Abstract
Copper-catalyzed azide–alkyne cycloaddition (CuAAC) is a widely utilized, reliable, and straightforward way for making covalent connections between building blocks containing various functional groups. It has been used in organic synthesis, medicinal chemistry, surface and polymer chemistry, and bioconjugation applications. Despite the apparent simplicity of the reaction, its mechanism involves multiple reversible steps involving coordination complexes of copper(i) acetylides of varying nuclearity. Understanding and controlling these equilibria is of paramount importance for channeling the reaction into the productive catalytic cycle. This tutorial review examines the history of the development of the CuAAC reaction, its key mechanistic aspects, and highlights the features that make it useful to practitioners in different fields of chemical science.
Introduction
Any chemical discipline, pure or applied, heavily depends on chemical transformations available to its practitioners. Reactions that reliably connect readily accessible building blocks in the presence of other functional groups and across a wide range of conditions are among the most useful tools for building new molecular architectures with useful functional properties. The speed with which practitioners outside of the field of synthetic organic chemistry adopt a new chemical transformation is a reliable indicator of its broad utility. The subject of this review, the Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) reaction is among the recently discovered transformations that have rapidly crossed traditional disciplinary boundaries.
The CuAAC process has emerged as the premier example of click chemistry, a term coined in 2001 by Sharpless to describe a set of ‘near-perfect’ bond-forming reactions useful for rapid assembly of molecules with desired function.1 Click transformations are easy to perform, give rise to their intended products in very high yields with little or no byproducts, work well under many conditions, and are unaffected by the nature of the groups being connected to each other. The potential of organic azides as highly energetic, yet very selective functional groups in organic synthesis was highlighted in the original publication, and their dipolar cycloadditions with olefins and alkynes were placed among the reactions fulfilling the click criteria. However, the inherently low reaction rate of the azide–alkyne cycloaddition did not make it very useful in the click context, and its potential was not revealed until the discovery of the reliable and broadly useful catalysis by copper(i).
The copper-catalyzed reaction was reported simultaneously and independently by the groups of Meldal in Denmark2 and Fokin and Sharpless in the U.S.3 It transforms organic azides and terminal alkynes exclusively into the corresponding 1,4-disubstituted 1,2,3-triazoles, in contrast to the uncatalyzed reaction, which requires much higher temperatures and provides mixtures of 1,4- and 1,5-triazole regioisomers. Meldal et al. used the transformation for the synthesis of peptidotriazoles in organic solvents starting from alkynylated amino acids attached to solid supports, emphasizing the requirement for immobilization of the alkyne on a resin for the success of the reaction, whereas the Scripps group immediately turned to aqueous systems and devised a straightforward and practical procedure for the covalent “stitching” of virtually any fragments containing an azide and an alkyne functionality, noting the broad utility and versatility of the novel process “for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks.”3
Numerous applications of the CuAAC reaction reported during the last several years have been regularly reviewed,4,5 and are continually enriched by investigators in many fields.6 Here we shall focus on the fundamental aspects of the CuAAC process and on its mechanistic features, with an emphasis on the qualities of in situ-generated copper(i) acetylides that best enable this unique mode of reactivity.
Azide–alkyne cycloaddition: the basics
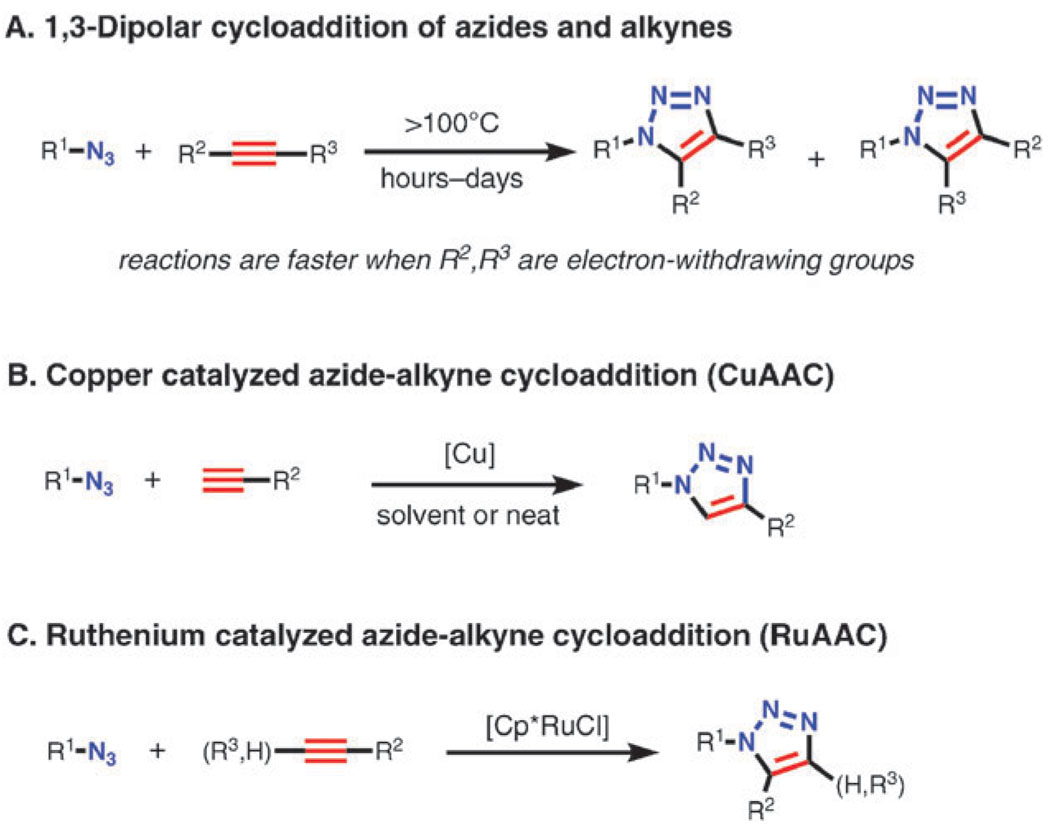
The thermal reaction of terminal or internal alkynes with organic azides (Scheme 1A) has been known for more than a century, the first 1,2,3-triazole being synthesized by A. Michael from phenyl azide and diethyl acetylene-dicarboxylate in 1893. The reaction has been most thoroughly investigated by Huisgen and coworkers in 1950s–70s in the course of their studies of the larger family of 1,3-dipolar cycloaddition reactions.7 Although the reaction is highly exothermic (ΔH0 between −50 and −65 kcal mol−1), its high activation barrier (approximately 25 kcal mol−1 for methyl azide and propyne8) results in exceedingly low reaction rates for unactivated reactants even at elevated temperature. Furthermore, since the differences in HOMO–LUMO energy levels for both azides and alkynes are of similar magnitude, both dipole-HOMO- and dipole-LUMO-controlled pathways operate in these cycloadditions. As a result, a mixture of regioisomeric 1,2,3-triazole products is usually formed when an alkyne is unsymmetrically substituted.
Scheme 1.
Thermal cycloaddition of azides and alkynes usually requires prolonged heating and results in mixtures of both 1,4-and 1,5-regioisomers (A), whereas CuAAC produces only 1,4-disubstituted-1,2,3-triazoles at room temperature in excellent yields (B). The RuAAC reaction proceeds with both terminal and internal alkynes and gives 1,5-disubstituted and fully, 1,4,5-trisubstituted-1,2,3-triazoles.
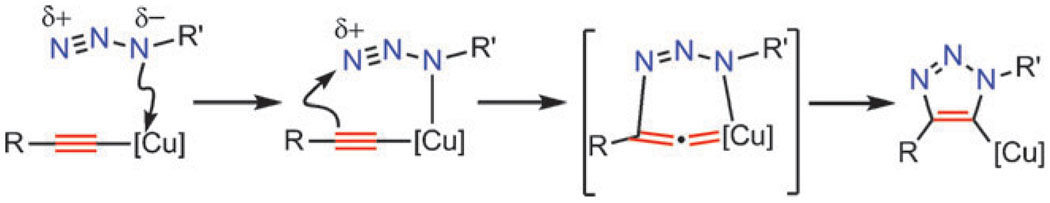
Copper catalysts (Scheme 1B) drastically change the mechanism and the outcome of the reaction, converting it to a sequence of discreet steps culminating in the formation of a 5-triazolyl copper intermediate (Scheme 2). The key C–N bond-forming event takes place between the nucleophilic, vinylidene-like β-carbon of copper(i) acetylide and the electrophilic terminal nitrogen of the coordinated organic azide. As will be discussed in detail, the reaction is far more complex than represented in the shorthand cartoons of Scheme 2.
Scheme 2.
Simplified representation of the proposed C–N bond-making steps in the reaction of copper(i) acetylides with organic azides. [Cu] denotes either a single-metal center CuLx or a di-/oligonuclear cluster CuxLy.
The rate of CuAAC is increased by a factor of 107 relative to the thermal process,8 making it conveniently fast at and below room temperature. The reaction is not significantly affected by the steric and electronic properties of the groups attached to the azide and alkyne centers, and primary, secondary, and even tertiary, electron-deficient and electron-rich, aliphatic, aromatic, and heteroaromatic azides usually react well with variously substituted terminal alkynes. The reaction proceeds in many protic and aprotic solvents, including water, and is unaffected by most organic and inorganic functional groups, therefore all but eliminating the need for protecting group chemistry. The 1,2,3-triazole heterocycle has the advantageous properties of high chemical stability (generally inert to severe hydrolytic, oxidizing, and reducing conditions, even at high temperature), strong dipole moment (4.8–5.6 Debye), aromatic character, and hydrogen bond accepting ability.9 Thus it can interact productively in several ways with biological molecules, organic and inorganic surfaces and materials, serving, for example, as a hydrolytically-stable replacement for the amide bond.
To date, copper stands out as the only metal for the reliable, facile, and 1,4-regiospecific catalysis of the azide–alkyne cycloaddition. Indeed, other metals known to catalyze various transformations of alkynes have not so far yielded effective catalysts for the conversion of azides and terminal alkynes to 1,4-triazoles. Surveys in our laboratories of complexes of all of the first-row transition elements as well as complexes of Ag(i), Pd(0/ii), Pt(ii), Au(i/iii), and Hg(ii), among others, have all failed to produce triazoles in synthetically useful yields; their effect on the rate and selectivity of the cycloaddition was at best marginally noticeable. The unique catalytic function of Cu(i) may be explained by the fortuitous combination of its ability to engage terminal alkynes in both σ- and π-interactions and the rapid exchange of these and other ligands in its coordination sphere, especially in aqueous conditions. When an organic azide is a ligand, the synergistic nucleophilic activation of the alkyne and electrophilic activation of the azide drives the formation of the first carbon-nitrogen bond.
In 2005, ruthenium cyclopentadienyl complexes were found to catalyze the formation of the complementary 1,5-disubstituted triazole from azides and terminal alkynes, and also to engage internal alkynes in the cycloaddition (Scheme 1C).10 As one would imagine from these differences, this sister process, designated RuAAC (ruthenium-catalyzed azide–alkyne cycloaddition), is mechanistically quite distinct from its cuprous cousin, although the underlying activation of the alkyne component appears to be fundamentally similar: the nucleophilicity of its π-system is increased by the back donation from the ruthenium center. While the scope and functional group compatibility of RuAAC are excellent,11 the reaction is more sensitive to the solvents and the steric demands of the azide substituents than CuAAC. Applications of RuAAC are only beginning to appear.
