetic SPG neurons innervate the trigeminal ganglion has not been previously reported. We have shown that approximately 39% of these neurons receive inputs from 5-HT1D receptor immunoreactive terminals. Taken together with data showing that these terminals are peptidergic and of trigeminal origin (see above), and reports that maxillary trigeminal ganglion neurons can be retrogradely labeled from the SPG20, this suggests that there are reciprocal connections between neurons in the trigeminal ganglion and the SPG. The function of these reciprocal connections between peripheral sensory and parasympathetic ganglia is not known, but we speculate that they may form part of a feedback loop that modulates trigeminal afferent activity in cluster headache and migraine (Figure 3).
We do not argue that other sites of action for the triptans are not possible or even more important than the mechanism suggested in the present study. Triptan activation of 5-HT1D receptors has been shown to decrease primary afferent and medullary dorsal horn neuronal excitability, and excitability in higher centers of the brain5, 7, 32. In addition, 5-HT1D receptors have been reported in somata of sympathetic ganglia of the head, such as the superior cervical ganglion17. The authors of this latter study postulated that some peripheral 5-HT1D receptor immunoreactive nerve terminals were of sympathetic postganglionic origin, and that activation of 5-HT1D receptors on sympathetic postganglionic terminals might result in a decreased intracranial vascular tone and therefore a reduced pain response in line with reduced activation of trigeminovascular neurons.
In summary, it is clear that many mechanisms might contribute to the efficacy of triptans in the treatment of cluster headache and migraine. We suggest that triptans may act on 5-HT1D receptors in the SPG to inhibit CGRP release from trigeminal primary afferent fibers, and therefore decrease the activation of postganglionic SPG neurons innervating the lacrimal gland and nasal mucosa. This may represent a novel site of action for triptans in the treatment of autonomic signs associated with cluster headache.
Acknowledgements
Financial support: This study was supported by the Brain Foundation (Australia), National Health and Medical Research Council #454606 (Australia) and, in part, with funding from the NIH NS047113 to AHA.
The authors thank Colin Anderson for his advice on early experiment design.
Abbreviations
- CGRP
calcitonin-gene related peptide
- SPG
sphenopalatine ganglion
- VAChT
vesicular acetylcholine transporter
Footnotes
Conflict of Interest: None
References
- 1.Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:251–257. doi: 10.1016/s1474-4422(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 2.Leroux E, Ducros A. Cluster headache. Orphanet J Rare Dis. 2008;3:20. doi: 10.1186/1750-1172-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nappi G, Micieli G, Cavallini A, Zanferrari C, Sandrini G, Manzoni GC. Accompanying symptoms of cluster attacks: their relevance to the diagnostic criteria. Cephalalgia. 1992;12:165–168. doi: 10.1046/j.1468-2982.1992.1203165.x. [DOI] [PubMed] [Google Scholar]
- 4.Obermann M, Yoon MS, Dommes P, et al. Prevalence of trigeminal autonomic symptoms in migraine: a population-based study. Cephalalgia. 2007;27:504–509. doi: 10.1111/j.1468-2982.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of Migraine. Neuroscience. 2009;161:327–341. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 7.Moskowitz MA. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends in pharmacological sciences. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- 8.Hardebo JE, Elner A. Nerves and vessels in the pterygopalatine fossa and symptoms of cluster headache. Headache. 1987;27:528–532. doi: 10.1111/j.1526-4610.1987.hed2710528.x. [DOI] [PubMed] [Google Scholar]
- 9.Ekbom K, Krabbe A, Micieli G, et al. Cluster headache attacks treated for up to three months with subcutaneous sumatriptan (6 mg). Sumatriptan Cluster Headache Long-term Study Group. Cephalalgia. 1995;15:230–236. doi: 10.1046/j.1468-2982.1995.015003230.x. [DOI] [PubMed] [Google Scholar]
- 10.Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand. 1993;88:63–69. doi: 10.1111/j.1600-0404.1993.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 11.Hardebo JE. Subcutaneous sumatriptan in cluster headache: a time study of the effect on pain and autonomic symptoms. Headache. 1993;33:18–21. doi: 10.1111/j.1526-4610.1993.hed3301018.x. [DOI] [PubMed] [Google Scholar]
- 12.Gobel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology. 1998;51:908–911. doi: 10.1212/wnl.51.3.908. [DOI] [PubMed] [Google Scholar]
- 13.Torelli P, Manzoni GC. Cluster headache: symptomatic treatment. Neurol Sci. 2004;25 Suppl 3:S119–S122. doi: 10.1007/s10072-004-0267-7. [DOI] [PubMed] [Google Scholar]
