Abstract
This study examined functional brain abnormalities in dyslexic German readers who – due to the regularity of German in the reading direction – do not exhibit the reading accuracy problem of English dyslexic readers, but suffer primarily from a reading speed problem. The in-scanner task required phonological lexical decisions (i.e., Does xxx sound like an existing word?) and presented familiar and unfamiliar letter strings of existing phonological words (e.g., Taxi-Taksi) together with nonwords (e.g., Tazi). Dyslexic readers exhibited the same response latency pattern (words < pseudohomophones < nonwords) as nonimpaired readers, but latencies to all item types were much prolonged. The imaging results were suggestive for a different neural organization of reading processes in dyslexic readers. Specifically, dyslexic readers, in response to lexical route processes, exhibited underactivation in a left ventral occipitotemporal (OT) region which presumably is engaged by visual-orthographic whole word recognition. This region was also insensitive to the increased visual-orthographic processing demands of the sublexical route. Reduced engagement in response to sublexical route processes was also found in a left inferior parietal region, presumably engaged by attentional processes, and in a left inferior frontal region, presumably engaged by phonological processes. In contrast to this reduced engagement of the optimal left hemisphere reading network (ventral OT, inferior parietal, inferior frontal), our dyslexic readers exhibited increased engagement of visual occipital regions and of regions presumably engaged by silent articulatory processes (premotor/motor cortex and subcortical caudate and putamen).
Keywords: Developmental dyslexia, fMRI, Reading, Phonological lexical decision, Dual-route
1. Introduction
A number of studies over the last 15 years have provided converging evidence showing that dyslexic readers of languages with orthographies more regular than English suffer from a pervasive and persistent reading speed deficit, but much less from the reading accuracy problem which is characteristic for dyslexic children learning to read English (e.g., Dutch: Van den Bos et al., 1998; Yap and Van der Leij, 1993; German: Wimmer, 1993; Italian: Zoccolotti et al., 1999; Spanish: Gonzalez and Valle, 2000; Norwegian: Lundberg and Hoien, 1990; Greek: Porpodas, 1999). The reading accuracy advantage of dyslexic children in regular orthographies was substantiated in direct English and German dyslexia comparisons which used similar words in the two orthographies (Landerl et al., 1997; Ziegler et al., 2003). To illustrate, the English dyslexic children (11-year-olds) studied by Landerl et al. had a problem with the word character. Some refused to read it, and others produced misreadings ranging from chancellor and calendar to nonwords such as tschraekter. Their German peers produced few misreadings for Charakter (all nonwords close to the target), but their reading time was between 2 and 3 times longer than normal. With respect to regularity, it should be noted that German – like many other alphabetic orthographies – is more regular in the reading (grapheme-to-phoneme) direction than in the writing (phoneme-to-grapheme) direction. This asymmetry has the effect that, in a substantial number of cases, accurate but slow reading is accompanied by incorrect, but phonetically acceptable spellings. This profile, in terms of dual-route theorizing, suggests that fully specified memory representations of the letter sequences of words (i.e., orthographic lexicon entries) are necessary for correct spellings, but not for correct readings.
A recent cognitive analysis by Bergmann and Wimmer (2008) – based on both orthographic and phonological lexical decisions – localized the source of the reading speed problem of German dyslexic readers in both the lexical and the sublexical route of the well-known dual-route model of visual word processing (Coltheart et al., 2001). This model specifies two routes through which word phonology is accessed from letter strings. The lexical route leads to phonology via orthographic whole-word recognition units which get instantiated by familiar letter strings and provide direct access to whole-word phonology and meaning. The sublexical route of the dual-route model leads to word phonology via serial conversion of graphemes into phonemes. In an orthographic lexical decision task (i.e., Is xxx correctly written?), Bergmann and Wimmer found that dyslexic readers exhibited major difficulty with the orthographic distinction between words and pseudohomophones (e.g., Taxi and Taksi), indicating that their orthographic word lexicon contained fewer fully specified orthographic word recognition units. However, even when such recognition units were available and used, the speed of access to word phonology was markedly impaired. This was evident from the latencies in the subsequent phonological lexical decision task. Moreover, the sublexical route was found to be even more speed impaired than the lexical route. A hallmark of impaired functioning of the sublexical route is the dramatic increase of reading onset time with each additional letter of a word or nonword (Marinus and De Jong, 2010, this issue; Moll et al., 2005; Spinelli et al., 2005; Ziegler et al., 2003; Zoccolotti et al., 2005). Based on a review of cognitive deficits associated with dyslexia in regular orthographies, Bergmann and Wimmer hypothesized that the underlying problem of slow functioning of both the lexical and the sublexical route resides in slow access to phonology, that is, in slow access from orthographic to phonological word representations (lexical route) and in slow access from graphemes to phonemes (sublexical route). Recently, the manifestation of slow lexical and sublexical route functioning in dyslexic readers was also examined in a study of eye-movements (Hawelka et al., 2010).
The present study extended the work by Bergmann and Wimmer (2008) by using their phonological lexical decision task for measuring brain activity in German dyslexic readers (adolescents and adults). The task requires evaluation of whether letter strings sound like an existing word, and it presents familiar strings of existing words, unfamiliar strings of the very same words (i.e., pseudohomophones) and unfamiliar strings of nonwords. Examples are Taxi, Taksi and Tazi. In terms of the dual-route model, processing of familiar strings should primarily depend on the lexical route, and processing of the unfamiliar strings should involve the sublexical route. A methodological advantage is that both the familiar and the unfamiliar letter string of an existing phonological word result in the same YES response. We expected that abnormalities of the brain response in specific regions will specify the rather broad dual-route explanation of the speed impairment of our dyslexic readers.
For expectations, the fMRI results of a preceding study from our group are important (Kronbichler et al., 2007), because this study used the present phonological lexical decision task, and the nonimpaired participants of Kronbichler et al. serve as control group in the present work. Kronbichler et al. identified a left hemisphere reading network consisting of occipitotemporal (OT), parietal and frontal regions which were engaged by both the lexical and the sublexical routes, but with increased demands posed by the sublexical route. Importantly, the left OT region corresponded closely to the Visual Word Form Area (VWFA) of Cohen et al. (2002). A straightforward expectation is that the present dyslexic readers may exhibit activation abnormalities in one or several of the mentioned regions engaged by efficient lexical and sublexical route processes in nonimpaired readers. Given that our dyslexic readers suffer primarily from impaired reading speed, a main candidate region for abnormality is the VWFA in the left OT cortex, which was originally conceptualized as brain region recruited by highly efficient letter string processing in competent readers (Cohen et al., 2002). Underactivation of the left OT cortex is a common finding as shown in a review by McCandliss and Noble (2003) and in a quantitative meta-analysis of imaging findings by our group (Richlan et al., 2009). However, the majority of imaging studies with dyslexic participants found underactivation of left temporoparietal regions (i.e., posterior aspect of the superior temporal gyrus/sulcus, supramarginal gyrus) as the main brain signature of dyslexia (see Richlan et al., for a complete list of studies). In reviews of imaging findings, the dysfunction of left posterior language regions is linked to the phonological deficit explanation of dyslexia (McCandliss and Noble; Pugh et al., 2000; Sandak et al., 2004).
2. Methods
2.1. Participants
Twenty German-speaking dyslexic readers (19 males, 1 female) were added to the sample of nonimpaired readers of the Kronbichler et al. (2007) study. However, for matching purposes, and because of partial data loss in one case, the present sample of nonimpaired readers (i.e., the control group) included the data from only 19 (17 males, 2 females) of the original 24 participants of Kronbichler et al. (2007). Both groups included adult and adolescent participants. The dyslexic group consisted of 12 adolescents (age range: 15–17 years) and 8 young adults (age range: 18–34 years) and the control group consisted of 11 adolescents and 8 adults in the same age range. The adolescents were recruited from a longitudinal study and were invited to participate based on a marked reading fluency deficit on previous assessments. The adult dyslexic participants were university students who volunteered to take part in the study. They reported a childhood history of reading and/or spelling problems and still felt that their reading speed and spelling were not adequate. However, only few participants of the adult group had received a formal dyslexia diagnosis. All participants were right-handed and had normal or corrected-to-normal vision. The study was approved by the ethical committee of the University of Salzburg. All participants gave written informed consent and were paid for participation.
