Huntington's disease (HD) is a dominant, monogenic, neurodegenerative disorder affecting 1 in 10,000 individuals. The progressive loss of gross motor skills, and the development of chorea – which consists of abrupt, involuntary movements – are considered the most prominent clinical manifestations of HD. However, motor defects are not limited to such gross abilities, and fine motor skills deteriorate earlier in the course of HD. Other important neurological signs include neuropsychiatric symptoms and cognitive impairment. However, effective therapies for this devastating disease are not currently available and, after a typical latency of 20 years from diagnosis, HD invariably culminates in premature death [1].
HD is characterised by the accumulation of mutated huntingtin (mu-htt) in intracellular inclusions. The accumulation of misfolded proteins is a feature common to many age-related neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, HD and amyotrophic lateral sclerosis [2, 3]. The intracellular degradation of these misfolded proteins is carried out by two main systems, the ubiquitin–proteasome and the autophagy–lysosome systems, and alteration of both has been demonstrated in the course of the pathogenesis of neurodegenerative diseases [4]. In particular, autophagy is responsible for the degradation of long-lived proteins and organelles, and increases in response to nutrient deprivation, hypoxia and mitochondrial dysfunction. In HD, the mitochondrial respiratory process is defective, and this condition leads to increased production of reactive oxygen species (ROS) [5]. These alterations may produce cell injury that may then result in cell death due to excessive autophagy or apoptosis. Type 2 transglutaminase (TG2) accumulates at high levels in cells under stressful conditions, and plays an important role both in apoptosis and autophagy regulation [6]. TG2 belongs to the transglutaminase (TGase) family of calcium-dependent transamidation acyltransferases, catalysing the post-translational modification of proteins [7].
In an effort to define novel therapeutic avenues, here we will discuss the relevance of TG2 to the pathogenesis of HD. TG2 inhibition, achieved either via drug treatments or genetic approaches, has been shown to be beneficial for the treatment of HD in animal models [8, 9]. We will therefore highlight those features of the pathogenesis that might be relevant for HD therapy, and cite other reports for a more comprehensive description of this disease.
Huntington's disease
HD is classified as a pathology of the basal ganglia, which affects principally the caudate nucleus and the putamen of the striatum. The pathology is selective for certain neuronal populations, including medium-sized projection spiny neurons (MSNs), whereas others such as aspiny interneurons are relatively spared [10-12]. MSNs use the neurotransmitter gamma-aminobutyric acid GABA and exert an inhibitory effect in the basal ganglia circuits; the uncontrolled choreic movements typical of HD have been ascribed to the loss of this inhibitory input [13]. Recent findings provided important insights into the mechanisms leading to the cognitive defects in HD, showing that these symptoms correlate with cortical atrophy; these findings demonstrate that HD pathology also involves brain areas other than the striatum [14]. Despite the predominance of neurological symptoms, it is now clear that HD is also associated with serious systemic defects; these alterations are not simply the consequence of the neurological problems, but are systemic components accompanying the neurological symptoms. Metabolic alterations [15, 16], impaired glucose metabolism [17, 18] and severe weigh loss [19, 20] are just some of the peripheral abnormalities associated with HD [21].
The genetic defect responsible for HD is the expansion of an unstable tract of CAG repeats in the 5′ region of the gene IT15 that encodes the protein htt, which is translated in a stretch of polyglutamine (polyQ) [22]. The function of wild-type htt is still poorly understood, with evidence pointing to a role in fundamental cellular functions such as trafficking, endocytosis and regulation of transcription [23, 24]. On the other hand, it is well established that the presence of elongated polyQ confers toxic properties to the protein. It is interesting that the toxicity of polyQ does not depend upon the nature of the protein containing the expansion; in fact, a total of nine genetic polyQ diseases have been described, and the expanded tract is the only common point identified so far in the mutated proteins, which are otherwise unrelated [25]. This observation strongly suggests that the presence of an expanded polyQ is sufficient to generate pathogenesis, regardless of the gene affected [26]. This notion is also supported by transgenic animal models, in which the expression of short fragments of the mutated proteins containing little more than the polyQ tract is enough to cause the development of neurological symptoms [27, 28]. Of importance, the length of polyQ positively correlates with the age of disease onset, as well as with the severity of the symptoms [29].
An important feature conferred by the presence of expanded polyQ is the development of aggregates, which is a hallmark of the diseases associated with this, including HD. Initially aggregates were classified on the basis of their subcellular localisation and microscopic morphology, and were distinguished as nuclear intracellular aggregates (NII) or protein aggregates in dystrophic neuritis [30] (Figure 2). However, these different varieties of inclusions probably represent a late form of aggregation, which does not seem to correlate with the progression of the pathogenesis. In fact, some mu-htt transgenic mice models show extensive aggregates in the brain without any sign of pathology [31]; moreover, we have shown that targeted deletion of the TG2 gene in R6/1 mice ameliorated the symptoms while leading to an increased number of aggregates [9]. Subsequent and more detailed biophysical studies provided important insights into the states that lead from the monomeric htt fragment to the aggregate forms, and have shown that mu-htt can generate amyloid fibrils [32], as well as oligomeric and protofibrillar structures [33, 34]. These small species are considered more toxic than large aggregates. As far as the mechanism of aggregation is concerned, biophysical studies have demonstrated that isolated polyQ can aggregate spontaneously through an enzyme-independent nucleation mechanism [35, 36]. However, the abundance of polyQ suggested that these amino acid tracts could be ideal substrates of cross-linking enzymes such as TGases. This suggestion was based on the observation that in the skin, polyQ-rich proteins such as involucrin are cross-linked by epidermal TGases to form the cornified layer of the epidermis [37]. The ability of TGases to cross-link polyQ has been demonstrated independently in several studies [38-40], and this finding led to more detailed investigations of the role of these enzymes in HD pathogenesis; this will be described in greater detail below. Although there is some debate about which mechanism better reflects the aggregation dynamics in vivo, it is likely that both hypothesised models – enzymatic or non-enzymatic driven aggregation - have a role.
Figure 2.
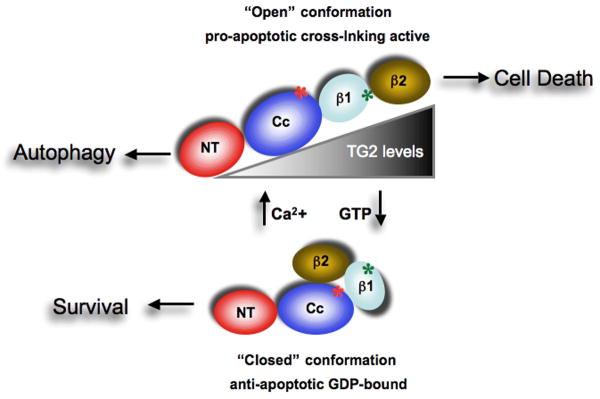
Type 2 transglutaminase (TG2) structural changes in relationship to its functions. Graphic representation of the four domains of TG2. The following regions are highlighted: C277, active site cysteine (red asterisk); GTP-binding motif (red asterisk). It has been proposed that Ca2+ concentration may control the shift between the ‘open’ pro-apoptotic [high (Ca2+)] and the ‘closed’ pro-survival [low (Ca2+)] conformations of TG2. The expression of high TG2 levels in the presence of calcium will favour apoptosis whilst lower enzyme levels promote autophagy. The pro-survival and pro-death features of TG2 also rely on its localization in the cytosol or in the nucleus.
