Abstract
The innate immune system plays a critical role in host defense against mucosal bacteria. Campylobacter jejuni is a major cause of human gastroenteritis that usually resolves spontaneously within several days, suggesting that innate mechanisms are important to control the infection. However, the specific means by which this occurs is not well understood. While diarrheal isolates of C. jejuni usually are susceptible to human serum, we found that a systemic strain of C. jejuni, isolated from the cerebrospinal fluid of an infant with meningitis, is relatively more resistant to human serum, the Bactericidal/Permeability-Increasing Protein (BPI), an endogenous cationic antimicrobial protein, and the cationic peptide antibiotic polymyxin B. To test the hypothesis that the surface properties of this strain contributed to its ability to withstand these innate host defenses, we constructed isogenic mutants in capsule (kpsM) and lipooligosaccharide (waaF) and complemented these mutants by insertion of the complementation construct in trans into hipO, a chromosomal locus. We found that capsule expression was essential for serum resistance, whereas lipooligosaccharide played no substantial role. In contrast, the lipooligosaccharide mutant showed increased sensitivity to polymyxin B, α-defensins, cathelicidins and BPI. These findings suggest that the polysaccharides of C. jejuni strains contribute differently to resistance against host innate immunity, whereby capsule is more important for resisting human complement and lipooligosaccharide is more important for protection against killing mediated by cationic antimicrobial peptides and proteins.
Key words: campylobacter, innate immunity, host defense, lipooligosaccharide, capsule, complementation, serum, cationic antimicrobial
Introduction
Campylobacter jejuni is a microaerophilic Gram-negative rod that is a common cause of food-borne diarrheal illness in humans.1,2 This self-limited disease is characterized by fever, abdominal pain and bloody diarrhea that usually resolves within several days of onset of symptoms,2 suggesting that host innate defenses contribute to the resolution of infection.3,4 Although the illness is often mild, C. jejuni infections occasionally lead to complications such as bacteremia, post-infectious reactive arthritis or Guillain-Barré Syndrome.2,5,6 Despite the observation that these bacteria are able to persistently colonize a variety of livestock, particularly fowl,7 relatively little is known about the mechanisms they use to evade host innate immune mechanisms.
Numerous studies have examined the role of bacterial surface structures, protein glycosylation, toxins and the invasion of intestinal epithelial cells that are important for C. jejuni diarrheal pathogenesis.8–11 However, less is known about the pathogenesis of C. jejuni infections that gain access to the vascular space and cause systemic infection. When a systemic C. jejuni infection occurs in an apparently immunocompetent individual, the recovered isolate is likely to have features promoting persistence in the bloodstream that distinguish it from diarrheal C. jejuni strains.12
The vertebrate intestinal tract relies on both innate and adaptive immune defenses to limit both the residential and the acquired, often pathogenic, microbiota from traversing the intestinal mucosa and entering the host bloodstream.13,14 Examples of important components of the innate immune system with direct bactericidal activity include cationic antimicrobial peptides and proteins (CAPs) and complement.13,15–17 For example, in the intestine, the inducible secretion of one family of CAPs known as defensins contributes to innate defenses.15,18 A second family of CAPs, the cathelicidins, is produced principally by neutrophils and effect host defense both in the vascular space and in tissue sites of infection after neutrophil recruitment.19,20 Another cationic neutrophil-derived antimicrobial is the 55 kDa Bactericidal/Permeability-Increasing Protein (BPI), which is stored in the primary granules of neutrophils and shows potent and selective antimicrobial activity against Gram-negative bacteria via its affinity for the lipid A portion of lipopolysaccharide (LPS) and lipooligosaccharide (LOS).17,21 The importance of CAPS in host defense against C. jejuni is supported by the observation that the diarrheal strain 11168 stimulates upregulation of human β-defensins in intestinal epithelial cells in vitro and is susceptible to their activity.4
C. jejuni has a highly expressed and varied surface glycome, and many glycosylated structures are associated with surface components including the capsule polysaccharide and the oligosaccharide side-chains of its LOS.22 Such surface structures are in direct contact with components of innate host immunity, and so likely play biologically important roles in C. jejuni. The importance of complement in defense against bloodstream pathogens in general23 and diarrheal C. jejuni strains specifically are wellknown,2,24 and has been attributed to both capsule and LOS in these diarrheal isolates.24,25 Specifically, truncation of sialic acid from the LOS cores of certain diarrheal strains lead to increased sensitivity to CAPs such as the cathelicidin LL-37 and polymyxin B.24 In the current work, we explore how the surface structures of systemic C. jejuni isolated affect susceptibility to complement and CAPs.
To achieve this, we focused on an invasive C. jejuni strain 84-25, which was isolated from the cerebrospinal fluid of an apparently immunologically normal child with meningitis.12 We also examined strain 84-19, another C. jejuni isolate from the cerebrospinal fluid of an infant with meningitis, with the expectation that both strains have similar surface characteristics. Although antecedent bacteremia was not documented in these children with Campylobacter meningitis, hematogenous spread to the meninges from the gut is the most likely sequence of events.
We hypothesized that capsule and LOS from strain 84-25 contribute to its virulence via resistance against serum and CAPs. By construction of isogenic acapsular and LOS truncation mutants of this systemic isolate, we assessed the contribution of its glycome to evasion of the antimicrobial properties of serum and cationic antimicrobial effectors. To complement these mutants, we developed a chromosomal complementation method. We used the hipO locus, encoding the non-essential enzyme hippurate hydrolase, which degrades hippuric acid into benzoic acid and glycine.26 Since this enzyme is highly conserved in Campylobacter,27 this complementation strategy can be used in nearly all C. jejuni strains and so represents a potential advance over previously published methods.8,11,28–30
Results
Characterization of capsule expression in the C. jejuni kpsM mutants.
Capsule produced by the wild-type strain 84-25, its capsule mutant (kpsM), and in trans complemented mutant (kpsMT/comp) were characterized by SDS-PAGE, followed by Alcian Blue staining (Fig. 1). As expected, the kpsM mutant showed decreased capsule expression compared to the wild-type strain. A Shine-Dalgarno sequence is present upstream of kpsM but not kpsT, suggesting translational coupling;31 therefore, complementation with kpsM alone did not restore capsule expression (data not shown) whereas complementation with kpsMT did, as noted by others in strain 81-17625 (Fig. 1). We also examined strain 84-19, another C. jejuni isolate from the cerebrospinal fluid of an infant with meningitis. Although the capsules of strains 84-25 and 84-19 display nonidentical electrophoretic mobility (data not shown) and are Penner serotype 2 and 13, respectively,12 similar results were observed in strain 84-19 and its isogenic kpsM mutant (data not shown). These results demonstrate that kpsM participates in capsule assembly of strain 84-25, as has been shown in other C. jejuni strain backgrounds.31
Figure 1.
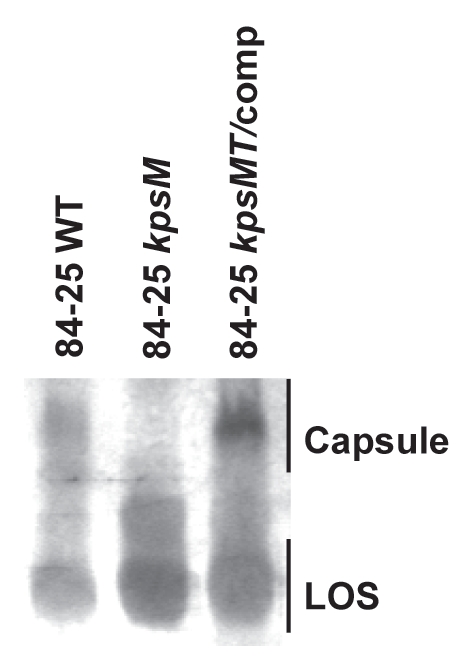
Analysis of capsule and LOS expression in a systemic C. jejuni strain. Whole cell proteinase K-digested lysates of wild-type C. jejuni 84-25 (84-25 WT), capsule mutant (84-25 kpsM), and the complement (84-25 kpsMT/comp) were separated on 12% SDS-PAGE and stained with 0.5% Alcian Blue to reveal capsular polysaccharides and with silver to stain LOS.