Final group assignment relied on a reading fluency test which is under development in our lab. This test presents a list of sentences (all of simple content) for 1 min with the instruction to mark as many sentences as possible as true (i.e., making sense) or false. Example items are “Dolphins and whales live in the sea”, or “Basketball can be played only during winter”. The format of this test corresponds to the reading fluency subtest of the Woodcock-Johnson III (WJ III) Test of Cognitive Abilities (COG; Woodcock et al., 2001). The test score is number of correctly marked sentences. Participants were included in the dyslexic sample when their reading speed score was below the 10th percentile. A score above the 15th percentile qualified for the control group. These thresholds were chosen based on preliminary norm samples of about 300 university students for the adult participants and about 200 adolescents for the younger participants.
Table 1 shows that the mean score of the dyslexic group on the sentence reading test was only about half of the score of the controls, and that this mean corresponds to a reading quotient of about 2 SDs below norm. The reading quotient was scaled like the IQ score (M = 100 and SD = 15). The adult dyslexic subgroup tended to score somewhat higher than the adolescent dyslexic subgroup (means of 13.9 and 10.0 sentences, respectively, pooled SD = 2.5) and this was also the case for the nonimpaired subgroups (means of 24.9 and 21.3 sentences, respectively, pooled SD = 4.1). The close to perfect sentence accuracy in Table 1 of the dyslexic group speaks against the possibility of the low sentence reading scores reflecting a deficit in vocabulary or knowledge required for evaluating the sentences. Table 1 further shows reading speed (syllables per second) and accuracy scores for reading aloud a short text consisting of 137 words. Unfortunately, data are missing for three nonimpaired and one dyslexic reader on this test. Dyslexic readers – similar to their slow performance on the sentence processing test – read with only about half of the speed of the controls. Again, text reading accuracy was close to ceiling even for the dyslexic individuals. Furthermore, the scores for sentence processing and reading aloud were substantially associated: r(35) = .87 for the combined groups and also within groups (dyslexic readers: .72, nonimpaired readers: .68).
Table 1.
Characteristics of the participants.
| Measures | Nonimpaired |
Dyslexics |
t (38) | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Age (years) | 20.87 | 6.85 | 20.41 | 6.75 | .21 |
| Sentence reading | |||||
| Speed (N/min) | 22.79 | 4.43 | 11.55 | 3.12 | 9.20∗∗∗ |
| Accuracy (% correct) | 97.85 | 4.14 | 96.60 | 6.84 | .69 |
| Reading quotient | 103.65 | 12.83 | 71.08 | 9.04 | 9.20∗∗∗ |
| Text reading | |||||
| Speed (syl/sec) | 4.99 | 1.20 | 2.71 | 1.02 | 6.09∗∗∗ |
| Accuracy (% correct) | 98.58 | 1.55 | 96.08 | 3.49 | 2.65∗ |
| Spelling test (% correct) | 78.00 | 18.99 | 32.80 | 15.93 | 8.07∗∗∗ |
| WAIS-III R subtests | |||||
| Vocabulary | 13.71 | 2.37 | 11.44 | 2.13 | 2.89∗∗ |
| Similarities | 13.47 | 2.50 | 12.88 | 2.99 | .62 |
| Block design | 12.88 | 2.40 | 12.50 | 2.48 | .45 |
| Object assembly | 12.88 | 2.85 | 12.88 | 1.86 | .01 |
| Estimated IQ | 116.18 | 9.89 | 112.11 | 7.99 | 1.29 |
| Early measuresa | t (22) | ||||
| Reading fluency (syl/min) | |||||
| 1st Grade | 66.34 | 30.27 | 23.87 | 9.46 | 4.63∗∗∗ |
| 3rd Grade | 152.05 | 13.15 | 60.55 | 16.72 | 14.49∗∗∗ |
| Reading accuracy (% correct) | |||||
| 1st Grade | 92.27 | 10.34 | 79.58 | 28.72 | 1.38 |
| 3rd Grade | 96.08 | 4.45 | 93.59 | 4.27 | 1.37 |
| Rapid naming (syl/min) | 48.24 | 11.75 | 39.46 | 7.49 | 2.16∗ |
| Peg moving (pegs/min) | 41.14 | 4.40 | 45.63 | 4.59 | −2.39∗ |
| Coherent motion detection (% dots) | 11.05 | 3.08 | 10.32 | 6.89 | .30 |
Notes: ∗∗∗p < .001, ∗∗p < .01, ∗p < .05.
Data from adolescent subsample only.
Table 1 further shows that the present dyslexic readers were not only slow readers but also poor spellers. For spelling assessment, a standardized test (Kersting and Althoff, 2004) was used. The percentages correct are based on 68 words. One may wonder how there can be massive spelling problems in an orthography which is characterized as regular. The answer is that German, like other orthographies, is more regular in the reading direction than in the writing direction, with the effect – evident in Table 1 from the comparison of the reading and spelling accuracy – that reading accuracy tends to be perfect and spelling accuracy tends to be comparatively low.
A further inclusion criterion for the dyslexic sample was a nonverbal IQ score in the normal range. This score was based on two subtests (Object Assembly and Block Design) of the German adaptation (Tewes, 1991) of the Wechsler Adult Intelligence Scale-Revised (WAIS-R). Averaged over the two subtests, a mean standard score of higher than 7 was required, which would correspond to a performance IQ of higher than 85. The subtests of the WAIS-R are standardized with a mean of 10 and a standard deviation of 3. In addition to the Performance Scale subtests, two subtests (Vocabulary and Similarities) of the Verbal Scale were also presented. The means in Table 1 show that the dyslexic participants tended to score above average on all four subtests. Only for the vocabulary subtest, their mean performance was reliably lower than that of the controls. The mean estimated IQ (based on the 4 subtests) in Table 1 shows that the dyslexic readers, similar to controls, tended to score above average.
The lower section of Table 1 shows measures from earlier assessments of the longitudinal participants (i.e., 12 dyslexics, 11 controls). Before the beginning of systematic reading instruction in Grade 1 – there is no reading preparation involving letters in kindergarten – a Rapid Automatized Naming (RAN) task, modelled after Denckla and Rudel (1976), was administered, which required quick naming of a sequence of pictured objects. Furthermore, a peg moving task, modelled after Annett (1985), required participants to quickly move 10 pegs from one line of holes in a frame into the holes of the line closer to the child (for details, see Mayringer and Wimmer, 2002). At the end of Grade 1, the longitudinal participants were instructed to read aloud a list of 10 words and a list of 10 nonwords quickly and accurately. In Grade 3, a list of 24 nonwords was presented with the same instruction. The mean syllables per minute scores in Table 1 show that the dyslexic participants exhibited slow RAN performance and marked early reading fluency impairments. However, even at the end of Grade 1, reading accuracy was rather high with about 80% correct. Importantly, Table 1 shows that on the peg moving task, the later dyslexic readers performed faster than the controls. This speaks against a general speed impairment as cause of their slow RAN performance and of their slow reading. On a visual coherent motion detection task, which was part of a cognitive assessment in Grade 3 (for details see Kronbichler et al., 2002), there was also no dyslexic deficit. This speaks against a visual magnocellular deficit as cause of the reading speed problem of the present dyslexic sample.
2.2. Stimuli and task
Stimuli and task were identical to Kronbichler et al. (2007). The 180 stimuli consisted of 60 orthographically familiar forms of German nouns, 60 orthographically unfamiliar forms of the same words (i.e., pseudohomophones) and 60 nonwords. Examples for the three item types are Taxi – Taksi – Tazi or Chaos – Kaos – Kuse. Quantitative information about item characteristics is provided in Table 2 of Kronbichler et al. (2007). The familiar forms consisted of 4–9 letters and began with a consonant (in upper case following German spelling convention for nouns). The familiar forms and the pseudohomophones did not differ in number of letters, syllables, bigram frequency, or in number of orthographic neighbours (i.e., words of the same length differing by one letter). The mean frequency of 86 occurrences per million according to the CELEX database (Baayen et al., 1993) indicates that the majority of words was of moderate to high frequency. Nonwords were generated in such a way that they could not be distinguished from pseudohomophones by superficial characteristics such as absence of vowel letters or length.