In general, the cellular conditions occurring during HD pathogenesis are very favorable for TGases activity, and the presence of good substrates as polyQ does not represent the sole element supporting the involvement of these enzymes in HD. In fact, their cross-linking activity depends upon calcium levels, and neuronal calcium homeostasis is highly perturbed in the presence of mu-htt [41].
A major source of calcium influx into neurons is the N-methyl-D-aspartic acid (NMDA) subclass of ionotropic glutamate receptors (NMDAr). Although NMDAr are essential for proper development and synaptic plasticity, their hyperactivation leads to cell death by excitotocixity [42, 43]. Dysfunction of NMDA receptors has been well documented in HD [44]. Initial studies based on radioactive ligand assays showed a dramatic decrease in signal in the striatum of patients with early stage disease and even in pre-symptomatic patients, suggesting that neurons with high levels of NMDA receptors were preferentially lost [45]. Subsequent studies demonstrated that the subunit composition of the NMDA receptor, which is critical for modulation of the activity of the receptor through gating properties [46], changes during the pathogenesis in animal models [44]. Perhaps more importantly, some findings suggest that the expression levels of NMDAr are a determinant of the age of onset of HD [47]. Finally, the neuropathological and behavioural symptoms of HD can be accurately reproduced by injecting agonists of the NDMAr such as quinolinic acid into the striatum of rodents and non-human primates [48, 49]. These alterations in the NMDAr are due, at least in part, to the polyQ-mediated disruption of the interaction between the NMDAr and the partner protein PSD95 [50]. Abnormalities of calcium homeostasis in HD, however, cannot be ascribed uniquely to the NMDAr. Indeed, in the presence of expanded polyQ, htt N-terminal fragments can be engaged in abnormal interactions with mitochondria, impairing the ability of the organelles to buffer calcium [51-53]. In such conditions, mitochondria are less resistant to calcium challenges, and are quicker to depolarise in response to the opening of the permeability transition pore. Moreover, mu-htt can also sensitise inositol (1,4,5)-triphosphate receptors on the endoplasmic reticulum to their agonist, thus facilitating further calcium release [41, 54]. The overall conclusion from these observations is that the calcium defects in HD are the consequence of the mobilisation of extracellular as well as intracellular stores. Calcium mishandling results in increased cytoplasmic levels, as evidenced by activation of calcium-dependent enzymes, including calpains [55] and TGases [9].
“Tissue” or type 2 transglutaminase
In mammals, nine distinct TGase genes have been identified [6, 7]. The enzymes encoded by these different genes display a wide structural homology being members of the papain-like superfamily of cysteine proteases [6, 7]. TG2, the most ubiquitous member of the TGase family, is a versatile multifunctional protein involved in a variety of biochemical functions at various cellular locations [6]. Depending upon the substrates, the Ca2+-dependent cross-linking activity of TG2 is responsible for different related protein post-translational modifications such as incorporation of di- and polyamines into proteins, protein–protein cross-linking and site-specific deamidation (if the involved group is a water molecule instead of an amine) (for an extensive biochemical description see references 6 and 7). TG2 can also act extracellularly, in fact, under certain circumstances the enzyme can be exposed on the external leaflet of the plasma membrane or released outside the cells, where it has been shown to mediate the interaction between integrins and fibronectin and the extracellular matrix (ECM) [6, 7].
In addition TG2 acts as a G-protein in the presence of low calcium levels, binding and hydrolysing GTP with an affinity and a catalytic rate similar to the alpha subunit of large heterotrimeric G-proteins and small Ras-type G-proteins [56]. Under such circumstances, TG2 couples α1b and α1d adrenoreceptors, thromboxane and oxytocine receptors to phospholipase C delta1 [6, 7, 57]. When the enzyme is in a GTP-/GDP-bound form, it does catalyse transamidation reactions [6, 7, 57]. This inhibition is abolished by Ca2+, which plays a role as a molecular switch between these two functions [58] (Figure 1). Finally, based both on in vitro and in vivo studies, a Ca2+-independent protein disulphide isomerase (PDI) function has been proposed for this enzyme [59, 60]. The PDI activity, which requires cysteine residues to occur, relies on an active domain independent from that used for the cross-linking activity; in fact, site-specific mutagenesis experiments demonstrated that the cysteine in the cross-linking active site is not necessary for the PDI activity itself [59, 60].
Figure 1.
Morphological characterization of dying cells in the brain of Huntington's disease R6/1 transgenic mice. (a) Ubiquitin immunostaining showing many huntingtin containing aggregates (NII) appearing as red dots inside the nuclei of neurons in the cortex of a 25- week-old R6/1 mice. (b–c) Electron micrographs of condensed striatal neurons detected in the cortex of 25- week-old R6/1 transgenic mice. An abnormal neuron in an advanced stage of condensation is shown in panel C (4000×). In panel C, the presence of a large autophagosome in a condensed abnormal neuron is clearly visible (arrow, 8000×). Note the absence in this condensed neuron of any morphological features of the apoptosis (such as chromatin condensation and margination, apoptotic bodies formation, detachment from neibouring cells).
This particular ability of TG2 to catalyse all these enzymatic activities together with its various subcellular locations and protein partners clearly suggest multiple biological functions of this enzyme, but although extensive analysis has been carried out in different cellular populations under physiological as well as pathological settings, a unified view is lacking [6, 7]. Endothelial, mesangial and smooth muscle cells constitutively express high TG2 levels [61]; other cell types do not. Gene expression of TG2 is induced by several distinct signalling pathways which target specific response elements in the regulatory region of the gene [62]. The regulation of the various TG2 enzymatic activities is also regulated by many heterogeneous factors. It has been proposed that high Ca2+ levels can induce the release of GTP/GDP molecules promoting the transamidation activity [58]. It is interesting that these two opposite functions are associated with a major transition in the protein 3D structure varying from an “open” GDP-/GTP-bound conformation to a “closed” Ca2+-dependent conformation (Figure 1). The Ca2+ requirement for such a transition and the cross-linking enzymatic activity can be reduced by the interaction of TG2 with specific molecules, such as sphingosylphoshocholine [63]. Nitric oxide can also strongly influence TG2 activity as up to 15 of the 18 cysteine residues in the protein can be nitrosylated and denitrosylated in a Ca2+-dependent manner, inhibiting and activating the enzyme, respectively [64].