Catalysts and ligands
A wide range of experimental conditions for the CuAAC have been employed since its discovery, underscoring the robustness of the process and its compatibility with most functional groups, solvents, and additives regardless of the source of the catalyst. The choice of the catalyst is dictated by the particular requirements of the experiment, and usually many combinations will produce desired results. The most commonly used protocols and their advantages and limitations are discussed below.
Different copper(i) sources can be utilized in the reaction, as recently summarized in necessarily partial fashion by Meldal and Tornøe.5 Copper(i) salts (iodide, bromide, chloride, acetate) and coordination complexes such as [Cu(CH3CN)4]PF6 and [Cu(CH3CN)4]OTf have been commonly employed. In general, however, we recommend against the use of cuprous iodide because of the ability of iodide anion to act as a bridging ligand for the metal, resulting in the formation of polynuclear acetylide complexes which interfere with the productive catalytic cycle by tying up the catalyst. Furthermore, under certain conditions copper(i) iodide may result in the formation of 1-iodoalkynes and, consequently, 5-iodotriazoles.12 High concentrations of chloride ion in water (0.5 M or above) can also be deleterious, although the inhibitory effect of the chloride is far less pronounced comparing to the iodide. Therefore, for reactions performed in aqueous solvents, cuprous bromide and acetate are favored, as is the sulfate from in situ reduction of CuSO4; for organic reactions, the cuprous acetate salt is generally a good choice.
Copper(ii) salts and coordination complexes are not competent catalysts, and reports describing Cu(ii)-catalyzed cycloadditions13 are not accurate. Cupric salts and coordination complexes are well-known oxidizing agents for organic compounds.14 Alcohols, amines, aldehydes, thiols, phenols, and carboxylic acids may readily be oxidized by the cupric ion, reducing it to the catalytically active copper(i) species in the process. Especially relevant is the family of oxidative acetylenic couplings catalyzed by the cupric species,15 with the venerable Glaser coupling being the most studied example. Since terminal acetylenes are necessarily present in CuAAC reactions, their oxidation is an inevitable side process which could, in turn, produce the needed catalytically active copper(i) species.
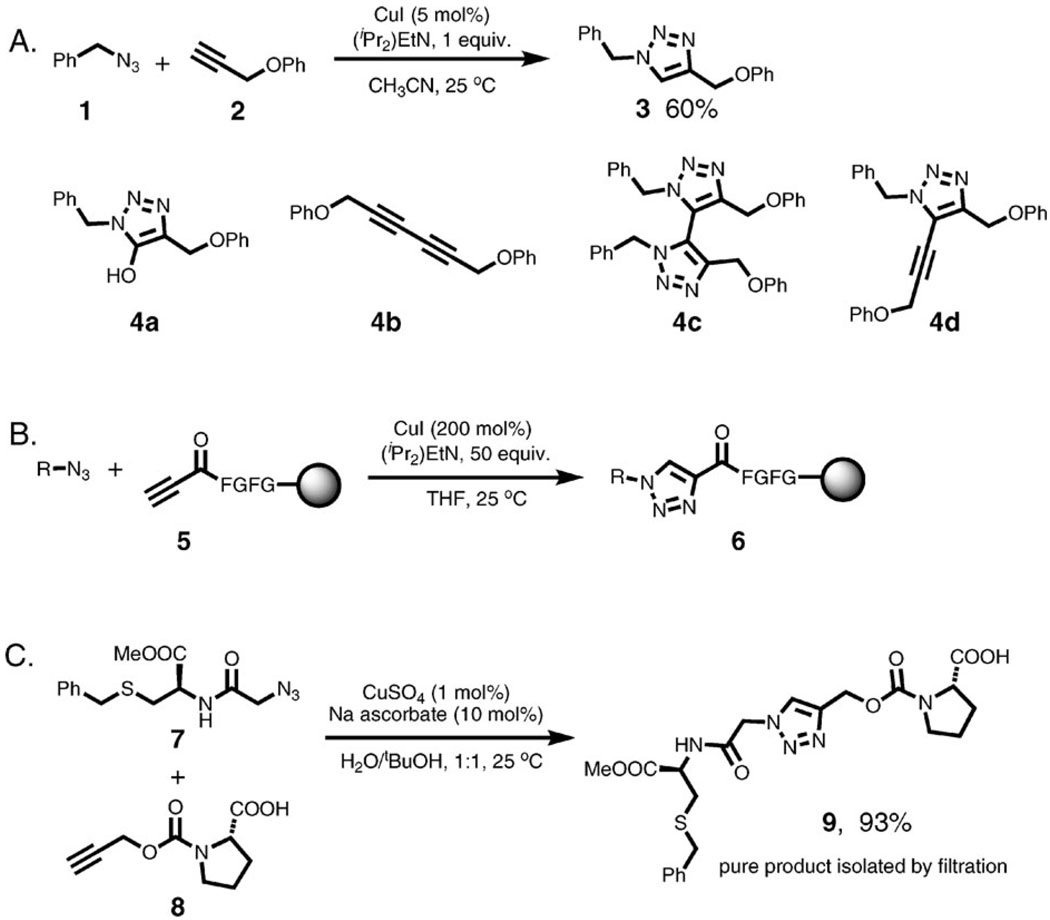
Among the three most common oxidation states of copper (0, +1, and +2), +1 is least thermodynamically stable. Cuprous ion can be oxidized to catalytically inactive Cu(ii) species, or can disproportionate to a mixture of Cu(ii) and Cu(0). The standard potential of the Cu2+/Cu+ couple is 159 mV, but can vary widely depending on the solvent and the ligands coordinated to the metal, and is especially complex in water.16 When present in significant amounts, the ability of Cu(ii) to mediate the aforementioned Glaser-type alkyne coupling processes can result in the formation of undesired byproducts while impairing triazole formation. When the cycloaddition is performed in orgsanic solvents using copper(i) halides as catalysts, the reaction is plagued by the formation of oxidative coupling byproducts 4a–d unless the alkyne is bound to a solid support (5, Scheme 3A–B).2 When the alkyne is dissolved and the azide is immobilized on the resin, only traces of the desired triazole product are formed. Therefore, when copper(i) catalyst is used directly, whether by itself or in conjunction with amine ligands, exclusion of oxygen may be required. Contrariwise, performing the reaction in the presence of mild oxidants allows capture of the triazolyl copper intermediates and isolation of the products derivatized at the 5-position in synthetically useful yields.17
Scheme 3.
(A) Oxidative coupling byproducts in the CuAAC reactions catalyzed by copper(i) salts in the presence of oxygen; (B) CuAAC with immobilized alkyne avoids the formation of the oxidative byproducts but requires large excess of the catalyst; reactions with immobilized azide fail; (C) solution-phase CuAAC in the presence of sodium ascorbate.
Ascorbate, a mild reductant, was introduced by Fokin and co-workers3 as a convenient and practical alternative to oxygen-free conditions. Its combination with a copper(ii) salt, such as the readily available and stable copper(ii) sulfate pentahydrate or copper(ii) acetate, has been quickly accepted as the method of choice for preparative synthesis of 1,2,3-triazoles. Water appears to be an ideal solvent capable of supporting copper(i) acetylides in their reactive state, especially when they are formed in situ (in fact, examination of the reactivity of in situ-generated, and hence less aggregated copper acetylides was the main impetus for the discovery and development of the ascorbate procedure). The formation of byproducts resulting from copper-mediated oxidative side reactions is suppressed as any dissolved dioxygen is rapidly reduced. The “aqueous ascorbate” procedure often furnishes triazole products in nearly quantitative yield and greater than 90% purity, without the need for ligands or additives or protection of the reaction mixture from oxygen (Scheme 3C). Of course, copper(i) salts can also be used in combination with ascorbate, wherein it converts any oxidized copper(ii) species back to the catalytically active +1 oxidation state.
The reaction can also be catalyzed by Cu(i) species supplied by elemental copper, thus further simplifying the experimental procedure—a small piece of copper metal (wire or turning) is all that is added to the reaction mixture, followed by shaking or stirring for 12–48 h.3,8,18 Aqueous alcohols (methanol, ethanol, tert-butanol), tetrahydrofuran, and dimethylsulfoxide can be used as solvents in this procedure. Cu(ii) sulfate may be added to accelerate the reaction; however, this is not necessary in most cases, as copper oxides and carbonates, the patina on the metal surface, are sufficient to initiate the catalytic cycle. Although the procedure based on copper metal requires longer reaction times when performed at ambient temperature, it usually provides access to very pure triazole products with low levels of copper contamination. Alternatively, the reaction can be performed under microwave irradiation at elevated temperature, reducing the reaction time to 10–30 min.18,19
The copper metal procedure is experimentally very simple and is particularly convenient for high-throughput synthesis of compound libraries for biological screening. Triazole products are generally isolated in >85–90% yields, and can often be submitted for biological assays without purification due to the very low copper contamination. When required, trace quantities of copper remaining in the reaction mixture can be removed with an ion-exchange resin or using solid-phase extraction techniques. Other heterogeneous copper(0) and copper(i) catalysts, such as copper nanoclusters,20 copper/cuprous oxide nanoparticles,21 and copper nanoparticles adsorbed onto charcoal22 have also shown good catalytic activity.