To examine differences in the BOLD response to the three item types, an event-related design was used. Each item was displayed for 1600 msec with an inter-stimulus interval of 2100 msec during which a fixation cross was shown. This stimulus onset asynchrony of 3700 msec is not a multiple of the TR of 2000 msec (see below) which enhances the efficiency of the design by sampling the haemodynamic response at different time-points. The 180 stimuli were presented in two pseudo-randomized lists, and each list was divided into two runs of 90 items, each composed of 30 items per stimulus type. In addition, 10 null-events of 3700 msec duration with a fixation cross were included in each run to improve evaluation of stimulus related activation relative to baseline. The two runs were separated by a short (1–2 min) break. The order of the 90 stimuli and of the 10 null events within each run was determined by a genetic algorithm (Wager and Nichols, 2003) which selects the most efficient sequence for testing stimulus contrasts. A critical feature for creating the two pseudo-randomized lists was the sequencing of the familiar and the unfamiliar forms of the same phonological word. When in list one the familiar form was presented in the first run, which was the case for half of the words, then this order was reversed in list two. Item order was varied between participants so that the familiar form was equally often presented before and after the unfamiliar form of the same word. Participants also never received three stimuli of the same type in immediate succession.
Participants were familiarized with the phonological lexical decision task (i.e., “Does it sound like an existing word?”) and with the response mode outside the scanner. Participants responded with the index finger (“yes”) and middle finger (“no”) of their right hand. Stimulus delivery and response registration were controlled by Presentation (Neurobehavioral Systems Inc., Albany, CA, USA).
2.3. fMRI data acquisition and analysis
During each of the two runs, 190 functional images sensitive to blood oxygenation level dependent (BOLD) contrast were acquired with a T2* weighted echo-planar imaging (EPI) sequence (TE – echo time, 40 msec, TR – repetition time, 2000 msec, FA – flip angle, 86°, 21 slices with a thickness of 6 mm, 220 mm FOV – field of view, with a 64 × 64 matrix resulting in 3.44 × 3.44 mm in plane resolution). Additionally, a low (3.5 × 3.5 × 6 mm) and a high resolution (1 × 1 × 1.3 mm) structural scan were acquired from each participant with T1 weighted MPRAGE sequences. A Philips 1.5 Tesla Intera Scanner (Philips Medical System, Best, The Netherlands) was used for MR imaging.
Data analysis used SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Functional images were realigned, unwarped and slice time corrected. Then the functional images were first co-registered to the low resolution structural image and, subsequently, the functional and the structural images were coregistered to the high resolution structural image. This two-step procedure was found to obtain higher coregistration accuracy for previous data sets from this scanner than directly coregistering functional and high resolution structural images. The high resolution structural image was normalized to the Montreal Neurological Institute (MNI) T1 template image, and the resulting parameters were used for normalisation of the functional images, which were re-sampled to isotropic 3 mm3 voxels and smoothed with a 9 mm full width at half maximum (FWHM) Gaussian kernel. We also characterized brain activation in regions of interest (ROIs) – based on results of the voxel-based analysis. For ROI analyses, parameter estimates of stimulus effects versus fixation were extracted with SPM. Regions were defined as spheres of 5 mm radius centered on peak coordinates from group comparisons (see Results section).
Voxel-based analysis was performed in a two stage mixed effects model. In the subject-specific first level model, each stimulus type was modelled by a canonical haemodynamic response function and its temporal derivative. The incorrectly answered and missed items were modelled as covariates of no interest. The functional data in these first level models were high pass filtered with a cut-off of 128 sec, and corrected for autocorrelation by an AR(1) model (Friston et al., 2002). The parameter estimates reflecting signal change for each item type versus fixation baseline (which consisted of the interstimulus interval and the null events) were calculated in the context of a general linear model (GLM) (see Henson, 2004). The subject specific contrast images were used for the second level random effects analyses.
Group differences and item type effects were examined by t-tests. These comparisons were thresholded at p < .005, uncorrected, in conjunction with a cluster size threshold of at least 10 voxels. These rather liberal thresholds were applied to reduce the risk of missing dyslexic abnormalities. Furthermore, these thresholds allow comparison with other recent fMRI studies which also used uncorrected thresholds (Booth et al., 2004; Brambati et al., 2006; Cao et al., 2006; Schulz et al., 2008; Temple et al., 2000). We also provide information about which of the liberally identified regions survive a more conservative False Discovery Rate (FDR) corrected threshold (Genovese et al., 2002). Group comparisons were restricted to a mask, which was created in two steps. First, brain activity against baseline (averaged across the three item types, p < .001, uncorrected) was computed separately for each group. Second, reliable activations of each group were combined, so that the mask contained all voxels which were activated in at least one of the two groups. Such a mask precludes that a group difference results from deactivations against baseline.
3. Results
3.1. Behavioral results
Table 2 shows accuracy and latency data for the in-scanner phonological lexical decision task. Dyslexic readers exhibited only minor accuracy problem for pseudohomophones and nonwords. Even for these more difficult item types, their accuracy was about 85% correct. Only for nonwords was the group difference reliable. Table 2 further shows that dyslexic readers exhibited markedly prolonged latencies for correct decisions on all item types and that these group differences were larger for pseudohomophones and nonwords than for words. The main effects of group and item type were reliable, F(1, 35) = 13.80, p < .01, and F(2, 70) = 190.08, p < .001, respectively. The group by item type interaction was also reliable, F(2, 70) = 9.63, p < .01. There was no main effect of age, F(1, 35) < 1, n. s., and none of the interactions involving age were reliable, Fs(2, 70) < 1, n. s. A possible concern is that the group by item type interaction on latencies may reflect an over-additivity effect resulting from overall higher latencies of the dyslexic group. Following Faust et al. (1999), we standardized the item type latencies of each individual (i.e., item type latency minus average of the three latencies divided by standard deviation of these latencies). For these transformed scores, the group by item type interaction was no longer reliable, F(2, 70) < 1, n. s.
Table 2.
Phonological lexical decision task: performance measures.
| Item type | Nonimpaired readers |
Dyslexic readers |
t (37) | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Accuracy (% correct) | |||||
| Words | 96.0 | 10.3 | 95.1 | 5.3 | – |
| Pseudohomophones | 91.5 | 11.8 | 86.2 | 8.4 | 1.63 |
| Pseudowords | 94.7 | 6.3 | 83.8 | 12.6 | 3.39∗∗ |
| Latencies (msec) | |||||
| Words | 973 | 383 | 1269 | 373 | 2.44∗ |
| Pseudohomophones | 1113 | 367 | 1571 | 326 | 4.13∗∗∗ |
| Pseudowords | 1404 | 360 | 1948 | 338 | 4.87∗∗∗ |
Notes: ∗∗∗p < .001, ∗∗p < .01, ∗p < .05.
In summary, the unfamiliar letter strings (pseudohomophones and nonwords) which required sublexical route processes led to more decision errors in dyslexics than in controls, although accuracy was still high for the dyslexic group. For words, accuracy was close to perfect in both groups. Dyslexic readers, similar to their slow performance on the reading tests, exhibited substantially prolonged decision latencies for all item types. Importantly, for both accuracy and latency of phonological lexical decision, dyslexics profited at least as much as nonimpaired readers when presented with familiar compared to unfamiliar letter strings of existing words (e.g., Taxi vs Taksi). This finding is suggestive for reliance on the lexical route for familiar letter strings, and on the sublexical route for unfamiliar letter strings.