In silico analysis of LOS biosynthetic loci.
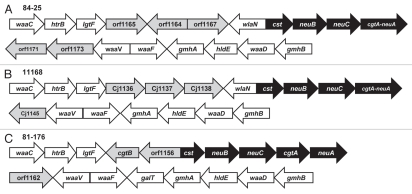
To study the contribution of the LOS core structures of diarrheal and systemic strains to resistance to host defenses, we first performed an in silico analysis of the LOS biosynthetic loci. We found that strain 84-25 and NCTC 11168 share greater than 98% average identity among homologous glycosyltransferases and sialic acid synthesis genes (Fig. 2). Although orf1164 of strain 84-25 is present on the minus strand, there was 100% identity between CJ1137 (strain 11168) and orf11644, suggesting they are orthologs. Therefore, strain 84-25 possesses a class C LOS locus,32,33 and likely expresses an inner-core LOS that is nearly identical to the diarrheal strain 11168.34 The genomic features that distinguish strains 84-25 and 81-176 include the presence of galT in 81-176 and the presence of wlaN in strain 84-25. We noted that orf1156 in strain 81-176 showed partial identity to the 5′ region of wlaN, however there was no corresponding 3′ region present for it to be considered an ortholog by ClustalX alignment. However, both galT and wlaN attach carbohydrate moieties to the outer-core region of LOS, whereas the inner LOS cores of the diarrheal strains and the systemic strain 84-25 are likely to be nearly identical, based on published structures.9,34
Figure 2.
Schematic diagram of the LOS biosynthetic loci. C. jejuni strain, 84-25 (A) and 1116868 (B), and 81-17669 (C), were analyzed in silico to determine homology within the LOS biosynthetic loci. The nomenclature reflects the open reading frame annotations of the respective strains, except where homology has been noted in the NCBI records (i.e., as in waaC and hldE). Pair-wise alignment revealed average identity of 98–99% among homologous glycosyltransferase (white arrows) and sialic acid synthesis genes (black arrows). Hypothetical proteins are shaded in grey. In strains 84-25 and 11168, the cgtA and neuA genes occur as an in-frame fusion ORF.9,32,70
Characterization of the LOS of the C. jejuni 84-25 waaF and 84-19 waaF mutants.
The LOS molecules produced by wild-type strains 84-25 and 84-19 and their waaF mutants were characterized by Tricine-PAGE. As expected,35 silver staining revealed that the LOS produced by the waaF mutant strains was truncated compared to that produced by their respective wild-type cells (Fig. 3). Complementation of the 84-25 mutant restored expression of full-length LOS. The results demonstrate that disruption of waaF in C. jejuni 84-25 and 84-19 leads to expression of a truncated LOS, which can be restored to full-length in strain 84-25 by complementation of waaF in the hipO locus.
Figure 3.
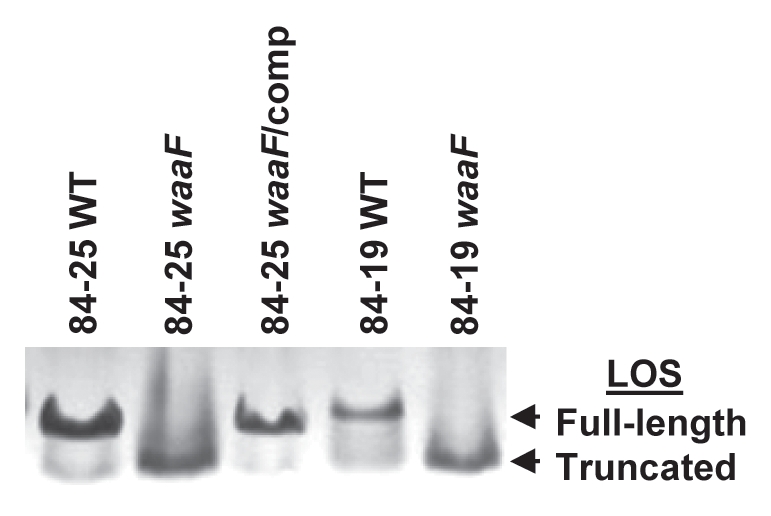
Analysis of LOS expression in C. jejuni strains. Bacterial lysates of the strains in Figure 1 and wild-type C. jejuni 84-19 (84-19 WT) and the LOS mutant (84-19 waaF) were resolved by 15% Tricine SDS-PAGE and silver-stained to reveal LOS.
Effect of LOS truncation and capsule loss on resistance to antimicrobial agents.
To cause illness, enteric bacteria must survive exposure to host mucosal innate defenses. CAPs play a major role in defending mucosal surfaces from pathogens,36 some of which have developed strategies to overcome these innate defenses such as encapsulation and/or production of full-length LPS or LOS.15,16,36,37 To test the hypothesis that such bacterial phenotypes would contribute to Campylobacter resistance to CAPs, we first tested the antimicrobial property of polymyxin B, a cyclic, lipid A-interactive peptide antibiotic because it is a model compound for assessing resistance to CAPs.38 Using an agar-based assay, the systemic C. jejuni strains 84-19 and 84-25 were two- to ten-fold more resistant to polymyxin B compared to diarrheal strains 81-176 and 79-193 (Table 3). Of the systemic strains, their corresponding LOS-truncation mutants showed four- to six-fold increased sensitivity to polymyxin B, as indicated by their lower MICs (p < 0.005). These data are consistent with findings by others using the diarrheal strain 81-176 and LOS truncation mutants.24 Complementation of waaF in strain 84-25 restored most polymyxin B resistance. Disruption of capsule or LOS expression did not affect susceptibility of mutants to streptomycin or nalidixic acid, which kill by inhibition of protein synthesis and DNA replication, respectively. Furthermore, polymyxin B sensitivity was unchanged in the capsule mutant of 84-25 compared to the wild-type strain, suggesting that this surface constituent does not play a major role in polymyxin B resistance.
Table 3.
Susceptibility of C. jejuni strains to antimicrobial agents
| Strain | MIC (µg/ml)a | ||
| Polymyxin B | Streptomycin | Nalidixic acid | |
| 79-193 | 10 | NDb | ND |
| 81-176 | 3 | ND | ND |
| 84-25 | 30 | 5 | 2 |
| 84-25 kpsM | 30 | 5 | 2 |
| 84-25 kpsM/comp | 30 | ND | ND |
| 84-25 waaF | 5c | 5 | 2 |
| 84-25 waaF/comp | 20c | 5 | 2 |
| 84-19 | 20d | 5 | 2 |
| 84-19 waaF | 5d | 5 | 2 |
Growth is shown as measured in agar dilution MIC assays, using an inoculum of 107 C. jejuni cells/ml. Results are the mean of two or more determinations of four replicates each.
ND not determined.
Statistically significant differences (p < 0.005) using the Student's two-tailed t-test.
Effect of LOS truncation on susceptibility to host CAPs.