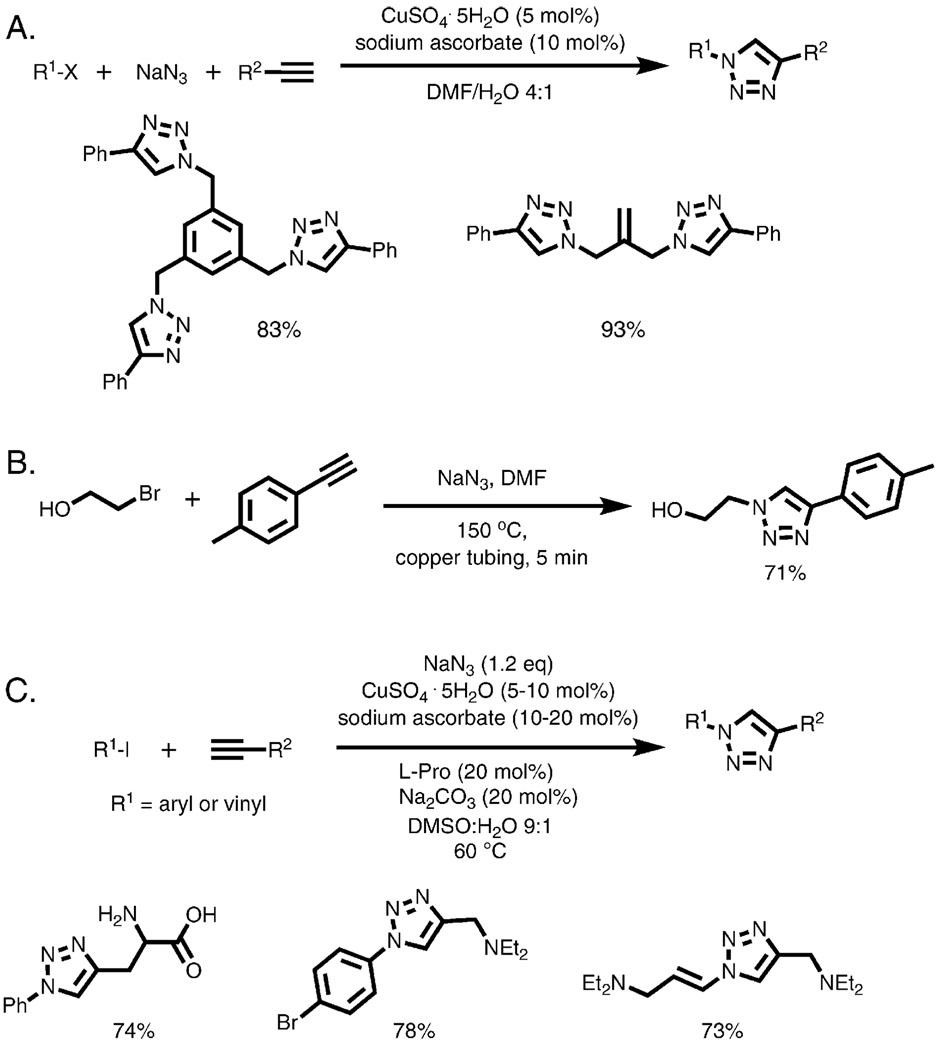
Although organic azides are generally stable and safe compounds, those of low molecular weight can spontaneously decompose and, therefore, could be difficult or dangerous to handle. This is especially true for small molecules with several azide functionalities that are important in the synthesis of polyfunctionalized structures. Indeed, small-molecule azides should never be isolated away from solvent, for example by distillation, precipitation, or recrystallization. Fortunately, the CuAAC reaction is highly tolerant of all manner of additives and spectator compounds, including inorganic azide even in large excess. The process can therefore be performed in a one-pot two-step sequence, whereby an in situ generated organic azide is immediately consumed in a reaction with a copper acetylide (Scheme 4). We have implemented this process many times in our laboratories starting from alkyl halides or arylsulfonates by SN2 reaction with sodium azide (Scheme 4A). In a recent example, Pfizer chemists developed a continuous flow process wherein a library of 1,4-disubstituted 1,2,3-triazoles was synthesized from alkyl halides, sodium azide, and terminal acetylenes, with the copper catalyst required for cycloaddition being supplied from the walls of the heated copper tubing through which the reaction solution was passed (Scheme 4B).23
Scheme 4.
One-pot syntheses of triazoles from halides at (A,B) sp3 and (C) sp2 carbon centers. Reaction B was performed in a flow reactor in 0.75 mm diameter Cu tubing with no added copper catalyst.
Aryl and vinyl azides can also be accessed in one step from the corresponding halides or triflates via a copper-catalyzed reaction with sodium azide in the presence of catalytic amount of l-proline (Scheme 4C).24 In this fashion, a range of 1,4-disubstituted 1,2,3-triazoles can be prepared in excellent yields.25 This reaction sequence can be performed at elevated temperature under microwave irradiation, reducing the reaction time to 10–30 min.18 Anilines can be also converted to aryl azides by the reaction with tert-butyl nitrite and azidotrimethylsilane.26 The resulting azides can be submitted to the CuAAC conditions without isolation, furnishing triazole products in excellent yields. Microwave heating further improves the reaction, significantly reducing reaction time.19
Many other copper complexes involving ligands have been reported as catalysts or mediators of the CuAAC reaction. It would be accurate to say that finding a copper(i) catalyst that is not active is more difficult than improving those that catalyze the reaction. Many reported reactions are performed under widely differing conditions, making quantitative comparisons of ligands performance difficult. Nevertheless, it may be useful to organize them into “soft” and “hard” classes by virtue of the properties of their donor centers.27 Cu(i) is a “borderline soft” Lewis acid,28 and therefore can partner with a wide variety of potentially effective ligands. A representative sample of reported systems, which is far from comprehensive, follows below.
The “soft” ligand class is exemplified by phosphine-containing catalytically active species such as the simple coordination complexes Cu(P(OMe)3)3Br29 and Cu(PPh3)3Br.30 These species are often used in reactions in organic solvents, in which cuprous salts have limited solubility. A recent report describes the bis(phosphine) complex Cu(PPh3)2OAc as an excellent catalyst for the CuAAC reaction in toluene and dichloromethane.31 Monodentate phosphoramidite and related donors have also been evaluated.32 Chelating complexes involving phosphines have not found favor, except for bidentate combinations of phosphine with the relatively weakly-binding triazole unit.33 Thiols are a potent poison of the CuAAC reaction in water, but thioethers show promise, although they remain an underexplored member of the “soft” ligand class.34
Several Cu(i) complexes with N-heterocyclic carbene ligands have been described as CuAAC catalysts at elevated temperature in organic solvents, under heterogeneous aqueous conditions (when both reactants are not soluble in water), and under neat conditions.35 These catalysts are very active under the solvent-free conditions, achieving turnover numbers as high as 20 000. However, their activity in solution-phase reactions is significantly lower than of other catalytic systems (for example, a stoichiometric reaction of the isolated copper(i) acetylide/NHC complex with benzhydryl azide required 12 h to obtain 65% yield of the product,36 whereas under standard solution conditions even a catalytic reaction would be complete within an hour). As an important footnote, we strongly advise against running highly exothermic reactions without a solvent, as runaway reactions and explosions can easily occur.6 Furthermore, performance of a catalyst under the solvent-free conditions is, at best, loosely correlated with its true activity, simply because most copper(i) species effortlessly catalyze such reactions.
The category of “hard” donor ligands of CuAAC-active systems is dominated by amines (see ref. 5 and the Supporting Information of ref. 37). In many cases, amines are labeled as “additives” rather than “ligands,” since it is often the intention to aid in the deprotonation of the terminal alkyne rather than to coordinate to the metal center. However, this assumption is not accurate, as formation of copper(i) acetylides is so facile that it occurs even in strongly acidic media (up to 20–25% H2SO4).38 Instead, the primary role of amine ligands can be (a) to prevent the formation of unreactive polynuclear copper(i) acetylides; (b) to facilitate the coordination of the azide to copper center at the ligand exchange step (vide infra); and (c) to increase the solubility of the copper complex to deliver higher solution concentrations of the necessary Cu(i)-species. In several cases of amine-based chelates, it is probable that metal binding is at least part of the productive role of the polydentate ligand. An example is the use of a hydrophobic Tren ligand for CuAAC catalysis in organic solvent at elevated temperature.39
As befits the borderline nature of the Cu(i) ion, by far the largest and most successful class of ligands are those of intermediate character between “hard” and “soft,” particularly those containing heterocyclic donors. With rare exceptions, these also contain a central tertiary amine center, which can serve as both a coordinating donor and a base. The need for these ligands was particularly evident for reactions involving biological molecules that are handled in water in low concentrations and are not stable to heating. Chemical transformations used in bioconjugations impose specific demands: they must be must be exquisitely chemoselective, biocompatible, and fast. Despite the experimental simplicity and efficiency of the “ascorbate” procedure, the rate of the CuAAC reaction in the absence of an accelerating ligand is simply not high enough when concentrations of reactants are low.
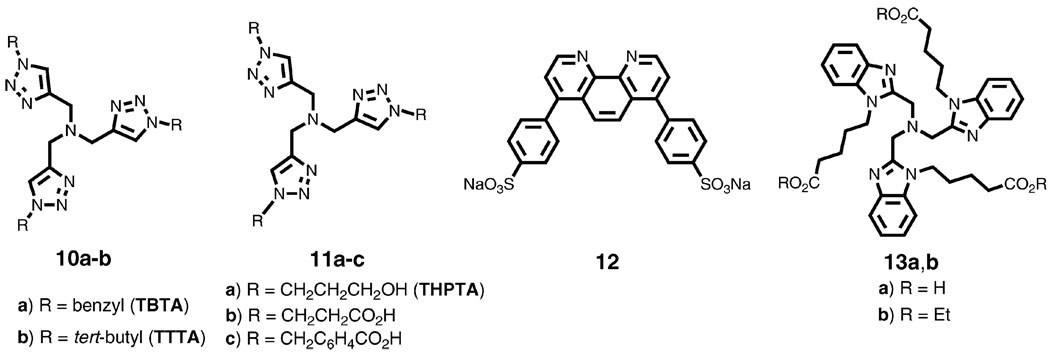
The first general solution to the bioconjugation problem was provided by tris(benzyltriazolyl)methyl amine ligand 10 (TBTA, Scheme 5), prepared using the CuAAC reaction and introduced shortly after its discovery.40 This ligand was shown to significantly accelerate the reaction and stabilize the Cu(i) oxidation state in water-containing mixtures. After its utility in bioconjugation was demonstrated by the efficient attachment of 60 alkyne-containing fluorescent dye molecules to the azide-labeled cowpea mosaic virus,41 it was widely adopted for use with such biological entities as nucleic acids, proteins, E. coli bacteria, and mammalian cells. A resin-immobilized version of TBTA42 has also been shown to be very useful in library synthesis and conjugation experiments when contamination of products by copper needs to be minimized. A recent addition to the family of triazolyl amine ligands, the tris(tert-butyl) analog, TTTA, 10b, shows superior activity to TBTA across the board.
Scheme 5.
CuAAC-accelerating ligands of choice: tris(1,2,3-triazolyl)methyl amine (TBTA, 10a) and its tert-butyl analog (TTTA, 10b), water-soluble analogs 11, sulfonated bathophenanthroline 12, and tris(benzimidazole)methyl amine (TBIA) 13.
The poor solubility of TBTA in water prompted the development of more polar analogues such as 11a–c (Scheme 5).43,44 At the same time, a combinatorial search for alternatives led to the identification of the commercially-available sulfonated bathophenanthroline 12 as the ligand component of the fastest water-soluble CuAAC catalyst under dilute aqueous conditions.45 The unmatched activity of this system made it very useful for demanding bioconjugation tasks. However, Cu·12 complexes are strongly electron-rich and are therefore highly susceptible to oxidation in air. Ascorbate can be used to keep the metal in the +1 oxidation state, but when exposed to air, reduction of O2 is very fast and can easily use up all of the available reducing agent. Thus, 12 is usually used under inert atmosphere, which can be inconvenient, particularly when small amounts of biomolecule samples are used. A procedural solution was found in the use of an electrochemical cell to provide the reducing equivalents to scrub O2 out of such reactions and maintain Cu·12 in the cuprous oxidation state, but this was again less than optimal due to the need for extra equipment and electrolyte salts.44 The polydentate trimethylamine theme has been extended to benzimidazole, benzothiazole, oxazoline, and pyridine substituents.37,46 Several have provided significantly faster catalysis when quantitative rates are measured, particularly the pendant ester and water-soluble acid derivatives of the tris(benzimidazole) motif, 13a,b.