The cytoplasm does not represent the only intracellular location for TG2, as the enzyme possesses nuclear localisation sequences allowing its translocation into the nucleus, presumably with the help of importin alpha 3 [65]. TG2 is also localised in the mitochondrial inter-membrane space where, through its PDI activity, it regulates the assembly and consequently the activity of some members of the respiratory chain complexes as well as of adenine nucleotide translocator 1 (ANT1) [66]. In keeping with these findings, the ablation of the TG2 gene in mice leads to an impairment of the respiratory complex chain resulting in a decrease in ATP production and an effect on the sensitivity of the cell to induce death [67].
TG2 plays an important role in the regulation of cell death and survival
TG2 is one of a number of genes that are selectively induced in dying cells during programmed cell death in vivo [68, 69]. In untransformed cells its overexpression potentiates apoptosis and, conversely, its silencing through anti-sense technology or inhibition reduces the onset of cell death [68]. The activation of the transmidation activity of TG2 in dying cells induces extensive post-translational modifications of intracellular proteins, including actin, histones and retinoblastoma protein [70], leading to the assembly of detergent-insoluble protein scaffolds, which contribute to the stabilisation of the structure of the dying cell prior to its clearance by phagocytosis [71]. In keeping with this finding, TG2-catalysed cross-linking in dying cells limits the leakage of intracellular components, thus preventing the release of soluble, harmful and immunogenic cellular degradation products, and so limiting inflammatory and autoimmune reactions [6].
It has been shown that the overexpression of TG2 in neural cells determines the imbalance of the redox status of cells leading to the accumulation of ROS associated with depletion of glutathione (GSH) [72]. Consistent with this, the enzyme glutathione-S-transferase pi-1 GST-P1-1 - which participates to cellular detoxification using GSH as a substrate - acts as a very efficient acyl donor as well as an acceptor substrate of TG2 both in cells and in vitro (Figure 3) [72]. The TG2-dependent polymerisation of GST-P1-1 leads to its functional inactivation and is effectively inhibited by GSH [72]. It is interesting that GSH depletion characterises the early phases of apoptosis, and the fact that GST-P1-1 might be functionally inactivated by TG2-catalysed oligomerisation indicates a potential pro-apoptotic role for TG2 in antagonising the cytoprotective effect of the elimination of ROS originating from oxidative metabolism [72].
Figure 3.
Type 2 transglutaminase (TG2) has been shown to be involved in many pathologies, the enzyme acting as cross-linking, protein disulphide isomerase (PDI), kinase and G-protein, posttranslationally modifies different protein substrates in the cytosol, nucleus and at mitochondrial level.
Important evidence supporting the pro-apoptotic function of TG2, and its effect on mitochondria, has been reported in pancreatic ductal adenocarcinoma. The activation of endogenous TG2, by the calcium ionophore A23187, in these transformed cells results in rapid and spontaneous apoptosis associated with the release of the apoptosis-inducing factor (AIF). The translocation of AIF from mitochondria to the nucleus leads to the induction of a caspase-independent form of apoptosis [73]. The extensive action exerted by TG2 at the mitochondrial level highlights the contribution of this multifunctional enzyme both to the maintenance of mitochondrial physiology and to the modifications that occur during the onset and execution of apoptosis [74]. This aspect is of particular relevance for those pathologies, such as neurodegenerative diseases, in which the mitochondrial functionality plays a crucial role in the decision between cell survival and death.
It is interesting to note that although TG2 acts a pro-apoptotic factor under physiological conditions, in transformed cells the enzyme has an opposite role acting as an anti-apoptotic factor (Figure 1). Nevertheless, during recent years various studies have demonstrated the ability of TG2 to act as a G-protein [56], a kinase [75], and a modulator of the cell/ECM adhesion process [76]. The prevalence of a specific activity is associated with a different localisation both inside and outside the cell, and is very likely to influence the switch between its pro- or anti-apoptotic functions [74, 77-80]. In fact, it has been shown that both the intracellular localisation and the activation of its transamidation activity are important factors in the modulation of the effects of TG2 on induction of apoptosis. Transfection studies with cDNAs coding for the wild-type or mutant TG2 lacking the transamidation activity targeted to different intracellular compartments, confirmed the pro-apoptotic nature of the cytosolic form of TG2. Nevertheless, the nuclear localisation of the cross-linking-inactive TG2 reduced apoptosis, thus indicating how the intracellular localisation influences its effect on cell death [79].
This differential regulation of cell death by TG2 is highly relevant in cancer, where the anti-apoptotic activity of TG2 might lead to cell survival. Some clues about the possible mechanisms of this pro-survival function of TG2 have been recently reported [79, 81-84]. It has been shown that the exposure of TG2-expressing cells to the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 reduces the ability of the enzyme to bind GTP. This observation suggests that PI3K regulates the shift between the transamidation and the GTP-binding activity of TG2, thus inhibiting the cross-linking activity of TG2 [85]. It is interesting that the opposing pro- and anti-apoptotic functions of TG2 are regulated by conformational changes in the protein (Figure 1). In fact, the binding of GTP can convert the enzyme from an “open”, cell death-promoting, to a “closed” protein conformation, which is able to provide protection against apoptotic stimuli (Figure 1) [86]. This hypothesis has been supported by the observation that the expression of full-length TG2 in tumour cells confers protection against cell death, whereas the expression of a shorter version of TG2, truncated at the 3′ end and unable to bind GTP, is cytotoxic [87]. The pro-apoptotic activity of the short form of TG2 does not rely on its transamidation activity, because the mutation in the cysteine 277 residue, essential for catalysing this reaction, does not compromise the ability of this short form of TG2 to induce cell death. It is notable that a shorter TG2 transcript encoding for a truncated form of TG2 (TGase-S), with a strong pro-apoptotic activity, has been identified in the brain of patients with Alzheimer's disease [88]. This TGase-S exhibits no detectable GTP-binding capability, further suggesting that the ability of TG2 to induce cell death is due to its inability to bind GTP. These results are particularly relevant considering the well-known involvement of TG2 in neurodegenerative diseases (see below).
HD, TG2 and mitochondria
The mechanism(s) by which mu-htt causes selective neuronal death remains obscure. A recently proposed hypothesis is that mu-htt impairs mitochondrial function, resulting in increased oxidative stress and consequent cytotoxicity [51-53, 89]. Several lines of evidence support the notion that impairment of energy production and mitochondrial dysfunction play a key role in the pathogenesis of HD [90].
Mitochondria are essential organelles for life and death of the cell, as they provide most ATP and regulate several cellular pathways, from Ca2+ signalling to apoptosis [91]. During apoptosis, they integrate both intrinsic and extrinsic signals by releasing protein cofactors from their inter-membrane space to the cytosol, where they are required for the activation of effector caspases [91]. These events are associated with the alteration of mitochondrial functionality, including loss of mitochondrial membrane potential (ΔΨ) [92], which is also commonly detected during autophagy [93]. During the induction of autophagy, mitochondria undergo membrane depolarisation before confinement within the autophagosomes [94]. Hence, ΔΨ loss may represent either an early event in apoptosis or a signal associated with autophagocytosis of mitochondria [95].