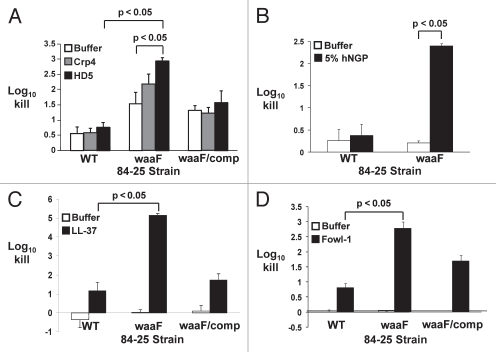
Given our finding that full-length LOS confers resistance against polymyxin B-mediated bacterial killing (Table 3), we hypothesized that LOS would similarly provide protection against CAP-dependent killing. Alpha defensins are a class of CAPs specifically secreted by Paneth cells into the lumen of the small intestine in response to bacterial surface structures,39 and likely would be the first and most potent intestine-derived CAPs to engage bile-resistant C. jejuni. Whereas wild-type C. jejuni 84-25 was relatively resistant to human α-defensin-5 and its murine homolog Crp4,40 the LOS mutant showed 2–3 log10 increased killing by Crp4 and human α-defensin-5 (Fig. 4A; p < 0.05 for the latter compared to buffer alone). As with polymyxin B, these data are consistent with findings by others using the diarrheal strain 81-176 and LOS truncation mutants.24 Complementation restored survival to near-wild-type levels. These data indicate that the distal LOS afforded protection against these CAPs.
Figure 4.
Killing of C. jejuni strains by human neutrophil granule extract and cationic antimicrobial peptides. The wild-type strain of C. jejuni 84-25 (WT), the LOS mutant (waaF) or the complement (waaF/comp) were incubated with cationic antimicrobial peptides (HD-5, Crp4, LL-37, Fowl-1; black bars), human neutrophil granule protein extract (hNGP; black bars) or buffer (white bars). Data are expressed as the log10 kill. (A) Wild-type C. jejuni strain 84-25 (WT), the LOS mutant (waaF) or the complement (waaF/comp) were incubated with the murine α-defensin homolog cryptdin-4 (Crp4), human α-defensin-5 (HD-5) (both 10 µg/ml) or buffer × 120 min. (B) C. jejuni 84-25 (WT) or the LOS mutant (waaF) were incubated with 5% hNGP or buffer × 30 min. (C) The strains in (A) were incubated with the human cathelicidin LL-37 (10 µg/ml) or buffer × 30 min. (D) The strains in (A) were incubated with the chicken cathelicidin homolog fowlicidin-1 (10 µg/ml; fowl-1) or buffer × 60 min. Data are the mean ± SD of at least two replicates performed 3–7 times. Significance (p < 0.05) was determined by student's two-tailed t-test.
Effect of LOS truncation on susceptibility to neutrophil-derived CAPs.
Bacteria that evade intestinal lumenal defenses may next invade the mucosa and gain systemic access, where they are exposed to additional cellular and humoral elements of the innate immune system. A key component of the cellular arm of innate immunity includes neutrophils in humans and heterophils in chickens. Neutrophils in humans are the major source of cathelicidins such as LL-37,41 and in chickens, the heterophil-derived cathelicidin homolog fowlicidin-1.42 To counteract these host defenses, some microbes have evolved surface-dependent virulence strategies; for example, in Neisseria meningitidis, LOS limits the access of the CAP LL-37 to susceptible hydrophobic membranes and mitigates killing compared to an LOS-deficient strain.43 Therefore, as a first step towards determining whether LOS plays a similar role in C. jejuni, we assessed the susceptibility of wild-type and LOS-truncated C. jejuni 84-25 measured the antimicrobial activity of human neutrophil granule protein (hNGP) extract, a complex mixture that contains, in addition to defensins, the majority of the host's cathelicidins and BPI.15,21,44 We found that the 84-25 waaF mutant was >100-fold more sensitive than the wild-type cells to hNGP (p < 0.05) (Fig. 4B). Based on this finding, we next determined whether the distal LOS protected C. jejuni from specific CAPs from humans and other species. The human CAP cathelicidin LL-37 caused >10-fold increased killing of the LOS-truncated strain of 84-25 versus the wild-type strain (Fig. 4C). Similarly, the LOS mutant showed ∼3 log10-fold increased susceptibility to the chicken cathelicidin homolog fowlicidin-1 (p < 0.05) compared to the wild-type strain (Fig. 4D). Complementation of the waaF gene conferred near-complete restoration of resistance to these cathelicidins (p < 0.05) (waaF/comp, Fig. 4C and D). Thus, similar to findings for the diarrheal strain 81-176 shown by others,24 full-length LOS provided considerable protection against the CAP constituents of innate immunity for these systemic C. jejuni isolates.
Susceptibility of diarrheal and systemic strains to neutrophil-derived rBPI-21 and effect of LOS truncation.
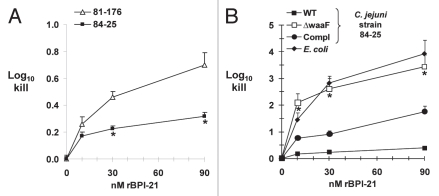
In addition to defensins and cathelicidins, neutrophils are the major source of the cationic protein BPI, which, like other CAPs, exerts selective activity against Gram-negative bacteria via its binding affinity for the lipid A portion of LPS/LOS.17 Since all of the antimicrobial activity of BPI is contained within its N-terminal half,45 we used this 21 kDa fragment in recombinant form (rBPI-21) to compare the susceptibility of a diarrheal strain (81-176) and our systemic strain 84-25 to BPI-mediated killing. Our positive control for BPI-21 antimicrobial activity was E. coli strain K1/r, known to be susceptible to BPI-mediated killing46 and which showed 1–4 log10 killing under these conditions (Fig. 5B). When we compared killing of the C. jejuni diarrheal and systemic strains at the highest BPI dose, we found 0.4 log10 increased killing of the diarrheal strain (p < 0.0001); the difference also was significant at the 30 nM dose (Fig. 5A). To measure the contribution of the distal LOS to the resistance of strain 84-25 to BPI-mediated killing, we next measured the effect of LOS truncation. We found dose-dependent, 2–3 log10 increased killing of the LOS mutant compared to the wild-type strain (p < 0.005), a phenotype partially restored by complementation of waaF (Fig. 5B). These data are consistent with our data showing increased sensitivity of the LOS mutant to human neutrophil granule protein extract and CAPs (Fig. 4). Overall, these results are consistent with studies in the E. coli showing that LPS chain length determines susceptibility to BPI-dependent killing,47,48 and indicate that the distal LOS of strain 84-25 contributes to protection against both lumenal and systemic CAPs.
Figure 5.
Killing of C. jejuni strains by the recombinant N-terminal fragment of human Bactericidal/Permeability-Increasing Protein (rBPI-21). (A) The C. jejuni systemic strain 84-25 or the diarrheal strain 81-176 were incubated with increasing doses of rBPI-21 or buffer as indicated. *p < 0.0001 vs. the diarrheal strain 81-176. (B) The wild-type strain of C. jejuni 84-25 (WT), the LOS mutant (waaF), the complemented strain (waaF/comp) or the control strain E. coli K1/r were incubated with rBPI-21 as in (A). All data are the mean + SD. 3–8 experiments performed in duplicate to quadruplicate. *p < 0.005 for C. jejuni waaF vs. WT or complement.
Effect of LOS truncation and capsule loss on complement-mediated bacterial killing by serum.