3.2. fMRI results
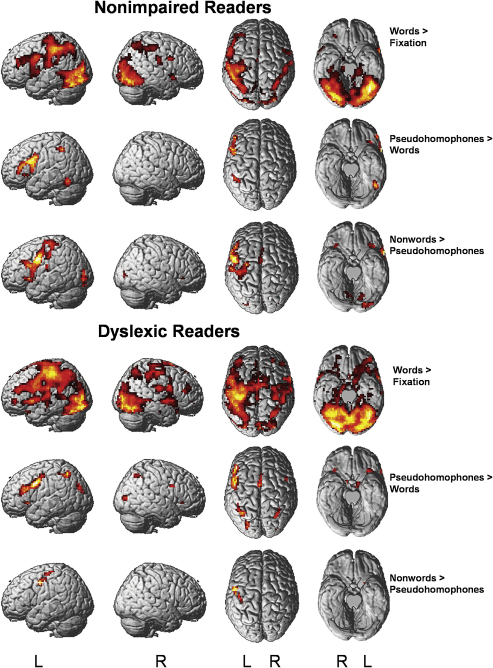
Because of the similar in-scanner performance of adolescents and adults, age was not used as a separate factor in the analyses of the fMRI results. For nonimpaired readers, the renders of Fig. 1 (first section) show that word items activated large bilateral occipital regions which were accompanied by bilateral frontal and parietal regions, including the motor cortex. The frontal and parietal regions were of larger extent in the left hemisphere. The unfamiliar letter strings of pseudohomophones compared to words led to an activation increase in three left hemisphere regions (OT, inferior parietal, inferior frontal). Nonwords compared to pseudohomophones led to a further increase in occipital regions and in large left frontal regions reaching from the inferior frontal gyrus (IFG) to precentral regions.
Fig. 1.
Brain regions identified by the contrasts of interest (see rightmost column) in nonimpaired and dyslexic readers, respectively. Activations are thresholded at p < .005, uncorrected, with a cluster size threshold of 10 voxels.
In response to words, dyslexic readers activated largely the same regions as the nonimpaired readers, but the extent of the left parietal and left frontal activations was larger, and there were additional activations in several right hemisphere regions. The pseudohomophone–word contrast identified a larger number of regions than in nonimpaired readers: bilateral middle occipital, bilateral parietal, bilateral frontal and insula regions. The largest regions with increased activity to pseudohomophones compared to words were localized in left inferior frontal regions and in the supplementary motor area (SMA). Importantly, the left OT region identified by the pseudohomophone–word contrast for nonimpaired readers was not identified for dyslexic readers. The nonword–pseudohomophone contrast in dyslexic readers identified a left precentral region and the left putamen, but did not identify the large left inferior frontal region found in nonimpaired readers.
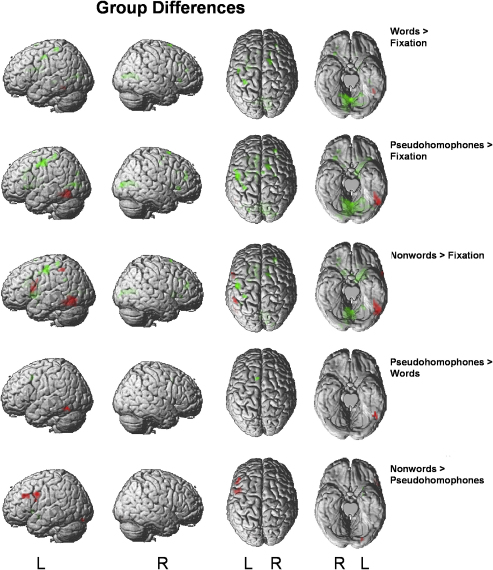
The results of the group comparisons are shown in Fig. 2 and in Table 3. For each of the three item types, dyslexic readers exhibited underactivation with an identical peak voxel in a left OT region. The extent of this underactivation increased from words to pseudohomophones and from pseudohomophones to nonwords in the posterior and lateral directions. In response to nonwords, but not in response to pseudohomophones, this left OT underactivation was accompanied by underactivation in a left inferior parietal and a left inferior frontal opercular region. These few regions with underactivation in dyslexic readers stood in contrast to a substantial number of regions with overactivation. For each item type, dyslexic readers exhibited overactivation in a large medial occipital region, a left postcentral region and in the left caudate. In response to words, there was additional overactivation in bilateral frontal and cingulum regions. In response to pseudohomophones and nonwords, a substantial number of regions with overactivation in frontal and subcortical regions were observed. For pseudohomophones, the largest anterior overactivations were in the left primary motor cortex and in the left cingulum. For nonwords, again large overactivations were identified in the left primary motor cortex and in the left cingulum and also in the left putamen.
Fig. 2.
Group differences in brain activity for contrasts of interest (see rightmost column). Red colour indicates higher activity for nonimpaired, green indicates higher activity for dyslexic readers. Activations are thresholded at p < .005, uncorrected, with a cluster size threshold of 10 voxels. For viewing purposes these activations are displayed here with a threshold of p < .01.
Table 3.
Brain regions identified by group differences.
| Region | MNI coordinates |
t | Voxel extent | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Words > baseline | |||||
| Nonimpaired readers > dyslexic readers | |||||
| L OT | −45 | −48 | −15 | 3.44 | 13 |
| Dyslexic readers > nonimpaired readers | |||||
| L postcentral | −33 | −33 | 63 | 3.09 | 11 |
| L precentral | −24 | −3 | 45 | 3.53 | 15 |
| L caudate | −12 | −3 | 21 | 3.04 | 12 |
| L inferior frontal, opercular | −30 | 9 | 24 | 3.48 | 22 |
| L anterior cingulum | −6 | 24 | 21 | 3.50 | 10 |
| R calcarine | 12 | −72 | 12 | 3.67 | 159 |
| R superior frontal | 15 | 12 | 72 | 3.82 | 11 |
| R middle cingulum | 15 | 24 | 33 | 3.62 | 10 |
| Pseudohomophones > baseline | |||||
| Nonimpaired readers > dyslexic readers | |||||
| L OT | −45 | −48 | −15 | 5.38# | 85 |
| Dyslexic readers > nonimpaired readers | |||||
| L postcentral | −33 | −36 | 63 | 3.46# | 13 |
| L primary motor cortex | −48 | −12 | 51 | 4.13# | 56 |
| Dyslexic readers > nonimpaired readers | |||||
| L precentral | −24 | −3 | 42 | 3.75# | 23 |
| L caudate | −12 | −6 | 18 | 3.31# | 17 |
| L pallidum | −9 | 0 | 3 | 3.29# | 16 |
| L caudate | −18 | 6 | 24 | 3.63# | 15 |
| L putamen | −18 | 15 | 3 | 2.96# | 15 |
| L middle cingulum | −12 | 21 | 36 | 3.63# | 59 |
| L Insula | −27 | 24 | 18 | 3.11# | 13 |
| R calcarine | 12 | −72 | 12 | 4.44# | 346 |
| R superior frontal | 15 | 9 | 72 | 3.94# | 16 |
| R Insula | 33 | 27 | 0 | 3.75# | 21 |
| R middle frontal | 27 | 42 | 21 | 3.71# | 27 |
| Nonwords > baseline | |||||
| Nonimpaired readers > dyslexic readers | |||||
| L OT | −45 | −48 | −15 | 6.49# | 139 |
| L inferior parietal | −51 | −45 | 54 | 4.23# | 15 |
| L inferior frontal, operculum | −57 | 12 | 12 | 3.06 | 22 |
| Dyslexic readers > nonimpaired readers | |||||
| L postcentral | −33 | −36 | 63 | 3.45 | 12 |
| L primary motor cortex | −48 | −12 | 51 | 4.23 | 47 |
| L caudate | −18 | 3 | 21 | 3.40 | 20 |
| L putamen | −18 | 12 | 3 | 3.81 | 140 |
| L anterior cingulum | −6 | 24 | 21 | 3.61 | 14 |
| L middle cingulum | −15 | 24 | 33 | 3.52 | 34 |
| R calcarine | 12 | −72 | 12 | 3.86 | 132 |
| R caudate | 15 | 12 | 6 | 3.42 | 32 |
| R middle cingulum | 12 | 24 | 33 | 3.73 | 12 |
| R middle frontal | 27 | 39 | 21 | 3.73 | 34 |
| Pseudohomophones > words | |||||
| Nonimpaired readers > dyslexic readers | |||||
| L OT | −45 | −51 | −18 | 2.77 | 3* |
| Dyslexic readers > nonimpaired readers | |||||
| L SMA | −9 | 12 | 45 | 3.49 | 13 |
| Nonwords > pseudohomophones | |||||
| Nonimpaired readers > dyslexic readers | |||||
| L lingual | −21 | −87 | −18 | 3.12 | 10 |
| L precentral | −45 | 6 | 36 | 3.56 | 61 |
| L inferior frontal, triangular | −48 | 27 | 33 | 3.79 | 27 |
| Dyslexic readers > nonimpaired readers | |||||
| L pallidum | −21 | 0 | −9 | 3.80 | 12 |
| L putamen | −21 | 15 | 0 | 3.32 | 10 |
Note: only regions with a reliable group difference of puncorrected < .005 and a cluster extent of >10 voxels are reported (a single exception is marked with *). #pFDR-corrected < .05.