- 14.Longmore J, Shaw D, Smith D, et al. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- 15.Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic Nociceptors of Both Trigeminal and Dorsal Root Ganglia Express Serotonin 1D Receptors: Implications for the Selective Antimigraine Action of Triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain research. 2001;909:112–120. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- 17.Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008;28:933–944. doi: 10.1111/j.1468-2982.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JS, Denaro FJ, Olazabal UE, Leard HO. Stereotaxic atlas of the trigeminal ganglion in rat, cat, and monkey. Brain Res Bull. 1981;7:93–95. doi: 10.1016/0361-9230(81)90103-9. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular vasoactive intestinal polypeptide-positive nerves in rat. J Cereb Blood Flow Metab. 1988;8:697–712. doi: 10.1038/jcbfm.1988.117. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Hardebo JE, Owman C. Trigeminal fibre collaterals storing substance P and calcitonin gene-related peptide associate with ganglion cells containing choline acetyltransferase and vasoactive intestinal polypeptide in the sphenopalatine ganglion of the rat. An axon reflex modulating parasympathetic ganglionic activity? Neuroscience. 1989;30:595–604. doi: 10.1016/0306-4522(89)90154-1. [DOI] [PubMed] [Google Scholar]
- 21.Weihe E, TaoCheng JH, Schafer MKH, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuhara O, Aimi Y, Shibano A, Kimura H. Primary sensory neurons containing choline acetyltransferase of the peripheral type in the rat trigeminal ganglion and their relation to neuropeptides-, calbindin- and nitric oxide synthase-containing cells. Brain research. 2007;1141:92–98. doi: 10.1016/j.brainres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- 24.Ito T, Hioki H, Nakamura K, et al. Gamma-aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion. J Comp Neurol. 2007;502:113–125. doi: 10.1002/cne.21309. [DOI] [PubMed] [Google Scholar]
- 25.Motosugi H, Chiba T, Konno A, Kaneko T. Distribution of neuropeptides in rat pterygopalatine ganglion: light and electron microscopic immunohistochemical studies. Archives of histology and cytology. 1992;55:513–524. doi: 10.1679/aohc.55.513. [DOI] [PubMed] [Google Scholar]
- 26.Beckers HJ, Klooster J, Vrensen GF, Lamers WP. Ultrastructural identification of trigeminal nerve terminals in the pterygopalatine ganglion of rats: an anterograde tracing and immunohistochemical study. Brain research. 1991;557:22–30. doi: 10.1016/0006-8993(91)90111-8. [DOI] [PubMed] [Google Scholar]
- 27.Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. Journal of Comparative Neurology. 2005;493:9–14. doi: 10.1002/cne.20688. [DOI] [PubMed] [Google Scholar]
- 28.Lundblad L, Lundberg JM, Brodin E, Anggard A. Origin and distribution of capsaicin-sensitive substance P-immunoreactive nerves in the nasal mucosa. Acta Otolaryngol. 1983;96:485–493. doi: 10.3109/00016488309132735. [DOI] [PubMed] [Google Scholar]
- 29.Uddman R, Malm L, Sundler F. Substance-P-containing nerve fibers in the nasal mucosa. Arch Otorhinolaryngol. 1983;238:9–16. doi: 10.1007/BF00453736. [DOI] [PubMed] [Google Scholar]
- 30.Buzzi MG, Moskowitz MA. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. British Journal of Pharmacology. 1990;99:202–206. doi: 10.1111/j.1476-5381.1990.tb14679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limmroth V, Katsarava Z, Liedert B, et al. An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain. 2001;92:101–106. doi: 10.1016/s0304-3959(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 32.Jennings EA, Ryan RM, Christie MJ. Effects of sumatriptan on rat medullary dorsal horn neurons. Pain. 2004;111:30–37. doi: 10.1016/j.pain.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Uddman R, Hara H, Edvinsson L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. Journal of the autonomic nervous system. 1989;26:69–75. doi: 10.1016/0165-1838(89)90109-4. [DOI] [PubMed] [Google Scholar]
- 34.Edvinsson L, Hara H, Uddman R. Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: colocalization with different peptides. J Cereb Blood Flow Metab. 1989;9:212–218. doi: 10.1038/jcbfm.1989.31. [DOI] [PubMed] [Google Scholar]
- 35.Hara H, Zhang QJ, Kuroyanagi T, Kobayashi S. Parasympathetic cerebrovascular innervation: an anterograde tracing from the sphenopalatine ganglion in the rat. Neurosurgery. 1993;32:822–827. doi: 10.1227/00006123-199305000-00016. discussion 827. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene-related peptide in rat. Neuroscience. 1989;31:427–438. doi: 10.1016/0306-4522(89)90385-0. [DOI] [PubMed] [Google Scholar]
- 37.Edvinsson L, Elsas T, Suzuki N, Shimizu T, Lee TJ. Origin and Co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microscopy research and technique. 2001;53:221–228. doi: 10.1002/jemt.1086. [DOI] [PubMed] [Google Scholar]