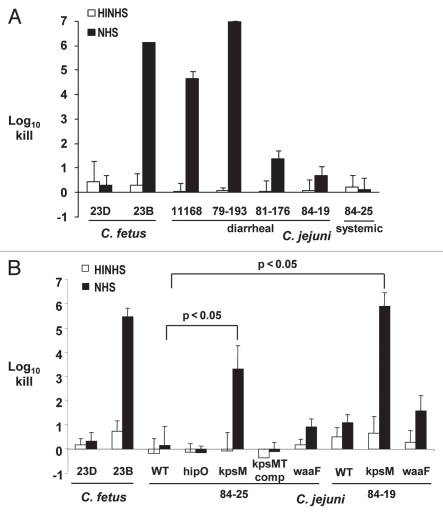
The bactericidal activity of complement present in serum is an important innate defense against bacteria that gain access to the vascular compartment. The three diarrheal strains, 11168, 79-193 and 81-176, were substantially more susceptible to human serum, with greater than 1 to 7 log10 increased killing compared to the two systemic strains 84-19 and 84-25 (p < 0.02) (Fig. 6A). Since removal of sialic acid residues from the distal LOS in C. jejuni strains 81-176 and MSC57360 increased its susceptibility to the bactericidal activities in normal human serum (NHS),8,9 we asked whether the distal LOS may play a similar role in systemic C. jejuni strains. However, there were no significant differences between wild-type 84-19 and 84-25 and their respective isogenic waaF mutants, lacking the outer-core of LOS (Fig. 6B). Next, to assess the role of capsule in mediating the relative serum-resistance of the systemic strains, we examined survival of the wild-type and capsule mutants of the systemic strains in the presence of NHS (Fig. 6B). Compared to the wild-type strains, the capsule mutants of 84-19 and 84-25 showed 4–5 log10 increased serum-dependent killing. Complementation of the 84-25 mutant with kpsMT in trans in hipO restored the serum-resistant phenotype of this strain. As a control, insertion of a cat cassette into hipO showed no loss of serum resistance compared with wild-type. In summary, these results show that while capsule contributed substantially to serum resistance of the systemic strains, LOS chain length did not.
Figure 6.
Complement-dependent killing of Campylobacter strains by normal human serum. The indicated Campylobacter strains were incubated with 10% serum (NHS, normal human serum; black bars) or serum previously heated to inactivate complement (HINHS, heat-inactivated NHS; white bars). C. fetus strains 23B and 23D are the serum-sensitive and -resistant controls, respectively. (A) Susceptibility of C. jejuni diarrheal (11168, 79–193 and 81–176) and systemic strains (84-25 and 84-19) to 10% NHS versus control (HINHS). (B) Susceptibility of kpsM and waaF mutants in C. jejuni systemic strain backgrounds (84-25 and 84-19). Bars represent the mean + SD for at least three replicate experiments. Brackets indicate statistically significant differences in survival between wild-type and kpsM mutants (p < 0.05) using the Student's two-tailed t-test.
Discussion
In this work, we sought to expand the understanding of the host-microbe interactions that affect the pathogenesis of C. jejuni, an important but under-studied human pathogen. We focused on the interplay between components of the host's innate immune system and C. jejuni's oligosaccharide surface structures, using a systemic (84-25) isolate. We hypothesized that this virulent strain might possess unique surface characteristics that assisted its apparent ability to evade not only mucosal/lumenal defenses (i.e., CAPs) but also systemic defenses such as complement and the neutrophil-derived antimicrobial protein BPI. With regard to LOS, in silico analysis (Fig. 2) suggested that strain 84-25 likely expresses an LOS inner-core structure similar to the diarrheal strains we tested. Based on this preliminary evidence, truncations of the LOS outer-core might therefore provide insights into the basis for altered susceptibility to innate defenses.
To address these questions, we constructed isogenic capsule and LOS mutants and their respective complements (Figs. 1 and 3). While complementation of C. jejuni has been challenging since many strains are refractory to the introduction of exogenous genes probably due to restriction barriers,49–51 we have devised a chromosomal approach that overcomes limitations associated with shuttle vectors or previously published methods8,11,28–30 through novel use of the hipO locus. Compared to using one of the ribosomal RNA loci (rpsL) for complementation,28 the single-copy hipO locus allows precise identification of the chromosomal location of the exogenous gene cassette. In addition, the hipO locus is not as prone to recombination as are the multiple rpsL loci.28 Finally, the ubiquity of the hipO locus27 provides a complementation site for nearly all C. jejuni strains compared to loci found only in some strains such as the arylsulfatase locus.29
Introduction of the kpsMT genes into the non-essential hipO locus on the C. jejuni chromosome yielded partial restoration of capsule and full-length LOS expression. Such partial complementation may have occurred if regulation of waaF and kpsMT in the hipO locus was not identical to that in their native loci, perhaps leading to diminished transcription. Nonetheless, the degree of functional complementation that we could achieve facilitates manipulation of this important microorganism that has low genetic tractability in general.
To assess the contribution of capsule and full-length LOS to resistance to innate immune defenses, we measured the bacterial killing of wild-type, mutant and complemented strains of the systemic strain 84-25 after exposure to cationic antimicrobial peptides and a protein, rBPI-21, examples of innate immune defenses that C. jejuni would encounter during host infection. As an initial approach to testing C. jejuni sensitivity to CAPs, we measured its sensitivity to polymyxin B, a clinically useful antibiotic that also is a cationic, lipid A-interactive peptide (Table 3). Because polymyxin B shares these attributes with endogenous CAPs, it may be used as an inexpensive and easily obtained surrogate marker for CAP sensitivity. The four- to six-fold increased polymyxin B susceptibility of two, LOS-truncated systemic strains (84-25 and 84-19) relative to the isogenic wild-type strains (Table 3) supports the hypothesis that the distal LOS limited access to lipid A, the target of polymyxin B bioactivity.52 The equal susceptibility of the wild-type strain and its acapsular mutant suggests that the sugars present in the capsule do not provide similar protection from polymyxin B.
Extending this observation to naturally-occurring CAPs produced by Paneth cells of the intestinal epithelium, we found that both the human α-defensin and its murine homolog Crp4 (Fig. 4A) produced substantially greater killing of the LOS truncated mutant compared to the wild-type strain. In these experiments, the ∼1 log10 excess killing of the LOS mutant in the presence of buffer alone likely reflects the more fragile nature of this strain which became manifest over the extended time course (120 min) of these experiments. Nonetheless, the approximately ten-fold increased killing of the LOS mutant by human α-defensin-5 was significantly greater (p < 0.05) compared to buffer alone (Fig. 4A). Overall, our data showing that the LOS truncated mutant had increased susceptibility to killing by α-defensins (Fig. 4A) compared to the wild-type strain supports the hypothesis that sugars present in the distal LOS confer a level of protection against mucosal innate immune defenses.
C. jejuni strains that withstand these defenses have the opportunity to gain access to the vascular space and cause systemic infection.12 Among the multiple innate immune effectors present in blood, neutrophils and complement are key cellular and soluble components, since when they are present in insufficient levels or function aberrantly, therefore serious clinical sequelae.23,53,54 After exposure to a mixture of neutrophil granule-derived peptides and proteins (hNGP), the enhanced killing of the LOS-truncated mutant compared to the wild-type strain indicates that full-length LOS is necessary for protection against hNGP (Fig. 4B) This result was particularly striking given the low concentration of hNGP extract used. Since neutrophils are the major source of the cathelicidin family of CAPs,41 we also measured bacterial survival after exposure to LL-37 (Fig. 4C). The greater than 1,000-fold difference in killing between the LOS-truncated mutant and both the wild-type and complemented strains suggests that the distal LOS sugars are an impediment to the binding of this CAP to lipid A, thereby providing protection against CAP-dependent killing. The similar pattern of results obtained with the chicken cathelicidin homolog fowlicidin-1 (Fig. 4D) indicates that C. jejuni uses the same strategy to evade poultry innate immune defenses. Although our results with human and chicken cathelicidin were similar, further study of poultry innate immunity may reveal differences that underlie why C. jejuni is a commensal in poultry and a pathogen in humans.