Mechanistic aspects of the CuAAC
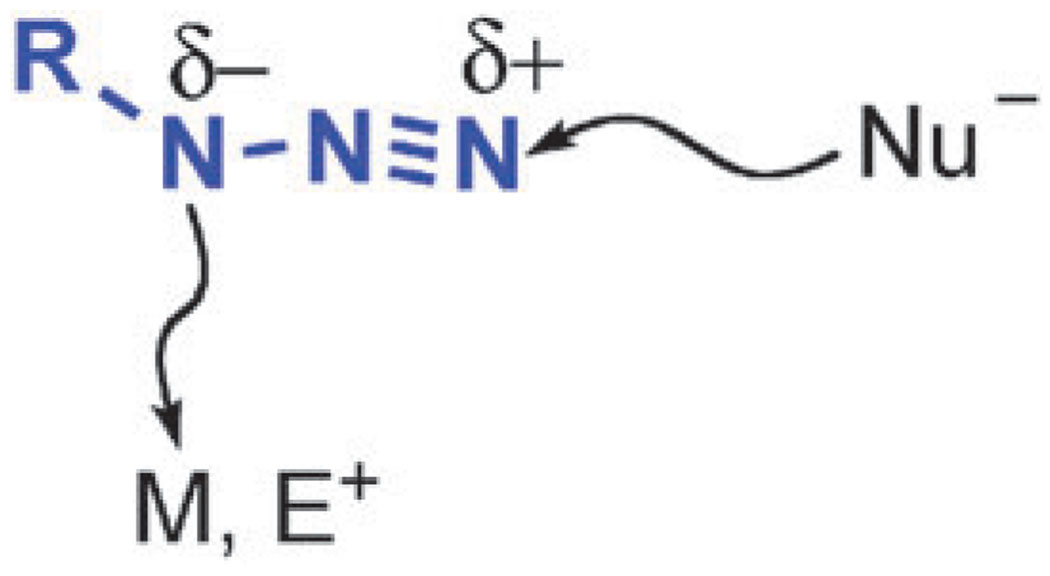
Before considering possible mechanistic pathways of the CuAAC process, it is important to highlight fundamental reactivity of the players: organic azides and copper(i) acetylides. The reactivity of organic azides, with the exception of the thermal and photochemical decomposition, is dominated by reactions with nucleophiles at the terminal N3 atom. Examples of reactions with electrophiles reacting at the proximal N1 (Scheme 6) are also known, although those are less common. These reactivity patterns are in agreement with theoretical studies of the electronic structure of the azido group. Coordination chemistry of organic azides follows the same trend, and the azide usually behaves as an L-type σ-donor via its N1 nitrogen atom, with a few exceptions when electron-rich π-basic metals engage the terminal N3 atom in back donation or when coordination via the terminal nitrogen is forced due to steric or geometrical constraints.47
Scheme 6.
Common reactivity patterns of organic azides. Nucleophiles attack at the electrophilic terminal nitrogen, whereas the more electron-rich N1 can react with electrophiles and coordinate to transition metals.
The history of copper(i) acetylides dates back as far as Glaser’s discovery in 1869 of oxidative dimerization of Cu-phenylacetylide. However, the precise nature of the reactive alkynyl copper species in CuAAC (as well as in many other reactions involving copper(i) acetylide complexes) is not well understood. The chief complications are the tendency of copper species to form polynuclear complexes38,48 and the great facility of the ligand exchange at the copper center. As a result, mixtures of Cu(i), terminal alkynes, and other ligands usually contain multiple organocopper species in rapid equilibrium with each other. While this may make elucidation of the exact mechanism difficult, the dynamic nature of copper acetylides is undoubtedly a major contributor to the remarkable adaptability of the reaction to widely different conditions. Whatever the details of the interactions of Cu with alkyne during the CuAAC reaction, it is clear that copper acetylide species are easily formed and are productive components of the reaction mechanism.
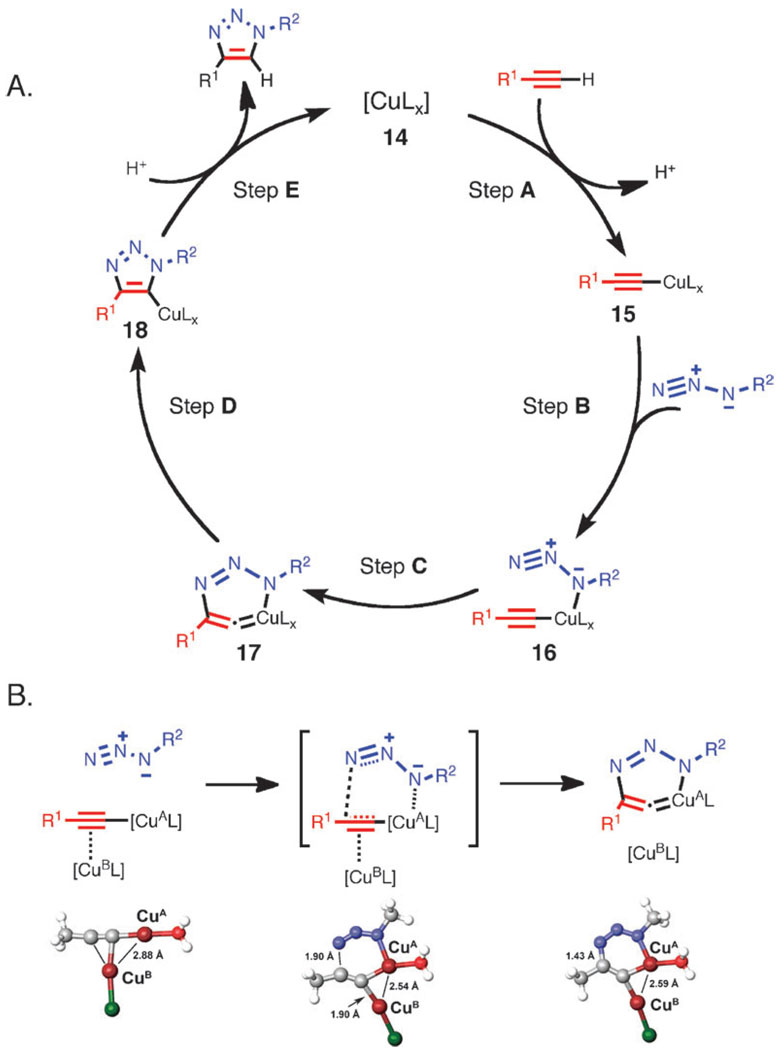
The initial computational treatment of CuAAC focused on the possible reaction pathways available to mononuclear copper(i) acetylides and organic azides; propyne and methyl azide were chosen for simplicity (Scheme 7). Formation of copper(i) acetylide 15 (step A) was calculated to be exothermic by 11.7 kcal mol−1, consistent with the well-known facility of this step which probably occurs through a π-alkyne copper complex intermediate. π-Coordination of alkyne to copper significantly acidifies terminal hydrogen of the alkyne, bringing it into the proper range to be deprotonated in an aqueous medium and resulting in the formation of a σ-acetylide. The azide is then activated by coordination to copper (step B), forming intermediate 16. This ligand exchange step is nearly thermoneutral computationally (2.0 kcal mol−1 uphill when L is water), and the coordination event is synergistic for both reactive partners: coordination of the azide reveals the β-nucleophilic, vinylidene-like properties of the acetylide, whereas the azide’s terminus becomes even more electrophilic. In the next step (C), the first C–N bond-forming event takes place, and a strained copper metallacycle 17 forms. This step is endothermic by 12.6 kcal mol−1 with a calculated barrier of 18.7 kcal mol−1, which corresponds roughly to the observed rate increase and is considerably lower than the barrier for the uncatalyzed reaction (approximately 26.0 kcal mol−1), thus accounting for the observed rate acceleration accomplished by Cu(i). The formation of copper triazolide is very facile and energetically favorable. When protected by steric bulk, Cu–triazolyl complexes can be isolated from CuAAC reactions,36 and in rare cases of low catalyst loading and high catalytic rate, step E can be turnover-limiting.46 Alternative pathways, including the concerted cycloaddition of azide and copper acetylide, were ruled out. The coordination of the azide to copper via the terminal nitrogen, proposed in a recent review article by Meldal and Tornøe,5 had been also examined and found to be energetically unfavorable (no feasible intermediates could be identified). There is no experimental evidence to support this proposal either.
Scheme 7.
(A) Early proposed catalytic cycle for the CuAAC reaction based on DFT calculations. (B) Introduction of a second copper(i) atom favorably influences the energetic profile of the reaction (L = H2O in DFT calculations). At the bottom is shown the optimized structures for dinuclear Cu forms of the starting acetylide (left, corresponding to 15), transition state for the key C–N bond-forming step (middle), and the metallacycle 17. The calculated structures are essentially identical when acetylide instead of chloride is used as the ancillary ligand on the second copper center (CuB).
The DFT investigation described above was soon followed by a study of the kinetics of the copper-mediated reaction between benzyl azide and phenylacetylene in DMSO under pseudo-first order conditions. With low catalyst loadings, saturating alkyne concentrations, and in the presence of the triazole product, the initial rate law was found to be second order in metal.49 Second-order dependence on catalyst concentration was also observed for CuAAC reactions accelerated by tris(benzimidazolyl)methyl amine ligands such as 13 in Tris buffer solutions.36,46 The 2nd order rate law in the catalyst during the initial stages of the reaction did not, of course, unequivocally indicate the involvement of two copper atoms in the bond-forming events in the catalytic cycle. Indeed, we have learned since then that the mechanism of the reaction is more complex, and the reaction often exhibits discontinuous kinetic behavior (specifically, rate law orders in the catalyst and reagents change as the reaction progresses). However, the implication that multinuclear copper species were involved in catalysis prompted us to examine such possibility both computationally and experimentally.
When the CuAAC pathway was investigated by DFT taking into account the possibility of the involvement of dinuclear copper(i) acetylides, a further drop in the activation barrier (by approximately 3–6 kcal mol−1) was revealed (Scheme 7B).50,51 In the transition state, a second copper(i) atom, CuB, strongly interacts with the proximal acetylide carbon (C1), as indicated by the short Cu–C distances of 1.93 and 1.90 Å. A computational study reported by Straub compared dinuclear complexes with higher order aggregates and concluded that dinuclear intermediates were favored over the tetranuclear complexes.51
Additional experiments further expanded our mechanistic understanding of this process and helped us formulate experimental guidelines that we would like to share with the readers. Since isolated copper(i) acetylides normally exist in highly aggregated form, preparation of the reactive copper(i) acetylides in situ, i.e. in the presence of an organic azide, is critical for the success of the reaction. Although organic azides are weak ligands for copper, their interaction with the metal center appears to be sufficient to prevent the formation of the unproductive polymeric acetylides and channel the acetylides into the productive catalytic cycle. This is demonstrated by a simple experiment which can be followed visually: the addition of 5 mol% of copper(ii) sulfate to the 0.1 M solution of phenyl acetylene in tert-butanol/water (2 : 1) results, within minutes, in the formation of dark yellow phenyl copper acetylide. Removal of the precipitate after 15 min leaves the supernatant virtually copper free, and the isolated phenyl copper acetylide does not react with azides. In fact, once the dark yellow acetylide precipitate is formed, the addition of the azide does not revive the catalysis. When the same experiment is performed in the presence of 1 equiv. of benzyl azide, the light yellow color that develops upon the addition of CuSO4 disappears within ca. 20 min, but the reaction is still far from completion (ca. 40% conversion in the case at hand). It is clear that the catalyst is undergoing reorganization while the catalytic cycle is turning over during the initial 20 min after the start of the reaction. Before the catalytic cycle enters the steady state regime, a number of turnovers occurs, and both the identity and concentration of the catalyst undergo significant changes. Such behavior is not uncommon for catalytic reactions, but clearly complicates interpretation of the initial rates results.52
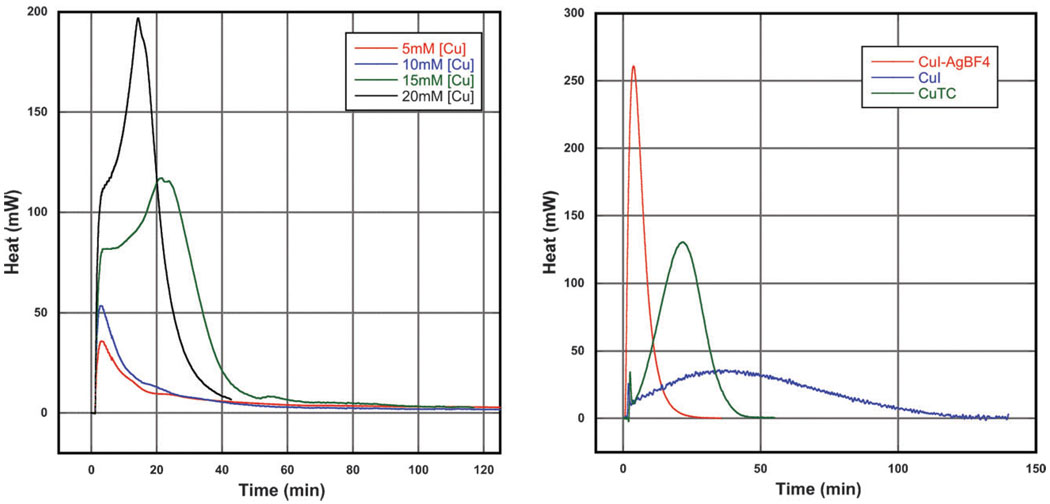
The multimodal kinetic profile of the reaction is confirmed by following its heat output using heat flow calorimetry. A representative thermogram of a CuAAC reaction is shown in Scheme 8A.