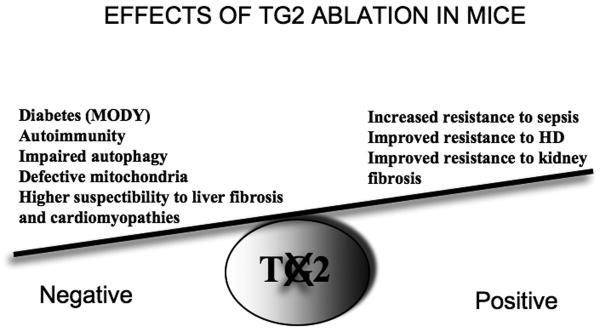
The functional versatility of mitochondria is also suggested by their complex morphology. Mitochondria display an intricate ultrastructure and, in the cytosol of many cells, they appear interconnected. The shape of the mitochondrial network and of the individual mitochondria results from the balance of fusion and fission processes, regulated by a growing family of mitochondria-shaping proteins [96]. This fusion/fission equilibrium is tightly controlled: during mitosis mitochondria fragment in order to be equally distributed into daughter cells; during apoptosis Dynamin-related Protein 1 (DRP–1) is recruited to the mitochondria to induce fission [97, 98] and mitochondrial cristae fuse and their narrow tubular junctions widen to increase the availability of cytochrome c for release [99]. Taken together, these results highlight a crucial role for mitochondrial functional and structural changes during the early events leading to neurodegeneration. TG2 is emerging as a novel player in mitochondrial homeostasis. It has been demonstrated that TG2 knockout mice show impaired glucose-stimulated insulin secretion and intolerance after glucose loading (Figure 4). The resulting phenotype resembles that of maturity onset diabetes of the young (MODY), thus suggesting that TG2 might play a physiological role in glucose tolerance [6]. Defects in glucose-induced insulin release have been widely related to defective respiratory chain activation and loss of ATP production [6]. In keeping with these findings, we have previously demonstrated that TG2 co-localises with mitochondria and that its overexpression leads to mitochondrial dysfunction [100]. We showed that TG2-/- cardiomiocytes are more sensitive to ischaemia/reperfusion injury than their wild-type counterparts [67]. In addition, as the result of impaired mitochondrial function, we demonstrated a significant decrease in the levels of adenine nucleotides in the hearts of TG2-/- mice [67]. These results highlight an unexpected role for TG2 in the modulation of mitochondrial physiology [67, 74]. In fact, TG2-/- mice show a constitutive defect in the activity of mitochondrial respiratory complex I, which protects them against nigro-striatal damage induced by the complex I inhibitor, 1-methyl-4-phenyl-pyridinium ion (MPP+). By contrast, TG2-/- mice are more vulnerable to damage induced by methamphetamine or by the complex II inhibitor, 3-nitropropionic acid [101].
Figure 4.
Graphic representation of the positive and negative effects elicited by the ablation of type 2 transglutaminase (TG2) in mice.
We have recently shown that the PDI activity of TG2 is involved in the regulation of mitochondrial physiology, by modulating the level of ANT1 polymers in the heart [66]. Our studies suggest a complex role for TG2 at the mitochondrial level. In fact, under physiological conditions, the PDI activity of the enzyme contributes to the stabilisation of the respiratory complex I and ANT1 and consequently to the overall homeostasis of ATP [66, 74]. In keeping with this assumption, TG2-/- mice show an impairment of ATP production which is partially buffered by the increased activity of both respiratory complex II and ANT1 [66, 74]. It is interesting that under stressful conditions, TG2 facilitates the recruitment of the pro-apoptotic Bcl-2–associated X protein (Bax) on mitochondria, and the Ca2+-dependent activation of its cross-linking activity leads to the polymerisation of ANT1, thus contributing to irreversible mitochondrial damage [74]. These findings open new avenues in the understanding of TG2 with regard to its physiological role(s) and potential application in the treatment of mitochondrial-dependent diseases, including neurodegenerative diseases.
Autophagy
Autophagy is one of two degradation mechanisms involved in the recycling and turnover of cytoplasmic constituents in eukaryotic cells [2, 3]. In yeast, autophagy is mainly involved in adaptation to starvation, but in multicellular organisms this process is emerging as a multifunctional pathway involved in a variety of additional cellular events [2, 3]. In fact, this process occurs at a basal level in most tissues and contributes to the routine turnover of cytoplasmic components of the cell [102, 103]. Autophagy is very important for cellular remodelling and development, and is also involved in preventing ageing and controlling cell growth [104]. Moreover, it is emerging as an important player in several human diseases and in particular in neurodegenerative disorders such as HD, Parkinson's disease and Alzheimer's disease [105, 106].
It is debatable whether autophagy plays a role in cell death mechanism (i.e. type II programmed cell death), even though it appears to be regulated, at least to some extent, in conjunction with apoptosis [107, 108].
To date, three forms of autophagy have been defined, according to how the lysosomes receive the material to be degraded [109, 110]. In macroautophagy, a double membrane structure, known as an autophagosome, envelops the cargo and fuses with the lysosome. In microautophagy, the lysosome directly engulfs the material via membrane invagination. In chaperone-mediated autophagy, heat shock cognate proteins deliver the substrates to the lysosome. The most characterised form of autophagy is macroautophagy in which cells form double-membrane autophagic vacuoles, containing cytoplasmic material (cytosol and/or organelles), which are then delivered to fuse with lysosomes, in order to achieve bulk degradation [111]. This process might occur in a generalised fashion; alternatively, it might target specific organelles, such as mitochondria and endoplasmic reticulum, thereby eliminating supernumerary, outlived or damaged structures in the stressed cell [112]. The initial steps of macroautophagy include the formation at the endoplasmic reticulum level (nucleation) and the expansion (elongation) of an isolation membrane, also known as a phagophore. The edges of the phagophore then fuse in order to form the autophagosome. This is followed by fusion of the autophagosome with a lysosome, in order to form an autolysosome, in which the captured material, together with the inner membrane, is degraded [104].
