Abstract
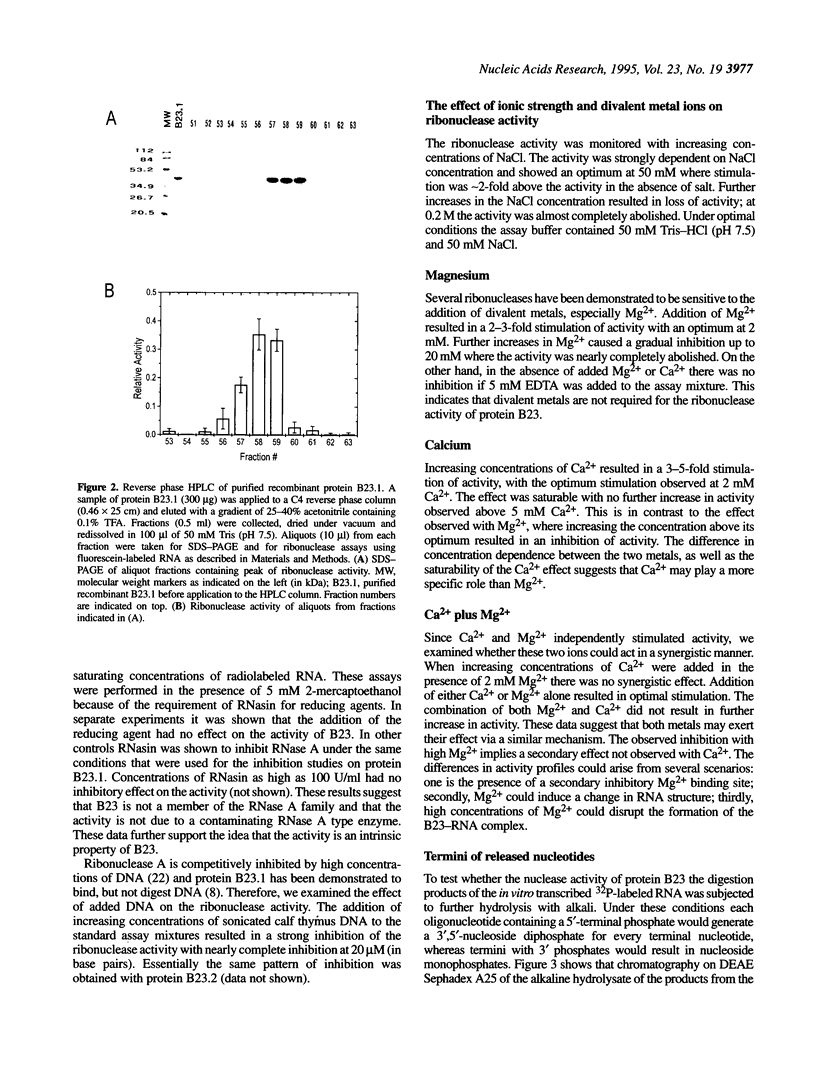
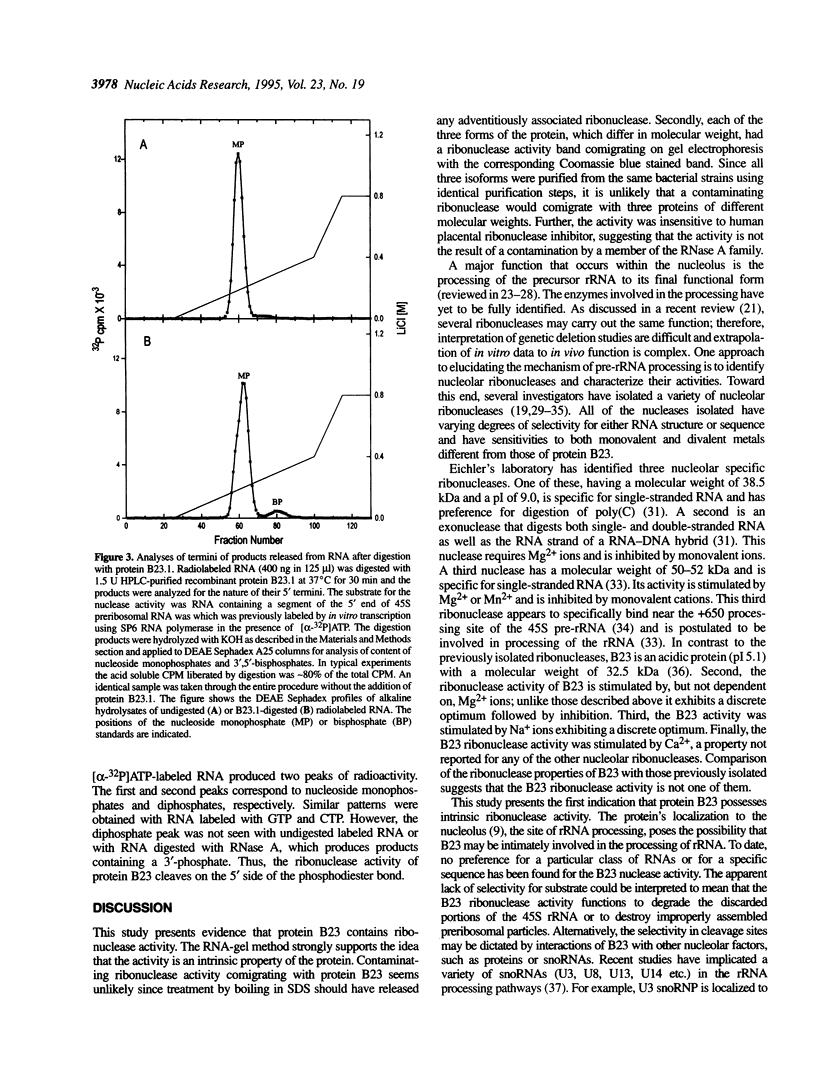
Protein B23 is an abundant nucleolar protein and putative ribosome assembly factor. The protein was analyzed for ribonuclease activity using RNA-embedded gels and perchloric acid precipitation assays. Three purified bacterially expressed forms of the protein, B23.1, B23.2 and an N-terminal polyhistidine tagged B23.1 as well as the natural protein were found to have ribonuclease activity. However, the specific activity of recombinant B23.1 was approximately 5-fold greater than that of recombinant B23.2. The activity was insensitive to human placental ribonuclease inhibitor, but was inhibited by calf thymus DNA in a dose dependent manner. The enzyme exhibited activity over a broad range of pH with an apparent optimum at pH 7.5. The activity was stimulated by but not dependent on the presence of low concentrations of Ca2+, Mg2+ or NaCl. The Ca2+ effect was saturable and only stimulatory in nature. In contrast, Mg2+ and NaCl exhibited optimal concentrations for stimulation and both inhibited the ribonuclease at concentrations above these optima. These data suggest that protein B23 has intrinsic ribonuclease activity. The location of protein B23 in subcompartments of the nucleolus that contain preribosomal RNA suggests that its ribonuclease activity plays a role in the processing of preribosomal RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltrame M., Tollervey D. Identification and functional analysis of two U3 binding sites on yeast pre-ribosomal RNA. EMBO J. 1992 Apr;11(4):1531–1542. doi: 10.1002/j.1460-2075.1992.tb05198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggiogera M., Fakan S., Kaufmann S. H., Black A., Shaper J. H., Busch H. Simultaneous immunoelectron microscopic visualization of protein B23 and C23 distribution in the HeLa cell nucleolus. J Histochem Cytochem. 1989 Sep;37(9):1371–1374. doi: 10.1177/37.9.2768807. [DOI] [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Chang J. H., Olson M. O. Structure of the gene for rat nucleolar protein B23. J Biol Chem. 1990 Oct 25;265(30):18227–18233. [PubMed] [Google Scholar]
- Deutscher M. P. Ribonuclease multiplicity, diversity, and complexity. J Biol Chem. 1993 Jun 25;268(18):13011–13014. [PubMed] [Google Scholar]
- Dumbar T. S., Gentry G. A., Olson M. O. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry. 1989 Nov 28;28(24):9495–9501. doi: 10.1021/bi00450a037. [DOI] [PubMed] [Google Scholar]
- Eichler D. C., Eales S. J. Isolation and characterization of a single-stranded specific endoribonuclease from Ehrlich cell nucleoli. J Biol Chem. 1982 Dec 10;257(23):14384–14389. [PubMed] [Google Scholar]
- Eichler D. C., Eales S. J. Purification and properties of a novel nucleolar exoribonuclease capable of degrading both single-stranded and double-stranded RNA. Biochemistry. 1985 Jan 29;24(3):686–691. doi: 10.1021/bi00324a022. [DOI] [PubMed] [Google Scholar]