Scheme 8.
(A) Calorimetry trace of the reaction of benzyl azide and phenyl acetylene (0.1 M in tert-butanol/water, 1 : 1, the indicated amount of copper sulfate, and two equivalents of ascorbate with respect to copper. The multimodal kinetic profile is especially easy to see in the black and green traces: the reactions take 12 and 25 min, respectively, to reach maximum rate. All four reactions proceeded to completion. (B) Calorimetry trace of the reaction of benzyl azide with phenyl acetylene (0.1 M in THF, 5 mM CuX as a 1 : 1 complex with TTTA ligand 10b). The reaction is significantly faster when the iodide is removed with silver(i) tetrafluoroborate (red trace) than in the presence of the iodide (blue trace). Copper thiophene carboxylate (CuTC)-catalyzed trace, which shows intermediate behavior, is in green.
As already mentioned, the nature of the copper counter ion also has a dramatic effect on the rate and efficiency of the reaction. As Scheme 8B demonstrates, the cuprous iodide-catalyzed reaction takes nearly 40 min to reach the maximum rate and over 100 min to reach full conversion, whereas the replacement of the iodide with much weaker coordinating tetrafluoroborate propels the reaction to completion within minutes.
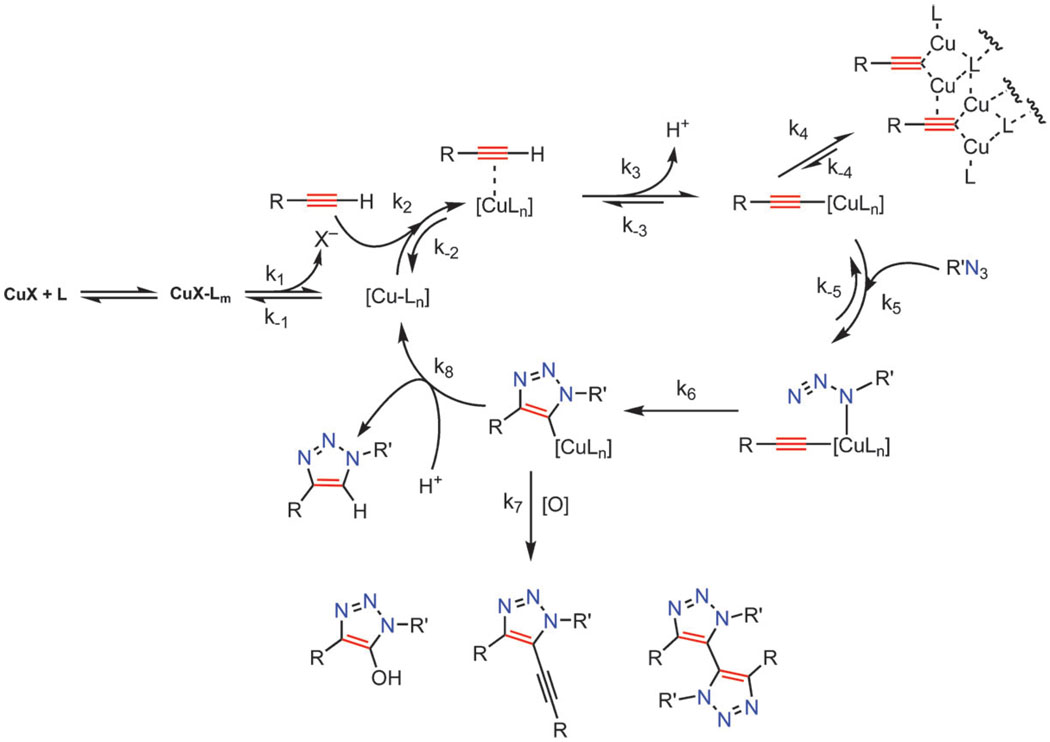
These mechanistic insights underscore the importance of taking into account every event affecting the fate of the catalytic cycle intermediates when analyzing complex catalytic processes involving multiple equilibria, competing pathways, and off-cycle dead ends (even if they are potentially reversible). Although the elementary steps for the key bond-making and bond-breaking events that were previously proposed and supported by DFT calculations8,50,51 still stand, we now have a better quantitative measure of catalyst activation and deactivation pathways and their effect on the overall process. The details of our studies are beyond the scope of this review and will be published in a full account shortly. Nevertheless, in Scheme 9, we offer our current mechanistic view of the process as gleaned from both the earlier studies and the more recent results of the rigorous kinetic analysis.
Scheme 9.
Several key equilibria and irreversible off-cycle pathways affect the productive CuAAC catalytic cycle. For example, formation of oligomeric copper acetylides may become a key event under certain conditions: if most of the catalyst is occupied in the form of unproductive polymeric copper acetylides, the rate of the re-entry of reactive copper acetylide into the catalytic cycle (k−4) would determine the overall rate of the process.
The main observations and hypotheses are as follows. First, the formation of higher order polynuclear copper acetylides has a detrimental effect on the rate and the outcome of the reaction. Therefore, solvents that promote ligand exchange (e.g. water and alcohols) are preferred over apolar, organic solvents which promote aggregation of copper species. Ill-defined catalysts perform well in the CuAAC precisely for this reason.
Second, dinuclear complexes may exhibit enhanced reactivity. Even though to date we have not seen an example of the reaction that exhibits uniform second order in the catalyst as it progresses, it is possible that nuclearity of the catalytic species is maintained throughout the catalytic cycle and, as a consequence, all elementary steps are effectively bimolecular, exhibiting the observed first order in the catalyst. As already mentioned, order of the reaction in catalyst is not an indicator of its molecularity nor of the composition of the catalyst.
Third, successful CuAAC ligands need to balance the competing requirements of binding Cu(i) strongly enough to prevent the formation of unreactive polymeric complexes, yet allowing azide to access the coordination sphere of the σ-acetylide Cu center. Too potent a binder would tie up the necessary Cu coordination sites and too weak a ligand would not prevent the formation of higher order aggregates. Tris-triazolyl amine ligands, such as TBTA 10a and TTTA 10b, and related tripodal ligands successfully meet these challenges by combining weak donor ligands on the “arms” of the structure with the stronger central nitrogen donor. Possible composition of a reactive complex is exemplified by structure 19. These ligands also accomplish the delicate task of providing enough electron density to the metal to promote the catalysis while not destabilizing the Cu(i) oxidation state. 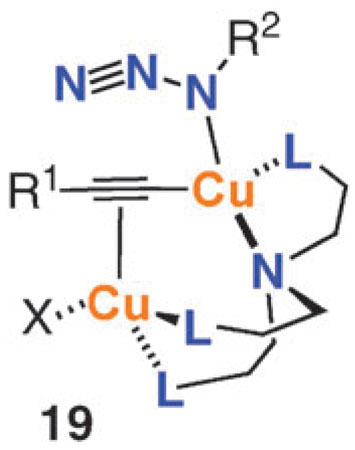
Reactions of 1-iodoalkynes
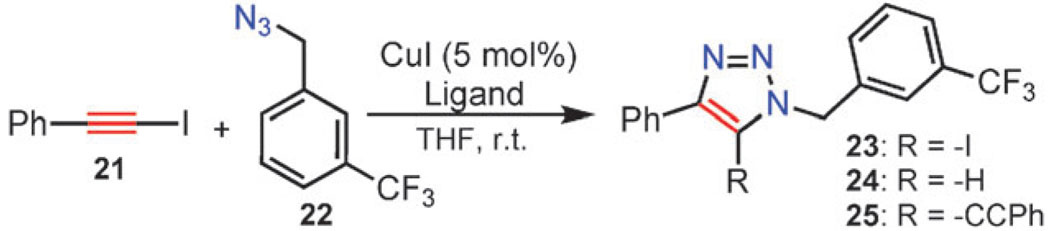
1-Iodoalkynes are stable and readily accessible internal acetylenes. As disclosed recently, they are exceptionally reactive partners with organic azides under copper(i) catalysis conditions (Scheme 10).53 In fact, their reactivity appears to surpass that of terminal alkynes. As an added benefit, the products of the reaction, 5-iodo-1,2,3-triazoles, are versatile synthetic intermediates amenable for further functionalization. The reaction is general, chemo- and regio-selective, and the experimental procedure is operationally simple. The 5-iodo-1,4,5-trisubstituted-1,2,3-triazoles are obtained in high yields from variously substituted organic azides and iodoalkynes. The process is catalyzed by copper(i) iodide in the presence of an amine ligand. In contrast to the CuAAC reaction, the observed rate and chemoselectivity are strongly dependent on the nature of the ligand, and the reaction does not proceed in its absence. Both TBTA (10a) and its tert-butyl analog, TTTA (10b) give 5-iodotriazoles 23 as exclusive products in excellent yield.
Scheme 10.
The iodoalkyne version of the CuAAC reaction (iCuAAC).
The competing pathways, which arise from dehalogenation of the iodoalkyne and the triazole product and lead to 5-proto 24 and 5-alkynyl triazoles 25, are far too slow in the presence of these ligands to be even detectable.
The CuI-TTTA catalyst system exhibits excellent functional group compatibility, and 5-iodotriazoles are obtained as exclusive products in high yield from structurally and functionally diverse azides and 1-iodoalkynes. Due to mild reaction conditions, high chemoselectivity, and low copper catalyst loading, reaction workup is usually as simple as trituration followed by filtration. The scale-up is also easy, and a number of 5-iodotriazoles have been prepared in multigram quantities.