In addition to being the major source of the host's cathelicidins, neutrophils are also the primary source of BPI, which is lethal to most strains of Gram-negative bacteria via its high affinity for the lipid A portion of LPS.45,55 BPI accumulates to levels as high as 1 ug/ml in sites of inflammation and infection.56–58 Rough (i.e., short-chain) LPS chemotypes are relatively more sensitive to BPI-mediated killing than are bacteria expressing smooth or long-chain LPS, although the smaller, N-terminal recombinant fragment represented by rBPI-21 seems less subject to this apparent steric hindrance.47 rBPI-21 dose-dependent increased killing of the diarrheal strain versus the systemic strain 84-25 (Fig. 5A) suggests that differences in the surface structures of these two strains contribute to their disparate susceptibility to innate defenses. Our finding that BPI mediated increased, potent and dose-dependent killing of the LOS truncated mutant versus the wild-type strain is consistent with the results obtained with hNGP and CAPs (Fig. 5B) indicating that C. jejuni 84-25 requires expression of distal LOS residues to evade the antibacterial effect of CAPs and BPI. Whether or not this effect is due to steric hindrance imparted by the distal LOS, involves binding of CAPs/BPI to sialic acid residues in the distal LOS region and away from their targets on lipid A, differences in other surface structures including capsular composition, or a combination of these factors, is unknown. Our in silico analyses of the LOS biosynthetic loci suggest that the inner-core LOS of the diarrheal strains 11168 and 81-176 are identical, and that the outer-cores are similar. Since waaF mutation would lead to loss of the outercore sugar residues including negatively-charged sialic acid, it is intriguing to speculate that sialic acid may act as a charge decoy leading to binding of CAPs and BPI at sites distant from lipid A on LOS. However, the mechanism of relative BPI-resistance afforded by distal LOS sugar residues in C. jejuni requires further study.
Complement-mediated bacterial killing is a key component of the humoral arm of innate immunity necessary to control systemic infection from mucosal sources. Encapsulated bacteria thwart the antibacterial effect of complement by preventing deposition of the membrane attack complex on bacterial membranes.59 That two systemic C. jejuni strains (84-25 and 84-19) were more resistant to complement-mediated killing compared to diarrheal strains (Fig. 6A) suggests that the capsules of these strains may have contributed to both their survival in the vascular compartment and ability to cause systemic disease. Although strains 11168 and 84-25 are both of the HS2 Penner serotype, their markedly different serum sensitivities may suggest that substituent modifications,60,61 which also may confer resistance to antimicrobial peptides, may not be sufficiently antigenic to be distinguished by Penner anti-sera.
Next, capsule mutants of both systemic strains were >1,000-fold more sensitive to complement-mediated killing compared to their respective wild-type strains (Fig. 6B). Interestingly, the LOS truncated mutants of C. jejuni 84-25 and 84-19 did not show increased susceptibility to complement-mediated killing (Fig. 6B), whereas an LOS mutant of another C. jejuni strain (MSC57360) lacking distal sialic acid residues became serum-sensitive.8 We speculate that differences in the chemical composition and physical presentation of the sugar residues in LOS MSC57360 versus 84-25 and 84-19 may afford differing resistance to complement-mediated killing; this unanswered question requires further study. As speculated with regard to BPI susceptibility, the presence of sialic acid alone may not be as important as the spatial configuration of those residues in the context of capsule versus LOS. Finally, it is important to note that bacterial virulence factors other than capsule or full-length LOS (such as secretion systems/effector, outer membrane proteins, etc.,) also likely contribute to the phenotypic differences between systemic and diarrheal C. jejuni strains.
In summary, we report the construction of capsule and LOS mutant strains of two systemic C. jejuni strains, and the development of a chromosomal-based complementation strategy utilizing the non-essential hipO locus. Our results with these mutants show that the distal LOS of these systemic C. jejuni strains contribute to resistance to CAPs and BPI, whereas capsule contributes to complement resistance. The combination of the specific distal LOS and capsule composition of these systemic strains contribute to the phenotype of greater resistance to innate defenses versus diarrheal isolates, allowing their evasion of the usually effective lumenal, mucosal and systemic innate immune mechanisms.
Materials and Methods
Strains and growth conditions.
The bacterial strains used in this study (Table 1) were obtained from the NYU Campylobacter/Helicobacter strain collection in our laboratory. E. coli XL-1blue was used for construction and cloning of plasmids and was grown in Luria-Bertani media at 37°C. C. jejuni cells were routinely cultured on Trypticase soy agar (TSA) plates with 5% sheep blood for 2–3 d at 37°C in a 5% CO2 atmosphere. Campylobacter strains were stored at −80°C in Brucella broth supplemented with 15% glycerol.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or ref. |
| Strains | ||
| Campylobacter jejuni | ||
| 11168 | Wild-type strain, diarrheal isolate | 68 |
| 81-176 | Wild-type strain, diarrheal isolate | 69 |
| 79-193 | Wild-type strain, diarrheal isolate | 12 |
| 84-25 | Wild-type strain, HS:2, meningitis isolate | 12 |
| 84-25 kpsM | kpsM with aphA insertion | This work |
| 84-25 kpsM/comp | kpsM::aphA complemented with kpsMT in hipO locus | This work |
| 84-25 waaF | waaF with cat insertion | This work |
| 84-25 waaF/comp | waaF::cat complemented with waaF in hipO locus | This work |
| 84-19 | Wild-type strain, meningitis isolate | 12 |
| 84-19 kpsM | kpsM with aphA insertion | This work |
| 84-19 waaF | waaF with cat insertion | This work |
| Campylobacter fetus | ||
| 23D | Wild-type strain, serum-resistant | 70 |
| 23B | 23D, with sapA promoter deletion | 70 |
| Plasmids | ||
| pTK802 | kpsM::aphA in pGEMT-Easy | This work |
| pTK1106 | waaF::cat in pGEMT-Easy | This work |
| pTK1311D | hipO::waaF-aphA in pGEMT-Easy | This work |
| pTK1330 | hipO::kpsMT-cat in pGEMT-Easy | This work |
| pTK1301B | hipO::cat in pGEMT-Easy | This work |
| pTK1301D | hipO::aphA in pGEMT-Easy | This work |
| pTK1302B | hipO::kpsM-cat in pGEMT-Easy | This work |
Construction of kpsM mutants.
To construct the kpsM mutant, amplification of kpsM was performed with oligonucleotides cj1449F23 and kpsTR640 (Table 2) according to a standard protocol with designated primers, as described in reference 62. The 3 kb PCR product was gel-purified and ligated to pGEM-T-Easy, producing pTK800. Unique BamHI sites were introduced into kpsM contained within pTK800 through inverse PCR with primers kpsMF481bam and kpsMR320bam, and a kanamycin resistance (aphA) cassette flanked by BamHI recognition sites was ligated to generate pTK802. This suicide vector (pTK802) was used to transform cells of C. jejuni strains 84-19 and 84-25 by electroporation, as described in reference 50. Transformants were confirmed by PCR to have insertion of the aphA cassette in the kpsM locus.
Table 2.