The lower section of Fig. 2 and Table 3 informs on regions showing group differences of item type effects. Important findings were that dyslexic readers failed to exhibit the pseudohomophone–word difference of the nonimpaired readers in the left OT and they failed to exhibit the nonword–pseudohomophone difference of the nonimpaired readers in left inferior frontal and precentral regions and in the left lingual gyrus. In contrast, dyslexic readers showed an increased pseudohomophone–word difference in the SMA and an increased nonword–pseudohomophone difference in subcortical regions (left putamen and pallidum). For dyslexic readers, we also searched for brain regions showing the inverse item type effects, that is, higher activity to words compared to pseudohomophones and higher activity to pseudohomophones compared to nonwords. In fact, a recent study by Pugh et al. (2008) found that dyslexic readers exhibited an activation pattern inverse to that of the nonimpaired readers (i.e., more activity to high compared to low frequency words). In the present study, however, no such region was identified for the word–pseudohomophone contrast. A left inferior parietal region was identified for the pseudohomophone–nonword contrast, which, however, resulted from differences in deactivation against baseline.
3.3. Brain activity in ROIs
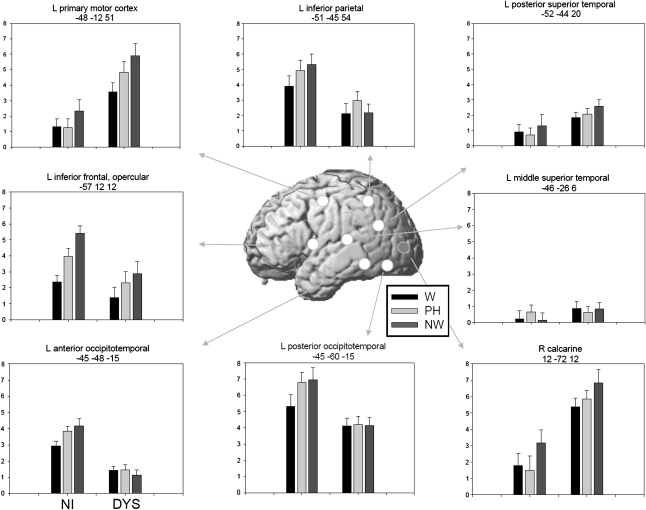
The brain activity estimates for critical ROIs in Fig. 3 illustrate main findings of the voxel-based analyses. As posterior regions, we selected an occipital and two left OT regions. The occipital ROI was centered around the peak of the large medial occipital region, where dyslexic readers exhibited higher activity compared to nonimpaired readers in response to all three item types. The anterior one of the two left OT ROIs (y = −48) exhibited reduced activity in dyslexic readers in response to all three item types, and the posterior one (y = −60) was selected to illustrate the posterior extent of this group difference in the case of pseudohomophones and nonwords. The mean estimates of brain activity in Fig. 3 illustrate the substantial size of these opposite dyslexic abnormalities, that is, overactivation in occipital and underactivation in OT regions. Furthermore, in the occipital ROI our dyslexic readers exhibited increased activity to nonwords compared to words. This was not the case for the OT ROIs, where dyslexic readers failed to exhibit the activation pattern of the nonimpaired readers (i.e., words < pseudohomophones = nonwords).
Fig. 3.
Brain activity in ROIs (see text). Estimates of brain activity (mean ± SEM) are given in arbitrary units. L = left, R = right, W = words, PH = pseudohomophones, NW = nonwords, NI = nonimpaired readers, DYS = dyslexic readers.
Fig. 3 also includes brain activity for two superior temporal gyrus regions and an inferior parietal region. The middle superior temporal ROI is centered around the maximum of dyslexic underactivation found in the letter-sound integration task of Blau et al. (2009) and the posterior temporal ROI is based a maximum of dyslexic underactivation in our quantitative meta-analysis (Richlan et al., 2009). The inferior parietal ROI is based on the present voxel-based finding of dyslexic underactivation in response to nonwords. In the middle superior temporal ROI there was generally little activity compared to baseline, but, interestingly, a tendency towards higher activity for dyslexic readers. In the posterior superior temporal ROI, activation levels were increased in dyslexic readers and the group difference was of borderline reliability (p = .07). For the inferior parietal ROI, the underactivation of the dyslexic readers was not limited to nonwords, but was also present for pseudohomophones and words.
Fig. 3 further includes two left frontal ROIs. The one in the left IFG, opercular part, was identified by reduced activity in dyslexic readers compared to nonimpaired readers in response to nonwords, but the means show that dyslexic underactivation was not limited to nonwords, as there was a tendency in this direction also for pseudohomophones and words. The left motor cortex ROI was identified by higher activity in dyslexic readers compared to nonimpaired readers in response to pseudohomophones and nonwords, but Fig. 3 shows dyslexic overactivation also in response to words.
We also examined correlations between individual reading speed scores outside the scanner (averaged over silent sentence evaluation and reading a text aloud) and individual brain activity estimates for the ROIs (averaged over the three item types). The rank correlations corresponded to the group differences. There was a positive correlation of ρ(35) = .56, p < .001, between reading speed and combined left OT activation (i.e., the higher the speed score, the higher the activation) and a negative association between reading speed and right occipital (calcarine) activation, ρ(35) = −.57, p < .001, as well as between reading speed and left motor cortex activation, ρ(35) = −.51, p < .01. Within each group, associations were lower and none was reliable.
The effect of age on brain activity was examined for the ROIs by analyses of variance (ANOVAs) with age (adolescents vs adults) and reading skill group (dyslexic vs nonimpaired) as between-subjects factors and item type as within-subjects factor. Corresponding to the absence of an age effect on response latencies for the in-scanner task, none of the main effects of age was reliable, Fs(1, 35) < 1, all n. s., and, with a single exception, none of the interactions involving age was reliable, Fs < 3.2, all n. s. The only exception was the age by group interaction for the left motor cortex ROI, F(2, 70) = 6.4, p < .05, which resulted from the higher brain activity of the dyslexic readers compared to the controls in the adolescent subsample, p < 01. This was not the case for the adult subsample.
4. Discussion
4.1. Behavioral evidence
The dyslexic participants of the present study exhibited the behavioral manifestation of dyslexia in regular orthographies, that is, they suffered from a severe impairment of reading speed but not of reading accuracy. The high accuracy in reading stood in contrast to the low accuracy for spelling. This pattern is expected from the asymmetric regularity of grapheme–phoneme correspondence in German, that is, high in the reading (grapheme-to-phoneme) direction and low in the spelling (phoneme-to-grapheme) direction. In terms of dual-route processes, the poor spelling performance is important. It indicates that dyslexic readers suffered from a poor orthographic lexicon, that is, they possessed memory representations containing all letters for only a reduced number of words. This reduced size of the orthographic lexicon may contribute to the reading speed problem, because it requires reliance on serial grapheme–phoneme processing for words, for which nonimpaired readers can rely on fast whole-word recognition based on orthographic word recognition units.
For the subsample of 12 adolescents coming from the longitudinal study, we additionally showed that accuracy for fast reading of a list of nonwords was about 90% correct even at the end of Grade 3. In contrast to English-based dyslexia findings, this early accuracy of sublexical reading most plausibly is due to the regular grapheme–phoneme relations of German and to reliance on a synthetic phonics teaching approach (for details, see Wimmer et al., 2000). The early acquisition of an accurately functioning sublexical reading route is important, as it can be seen as precondition of long-term storage of the letter-sequences of correctly decoded words (Share, 1995). A specific difficulty of dyslexic readers for such orthographic learning was found in training studies (Reitsma, 1983; Thaler et al., 2004). In contrast to these high levels of early reading accuracy, reading speed was impaired over all three longitudinal assessments, and this reading speed impairment was preceded by poor performance in a RAN task. Importantly, this early RAN impairment was not accompanied by a slow speed on a peg moving test which was part of the precursor assessment. Furthermore, on a coherent motion detection task – measuring visual magnocellular functioning – the dyslexics performed similarly to nonimpaired readers.