- Eichler D. C., Eales S. J. The effect of RNA secondary structure on the action of a nucleolar endoribonuclease. J Biol Chem. 1983 Aug 25;258(16):10049–10053. [PubMed] [Google Scholar]
- Eichler D. C., Liberatore J. A., Shumard C. M. Selection of a preribosomal RNA processing site by a nucleolar endoribonuclease involves formation of a stable complex. Nucleic Acids Res. 1993 Dec 11;21(24):5775–5781. doi: 10.1093/nar/21.24.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler D. C., Tatar T. F. Properties of a purified nucleolar ribonuclease from Ehrlich ascites carcinoma cells. Biochemistry. 1980 Jun 24;19(13):3016–3022. doi: 10.1021/bi00554a028. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Izaurralde E., Adachi Y., Wingfield P., Laemmli U. K. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: dissociation by the Rev response element. Mol Cell Biol. 1991 May;11(5):2567–2575. doi: 10.1128/mcb.11.5.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Mond J. J., Kinchington P. R., Hickey R., Karjalainen Lindsberg M. L., Hay I., Ruyechan W. T. Evidence for DNA binding activity of numatrin (B23), a cell cycle-regulated nuclear matrix protein. Biochim Biophys Acta. 1990 Oct 23;1087(2):127–136. doi: 10.1016/0167-4781(90)90196-9. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Maxwell E. S. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993 Apr;18(4):131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S. Nuclear transport. Curr Opin Cell Biol. 1989 Jun;1(3):441–446. doi: 10.1016/0955-0674(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A. Biogenesis of ribosomes in eukaryotes. Subcell Biochem. 1980;7:1–80. doi: 10.1007/978-1-4615-7948-9_1. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Nikolaev N. Maturation of ribosomal ribonucleic acids and the biogenesis of ribosomes. Prog Biophys Mol Biol. 1976;31(2):95–144. doi: 10.1016/0079-6107(78)90006-8. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Kwan C. N. Purification and characterization of an endoribonuclease from nucleoplasm and nucleoli of HeLa cells. J Biol Chem. 1976 Nov 25;251(22):7132–7136. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maser R. L., Calvet J. P. U3 small nuclear RNA can be psoralen-cross-linked in vivo to the 5' external transcribed spacer of pre-ribosomal-RNA. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6523–6527. doi: 10.1073/pnas.86.17.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Perry R. P. RNA processing comes of age. J Cell Biol. 1981 Dec;91(3 Pt 2):28s–38s. doi: 10.1083/jcb.91.3.28s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Klomp G. R., Schmoll D. J., Busch H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry. 1974 Apr 23;13(9):1945–1951. doi: 10.1021/bi00706a026. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., Lewis B. C., Busch H. Purification and properties of a nucleolar endoribonuclease from Novikoff hepatoma. Biochim Biophys Acta. 1973 Sep 7;319(3):323–335. doi: 10.1016/0005-2787(73)90172-x. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., Tonato M., Busch H. Low molecular weight RNA associated with 28 s nucleolar RNA. J Mol Biol. 1970 Feb 14;47(3):505–515. doi: 10.1016/0022-2836(70)90318-9. [DOI] [PubMed] [Google Scholar]