Oligonucleotide primers used in this study
| Primer designation | Genomic locationa | Primer Sequence (5′->3′)b | ORF | Orientation |
| gmhaF3b | 1082341-1082367 | TAC CCA AAT CGC TAA AGT AGG TGA GC | gmha | R |
| wlasaR3 | 1080361-1080392 | AAA CTG CAG CAC TTA GCC CAA ACC GAC CAG C | waaV | F |
| waaFR490bam2 | 1081397-1081317 | CGC GGA TCC GCG TTC CAA AGC TTG CAC CAG GGT TGA G | waaF | R |
| waaFF550bam2 | 1081458-1081482 | CGC GGA TCC GCG ATG ATA TTT TAA TCT TTG GTG CAG G | waaF | F |
| cj0984F339 | 918174-918196 | TAA TCT TTT TGG CAT TGT AAGG | Cj0984 | F |
| cj0987F913 | 920041-920019 | TTA GTA TTG ATA CAG ATT TTT GC | Cj0987 | R |
| hipOR565bam | 919168-919190 | CGC GGA TCC GCG TTC AAT ACT ATA ACT ATC CGA AG | hipO | R |
| hipOF574mfeb | 919131-919110 | AAC AAT TGT TAA GGC AAA AGA TCC TAT TTA TG | hipO | F |
| waaFpr1bamF | 1081867-1081844 | CGC GGA TCC GCG TCA TAG ATG AGA GTT TTT AAG TAA | waaV | F |
| waaFpr1bamR | 1080854-1080882 | CGC GGA TCC GCG TTC CTA AAT TTT GTT AAA ATA ATA AAA AC | gmhA | R |
| cj0984F681 | 918516-918534 | ACT CAA AGA AAT TCA AAT C | Cj0984 | F |
| pBSC103Fbam | NAc | CGC GGA TCC GCG ATC GTA TGG AGC GGA CAA CG | cat | F |
| waaFF1 | 1080908-1080930 | ATG AAA ATT TTT ATA CAT CTT CC | waaF | F |
| cj1449F23 | 1388207-1388232 | TTA GAA TTT ATA AAA AAT GAG CAG C | Cj1449 | F |
| kpsTR640 | 1385624-1385652 | TAT TCC TTC ATC TAC ATC ATC ATA AAC C | kpsT | R |
| kpsMF481bam | 1387224-1387201 | CGG GGA TCC CGA TTA TTT GGC ATT TTG TGG AAC C | kpsM | F |
| kpsMR320bam | 1387385-1387410 | CGG GAT CCC GTT CTA GCA ATA AAT ACA TGT ATA GG | kpsM | R |
| Cj0984F646 | 918842-918501 | AAA GAG CTT TTA GCA AAC C | Cj0984 | F |
| kpsMpr1bamR | 1387775-1387825 | CGG GAT CCC GAA CAA TGC TTT AGG ACT TAG | Cj1449 | R |
| kpsER120bam | 1386144-1386157 | CGG GAT CCC GTG GTG CTG CAA TC | kpsE | F |
| kpsMF1 | 1387679-1387699 | ATG TTA AAT GTA ATT TAT GC | kpsM | F |
Location based on the sequence of C. jejuni strain 11168.
Restriction sites underlined; BamHI (GGATCC) and MfeI (CAA TTG).
Not applicable.
Construction of waaF mutants.
A C. jejuni mutant expressing a truncated LOS, waaF, encoding a heptosyltransferase necessary for the addition of the second heptose beyond KDO,35 was constructed using insertional mutagenesis. First, amplification of waaF was performed with oligonucleotides gmhaF3b and wlasaR3 (Table 2), as described in reference 35. PCRs were performed according to a standard protocol with designated primers (Table 2), as described in reference 35. The 3 kb waaF PCR product was gel-purified and ligated to pGEM-T-Easy, producing pTK1100. Unique BamHI sites were introduced into waaF contained within pTK1100 through inverse PCR with primers waaFR490bam2 and waaFF550bam2, and a chloramphenicol acetyltransferase (cat) cassette flanked by BamHI recognition sites was ligated to generate pTK1106. This suicide vector (pTK1106) was used to transform cells of C. jejuni strains 84-19 and 84-25 by electroporation, as described in reference 50. Transformants were examined by PCR using primers gmhaF3b and pBSC103Fbam to confirm insertion of cat into waaF. Retention of capsule expression in candidate LOS mutants was confirmed by Alcian Blue staining of Tricine-SDS PAGE bacterial lysates.
Complementation of kpsM and waaF mutants.
Chromosomal DNA from C. jejuni strain 84-25 was extracted with the Wizard Genomic DNA Purification kit (Promega, Madison, WI). PCR amplification of a 3 kb fragment containing hipO and 1 kb of flanking sequence was performed with oligonucleotides designated Cj0984F646 and Cj0987F913 (Table 2). The amplified product was cloned into pGEM-T Easy (Promega), yielding pTK1300. Unique BamHI and MfeI restriction sites were introduced into hipO by inverse PCR, using oligonucleotides hipOR565bam and hipOF574mfeB. The inverse PCR product was digested with BamHI and MfeI, and then aphA was inserted into hipO to create pTK1301-D. PCR primers waaFpr1bamF and waaFpr1bamR were used to amplify waaF with its 73 bp upstream (intergenic) sequence that includes its likely promoter. The PCR-amplified product was purified using a Qiagen PCR purification kit, then digested with BamHI, and the product cloned into pTK1301-D and screened by PCR with primers Cj0984F681 and Cj0987F913; the plasmid containing waaF was designated pTK1311-D. Cells of C. jejuni mutant strain 84-25 waaF were electroporated with pTK1311-D and transformants selected for growth on Brucella agar containing kanamycin and chloramphenicol. Hippuricase-negative transformants were confirmed by PCR using primers Cj0984F339 and waaFF1, to show introduction of waaF into the hipO locus by allelic exchange.
PCR primers kpsMpr1bamR and kpsER120bam were used to amplify the kpsMT genes; the resulting PCR product, which includes 126 bp of flanking sequence upstream of kpsM, was cloned into the BamHI site of pTK1301-B to produce pTK1330. The C. jejuni kpsM mutant was electroporated with the appropriate complementation plasmids, pTK1302B or pTK1330, and transformants were selected for growth on Brucella agar containing kanamycin and chloramphenicol. Hippuricase-negative transformants were confirmed by PCR for allelic exchange in the hipO locus with oligonucleotides Cj0984F339 and kpsMF1.
Detection of capsule and LOS expression.
Wild-type and mutant C. jejuni cells were harvested following 48 h growth as previously described, washed in PBS, standardized to an optical density at 600 nm (OD600) of 2.0, then solubilized in 200 µl of sample buffer (2% sodium dodecyl sulfate, 4% 2-mercaptoethanol, 10% glycerol, 1 M Tris-Cl, pH 6.8 and 10 mg bromophenol blue), at 100°C for 10 min, and then incubated with 60 µg proteinase K for 1 h at 60°C. The whole cell lysates were fractionated on 12% SDS-PAGE or 15% Tricine sodium-dodecyl sulfate polyacrylamide gels at 35 mA for 10 h. Prestained protein markers were used (Biorad, Hercules, CA), and bands resolved with dual silver and Alcian Blue stains, as described in reference 63.
Determination of growth inhibition by antibiotics.
To determine susceptibilities of C. jejuni to antimicrobial agents, the Steer's replicator agar dilution procedure was repeated three or more times as described in reference 64. Briefly, bacteria were suspended in PBS and distributed in 150 µl aliquots in microtiter plate wells. A Steer's replicator was used to inoculate bacteria in quadruplicate onto Brucella agar supplemented with indicated amounts of antibiotics. The antimicrobial concentrations (µg/ml) were as follows: polymyxin B (0.5–50), streptomycin (0.5–10), nalidixic acid (1–20). Plates were incubated at 37°C for 3 d, and examined for growth. The minimal inhibitory concentration (MIC) was defined as the lowest concentration of antibiotic that resulted in complete inhibition of growth. All determinations were done at least in duplicate.
Susceptibility of C. jejuni strains to CAPs and BPI.