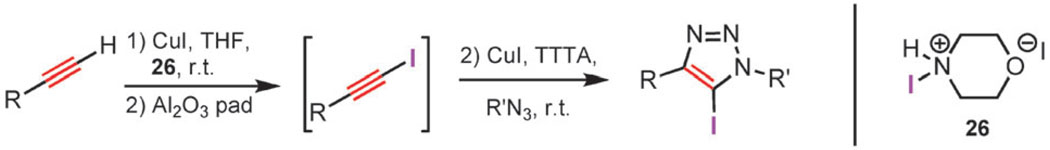
1-Iodoalkynes are readily obtained from terminal acetylenes by treating them with N-iodomorpholine 26 in the presence of CuI, giving the corresponding 1-iodoalkynes within 30 to 60 min. The products can be isolated by passing the reaction mixture through a pad of silica gel or alumina, yielding the desired 1-iodoalkyne in high yield. It can be submitted to the reaction with an azide in a one-pot, two-step procedure (Scheme 11). The 1-iodoalkyne is partially purified via filtration through neutral alumina prior to the introduction of the azide component. This method gives 5-iodotriazoles with an efficiency comparable to that observed with the isolated 1-iodoalkynes.
Scheme 11.
One-pot, two-step synthesis of 5-iodo-1,2,3-triazoles.
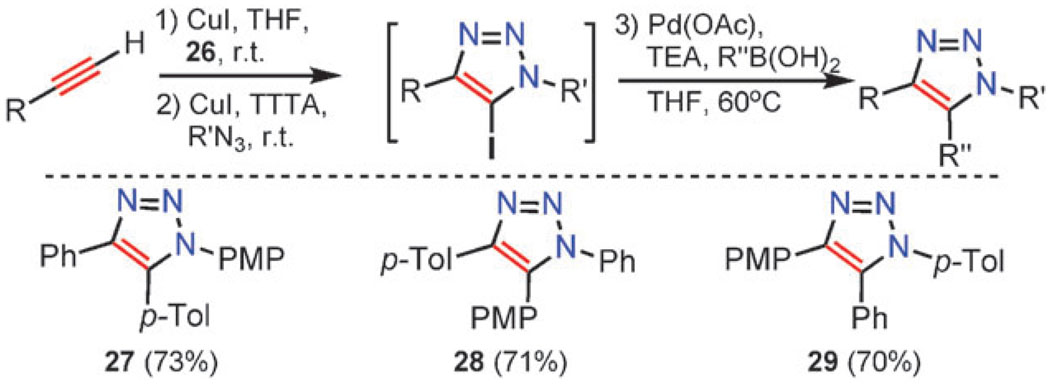
This sequence could be further extended to the synthesis of 1,4,5-triaryl-1,2,3-triazoles 27–29 (Scheme 12) by assembling the 5-iodotriazole and immediately employing Pd(0)-catalyzed cross-coupling with an appropriate arylboronic acid. This simple stepwise construction obviates purification of any intermediates and simultaneously provides complete control over the placement of substituents around the 1,2,3-triazole nucleus, allowing facile access to all regioisomeric permutations of trisubstituted triazoles 27–29. Similar regiocontrolled synthesis would not be possible via thermal or ruthenium-catalyzed routes due to the steric and electronic similarity of the aryl substituents (phenyl, tolyl, and p-methoxyphenyl).
Scheme 12.
One-pot, three-step synthesis of 1,4,5-triaryltriazoles. PMP = p-methoxyphenyl, p-Tol = p-methylphenyl.
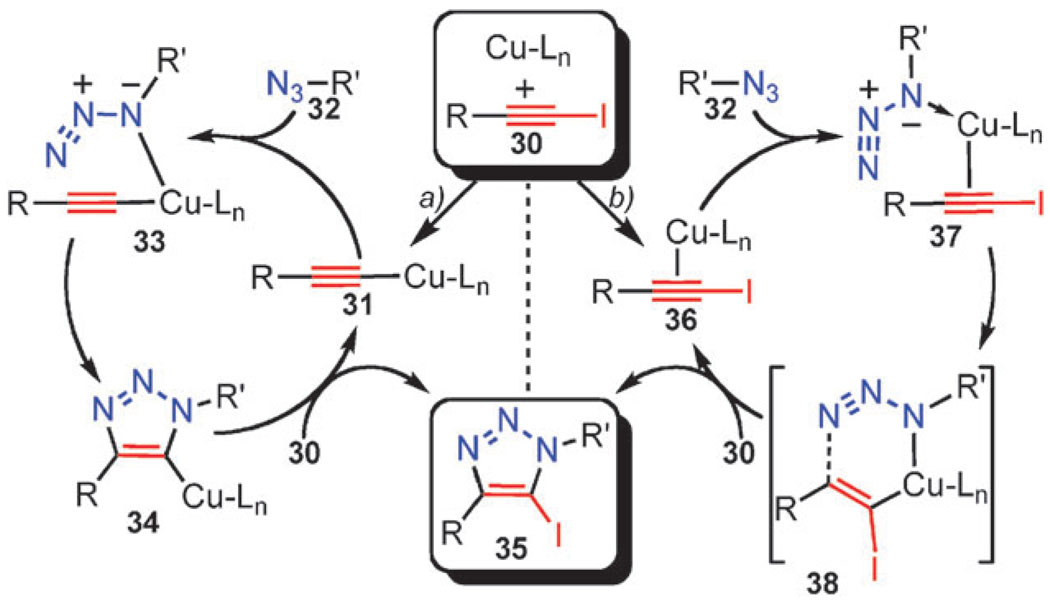
Beyond the immediate synthetic utility of the iodo–CuAAC reactions, examination of its mechanism will provide a better understanding of both the iodo and the parent CuAAC processes. Although both reactions clearly share common features, the activation of iodo- and terminal alkynes by copper are very different too. The current mechanistic proposals are outlined in Scheme 13. One of possible pathways is similar to that proposed for the CuAAC and involves the formation of the σ-acetylide complex 31 as the first key intermediate (Scheme 13a). Cu–I exchange via σ-bond metathesis with iodoalkyne 30 completes the cycle, liberating iodotriazole 35 and regenerating acetylide 31.
Scheme 13.
Proposed mechanistic pathways for the Cu(i)-catalyzed azide-iodoalkyne cycloaddition.
Alternatively, copper may activate the iodoalkyne via the formation of a π-complex intermediate (Scheme 13b), which then engages the azide, producing complex 37. Cyclization then proceeds via a now familiar vinylidene-like intermediate 38, to give iodotriazole 35. A similar transition state has been proposed to explain the involvement of di-copper intermediates in the CuAAC reaction.50,51 The distinctive feature of this pathway is that the carbon–iodine bond is never severed during the catalysis.
Preliminary studies and the results from reaction optimization experiments to date appear to favor pathway b in Scheme 13. The main observation supporting this hypothesis being the exclusive formation of the 5-iodotriazole even when the reaction is performed in protic solvents and/or with substrates containing acidic protons. If pathway a were operational, the cuprated triazole intermediate 34 could be trapped with other electrophiles, including a proton, thereby producing a mixture of the 5-iodo and 5-prototriazoles. Whatever the interactions of iodoalkynes with copper(i) are, they appear to be stronger than for terminal acetylenes: when the reaction is performed on a mixture of a terminal and 1-iodoalkyne, the formation of the 5-prototriazole does begin until all iodoalkyne is consumed. This behavior may indicate the complete catalyst monopoly by the iodo cycle and different catalyst resting states for the two competing processes.
Reactions of sulfonyl azides
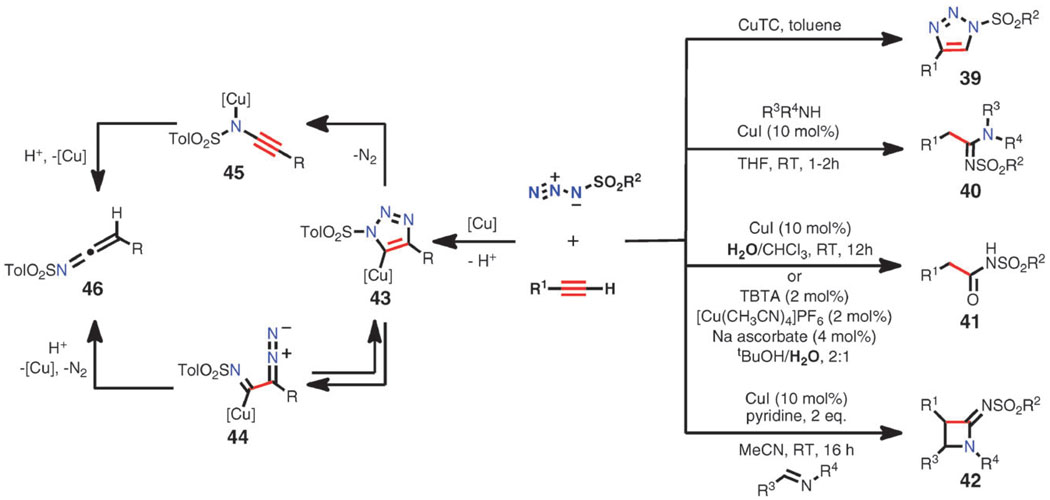
Sulfonyl azides participate in unique reactions with terminal alkynes under the copper catalysis conditions. Depending on the conditions and reagents, products other than the expected triazole (39)54 can be obtained, as shown in Scheme 14. These products are thought to derive from the cuprated triazole intermediate 43, which is destabilized by the strong electron-withdrawing character of the N-sulfonyl substituent. Ring-chain isomerization can occur to form the cuprated diazoimine 44 which, upon the loss of a molecule of dinitrogen, furnishes the N-sulfonyl ketenimine 46.55 Alternatively, copper(i) alkynamide 45 can be generated with a concomitant elimination of N2 and, after protonation, would again generate the reactive ketenimine species 46. In the absence of a nucleophilic reagent, ketenimine dimers (not shown) are isolated.55 When the reaction is conducted in the presence of amines, N-sulfonyl amidines (40) are formed.56 Under aqueous conditions, N-acyl sulfonamides (41) are the major products.57 In addition to amines and water, ketenimine intermediates can be trapped with imines, furnishing a N-sulfonyl azetidinimines 42 (Scheme 4).55
Scheme 14.
(Right) Products of CuAAC reactions with sulfonyl azides. (Left) Possible pathways leading to ketenimine intermediates.