The results of our previous studies, in the R6/1 mouse model of HD, suggested a possible involvement of TG2 in autophagy associated with the neurodegenerative process observed in this disease [9]. Indeed, the analysis of brains belonging to patients and animals suffering from CAG-repeat-based genetic diseases, such as HD, revealed clear neurodegenerative features not associated with any hallmark of classical apoptosis [29, 113]. These studies showed how degenerating neurons exhibited lysosome-associated responses, including induction of electron-dense lysosomes, thus indicating that the mu-htt could induce autophagy (Figure 2). Autophagy could therefore be the major route for clearance of intracellular misfolded protein aggregates which characterise this neurodegenerative disease [4]. Analysis of the TG2-/-/HD transgenic mouse model showed that HD onset is associated with a large reduction in non-apoptotic cell death and with an increased number of nuclear protein inclusions, suggesting an impairment of their clearance by autophagy [9]. In keeping with this hypothesis, ablation of TG2 protein has recently been shown to result in accumulation of pre-autophagic vesicles, suggesting a marked induction of autophagy [114]. By contrast, the formation of acidic vesicular organelles was very limited, as detected by the lack of acidification of the autophago-lysosomes, thus indicating a defect in the autophagosome maturation process. Indeed, a drastic reduction in the co-localisation between autophagosomes and lysosomes has been detected in TG2-/- cells, even in the presence inhibitors of intralysosomal protein degradation. It is worth noting that inhibition of the transamidation activity of TG2, by the specific inhibitor R283 [115], results in a marked decrease in the number of acidic vesicular organelles, further highlighting the important role in autophagy of the cross-linking activity of TG2. Although the molecular mechanism(s) by which TG2 can regulate autophagy is not yet known, one interesting finding is that several TG2 candidate protein substrates are enriched in the autophagosome, thus providing further evidence that TG2-catalysed post-translational modifications may have a role in the maturation of the autophagosomes [114]. Of particular interest is the recent finding of the importance of the valosin-containing protein (VCP), a TG2 substrate [116], in the regulation of autophagy. In fact, mutations in the VCP gene cause inclusion body myopathy associated with Paget's disease of bone and frontotemporal dementia (IBMPFD) [117]. Although, the mechanism of IBMPFD pathogenesis is unknown, it has recently been shown that muscle from patients with IBMPFD accumulates autophagosomes [117]. In addition, under basal conditions, the loss of VCP activity results in the accumulation of autophagosomes, which fail to mature into autolysosomes; these findings are surprisingly similar to our observations in TG2 knockout cells [114]. Of interest, it has been demonstrated that VCP acts as a polyQ-interacting protein in vitro and in vivo [118], and co-localization experiments found endogenous VCP in aggregates in cultured cells expressing polyQ, in nuclear inclusions in HD patient brains, and in Lewy bodies in patient samples [118]. Moreover, the expression of VCP mutants induces cytoplasmic vacuoles, followed by cell death. Very similar vacuoles were also induced by polyQ expression or proteasome inhibitor treatment [119]. This suggests that VCP functions as a recognition factor for abnormally folded proteins and may act as a pathological effector in neurodegenerative diseases. It is interesting that VCP acts similarly to the p62/sequestosome-1 protein which binds to both LC3, the mammalian homologue of yeast Atg8, and polyubiquitinated cargo proteins destined to undergo autophagy-mediated degradation. Recent results suggest that p62 selectively mediates the clearance of mu-htt [120]. Future studies should clarify whether TG2 cross-linking is involved in the molecular mechanism of action of the VCP-dependent effect on autophagosome maturation, and whether this interaction plays a role in HD pathogenesis.
The existence of an autophagic programme specific for mitochondria has recently been proposed [121]. This hypothesis is supported by the observation that a Bcl-2 antisense oligonucleotide can trigger mitochondrial membrane permeabilisation (MMP) and autophagic cell death (ACD) [121]. In several paradigms of ACD induction, the so-called death-associated protein (DAP) kinases have been shown to be essential [122]. Overexpression of constitutively active DAP kinase is sufficient to trigger ACD accompanied by MMP, and Bcl-2 can prevent DAP kinase-induced cell death, presumably through its capacity to interfere with MMP [122]. These findings further indicate the existence of a crosstalk between autophagic and apoptotic cell death. Recent findings indicate that multiple genes involved in apoptosis also act during ACD [123], supporting the notion that these two processes can utilise common pathways or pathway components. Of interest, TG2 regulates the functions of DLK, which is a member of the DAP-kinase family (47). Based on these findings, and considering its pathogenetic role in neurodegenerative diseases, it is possible that, under some circumstances, TG2 could be one of the molecules that participate in the physiological switch between ACD and apoptosis [124]. In fact, it has been demonstrated that when TG2 is over-expressed in cell lines, cell-death -- which would otherwise occur through the activation of classic apoptotic pathways -- occurs through caspase-independent mechanisms [125]. This switch from caspase-dependent to caspase independent actuation of cell-death in the presence of high levels of TG2 further suggests a potential pro-ACD function of TG2. It is also noteworthy that the persistence of a death stimulus and the impairment of mitochondrial function in the presence of an apoptosome block can lead to death by inducing autophagy [2-4, 124].
TG2 Inhibition as a Potential Treatment for HD
As an active component of the cell death machinery, TG2 is involved in the pathogenesis of several diseases (Figures 3-4), as the enzyme is activated in various disorders [6, 7, 9, 74]. TG2 cross-linking activity has been proposed to participate in protein aggregate formation in the major neurodegenerative diseases [74]. It is interesting that genetic deletion of TG2 did not prevent or reduce the number of NIIs, thus suggesting that TG2-mediated mu-htt cross-linking is not directly involved in the formation of NIIs in vivo [9]. By contrast, a significant increase in the NII/nuclei ratio in R6/1 TG2-/- brains was observed, compared with controls. This finding suggests that genetic deletion of TG2 impairs certain critical mechanisms that normally favour the degradation of these nuclear protein aggregates [9]. As previously discussed, autophagy might constitute a fundamental component of this process, as it has been shown to be a major route of degradation for the NII.
In previous studies, we [9] and others [113] have demonstrated that cell death in the cortex and striatum of HD transgenic mice is characterised by condensed neurons that do not display classical apoptotic features, but rather these dying cells undergo ACD (Figure 2). A dramatic reduction (60–70%) in the number of these dying cells in the neocortex and striatum of HD transgenic/TG2 knockout brains, as compared to HD transgenic animals, was observed (7, 9). It is important to note that TG2 gene deletion ameliorates the neurodegenerative process observed in HD mice both with regard to symptoms and survival of these animals; a significant increase in life span was observed [9]. Based on all these findings, a potential approach to HD treatment involving TG2 inhibition should include compound(s) able to stimulate autophagy, which is impaired in the absence of an active TG2 and is essential for the clearance of NII [114].
As previously described in neurodegenerative diseases, progressive oxidative stress is a major event that precedes neuronal death [126]. Oxidative stress is characterised by an imbalance between oxidants and antioxidants. This imbalance induces molecular and cellular damage, reducing cell viability. 3-nitropropionic acid causes oxidative stress, and other molecular and cellular changes similar to those observed in neurons of patients with HD [126]. It is emerging that compounds such as carvedilol and melatonin, which might act as free radical scavengers, prevent the increase in lipid peroxidation, the depletion of reduced GSH, and the reduction of antioxidative enzyme activities that are normally observed in cellular models of HD [127, 128]. In association with their antioxidant activity, carvedilol and melatonin also exert a cytoprotective function, as indicated by the decreased cell-death levels, and therefore could represent potential treatments for HD and other neurodegenerative diseases. It would be very interesting to verify whether a combined therapy utilising a TG2 inhibitor, together with an autophagy inducer (e.g. rapamycin) and antioxidants can play a synergist role in substantially delaying the onset of HD in a mouse model.