Human neutrophil granule protein acid extract (hNGP) was obtained from Yvette Z. Weinrauch (NYU School of Medicine, New York, NY),21 LL-37 was obtained from Panatecs (Tubingen, Germany), and fowlicidin-1 was a kind gift from Guolong Zhang (Oklahoma State University, Stillwater, OK). The α-defensin murine cryptdin-4 (Crp4) and human β-defensin-5 were kindly provided by Andre J. Ouellette (University of California, Irvine, CA). A 21 kDa recombinant N-terminal fragment of the Bactericidal/Permeability-Increasing Protein (rBPI-21), which retains all the bactericidal and LPS-neutralizing bioactivities of the holoprotein,45 was kindly provided by Dr. Jerrold Weiss (University of Iowa School of Medicine, Iowa City, IA).
In assays testing CAPs and hNGP, C. jejuni strains were harvested after 48 h of growth on TSA plates at 37°C under microaerophilic conditions and resuspended in Hank's balanced salt solution (without calcium or magnesium, supplemented with 0.3% casamino acid and 10 mM HEPES pH 7.4) to a final concentration of 108/ml. Bacteria were incubated with hNGP (5% vol/vol), fowlicidin-1 (10 µg/ml), LL-37 (10 µg/ml) or diluent (20 mM sodium acetate, pH 4) in a total volume of 100 µl at 37°C. Fifty µl aliquots were serially diluted in phosphate buffered saline and inoculated on TSA plates. For the rBPI-21 assay, C. jejuni strains were harvested as described above and resuspended in Hank's balanced salt solution (without calcium or magnesium, supplemented with 0.5% pyrogen-free human serum albumin and 10 mM HEPES pH 7.4) to a concentration of 1.1 × 106/ml in a volume of 90 ul. Bacteria were incubated with 10 ul of rBPI-21 or diluent (10 mM sodium acetate, pH 4.0) in a final volume of 100 ul at 37°C × 1 h. For all assays, at the stated intervals, aliquots were serially diluted in PBS, inoculated on TSA plates, incubated at 37°C in 5% CO2 for three days, and the number of colony forming units (cfu) counted. Log10 kill was determined by subtracting the difference between the number of cfu before and after exposure to CAPs, hNGP, rBPI-21 and their respective diluents.65
Serum bactericidal assays.
Normal human serum (NHS) was obtained from donors who had tested negative for anti-C. jejuni antibodies by ELISA, as described in reference 66. Fresh serum from three donors was pooled, sterilized by passage through a 0.22 µm filter, and stored at −70°C.
C. jejuni strains were harvested after 48 h growth on TSA as previously described, washed in sterile saline and resuspended from 101/ml to 106/ml in Medium 199 with Hank's balanced salt solution (with 0.01% glutamine). From each dilution, 150 µl aliquots of bacteria were transferred in triplicate to microtiter wells. To each well, 50 µl was added of either 40% NHS in Medium 199, 40% heat-inactivated (56°C for 30 min) NHS (HINHS) or Medium 199 (buffer alone). When present, the final serum concentration was 10%. After 60 min of incubation, 50 µl aliquots from the wells were inoculated onto TSA plates. The plates were incubated at 37°C in 5% CO2 for four days, and colony forming units counted. Log10 kill was determined by subtracting the difference between the number of cells before and after exposure to NHS or HINHS.65 Capsule and LOS expression in strain 81-176 was confirmed by Alcian Blue and silver staining (Sup. Fig. 1).
In silico analyses of C. jejuni LOS loci.
Nucleotide sequences of C. jejuni strains 81-176 (NC_008787.1), 84-25 (NZ_AANT00000000.2) and 11168 (NC_002163.1) were obtained from NCBI Entrez genome (www.ncbi.nlm.nih.gov/projects/genome/?db=genome). Identification of orthologs was performed with the Basic Local Alignment Search Tool (BLAST) proGram for microbial genomes (www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) and confirmed with ClustalX 2.0.12 analysis.67
Statistical analyses.
The Student's t-test was used in comparisons of strain characteristics, with p value < 0.05 considered significant.
Acknowledgements
We are grateful to Yvette Z. Weinrauch and Andre J. Ouellette (University of California, Irvine) for advice and reagents. This work was supported in part by grant R01 AI24145 from the National Institutes of Health, by the Medical Research Service of the Department of Veterans Affairs, by the Diane Belfer Program in Microbial Ecology and by the Saperstein Medical Scholars Program.
Abbreviations
- BPI
the bactericidal/permeability-increasing protein
- CAPs
cationic antimicrobial peptides and proteins
- cfu
colony-forming units
- Crp4
cryptdin-4
- HINHS
heat-inactivated normal human serum
- hNGP
human neutrophil granule protein extract
- LPS
lipopolysaccharide
- LOS
lipooligosaccharide
- MIC
minimal inhibitory concentration
- NHS
normal human serum
- TSA
trypticase soy agar
- rBPI-21
twenty-one kilodalton fragment of recombinant BPI
Supplementary Material
References
- 1.Preliminary Foodnet data on the incidence of infection with pathogens transmitted commonly through food—10 states 2009. MMWR Morb Mortal Wkly Rep. 2010;59:418–422. [PubMed] [Google Scholar]
- 2.Blaser MJ, Allos BM, Campylobacter jejuni and related species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005. pp. 2548–2557. [Google Scholar]
- 3.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrauch Y. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect Immun. 2008;76:986–993. doi: 10.1128/IAI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilbauer M, Dorrell N, Boughan PK, Harris A, Wren BW, Klein NJ, et al. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun. 2005;73:7281–7289. doi: 10.1128/IAI.73.11.7281-7289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 6.Skirrow MB. Campylobacter enteritis: A “New” Disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerry P, Ewing CP, Hickey TE, Prendergast MM, Moran AP. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect Immun. 2000;68:6656–6662. doi: 10.1128/iai.68.12.6656-6662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, et al. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun. 2002;70:787–793. doi: 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanipes MI, Holder LC, Corcoran AT, Moran AP, Guerry P. A deep-rough mutant of Campylobacter jejuni 81-176 is noninvasive for intestinal epithelial cells. Infect Immun. 2004;72:2452–2455. doi: 10.1128/IAI.72.4.2452-2455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlyshev AV, Everest P, Linton D, Cawthraw S, Newell DG, Wren BW. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiol. 2004;150:1957–1964. doi: 10.1099/mic.0.26721-0. [DOI] [PubMed] [Google Scholar]
- 12.Blaser MJ, Perez GP, Smith PF, Patton C, Tenover FC, Lastovica AJ, et al. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: Host factors and strain characteristics. J Infect Dis. 1986;153:552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- 13.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23:115–120. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 14.Diffenbach CW, Tramont EC. Innate (general or nonspecific) host defense mechanisms. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005. pp. 34–42. [Google Scholar]
- 15.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 16.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20:407–431. [PubMed] [Google Scholar]
- 17.Weiss J. Bactericidal/Permeability-Increasing Protein (BPI) and Lipopolysaccharide-Binding Protein (LBP): Structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans. 2003;31:785–790. doi: 10.1042/bst0310785. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann L. Innate immunity and mucosal bacterial interactions in the intestine. Curr Opin Gastroenterol. 2004;20:82–88. doi: 10.1097/00001574-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 20.Zanetti M, Gennaro R, Romeo D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 21.Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978;253:2664–2672. [PubMed] [Google Scholar]
- 22.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Frank MM. Complement deficiencies. Pediatr Clin North Am. 2000;47:1339–1354. doi: 10.1016/s0031-3955(05)70274-1. [DOI] [PubMed] [Google Scholar]