The performance of the dyslexic participants on the in-scanner phonological lexical decision task (i.e., Does xxx sound like an existing word?) corresponded to their reading performance outside the scanner. Similar to their generally high reading accuracy, their phonological lexical decisions were quite accurate, but latencies were prolonged. Importantly, similar to the nonimpaired readers, the dyslexic sample exhibited more accurate and faster YES responses to familiar strings such as Taxi compared to pseudohomophones such as Taksi. Actually, they profited at least as much from orthographic familiarity as nonimpaired readers. This marked orthographic familiarity effect suggests that dyslexic readers relied on the efficient lexical route to word phonology (via orthographic word recognition units) for a substantial number of the familiar letter strings. However, their shorter decision latencies in response to familiar compared to unfamiliar strings were still substantially prolonged compared to the latencies of the nonimpaired readers. Following Bergmann and Wimmer (2008), this speed impairment may emerge from two sources: One is absence of fully specified orthographic word recognition units for some words, so that dyslexic readers may have had to rely on the slower sublexical route to reach the YES response for these items. The poor spelling performance of our dyslexic readers speaks for this possibility. The second source is an original speed impairment of the lexical route. Bergmann and Wimmer showed that dyslexic readers exhibited prolonged phonological lexical decision latencies even when availability of orthographic whole-word recognition units could be inferred from their orthographic lexical decisions. Importantly, prolonged decision latencies on the in-scanner task were not limited to words, but were also observed for pseudohomophones and nonwords. Actually, in absolute terms the speed deficit was larger for the unfamiliar letter strings. This suggests an at least similar efficiency problem of the sublexical route as of the lexical route.
4.2. Left OT underactivation and absent orthographic familiarity effect accompanied by overactivation in occipital regions
Of relevance for interpreting the reading speed impairment in terms of the dual-route model is the finding that dyslexic readers exhibited underactivation in a left OT brain region in response to all three item types. The center of this region was identical at around x = −45, y = −48, z = −15 (MNI coordinates) for all three item types. This center in the OT cortex is slightly anterior to the classical VWFA of Cohen et al. (2002) at around x = −43, y = −54, z = −12. Our quantitative meta-analysis (Richlan et al., 2009) identified a maximum of dyslexic underactivation in the fusiform gyrus at x = −46, y = −50, z = −16. The extent of the left OT underactivation was relatively small for words and was enlarged in the posterior and lateral direction for pseudohomophones and nonwords. This left OT underactivation stood in contrast to dyslexic overactivation in a large medial occipital region. Therefore, it can be excluded that the left OT underactivation is a down-stream consequence of a posterior occipital dysfunction. Dyslexic overactivation in an occipital region (lingual gyrus) was also found in our quantitative meta-analysis.
Besides underactivation of the VWFA, dyslexic readers completely failed to exhibit the modulation of the VWFA shown by the nonimpaired readers, that is, increased activity to unfamiliar letter strings of pseudohomophones and nonwords compared to familiar letter strings of words. The failure of dyslexic readers to exhibit this modulation cannot be attributed to a lack of familiarity with the letter strings of words, because the effect of familiarity on response time (in absolute terms) was stronger for dyslexic than nonimpaired readers. The absence of orthographic familiarity effects on left OT activation of the present poor readers is not an isolated finding, but was also found in two other fMRI studies which used the present procedure (Bruno et al., 2008; Van der Mark et al., 2009). There is also correspondence with a recent fMRI study by Pugh et al. (2008). This study measured brain activity in response to reading aloud words of high or low frequency and found that dyslexic readers exhibited underactivation of the left OT cortex and failed to exhibit any modulation in response to word frequency. One may be concerned that the presently found underactivation of the VWFA in the left OT cortex and the absence of a familiarity effect in this region may simply reflect a delay in the acquisition of reading skill, which may be overcome with further reading development. This concern is certainly valid. However, we note that the present dyslexic readers were adolescents and adults who typically show little further gains in reading skill. Furthermore, a recent study by Hoeft et al. (2007) compared dyslexic and younger nonimpaired readers matched for reading ability, and, consistent with the present study, found underactivation in the left OT/fusiform gyrus, together with left inferior parietal and right OT underactivation.
In previous studies (Kronbichler et al., 2007, 2009; Schurz et al., 2010) with nonimpaired readers, we have found similar VWFA activation patterns as in the present study (i.e., words < pseudohomophones = nonwords). This was interpreted as recruitment of the VWFA by both lexical and sublexical coding processes. Specifically, we proposed that the lower activity in response to the familiar letter strings reflects efficient assimilation of whole-word strings by often used orthographic word recognition units, whereas the higher activity in response to unfamiliar letter strings reflects coding into grapheme sequences. In this perspective, the present dyslexic result pattern – reduced activity to all three items types, absence of orthographic familiarity related modulation – suggests that the VWFA was not recruited in dyslexic readers by lexical and sublexical orthographic coding processes. This failure to recruit the VWFA for lexical and sublexical route processes can be seen as a serious abnormality in the neural organization of reading processes. This interpretation is suggested by evidence showing that the VWFA is critically involved in highly efficient processing of letter string information (Cohen et al., 2002; Dehaene et al., 2005). In direct support of this function, disruption or deafferentation of the VWFA was found to result in letter-by-letter reading in formerly fluent readers (Cohen et al., 2004; Gaillard et al., 2006; Philipose et al., 2007). A different, but also critically important function of left OT regions in visual word processing was proposed by Price and Devlin (2003). These authors presented evidence for the position that the left OT cortex functions as an efficient interface which channels visual or tactile information to brain regions engaged by language and knowledge representation (see also Devlin et al., 2006).
4.3. No evidence for a left superior temporal dysfunction
As noted in the Introduction, reviews of dyslexic brain activation abnormalities summarize the largely English-based evidence as speaking for a primary dysfunction of left temporoparietal language regions (e.g., Pugh et al., 2000; Sandak et al., 2004, McCandliss and Noble, 2003; Shaywitz et al., 2007). Indeed, our quantitative meta-analysis of functional imaging research identified several maxima of dyslexic underactivation in left posterior temporal regions (Richlan et al., 2009). The mentioned reviews suggest that left temporoparietal regions are engaged primarily by the sublexical phonological reading route which is known to be specifically error-prone for (English) dyslexic readers. Recently, further support for a left superior temporal dysfunction was supplied by fMRI studies which used a letter-sound integration paradigm with Dutch adult dyslexic readers (Blau et al., 2009). The dyslexic readers – although knowing all relevant letter-sound associations – failed to exhibit modulation of left superior temporal areas in response to incongruent letter-sound pairs. In a more general perspective, underactivation of posterior temporal language areas in response to reading-related tasks is linked to the phonological deficit explanation of dyslexia, which assumes that underspecified phonological word representations give rise to a phonemic awareness deficit which hinders the extraction of grapheme–phoneme associations on which sublexical reading is dependent (e.g., Snowling, 2000).
The present findings stand in marked contrast to the position that sublexical reading engages a left temporoparietal reading system, and that dyslexic readers suffer from a primary dysfunction of these regions. In the whole-brain analysis, neither nonimpaired nor dyslexic readers exhibited reliable activation in left superior temporal regions in response to pseudohomophones and nonwords, despite a liberal statistical threshold. Specifically, the left superior temporal region, identified by Blau et al. (2009) as exhibiting dyslexic abnormalities in response to letter-sound matching, did not show reliable activation in the present study. Actually, we found the opposite from what is expected from a dysfunction of left superior temporal regions, because our dyslexic readers exhibited a tendency towards higher activity in the left posterior superior temporal gyrus. A rather obvious explanation of the present absence of left temporal activation in both nonimpaired and dyslexic readers is that our activation task was based on silent reading. However, one may note that the instruction required a judgement based on “sound” (i.e., Does xxx sound like a real word?) and presented pseudohomophones and nonwords (e.g., Taksi and Tazi) which can only be reliably distinguished by sublexical reading processes. Of course, the present negative finding on a dysfunction of the left temporoparietal cannot rule out that such a dysfunction would have become apparent if we had relied on a more demanding phonological task, for example, judging whether two visual words do or do not rhyme (see Richlan et al., 2009, for a list of activation task). Furthermore, there is evidence that the left supramarginal gyrus plays an important role in the early phase of learning to read (e.g., Church et al., 2008). From this finding one may infer that a left temporoparietal dysfunction may have been identified with a younger group of German dyslexic readers.