In conclusion, despite extensive investigation, the role played by TG2 in the pathogenesis of HD remains elusive. However, as discussed in this review, compelling evidence demonstrates that the enzyme is involved in the regulation of cell death/autophagy and in mitochondrial homeostasis, two essential cellular events that are altered in HD. As far as the role of TG2 in HD is concerned, several questions remain. (1) Is the enzyme indeed acting as PDI in vivo, and does this activity play a role in the mitochondrial damage observed in HD? (2) Is the regulation of mitochondrial homeostasis and/or autophagy the major physiological activity of TG2? (3) Is the beneficial activity observed as a result of TG2 ablation in HD mice due only to the absence of its cross-linking activity? (4) Is TG2 involved in the regulation of autophagy in the brain of HD patients? Future studies should address all these questions in addition to validating whether TG2 inhibition could be of clinical relevance as a feasible therapeutic avenue for a long-lasting treatment such as is needed for HD. However, we believe that an eventual clinical application involving TG2 inhibition should selectively target one of the activities of the enzyme as the total ablation of the protein in TG2 knockout mice has been shown to have detrimental effects (Figure 4).
Acknowledgments
This work was supported by grants from the Ministry of Health of Italy “Ricerca Corrente” and “Ricerca Finalizzata”, Banca S. Paolo di Torino, Associazione Italiana Ricerca sul Cancro (AIRC) and Fondazione Telethon to MP. The support of the Marie Curie grant “TRACKS” and the EU grant “Apo-Sys” to MP is also acknowledged. PGM was supported by a grant from the National Institutes of Health (K99-ES016352), an administrative supplement under the American Recovery and Reinvestment Act of 2009 from the National Institute of Environmental Health Sciences NIEHS, and a Marie Curie International Reintegration Grant.
Footnotes
Conflict of interest statement: No conflict of interest was declared.
References
- 1.Folstein SE. Huntington's disease: a disorder of families. 1989:251. [Google Scholar]
- 2.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annual review of pathology. 2008;3:427–55. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 3.Winslow AR, Rubinsztein DC. Autophagy in neurodegeneration and development. Biochim Biophys Acta. 2008;1782:723–9. doi: 10.1016/j.bbadis.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 5.Quintanilla RA, Johnson GVW. Role of mitochondrial dysfunction in the pathogenesis of Huntington's disease. Brain Res Bull. 2009;80:242–7. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–9. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 7.Lorand L, Graham R. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–56. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 8.Karpuj MV, Becher MW, Springer JE, et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–9. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 9.Mastroberardino PG, Iannicola C, Nardacci R, et al. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington's disease. Cell Death Differ. 2002;9:873–80. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante RJ, Beal MF, Kowall NW, Richardson EP, Martin JB. Sparing of acetylcholinesterase-containing striatal neurons in Huntington's disease. Brain Res. 1987;411:162–6. doi: 10.1016/0006-8993(87)90694-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferrante RJ, Kowall NW, Richardson EP, Bird ED, Martin JB. Topography of enkephalin, substance P and acetylcholinesterase staining in Huntington's disease striatum. Neurosci Lett. 1986;71:283–8. doi: 10.1016/0304-3940(86)90634-8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante RJ, Kowall NW, Beal MF, Richardson EP, Bird ED, Martin JB. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985;230:561–3. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- 13.Joel D. Open interconnected model of basal ganglia-thalamocortical circuitry and its relevance to the clinical syndrome of Huntington's disease. Mov Disord. 2001;16:407–23. doi: 10.1002/mds.1096. [DOI] [PubMed] [Google Scholar]
- 14.Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington's disease. J Psychiatry Neurosci. 2006;31:21–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Mochel F, Charles P, Seguin F, et al. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS ONE. 2007;2:e647. doi: 10.1371/journal.pone.0000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab. 2006;4:349–62. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Podolsky S, Leopold NA. Abnormal glucose tolerance and arginine tolerance tests in Huntington's disease. Gerontology. 1977;23:55–63. doi: 10.1159/000212174. [DOI] [PubMed] [Google Scholar]
- 18.Podolsky S, Leopold NA, Sax DS. Increased frequency of diabetes mellitus in patients with Huntington's chorea. Lancet. 1972;1:1356–8. doi: 10.1016/s0140-6736(72)91092-6. [DOI] [PubMed] [Google Scholar]
- 19.Djoussé L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington's disease. Neurology. 2002;59:1325–30. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 20.Stoy N, McKay E. Weight loss in Huntington's disease. Ann Neurol. 2000;48:130–1. [PubMed] [Google Scholar]
- 21.Sassone J, Colciago C, Cislaghi G, Silani V, Ciammola A. Huntington's disease: the current state of research with peripheral tissues. Exp Neurol. 2009;219:385–97. doi: 10.1016/j.expneurol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Group THDCR A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 23.Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci. 2005;6:919–30. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- 24.Caviston JP, Holzbaur ELF. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–55. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 26.Walker FO. Huntington's disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda H, Yamaguchi M, Sugai S, Aze Y, Narumiya S, Kakizuka A. Expanded polyglutamine in the Machado-Joseph disease protein induces cell death in vitro and in vivo. Nat Genet. 1996;13:196–202. doi: 10.1038/ng0696-196. [DOI] [PubMed] [Google Scholar]
- 28.Mangiarini L, Sathasivam K, Seller M, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 29.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–84. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 30.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–3. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 31.Slow EJ, Graham RK, Osmand AP, et al. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci USA. 2005;102:11402–7. doi: 10.1073/pnas.0503634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherzinger E, Lurz R, Turmaine M, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–58. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 33.Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem. 2002;277:41032–7. doi: 10.1074/jbc.M205809200. [DOI] [PubMed] [Google Scholar]
- 34.Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11:1215–22. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 35.Colby DW, Cassady JP, Lin GC, Ingram VM, Wittrup KD. Stochastic kinetics of intracellular huntingtin aggregate formation. Nat Chem Biol. 2006;2:319–23. doi: 10.1038/nchembio792. [DOI] [PubMed] [Google Scholar]
- 36.Thakur AK, Jayaraman M, Mishra R, et al. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol. 2009;16:380–9. doi: 10.1038/nsmb.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993;74:955–6. doi: 10.1016/0092-8674(93)90718-6. [DOI] [PubMed] [Google Scholar]