- Sekine H., Nakano E., Sakaguchi K. The interaction of DNA with pancreatic ribonuclease A. Biochim Biophys Acta. 1969 Jan 21;174(1):202–210. doi: 10.1016/0005-2787(69)90243-3. [DOI] [PubMed] [Google Scholar]
- Shumard C. M., Torres C., Eichler D. C. In vitro processing at the 3'-terminal region of pre-18S rRNA by a nucleolar endoribonuclease. Mol Cell Biol. 1990 Aug;10(8):3868–3872. doi: 10.1128/mcb.10.8.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Ochs R. L., Busch H. Silver staining, immunofluorescence, and immunoelectron microscopic localization of nucleolar phosphoproteins B23 and C23. Chromosoma. 1984;90(2):139–148. doi: 10.1007/BF00292451. [DOI] [PubMed] [Google Scholar]
- Stroke I. L., Weiner A. M. The 5' end of U3 snRNA can be crosslinked in vivo to the external transcribed spacer of rat ribosomal RNA precursors. J Mol Biol. 1989 Dec 5;210(3):497–512. doi: 10.1016/0022-2836(89)90126-5. [DOI] [PubMed] [Google Scholar]
- Szebeni A., Herrera J. E., Olson M. O. Interaction of nucleolar protein B23 with peptides related to nuclear localization signals. Biochemistry. 1995 Jun 27;34(25):8037–8042. doi: 10.1021/bi00025a009. [DOI] [PubMed] [Google Scholar]
- Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987 Dec 20;6(13):4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. A new interaction between the mouse 5' external transcribed spacer of pre-rRNA and U3 snRNA detected by psoralen crosslinking. Nucleic Acids Res. 1992 Oct 25;20(20):5375–5382. doi: 10.1093/nar/20.20.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekawa H., Chang J. H., Correia J. J., Wang D., Wingfield P. T., Olson M. O. Nucleolar protein B23: bacterial expression, purification, oligomerization and secondary structures of two isoforms. Cell Mol Biol Res. 1993;39(7):635–645. [PubMed] [Google Scholar]
- Wang D., Baumann A., Szebeni A., Olson M. O. The nucleic acid binding activity of nucleolar protein B23.1 resides in its carboxyl-terminal end. J Biol Chem. 1994 Dec 9;269(49):30994–30998. [PubMed] [Google Scholar]
- Wang D., Umekawa H., Olson M. O. Expression and subcellular locations of two forms of nucleolar protein B23 in rat tissues and cells. Cell Mol Biol Res. 1993;39(1):33–42. [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Yung B. Y., Busch H., Chan P. K. Translocation of nucleolar phosphoprotein B23 (37 kDa/pI 5.1) induced by selective inhibitors of ribosome synthesis. Biochim Biophys Acta. 1985 Dec 18;826(4):167–173. doi: 10.1016/0167-4781(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Yung B. Y., Busch R. K., Busch H., Mauger A. B., Chan P. K. Effects of actinomycin D analogs on nucleolar phosphoprotein B23 (37,000 daltons/pI 5.1). Biochem Pharmacol. 1985 Nov 15;34(22):4059–4063. doi: 10.1016/0006-2952(85)90387-9. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell. 1976 May;8(1):19–31. doi: 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]