4.4. Left inferior parietal underactivation
Consistent with an important role of the left parietal cortex in reading, our nonimpaired readers exhibited high activity in a left inferior parietal region, specifically in response to pseudohomophones and nonwords, and dyslexic readers exhibited reduced activity in this region. For interpretation, one may note that this inferior parietal cluster at x = −51, y = −45, z = 54 is quite distant from the left posterior superior temporal regions which are considered as core regions of phonological reading processes. Furthermore, the activation pattern shown by nonimpaired readers, that is, high activity in the inferior parietal cortex but no substantial activity in the posterior superior temporal area, speaks against the possibility that the inferior parietal activation reflects phonological processing (i.e., “hearing” of phonemes or assembled pronunciations). Following Milner and Goodale (1995), one may hypothesize that the left inferior parietal cortex in silent reading serves as attention guiding interface between visual-orthographic coding in OT regions and productive phonological processes in left IFG regions. However, one may note that the presently identified region with dyslexic underactivation in response to nonwords is part of an extended left parietal region which was activated by all item types in dyslexic and nonimpaired readers.
4.5. Underactivation in left inferior frontal regions accompanied by overactivation in premotor and motor regions
The only brain region where dyslexic readers, in addition to left OT and left inferior parietal regions, exhibited underactivation was identified in the left IFG, opercular part. Different from the left OT region which exhibited underactivation in response to all three item types, the left inferior frontal together with the left inferior parietal region were identified by the voxel-based analysis only for nonwords. This suggests that a dysfunction of these regions became apparent only for the most difficult item types for which sublexical processing did not find a phonological lexicon entry. However, the ROI analysis found reliable underactivation in the IFG not only for nonwords but also for pseudohomophones and a tendency was also apparent for words. Left IFG underactivation was also found in our meta-analysis (Richlan et al., 2009) where it was high-lighted as a new finding which is overlooked in narrative reviews of imaging studies. These reviews summarily speak of dyslexic overactivation in a left frontal reading system in order to compensate for underactivation in the left temporoparietal reading system (e.g., Pugh et al., 2000; Sandak et al., 2004). The present study differs from this pattern by finding left IFG underactivation without underactivation in left TP regions. The underactivation in the left IFG in response to pseudohomophones and nonwords is suggestive of a dysfunction in the efficient access to sublexical phonological segments.
The mentioned reviews summarily interpret the left frontal overactivation in dyslexic readers as reflection of compensatory silent articulatory processes in visual word processing. Although we did find the opposite of left frontal overactivation in the left IFG, there is specific support for this interpretation in our results. We did find dyslexic overactivation in pre- and post-central regions and in the primary motor cortex. These findings, together with overactivation in the caudate and putamen, speak for reliance on silent articulatory processes. Dyslexic abnormalities of item type effects strengthen this interpretation. Specifically, dyslexic readers showed an increased pseudohomophone–word difference in the SMA and an increased nonword–pseudohomophone difference in subcortical regions (left putamen and pallidum).
A predominance of dyslexic overactivation was also found in our preceding study which measured brain activity in response to sentence verification (Kronbichler et al., 2006) and in another German-based study which measured dyslexic brain activity in response to rhyme judgements of nonwords (Grünling et al., 2004). This predominance of overactivation in German-based dyslexia studies stands in contrast to a predominance of underactivation in a substantial number of English-based dyslexia studies (Richlan et al., 2009) and may be related to effortful sublexical route processes based on the reliable grapheme–phoneme relations of German. These orthography-related differences in dyslexic brain dysfunctions challenge current neurocognitive accounts of dyslexia, which assume that all dyslexic readers – irrespective of the particular writing system of their language – have the same underlying brain dysfunction (for a discussion, see Hadzibeganovic et al., 2010, this issue).
5. Conclusion
The behavioral in-scanner data suggested that the present German dyslexic readers, similar to nonimpaired controls, relied on lexical route processes (orthographic word recognition, direct access to word phonology) for familiar letter strings of words, and on sublexical route processes (grapheme–phoneme conversion) for unfamiliar letter strings of pseudohomophones and nonwords. However, both lexical and sublexical route processes were performed inefficiently although accurately. The imaging results were suggestive for a different neural organization of reading processes in dyslexic readers. Specifically, dyslexic readers, in response to lexical route processes, exhibited under-activation in a left OT region corresponding to the VWFA, presumably engaged by visual-orthographic whole word recognition. This region was also insensitive to the increased visual-orthographic processing demands of the sublexical route. Reduced engagement in response to sublexical route processes was also found in a left inferior parietal region, presumably engaged by attentional processes, and in a left inferior frontal region, presumably engaged by phonological processes. In contrast, to this reduced engagement of the “nonimpaired” reading network, our dyslexic readers exhibited increased engagement of visual occipital regions and of regions presumably engaged by silent articulatory processes (premotor/motor and subcortical caudate and putamen). Different from largely English-based imaging finding, no dyslexic abnormalities were found in left posterior temporal regions. These regions were not activated, neither in nonimpaired nor in dyslexic readers.
Acknowledgements
This research was supported by grant of the Austrian Science Foundation to Heinz Wimmer and Gunther Ladurner (Grant Number P18832-B02), and by a grant of the European Union (FP6-2004-LIFESCIHEALTH-5) to Karin Landerl and Heinz Wimmer. We are grateful to the members of the Department of Radiology for assistance and to reviewers for thoughtful comments.