- 38.Jeitner TM, Pinto JT, Krasnikov BF, Horswill M, Cooper AJL. Transglutaminases and neurodegeneration. J Neurochem. 2009;109 1:160–6. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahlem P, Green H, Djian P. Transglutaminase action imitates Huntington's disease: selective polymerization of Huntingtin containing expanded polyglutamine. Mol Cell. 1998;1:595–601. doi: 10.1016/s1097-2765(00)80059-3. [DOI] [PubMed] [Google Scholar]
- 40.Karpuj MV, Garren H, Slunt H, Price DL, Gusella J, Becher MW, Steinman L. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington's disease brain nuclei. Proc Natl Acad Sci USA. 1999;96:7388–93. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochem Biophys Res Commun. 2004;322:1310–7. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–37. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 43.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 44.Fan MMY, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 2007;81:272–93. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Young AB, Greenamyre JT, Hollingsworth Z, Albin R, D'Amato C, Shoulson I, Penney JB. NMDA receptor losses in putamen from patients with Huntington's disease. Science. 1988;241:981–3. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- 46.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 47.Arning L, Kraus PH, Valentin S, Saft C, Andrich J, Epplen JT. NR2A and NR2B receptor gene variations modify age at onset in Huntington disease. Neurogenetics. 2005;6:25–8. doi: 10.1007/s10048-004-0198-8. [DOI] [PubMed] [Google Scholar]
- 48.Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–71. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- 49.Hantraye P, Riche D, Maziere M, Isacson O. A primate model of Huntington's disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol. 1990;108:91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem. 2001;276:24713–8. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 51.Orr AL, Li S, Wang CE, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–92. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Archives of Biochemistry and Biophysics. 2003;410:1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- 53.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–6. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 54.Tang TS, Tu H, Chan EYW, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–39. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gafni J, Ellerby LM. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–9. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakaoka H, Perez D, Baek K, et al. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–6. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 57.Murthy S, Lomasney J, Mak E, Lorand L. Interactions of G(h)/transglutaminase with phospholipase Cdelta1 and with GTP. Proc Natl Acad Sci U S A. 1999;96:11815–9. doi: 10.1073/pnas.96.21.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Venere A, Rossi A, De Matteis F, Rosato N, Agro A, Mei G. Opposite effects of Ca(2+) and GTP binding on tissue transglutaminase tertiary structure. J Biol Chem. 2000;275:3915–21. doi: 10.1074/jbc.275.6.3915. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa G, Suwa M, Ichikawa Y, et al. A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J. 2003;373:793–803. doi: 10.1042/BJ20021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mastroberardino PG, Farrace MG, Viti I, et al. “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta. 2006;1757:1357–65. doi: 10.1016/j.bbabio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Thomazy V, Fesus L. Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res. 1989;255:215–24. doi: 10.1007/BF00229084. [DOI] [PubMed] [Google Scholar]
- 62.Szegezdi E, Szondy Z, Nagy L, Nemes Z, Friis R, Davies P, Fesus L. Apoptosis-linked in vivo regulation of the tissue transglutaminase gene promoter. Cell Death Differ. 2000;7:1225–33. doi: 10.1038/sj.cdd.4400751. [DOI] [PubMed] [Google Scholar]
- 63.Lai T, Bielawska A, Peoples K, Hannun Y, Greenberg C. Sphingosylphosphocholine reduces the calcium ion requirement for activating tissue transglutaminase. J Biol Chem. 1997;272:16295–300. doi: 10.1074/jbc.272.26.16295. [DOI] [PubMed] [Google Scholar]
- 64.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–10. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- 65.Peng X, Zhang Y, Zhang H, Graner S, Williams J, Levitt M, Lokshin A. Interaction of tissue transglutaminase with nuclear transport protein importin-alpha3. FEBS Lett. 1999;446:35–9. doi: 10.1016/s0014-5793(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 66.Malorni W, Farrace MG, Matarrese P, et al. The adenine nucleotide translocator 1 acts as a type 2 transglutaminase substrate: implications for mitochondrial-dependent apoptosis. Cell Death Differ. 2009;16:1480–92. doi: 10.1038/cdd.2009.100. [DOI] [PubMed] [Google Scholar]
- 67.Szondy Z, Mastroberardino PG, Váradi J, et al. Tissue transglutaminase (TG2) protects cardiomyocytes against ischemia/reperfusion injury by regulating ATP synthesis. Cell Death Differ. 2006;13:1827–9. doi: 10.1038/sj.cdd.4401889. [DOI] [PubMed] [Google Scholar]
- 68.Assisi L, Autuori F, Botte V, Farrace M, Piacentini M. Hormonal control of “tissue” transglutaminase induction during programmed cell death in frog liver. Exp Cell Res. 1999;247:339–46. doi: 10.1006/excr.1998.4358. [DOI] [PubMed] [Google Scholar]
- 69.Melino G, Annicchiarico-Petruzzelli M, Piredda L, Candi E, Gentile V, Davies P, Piacentini M. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994;14:6584–96. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oliverio S, Amendola A, Di Sano F, et al. Tissue transglutaminase-dependent posttranslational modification of the retinoblastoma gene product in promonocytic cells undergoing apoptosis. Mol Cell Biol. 1997;17:6040–8. doi: 10.1128/mcb.17.10.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fesus L, Thomazy V, Autuori F, Ceru M, Tarcsa E, Piacentini M. Apoptotic hepatocytes become insoluble in detergents and chaotropic agents as a result of transglutaminase action. FEBS Lett. 1989;245:150–4. doi: 10.1016/0014-5793(89)80210-8. [DOI] [PubMed] [Google Scholar]
- 72.Piredda L, Farrace M, Lo Bello M, Malorni W, Melino G, Petruzzelli R, Piacentini M. Identification of ‘tissue’ transglutaminase binding proteins in neural cells committed to apoptosis. FASEB J. 1999;13:355–64. doi: 10.1096/fasebj.13.2.355. [DOI] [PubMed] [Google Scholar]
- 73.Fok JY, Mehta K. Tissue transglutaminase induces the release of apoptosis inducing factor and results in apoptotic death of pancreatic cancer cells. Apoptosis. 2007;12:1455–63. doi: 10.1007/s10495-007-0079-3. [DOI] [PubMed] [Google Scholar]
- 74.Malorni W, Farrace MG, Rodolfo C, Piacentini M. Type 2 transglutaminase in neurodegenerative diseases: the mitochondrial connection. Curr Pharm Des. 2008;14:278–88. [PubMed] [Google Scholar]
- 75.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem. 2004;279:23863–8. doi: 10.1074/jbc.M311919200. [DOI] [PubMed] [Google Scholar]
- 76.Verderio EAM, Telci D, Okoye A, Melino G, Griffin M. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem. 2003;278:42604–14. doi: 10.1074/jbc.M303303200. [DOI] [PubMed] [Google Scholar]