1-Sulfonyl triazoles: convenient precursors of azavinyl carbenes
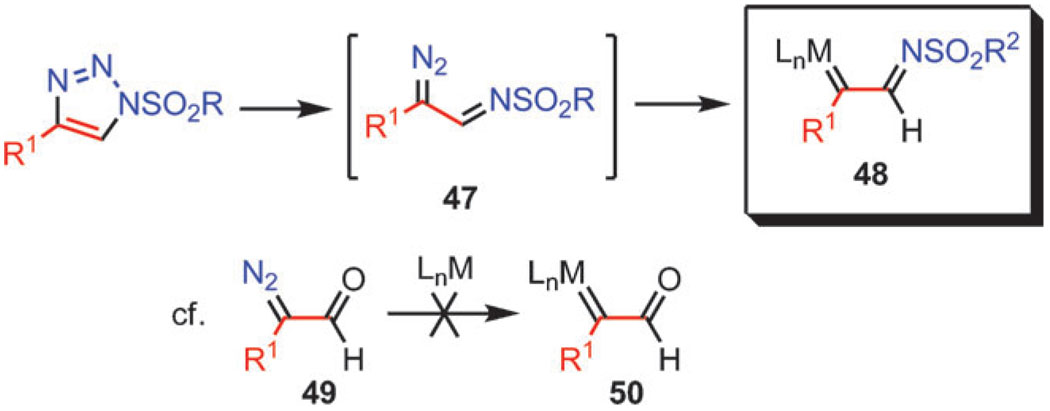
As already mentioned, 1,2,3-triazoles are very stable heterocycles. However, those that bear a strong electron-withdrawing group at N1 are known to undergo ring-chain tautomerization. The ring-chain isomerism of 1-sulfonyl 1,2,3-triazoles can be exploited in synthesis. Thus, 1-sulfonyl triazoles could serve as precursors to the diazoimine species 47 that, in turn, could be converted to metal carbene complexes 48 (Scheme 15). Rhodium(ii)-stabilized carbene complexes exhibit the wealth of reactivity, and this method of generating their diazo progenitors is particularly attractive considering that sulfonyl triazoles effectively become synthetic equivalents of α-diazo aldehydes 49, which are very unstable cannot be converted to the corresponding metal carbenes 50.
Scheme 15.
Azavinyl carbenes from diazoimines.
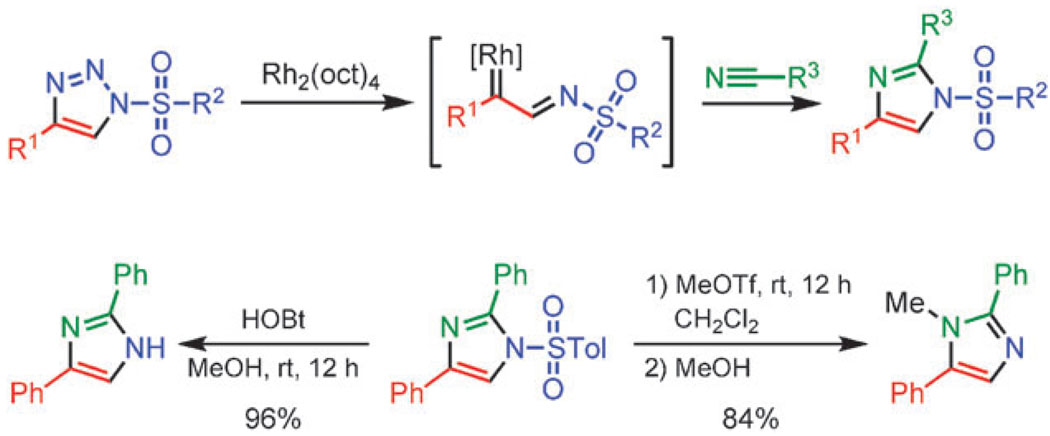
In the presence of a dirhodium(ii) tetraoctanoate catalyst, 1-sulfonyl triazoles react with nitriles, forming imidazoles.58 The reaction proceeds at 60–80 °C with conventional heating or can be performed in a microwave reactor and generally provides imidazoles in good to excellent yields (Scheme 16, top). The sulfonyl group can be readily removed, revealing the parent NH-imidazole. Alternatively, sulfonyl imidazoles can be converted to 1,2,5-trisubstituted imidazoles by simple alkylation (Scheme 16, bottom).
Scheme 16.
Synthesis of imidazoles via rhodium-catalyzed transannulation of 1-sulfonyl triazoles.
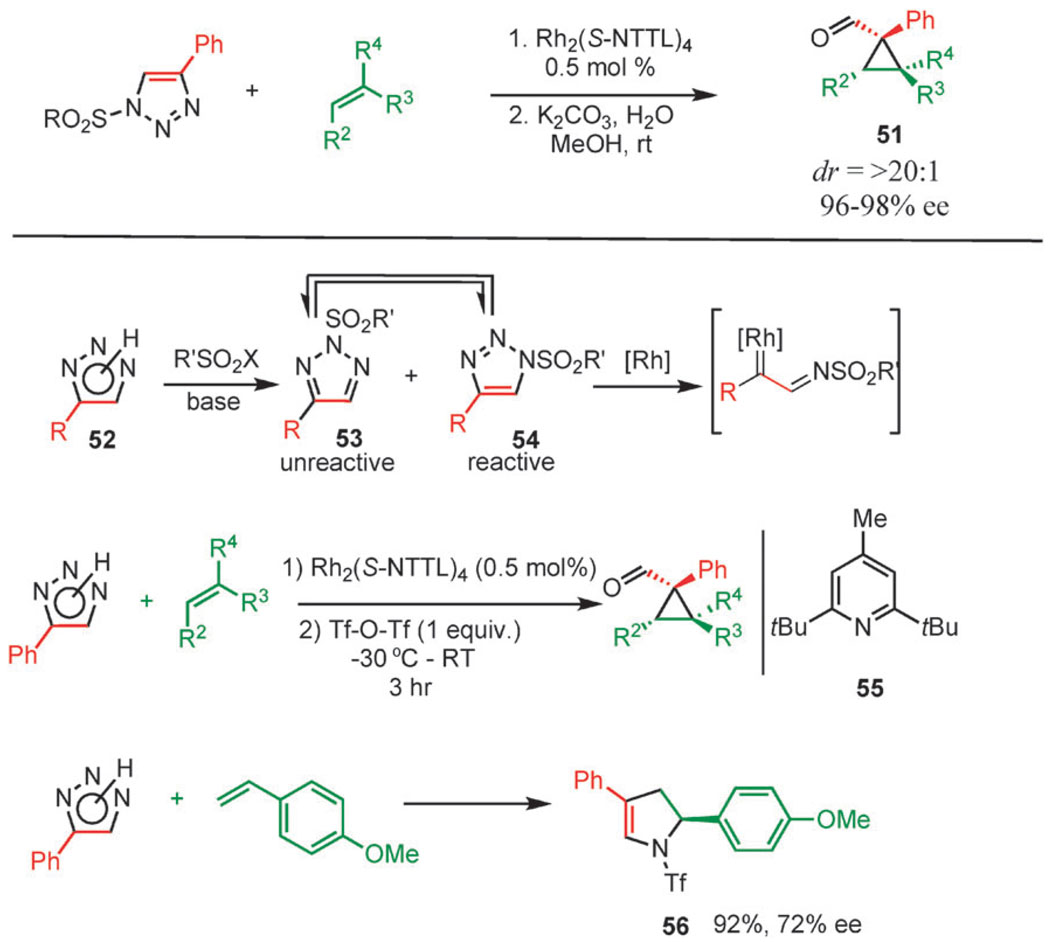
Another example of the exquisite reactivity of rhodium(ii) azavinyl carbenes is illustrated by their addition to olefins, which is a very facile process that proceeds under mild conditions with high diastereo and enantioselectivity. Chiral rhodium(ii) carboxylate complexes, such as Rh2(S-NTTL)4 and Rh2(S-NTV)4 are used in the reactions, which are usually performed at elevated temperature (Scheme 17, top) when isolated N-sulfonyl triazoles are used as starting materials.59 The N-sulfonyl imine group hydrolyzes quickly, revealing virtually enantiopure cyclopropane carboxaldehydes 51. These useful synthetic intermediates are difficult to obtain using other methods.
Scheme 17.
Cyclopropanation of olefins using 1-sulfonyl 1,2,3-triazoles.
In an alternative approach, very electron-deficient and highly reactive N-triflyl azavinyl carbenes can be prepared by in situ sulfonylation of NH-triazoles 52 with triflic anhydride in the presence of a hindered pyridine base 55 (Scheme 17).60 Although the sulfonylation step is not selective and results in the formation of both N1- and N2-sulfonylated triazoles (53 and 54, respectively), only the N1-isomer can undergo ring-chain isomerization and, therefore, form a reactive carbene intermediate (the N1 and N2 regioisomers are interconverting on the time scale of the reaction, so the yields are high). The resulting N-triflyl azavinyl carbenes exhibit exceptional reactivity towards olefins, resulting in the formation of cyclopropanes and 1,2-dihydropyrroles (when electron-rich olefins are used; Scheme 17, bottom) with excellent enantio- and diastereoselectivity. The ability to introduce extremely electron withdrawing groups into the azavinyl carbene, thereby controlling its electrophilicity, is a valuable feature of this approach.
Conclusions
The quick acceptance which the CuAAC reaction gained by practitioners of many chemical disciplines illustrates the immense need for transformations that are experimentally simple, robust, and reliable. CuAAC is a rare example of a reaction that meets those criteria and yet is mechanistically very complex. Designing new ligands and conditions for a catalytic transformation that already performs very well and whose mechanism is not well understood is a daunting task, and we hope that this tutorial review has clarified the main features known to date of these unusual and, above all, dynamic catalytic processes. Our aim was to capture the essential reactivity variables that are most likely to be useful to the users of CuAAC catalysis and to also facilitate efforts by fellow reaction developers looking to unearth new and useful transformations of transition metal acetylide species.
Acknowledgements
We are grateful to the many colleagues and coworkers who have contributed to the work cited here. Research in our laboratories described in this review has been supported in part by the National Institute of General Medical Sciences, National Institutes of Health (GM087620), National Science Foundation (CHE-0848982), and the Skaggs Institute for Chemical Biology.
Biographies

Jason E. Hein
Jason Hein received his BSc in biochemistry in 2000 from the University of Manitoba in Winnipeg, MB, Canada. He then began his PhD studies as an NSERC postgraduate fellow under the guidance of Professor Philip G. Hultin at the University of Manitoba. In 2005 he completed his graduate work, where he synthesized and studied a new family of soluble-supported chiral auxiliaries. He is currently an NSERC postdoctoral fellow at the Scripps Research Institute. His current interests include the design, development and study of new metal-catalyzed reactions.

Valery V. Fokin
Valery V. Fokin received his undergraduate education at the University of Nizhny Novgorod, Russia, and his PhD degree at the University of Southern California under the tutelage of Prof. Nicos A. Petasis. After a postdoctoral stint with Prof. K. Barry Sharpless at The Scripps Research Institute in La Jolla, California, he joined the Scripps faculty, where he is currently Associate Professor in the Department of Chemistry. His research is centered on the understanding of chemical reactivity of organometallic species and on applying it to the studies of macromolecular and biological phenomena. His research group is working on new reaction development, studies of the organic and organometallic mechanism, medicinal chemistry, synthesis of multifunctional probes for imaging and drug delivery, and smart polymeric materials.