References
- Annett M. Lawrence Associates Inc; Hillsdale: 1985. Left, Right Hand and Brain: The Right Shift Theory. [Google Scholar]
- Baayen R.H., Piepenbrock R., van Rijn H. Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1993. The Celex Lexical Database [Computer Software] [Google Scholar]
- Bergmann J., Wimmer H. A dual-route perspective on poor reading in a regular orthography: Evidence from phonological and orthographic lexical decisions. Cognitive Neuropsychology. 2008;25(5):653–676. doi: 10.1080/02643290802221404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16(7):1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau V., van Atteveldt N., Ekkebus M., Goebel R., Blomert L. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Current Biology. 2009;19:503–508. doi: 10.1016/j.cub.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Brambati S.M., Termine C., Ruffino M., Danna M., Lanzi G., Stella G. Neuropsychological deficits and neural dysfunction in familial dyslexia. Brain Research. 2006;1113:174–185. doi: 10.1016/j.brainres.2006.06.099. [DOI] [PubMed] [Google Scholar]
- Bruno J.L., Zumberge A., Manis F.R., Lu Z.L., Goldman J.G. Sensitivity to orthographic familiarity in the occipito-temporal region. NeuroImage. 2008;39:1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.-L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry. 2006;47(10):1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J.A., Coalson R.S., Lugar H.M., Petersen S.E., Schlaggar B.L. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18(9):2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L., Henry C., Dehaene S., Martinaud O., Lehéricy S., Lemer C. The pathophysiology of letter-by-letter reading. Neuropsychologia. 2004;42(13):1768–1780. doi: 10.1016/j.neuropsychologia.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehericy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., Ziegler J. DRC: A dual route cascade model of visual word processing and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Sigman M., Vinckier F. The neural code for written words: A proposal. Trends in Cognitive Sciences. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Denckla M., Rudel R.G. Rapid “automatized” naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Devlin J.T., Jamison H.L., Gonnerman L.M., Matthews P.M. The role of the posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M.E., Balota D.A., Spieler D.H., Ferraro F.R. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Glaser D.E., Henson R.N.A., Kiebel S., Phillips C., Ashburner J. Classical and Bayesian inference in neuroimaging: Applications. NeuroImage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Gaillard R., Naccache L., Pinel P., Clémenceau S., Volle E., Hasboun D. Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.E.J., Valle I.H. Word identification and reading disorders in the Spanish language. Journal of Learning Disabilities. 2000;33(1):44–60. doi: 10.1177/002221940003300108. [DOI] [PubMed] [Google Scholar]
- Grünling C., Ligges M., Huonker R., Klingert M., Mentzel H.J., Rzanny R. Dyslexia: The possible benefit of multimodal integration of fMRI- and EEG-data. Journal of Neural Transmission. 2004;111(7):951–969. doi: 10.1007/s00702-004-0117-z. [DOI] [PubMed] [Google Scholar]
- Hadzibeganovic T, van den Noort M, Bosch P, Perc M, van Kralingen R, Mondt K, et al. Cross-linguistic neuroimaging and dyslexia: A critical view. Cortex, 46(10): 1312–1316, 2010. [DOI] [PubMed]
- Hawelka S., Gagl B., Wimmer H.A. Dual-route perspective on eye movements of dyslexic readers. Cognition. 2010;115(3):367–379. doi: 10.1016/j.cognition.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R.N.A. Analysis of fMRI timeseries: Linear time-invariant models, event-related fMRI and optimal experimental design. In: Frackowiak R.S.J., Friston K.J., Frith C.D., Dolan R., Price C.J., Zeki S., editors. Human Brain Function. 2nd ed. Academic Press; London: 2004. pp. 793–822. [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the USA. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersting M., Althoff K. Hogrefe; Göttingen: 2004. Rechtschreibungstest (RT) [Google Scholar]
- Kronbichler M., Bergmann J., Hutzler F., Staffen W., Mair A., Ladurner G. Taxi vs. Taksi: On orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19:1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M., Hutzler F., Staffen W., Mair A., Ladurner G., Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44:1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kronbichler M., Hutzler F., Wimmer H. Dyslexia: Verbal impairments in the absence of magnocellular impairments. NeuroReport. 2002;13(5):617–620. doi: 10.1097/00001756-200204160-00016. [DOI] [PubMed] [Google Scholar]
- Kronbichler M., Klackl J., Richlan F., Schurz M., Staffen W., Ladurner G. On the functional neuroanatomy of visual word processing: Effects of case and letter deviance. Journal of Cognitive Neuroscience. 2009;211:1–8. doi: 10.1162/jocn.2009.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K., Wimmer H., Frith U. The impact of orthographic consistency on dyslexia: A German–English comparison. Cognition. 1997;63(3):315–334. doi: 10.1016/s0010-0277(97)00005-x. [DOI] [PubMed] [Google Scholar]
- Lundberg I., Hoien T. Patterns of information processing skills and word recognition strategies in developmental dyslexia. Scandinavian Journal of Educational Research. 1990;34:231–240. [Google Scholar]
- Marinus E and De Jong PF. Variability in the word-reading performance of dyslexic readers: Effects of letter length, phoneme length and digraph presence. Cortex, 46(10): 1259–1271, 2010. [DOI] [PubMed]
- Mayringer H., Wimmer H. No deficits at the point of hemispheric indecision. Neuropsychologia. 2002;40(77):701–704. doi: 10.1016/s0028-3932(01)00191-9. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Noble K.G. The development of reading impairment: A cognitive neuroscience model. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9(3):196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- Milner A.D., Goodale M.A. Oxford University Press; Oxford: 1995. The Visual Brain in Action. [Google Scholar]
- Moll K., Hutzler F., Wimmer H. Developmental dyslexia in a regular orthography: A single case study. Neurocase. 2005;11(6):433–440. doi: 10.1080/13554790500263537. [DOI] [PubMed] [Google Scholar]
- Philipose L.E., Gottesman R.F., Newhart M., Kleinman J.T., Herskovits E.H., Pawlak M.A. Neural regions essential for reading and spelling of words and pseudowords. Annals of Neurology. 2007;62(5):481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Porpodas C.D. Patterns of phonological and memory processing in beginning readers and spellers of Greek. Journal of Learning Disabilities. 1999;32(5):406–416. doi: 10.1177/002221949903200506. [DOI] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Frost S.J., Sandak R., Landi N., Rueckl J.G., Constable R.T. Effects of stimulus difficulty and repetition on printed word identification: A functional magnetic resonance imaging comparison of nonimpaired and reading disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20(7):1–15. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. Printed word learning in beginning readers. Journal of Experimental Child Psychology. 1983;36:321–339. [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandak R., Mencl W.E., Frost S.J., Pugh K.R. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8(3):273–292. [Google Scholar]
- Schulz E., Maurer U., Van der Mark S., Bucher K., Brem S., Martin E. Impaired semantic processing during sentence reading in children with dyslexia: Combined fMRI and ERP evidence. NeuroImage. 2008;41:153–168. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Schurz M., Sturm D., Richlan F., Kronbichler M., Ladurner G., Wimmer H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA) NeuroImage. 2010;49:2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Share D.L. Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition. 1995;55:151–218. doi: 10.1016/0010-0277(94)00645-2. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Skudlarski P., Holahan J.M., Marchione K.E., Constable R.T., Fulbright R.K. Age-related changes in reading systems of dyslexic children. Annals of Neurology. 2007;61(4):363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Snowling M.J. 2nd ed. Blackwell Publishers; Oxford: 2000. Dyslexia. [Google Scholar]
- Spinelli D., De Luca M., Di Filippo G., Mancini M., Martelli M., Zoccolotti P. Length effect in word naming in reading: Role of reading experience and reading deficit in Italian readers. Developmental Neuropsychology. 2005;27(2):217–235. doi: 10.1207/s15326942dn2702_2. [DOI] [PubMed] [Google Scholar]
- Thaler V., Ebner E.M., Wimmer H., Landerl K. Training reading fluency in dysfluent readers with high reading accuracy: Word specific effects but low transfer to untrained words. Annals of Dyslexia. 2004;54(1):89–113. doi: 10.1007/s11881-004-0005-0. [DOI] [PubMed] [Google Scholar]
- Temple E., Poldrack R.A., Protopapas A., Nagarajan S., Salz T., Tallal P. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the USA. 2000;97(25):13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewes U. 2nd ed. Huber; Bern: 1991. Hamburger-Wechsler Intelligenztest für Erwachsene Revision. [Google Scholar]
- Van den Bos K.P., Brand-Gruwel S., Aarnoutse C.A.J. Text comprehension strategy instruction with poor readers. Reading and Writing. 1998;10(6):471–498. [Google Scholar]
- Van der Mark S., Bucher K., Maurer U., Schulz E., Brem S., Buckelmüller J. Children with Dyslexia lack multiple specializations along the Visual Word-Form (VWF) System. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Nichols T.E. Optimization of experimental design in fMRI: A general framework using a genetic algorithm. NeuroImage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wimmer H. Characteristics of developmental dyslexia in a regular writing system. Applied Psycholinguistics. 1993;14(1):1–33. [Google Scholar]
- Wimmer H., Mayringer H., Landerl K. The double-deficit hypothesis and difficulties in learning to read a regular orthography. Journal of Educational Psychology. 2000;92(4):668–680. [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Riverside Publishing; Itasca, IL: 2001. Woodcock-Johnson III. [Google Scholar]
- Yap R., Van der Leij A. Word-processing in dyslexics – an automatic decoding deficit. Reading and Writing. 1993;5(3):261–279. [Google Scholar]
- Ziegler J.C., Perry C., Ma-Wyatt A., Ladner D., Schulte-Korne G. Developmental dyslexia in different languages: Language-specific or universal? Journal of Experimental Child Psychology. 2003;86(3):169–193. doi: 10.1016/s0022-0965(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Zoccolotti P., De Luca M., Di Pace E., Gasperini F., Judica A., Spinelli D. Word length effect in early reading and in developmental dyslexia. Brain and Language. 2005;93(3):369–373. doi: 10.1016/j.bandl.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Zoccolotti P., De Luca M., Di Pace E., Judica A., Orlandi M. Markers of developmental surface dyslexia in a language (Italian) with high grapheme–phoneme correspondence. Applied Psycholinguistics. 1999;20(2):191–216. [Google Scholar]