- 77.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893–900. doi: 10.1093/carcin/bgn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–70. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 79.Gundemir S, Johnson GVW. Intracellular localization and conformational state of transglutaminase 2: implications for cell death. PLoS ONE. 2009;4:e6123. doi: 10.1371/journal.pone.0006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lesort M, Attanavanich K, Zhang J, Johnson GV. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–4. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- 81.Antonyak MA, Singh US, Lee DA, et al. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against apoptosis. J Biol Chem. 2001;276:33582–7. doi: 10.1074/jbc.M105318200. [DOI] [PubMed] [Google Scholar]
- 82.Beck KE, De Girolamo LA, Griffin M, Billett EE. The role of tissue transglutaminase in 1-methyl-4-phenylpyridinium (MPP+)-induced toxicity in differentiated human SH-SY5Y neuroblastoma cells. Neurosci Lett. 2006;405:46–51. doi: 10.1016/j.neulet.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 83.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–33. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 84.Verma A, Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist Updat. 2007;10:144–51. doi: 10.1016/j.drup.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Antonyak MA, Boehm JE, Cerione RA. Phosphoinositide 3-kinase activity is required for retinoic acid-induced expression and activation of the tissue transglutaminase. J Biol Chem. 2002;277:14712–6. doi: 10.1074/jbc.M112259200. [DOI] [PubMed] [Google Scholar]
- 86.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antonyak MA, Jansen JM, Miller AM, Ly TK, Endo M, Cerione RA. Two isoforms of tissue transglutaminase mediate opposing cellular fates. Proc Natl Acad Sci USA. 2006;103:18609–14. doi: 10.1073/pnas.0604844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tee AEL, Marshall GM, Liu PY, et al. Opposing effects of two tissue transglutaminase protein isoforms in neuroblastoma cell differentiation. J Biol Chem. 2010;285:3561–7. doi: 10.1074/jbc.M109.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–73. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 90.Milakovic T, Johnson GVW. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280:30773–82. doi: 10.1074/jbc.M504749200. [DOI] [PubMed] [Google Scholar]
- 91.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 92.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 93.Boya P, González-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–40. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–7. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez-Enriquez S, He L, Lemasters JJ. Role of mitochondrial permeability transition pores in mitochondrial autophagy. Int J Biochem Cell Biol. 2004;36:2463–72. doi: 10.1016/j.biocel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Scorrano L. Proteins that fuse and fragment mitochondria in apoptosis: con-fissing a deadly con-fusion? J Bioenerg Biomembr. 2005;37:165–70. doi: 10.1007/s10863-005-6572-x. [DOI] [PubMed] [Google Scholar]
- 97.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 98.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–60. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 99.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 100.Piacentini M, Farrace MG, Piredda L, et al. Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J Neurochem. 2002;81:1061–72. doi: 10.1046/j.1471-4159.2002.00898.x. [DOI] [PubMed] [Google Scholar]
- 101.Battaglia G, Farrace MG, Mastroberardino PG, et al. Transglutaminase 2 ablation leads to defective function of mitochondrial respiratory complex I affecting neuronal vulnerability in experimental models of extrapyramidal disorders. J Neurochem. 2007;100:36–49. doi: 10.1111/j.1471-4159.2006.04140.x. [DOI] [PubMed] [Google Scholar]
- 102.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 103.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 104.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 105.Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6:1837–49. doi: 10.4161/cc.6.15.4511. [DOI] [PubMed] [Google Scholar]
- 106.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorski SM, Chittaranjan S, Pleasance ED, et al. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003;13:358–63. doi: 10.1016/s0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 108.Hou YCC, Hannigan AM, Gorski SM. An executioner caspase regulates autophagy. Autophagy. 2009;5:530–3. doi: 10.4161/auto.5.4.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–10. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 113.Turmaine M, Raza A, Mahal A, Mangiarini L, Bates G, Davies S. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2000;97:8093–7. doi: 10.1073/pnas.110078997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D'Eletto M, Farrace MG, Falasca L, et al. Transglutaminase 2 is involved in autophagosome maturation. Autophagy. 2009;5:1145–54. doi: 10.4161/auto.5.8.10040. [DOI] [PubMed] [Google Scholar]
- 115.Skill NJ, Johnson TS, Coutts IGC, et al. Inhibition of transglutaminase activity reduces extracellular matrix accumulation induced by high glucose levels in proximal tubular epithelial cells. J Biol Chem. 2004;279:47754–62. doi: 10.1074/jbc.M402698200. [DOI] [PubMed] [Google Scholar]
- 116.Orru S, Caputo I, D'Amato A, Ruoppolo M, Esposito C. Proteomics identification of acyl-acceptor and acyl-donor substrates for transglutaminase in a human intestinal epithelial cell line. Implications for celiac disease. J Biol Chem. 2003;278:31766–73. doi: 10.1074/jbc.M305080200. [DOI] [PubMed] [Google Scholar]
- 117.Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–88. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ju JS, Miller SE, Hanson PI, Weihl CC. Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J Biol Chem. 2008;283:30289–99. doi: 10.1074/jbc.M805517200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarkar S, Rubinsztein DC. Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS J. 2008;275:4263–70. doi: 10.1111/j.1742-4658.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 120.Tung YT, Hsu WM, Lee H, Huang WP, Liao YF. The Evolutionarily Conserved Interaction Between LC3 and p62 Selectively Mediates Autophagy-Dependent Degradation of Mutant Huntingtin. Cell Mol Neurobiol. 2010 doi: 10.1007/s10571-010-9507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldman SJ, Taylor R, Zhang Y, Jin S. Autophagy and the degradation of mitochondria. Mitochondrion. 2010 doi: 10.1016/j.mito.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 123.Fimia GM, Piacentini M. Regulation of autophagy in mammals and its interplay with apoptosis. Cellular and molecular life sciences : CMLS. 2010 doi: 10.1007/s00018-010-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robitaille K, Daviau A, Lachance G, Couture JP, Blouin R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 2008;15:1522–31. doi: 10.1038/cdd.2008.77. [DOI] [PubMed] [Google Scholar]
- 125.Griffin M, Verderio E. Tissue transglutaminase in cell death. Symp Soc Exp Biol. 2000;52:223–40. [PubMed] [Google Scholar]
- 126.Kumar P, Kalonia H, Kumar A. Huntington's disease: pathogenesis to animal models. Pharmacol Rep. 2010;62:1–14. doi: 10.1016/s1734-1140(10)70238-3. [DOI] [PubMed] [Google Scholar]
- 127.Srinivasan V, Pandi-Perumal SR, Maestroni GJ, Esquifino AI, Hardeland R, Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 128.Tasset I, Espínola C, Medina FJ, et al. Neuroprotective effect of carvedilol and melatonin on 3-nitropropionic acid-induced neurotoxicity in neuroblastoma. J Physiol Biochem. 2009;65:291–6. doi: 10.1007/BF03180581. [DOI] [PubMed] [Google Scholar]