Abstract
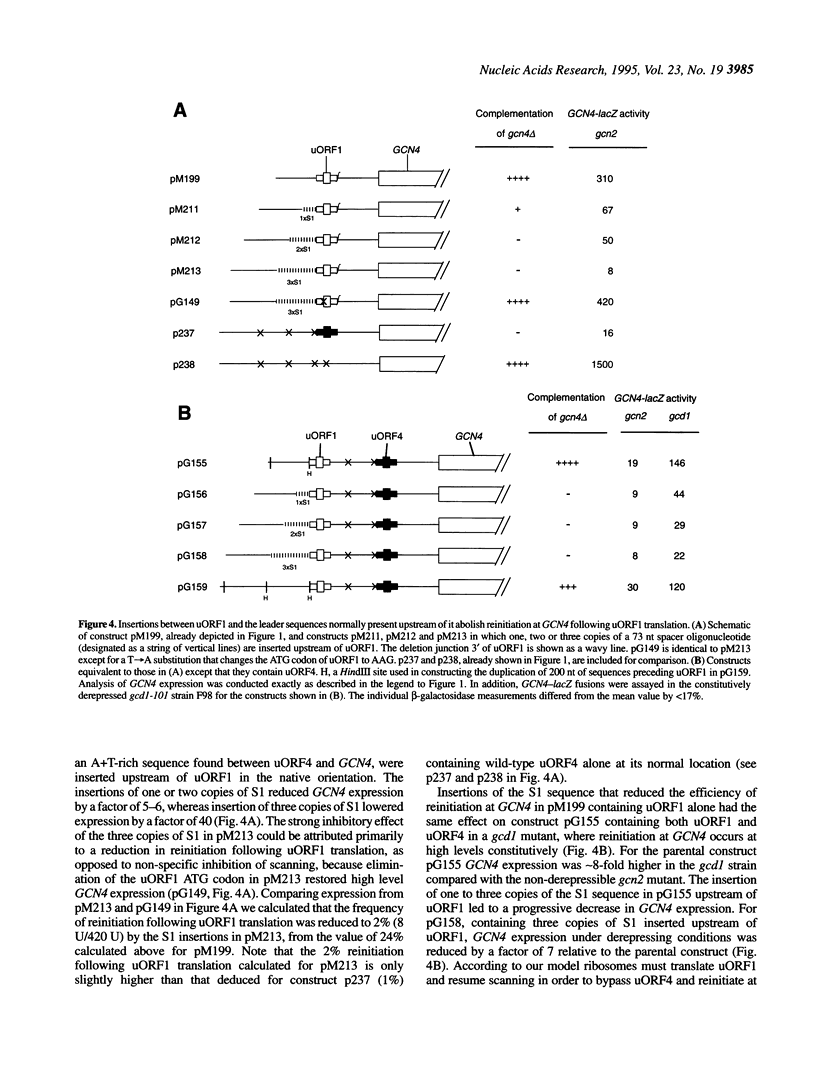
Translation of yeast GCN4 mRNA occurs by a reinitiation mechanism that is modulated by amino acid levels in the cell. Ribosomes which translate the first of four upstream open reading frames (uORFs) in the mRNA leader resume scanning and can reinitiate downstream. Under non-starvation conditions reinitiation occurs at one of the remaining three uORFs and GCN4 is repressed. Under starvation conditions, in contrast, ribosomes bypass the uORFs and reinitiate at GCN4 instead. The high frequency of reinitiation following uORF1 translation depends on an adequate distance to the next start codon and particular sequences surrounding the uORF1 stop codon. We present evidence that sequences 5' to uORF1 also strongly enhance reinitiation. First, reinitiation was severely inhibited when uORF1 was transplanted into the position of uORF4, even though the native sequence environment of the uORF1 stop codon was maintained, and this effect could not be accounted for by the decreased uORF1-GCN4 spacing. Second, insertions and deletions in the leader preceding uORF1 greatly reduced reinitiation at GCN4. Sequences 5' to uORF1 may influence the probability of ribosome release following peptide termination at uORF1. Alternatively, they may facilitate rebinding of an initiation factor required for reinitiation prior to resumption of the scanning process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Miller P. F., Jackson B. M., Hinnebusch A. G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991 Jan;11(1):486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Donahue T. F. Sequence and structural features associated with translational initiator regions in yeast--a review. Gene. 1987;59(1):1–18. doi: 10.1016/0378-1119(87)90261-7. [DOI] [PubMed] [Google Scholar]
- Grant C. M., Hinnebusch A. G. Effect of sequence context at stop codons on efficiency of reinitiation in GCN4 translational control. Mol Cell Biol. 1994 Jan;14(1):606–618. doi: 10.1128/mcb.14.1.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. M., Miller P. F., Hinnebusch A. G. Requirements for intercistronic distance and level of eukaryotic initiation factor 2 activity in reinitiation on GCN4 mRNA vary with the downstream cistron. Mol Cell Biol. 1994 Apr;14(4):2616–2628. doi: 10.1128/mcb.14.4.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. A hierarchy of trans-acting factors modulates translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Sep;5(9):2349–2360. doi: 10.1128/mcb.5.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Translational control of GCN4: an in vivo barometer of initiation-factor activity. Trends Biochem Sci. 1994 Oct;19(10):409–414. doi: 10.1016/0968-0004(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanker S., Bushman J. L., Hinnebusch A. G., Trachsel H., Mueller P. P. Autoregulation of the yeast lysyl-tRNA synthetase gene GCD5/KRS1 by translational and transcriptional control mechanisms. Cell. 1992 Aug 21;70(4):647–657. doi: 10.1016/0092-8674(92)90433-d. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. F., Hinnebusch A. G. Sequences that surround the stop codons of upstream open reading frames in GCN4 mRNA determine their distinct functions in translational control. Genes Dev. 1989 Aug;3(8):1217–1225. doi: 10.1101/gad.3.8.1217. [DOI] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Mueller P. P., Jackson B. M., Miller P. F., Hinnebusch A. G. The first and fourth upstream open reading frames in GCN4 mRNA have similar initiation efficiencies but respond differently in translational control to change in length and sequence. Mol Cell Biol. 1988 Dec;8(12):5439–5447. doi: 10.1128/mcb.8.12.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez de Aldana C. R., Wek R. C., Segundo P. S., Truesdell A. G., Hinnebusch A. G. Multicopy tRNA genes functionally suppress mutations in yeast eIF-2 alpha kinase GCN2: evidence for separate pathways coupling GCN4 expression to unchanged tRNA. Mol Cell Biol. 1994 Dec;14(12):7920–7932. doi: 10.1128/mcb.14.12.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. P., Mueller P. P., Hinnebusch A. G. The positive regulatory function of the 5'-proximal open reading frames in GCN4 mRNA can be mimicked by heterologous, short coding sequences. Mol Cell Biol. 1988 Sep;8(9):3827–3836. doi: 10.1128/mcb.8.9.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