Footnotes
Part of a themed issue reviewing the latest applications of click chemistry.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3062. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med. Res. Rev. 2008;28:278–308. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]; Lutz J-F, Zarafshani Z. Adv. Drug Delivery Rev. 2008;60:958–970. doi: 10.1016/j.addr.2008.02.004. [DOI] [PubMed] [Google Scholar]; Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]; Fokin VV. ACS Chem. Biol. 2007;2:775–778. doi: 10.1021/cb700254v. [DOI] [PubMed] [Google Scholar]; Johnson JA, Koberstein JT, Finn MG, Turro NJ. Macromol. Rapid Commun. 2008;29:1052–1072. [Google Scholar]; Bock VD, Hiemstra H, van Maarseveen JH. Eur. J. Org. Chem. 2006:51–68. [Google Scholar]; Wu P, Fokin VV. Aldrichimica Acta. 2007;40:7–17. [Google Scholar]
- 5.Meldal M, Tornoe CW. Chem. Rev. Vol. 108. Washington, DC, U. S.: 2008. pp. 2952–3015. [Google Scholar]
- 6.For a continuously updated list of applications of the CuAAC and a comprehensive list of reaction conditions, the reader is referred to http://www.scripps.edu/chem/fokin/cuaac
- 7.Huisgen R. Angew. Chem., Int. Ed. Engl. 1963;2:565–598. [Google Scholar]
- 8.Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 9.Tomé AC. In: Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Storr RC, Gilchrist TL, editors. vol. 13. Thieme, Stuttgart: 2004. pp. 415–601. Five-Membered Hetarenes with Three or More Heteroatoms. [Google Scholar]; Krivopalov VP, Shkurko OP. Russ. Chem. Rev. 2005;74:339–379. [Google Scholar]
- 10.Zhang L, Chen X, Xue P, Sun HHY, Williams ID, Sharpless KB, Fokin VV, Jia G. J. Am. Chem. Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
- 11.Boren BC, Narayan S, Rasmussen LK, Zhang L, Zhao H, Lin Z, Jia G, Fokin VV. J. Am. Chem. Soc. 2008;130:8923–8930. doi: 10.1021/ja0749993. [DOI] [PubMed] [Google Scholar]; Rasmussen LK, Boren BC, Fokin VV. Org. Lett. 2007;9:5337–5339. doi: 10.1021/ol701912s. [DOI] [PubMed] [Google Scholar]; Majireck MM, Weinreb SM. J. Org. Chem. 2006;71:8680–8683. doi: 10.1021/jo061688m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hein JE, Tripp JC, Krasnova LB, Sharpless KB, Fokin VV. Angew. Chem., Int. Ed. 2009;48:8018–8021. doi: 10.1002/anie.200903558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy KR, Rajgopal K, Kantam ML. Synlett. 2006:957–959. [Google Scholar]; Reddy KR, Rajgopal K, Kantam ML. Catal. Lett. 2007;114:36–40. [Google Scholar]; Fukuzawa S, Shimizu E, Kikuchi S. Synlett. 2007:2436–2438. [Google Scholar]
- 14.Nigh WG. In: Oxidation in Organic Chemistry. Part B. Trahanovsky WS, editor. New York: Academic Press; 1973. pp. 1–95. [Google Scholar]
- 15.Siemsen P, Livingston RC, Diederich F. Angew. Chem., Int. Ed. 2000;39:2632–2657. [PubMed] [Google Scholar]
- 16.Fahrni CJ. Curr. Opin. Chem. Biol. 2007;11:121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Angell Y, Burgess K. Angew. Chem., Int. Ed. 2007;46:3649–3651. doi: 10.1002/anie.200700399. [DOI] [PubMed] [Google Scholar]; Gerard B, Ryan J, Beeler AB, Porco JA., Jr Tetrahedron. 2006;62:6405–6411. [Google Scholar]
- 18.Appukkuttan P, Dehaen W, Fokin VV, Van der Eycken E. Org. Lett. 2004;6:4223–4225. doi: 10.1021/ol048341v. [DOI] [PubMed] [Google Scholar]
- 19.Moorhouse AD, Moses JE. Synlett. 2008:2089–2092. [Google Scholar]
- 20.Pachon LD, van Maarseveen JH, Rothenberg G. Adv. Synth. Catal. 2005;347:811–815. [Google Scholar]
- 21.Molteni G, Bianchi CL, Marinoni G, Santo N, Ponti A. New J. Chem. 2006;30:1137–1139. [Google Scholar]
- 22.Lipshutz BH, Taft BR. Angew. Chem., Int. Ed. 2006;45:8235–8238. doi: 10.1002/anie.200603726. [DOI] [PubMed] [Google Scholar]
- 23.Bogdan AR, Sach NW. Adv. Synth. Catal. 2009;351:849–854. [Google Scholar]
- 24.Zhu W, Ma D. Chem. Commun. 2004:888–889. doi: 10.1039/b400878b. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AK, Colasson B, Fokin VV. Org. Lett. 2004;6:3897–3899. doi: 10.1021/ol048859z. [DOI] [PubMed] [Google Scholar]
- 26.Barral K, Moorhouse AD, Moses JE. Org. Lett. 2007;9:1809–1811. doi: 10.1021/ol070527h. [DOI] [PubMed] [Google Scholar]
- 27.Pearson RG. J. Am. Chem. Soc. 1988;110:7684–7690. [Google Scholar]
- 28.Hathaway BF. In: Comprehensive Coordination Chemistry. Wilkinson G, editor. vol. 5. Pergamon, Oxford: 1987. p. 533. [Google Scholar]
- 29.Perez-Balderas F, Ortega-Munoz M, Morales-Sanfrutos J, Hernandez-Mateo F, Calvo-Flores FG, Calvo-Asin JA, Isac-Garcia J, Santoyo-Gonzalez F. Org. Lett. 2003;5:1951–1954. doi: 10.1021/ol034534r. [DOI] [PubMed] [Google Scholar]
- 30.Malkoch M, Schleicher K, Drockenmuller E, Hawker CJ, Russell TP, Wu P, Fokin VV. Macromolecules. 2005;38:3663–3678. [Google Scholar]; Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Angew. Chem., Int. Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 31.Gonda Z, Novák Z. Dalton Trans. 2010;39:726–729. doi: 10.1039/b920790m. [DOI] [PubMed] [Google Scholar]
- 32.Campbell-Verduyn LS, Mirfeizi L, Dierckx RA, Elsinga PH, Feringa BL. Chem. Commun. 2009:2139–2141. doi: 10.1039/b822994e. [DOI] [PubMed] [Google Scholar]
- 33.Detz RJ, Arevalo Heras S, De Gelder R, Van Leeuwen PWNM, Hiemstra H, Reek JNH, Van Maarseveen JH. Org. Lett. 2006;8:3227–3230. doi: 10.1021/ol061015q. [DOI] [PubMed] [Google Scholar]
- 34.Bai S-Q, Koh LL, Hor TSA. Inorg. Chem. 2009;48:1207–1213. doi: 10.1021/ic801690v. [DOI] [PubMed] [Google Scholar]
- 35.Díez-Gonzalez S, Correa A, Cavallo L, Nolan SP. Chem.-Eur. J. 2006;12:7558–7564. doi: 10.1002/chem.200600961. [DOI] [PubMed] [Google Scholar]; Díez-González S, Nolan SP. Angew. Chem. 2008;46:9013–9016. doi: 10.1002/anie.200803289. [DOI] [PubMed] [Google Scholar]
- 36.Nolte C, Mayer P, Straub BF. Angew. Chem., Int. Ed. 2007;46:2101–2103. doi: 10.1002/anie.200604444. [DOI] [PubMed] [Google Scholar]
- 37.Rodionov VO, Presolski SI, Diaz DD, Fokin VV, Finn MG. J. Am. Chem. Soc. 2007;129:12705–12712. doi: 10.1021/ja072679d. [DOI] [PubMed] [Google Scholar]
- 38.Mykhalichko BM, Temkin ON, Mys’kiv MG. Russ. Chem. Rev. 2000;69:957–984. [Google Scholar]
- 39.Candelon N, Lastecoueres D, Diallo AK, Aranzaes JR, Astruc D, Vincent JM. Chem. Commun. 2008:741–743. doi: 10.1039/b716306a. [DOI] [PubMed] [Google Scholar]
- 40.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 42.Chan TR, Fokin VV. QSAR Comb. Sci. 2007;26:1274–1279. [Google Scholar]
- 43.Chan TR, Fokin VV, Sharpless KB. Abstracts of Papers, 227th ACS National Meeting; March 28–April 1, 2004; Anaheim, CA, United States. 2004. ORGN-041. [Google Scholar]; Hong V, Presolski SI, Ma C, Finn MG. Angew. Chem., Int. Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong V, Udit AK, Evans RA, Finn MG. ChemBioChem. 2008;9:1481–1486. doi: 10.1002/cbic.200700768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis WG, Magallon FG, Fokin VV, Finn MG. J. Am. Chem. Soc. 2004;126:9152–9153. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]
- 46.Rodionov VO, Presolski SI, Gardinier S, Lim Y-H, Finn MG. J. Am. Chem. Soc. 2007;129:12696–12704. doi: 10.1021/ja072678l. [DOI] [PubMed] [Google Scholar]
- 47.Cenini S, Gallo E, Caselli A, Ragaini F, Fantauzzi S, Piangiolino C. Coord. Chem. Rev. 2006;250:1234–1253. [Google Scholar]
- 48.Vrieze K, van Koten G. Comprehensive Coordination Chemistry. vol. 2. Pergamon, Oxford: 1987. pp. 189–245. [Google Scholar]
- 49.Rodionov VO, Fokin VV, Finn MG. Angew. Chem., Int. Ed. 2005;44:2210–2215. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 50.Ahlquist M, Fokin VV. Organometallics. 2007;26:4389–4391. [Google Scholar]
- 51.Straub BF. Chem. Commun. 2007:3868–3870. doi: 10.1039/b706926j. [DOI] [PubMed] [Google Scholar]
- 52.Rosner T, Pfaltz A, Blackmond DG. J. Am. Chem. Soc. 2001;123:4621–4622. doi: 10.1021/ja005872f. [DOI] [PubMed] [Google Scholar]
- 53.Hein JE, Tripp JC, Krasnova LB, Sharpless KB, Fokin VV. Angew. Chem., Int. Ed. 2009;48:8018–8021. doi: 10.1002/anie.200903558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo EJ, Ahlquist M, Kim SH, Bae I, Fokin VV, Sharpless KB, Chang S. Angew. Chem., Int. Ed. 2007;46:1730–1733. doi: 10.1002/anie.200604241. [DOI] [PubMed] [Google Scholar]
- 55.Whiting M, Fokin VV. Angew. Chem., Int. Ed. 2006;45:3157–3161. doi: 10.1002/anie.200503936. [DOI] [PubMed] [Google Scholar]
- 56.Bae I, Han H, Chang S. J. Am. Chem. Soc. 2005;127:2038–2039. doi: 10.1021/ja0432968. [DOI] [PubMed] [Google Scholar]
- 57.Cassidy MP, Raushel J, Fokin VV. Angew. Chem., Int. Ed. 2006;45:3154–3157. doi: 10.1002/anie.200503805. [DOI] [PubMed] [Google Scholar]; Cho SH, Yoo EJ, Bae I, Chang S. J. Am. Chem. Soc. 2005;127:16046–16047. doi: 10.1021/ja056399e. [DOI] [PubMed] [Google Scholar]
- 58.Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J. Am. Chem. Soc. 2008;130:14972–14974. doi: 10.1021/ja805079v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J. Am. Chem. Soc. 2009;131:18034–18035. doi: 10.1021/ja908075u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimster NP, Zhang L, Fokin VV. J. Am. Chem. Soc. 2010;132:2510–2511. doi: 10.1021/ja910187s. [DOI] [PMC free article] [PubMed] [Google Scholar]