- 24.Naito M, Frirdich E, Fields JA, Pryjma M, Li J, Cameron A, et al. Effects of sequential Campylobacter jejuni 81-176 lipooligosaccharide core truncations on biofilm formation, stress survival and pathogenesis. J Bacteriol. 2010;192:2182–2192. doi: 10.1128/JB.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 26.Hani EK, Chan VL. Expression and characterization of Campylobacter jejuni benzoylglycine amidohydrolase (hippuricase) gene in Escherichia coli. J Bacteriol. 1995;177:2396–2402. doi: 10.1128/jb.177.9.2396-2402.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slater ER, Owen RJ. Restriction fragment length polymorphism analysis shows that the hippuricase gene of Campylobacter jejuni is highly conserved. Lett Appl Microbiol. 1997;25:274–278. doi: 10.1046/j.1472-765x.1997.00218.x. [DOI] [PubMed] [Google Scholar]
- 28.Karlyshev AV, Wren BW. Development and application of an insertional system for gene delivery and expression in Campylobacter jejuni. Appl Environ Microbiol. 2005;71:4004–4013. doi: 10.1128/AEM.71.7.4004-4013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao R, Burr DH, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]
- 30.Yao R, Guerry P. Molecular cloning and site-specific mutagenesis of a gene involved in arylsulfatase production in Campylobacter jejuni. J Bacteriol. 1996;178:3335–3338. doi: 10.1128/jb.178.11.3335-3338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for penner serotype specificity. Mol Microbiol. 2000;35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert M, Karwaski MF, Bernatchez S, Young NM, Taboada E, Michniewicz J, et al. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J Biol Chem. 2002;277:327–337. doi: 10.1074/jbc.M108452200. [DOI] [PubMed] [Google Scholar]
- 33.Parker CT, Gilbert M, Yuki N, Endtz HP, Mandrell RE. Characterization of lipooligosaccharide-biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: Evidence of mosaic organizations. J Bacteriol. 2008;190:5681–5689. doi: 10.1128/JB.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. Michael F, Szymanski CM, Li J, Chan KH, Khieu NH, Larocque S, et al. The structures of the lipooligosaccharide and capsule polysaccharide of Campylobacter jejuni genome sequenced strain NCTC 11168. Eur J Biochem. 2002;269:5119–5136. doi: 10.1046/j.1432-1033.2002.03201.x. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield NJ, Moran AP, Millar LA, Prendergast MM, Ketley JM. Characterization of the Campylobacter jejuni heptosyltransferase II gene, waaf, provides genetic evidence that extracellular polysaccharide is lipid A core independent. J Bacteriol. 2002;184:2100–2107. doi: 10.1128/JB.184.8.2100-2107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganz T. Defensins: Antimicrobial peptides of vertebrates. C R Biol. 2004;327:539–549. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis. 1986;154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 38.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 39.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 40.Ouellette AJ. Iv. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am J Physiol. 1999;277:257–261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 41.Cowland JB, Johnsen AH, Borregaard N. hCap-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 42.Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281:2858–2867. doi: 10.1074/jbc.M507180200. [DOI] [PubMed] [Google Scholar]
- 43.Jones A, Georg M, Maudsdotter L, Jonsson AB. Endotoxin, capsule and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol. 2009;191:3861–3868. doi: 10.1128/JB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 45.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, et al. Human Bactericidal/Permeability-Increasing Protein and a recombinant NH2-terminal fragment cause killing of serum-resistant Gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Invest. 1992;90:1122–1130. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iovine NM, Elsbach P, Weiss J. An opsonic function of the neutrophil Bactericidal/Permeability-Increasing Protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci USA. 1997;94:10973–10978. doi: 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss J, Beckerdite-Quagliata S, Elsbach P. Resistance of Gram-negative bacteria to purified bactericidal leukocyte proteins: Relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980;65:619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss J, Hutzler M, Kao L. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil Bactericidal/Permeability-Increasing Protein. Infect Immun. 1986;51:594–599. doi: 10.1128/iai.51.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassenaar TM, Fry BN, van der Zeijst BA. Genetic manipulation of campylobacter: Evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 50.Miller JF, Dower WJ, Tompkins LS. High-voltage electroporation of bacteria: Genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci USA. 1988;85:856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labigne-Roussel A, Harel J, Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: Development of shuttle vectors for genetic analysis of Campylobacter jejuni. J Bacteriol. 1987;169:5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochem. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 53.Johnson RJ, Nolan C, Wang SP, Shelton WR, Blaser MJ. Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann Intern Med. 1984;100:832–834. doi: 10.7326/0003-4819-100-6-832. [DOI] [PubMed] [Google Scholar]
- 54.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 55.Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, et al. High-affinity binding of the Bactericidal/Permeability-Increasing Protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bokarewa MI, Jin T, Tarkowski A. Intraarticular release and accumulation of defensins and Bactericidal/Permeability-Increasing Protein in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1719–1724. [PubMed] [Google Scholar]
- 57.Opal SM, Palardy JE, Marra MN, Fisher CJ, Jr, McKelligon BM, Scott RW. Relative concentrations of endotoxin-binding proteins in body fluids during infection. Lancet. 1994;344:429–431. doi: 10.1016/s0140-6736(94)91767-1. [DOI] [PubMed] [Google Scholar]
- 58.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, et al. Extracellular accumulation of potently microbicidal Bactericidal/Permeability-Increasing Protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Invest. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffitt MC, Frank MM. Complement resistance in microbes. Springer Semin Immunopathol. 1994;15:327–344. doi: 10.1007/BF01837364. [DOI] [PubMed] [Google Scholar]
- 60.McNally DJ, Lamoureux MP, Karlyshev AV, Fiori LM, Li J, Thacker G, et al. Commonality and biosynthesis of the o-methyl phosphoramidate capsule modification in Campylobacter jejuni. J Biol Chem. 2007;282:28566–28576. doi: 10.1074/jbc.M704413200. [DOI] [PubMed] [Google Scholar]
- 61.McNally DJ, Jarrell HC, Khieu NH, Li J, Vinogradov E, Whitfield DM, et al. The HS:19 serostrain of Campylobacter jejuni has a hyaluronic acid-type capsular polysaccharide with a nonstoichiometric sorbose branch and o-methyl phosphoramidate group. FEBS J. 2006;273:3975–3989. doi: 10.1111/j.1742-4658.2006.05401.x. [DOI] [PubMed] [Google Scholar]
- 62.Takata T, Aras R, Tavakoli D, Ando T, Olivares AZ, Blaser MJ. Phenotypic and genotypic variation in methylases involved in type II restriction-modification systems in Helicobacter pylori. Nucleic Acids Res. 2002;30:2444–2452. doi: 10.1093/nar/30.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karlyshev AV, Wren BW. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using Alcian Blue dye. J Clin Microbiol. 2001;39:279–284. doi: 10.1128/JCM.39.1.279-284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeCross AJ, Marshall BJ, McCallum RW, Hoffman SR, Barrett LJ, Guerrant RL. Metronidazole susceptibility testing for Helicobacter pylori: Comparison of disk, broth and agar dilution methods and their clinical relevance. J Clin Microbiol. 1993;31:1971–1974. doi: 10.1128/jcm.31.8.1971-1974.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaser MJ, Smith PF, Kohler PF. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985;151:227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- 66.Blaser MJ, Duncan DJ. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect Immun. 1984;44:292–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 68.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 69.Kanipes MI, Tan X, Akelaitis A, Li J, Rockabrand D, Guerry P, et al. Genetic analysis of lipooligosaccharide core biosynthesis in Campylobacter jejuni 81-176. J Bacteriol. 2008;190:1568–1574. doi: 10.1128/JB.01696-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilbert M, Brisson JR, Karwaski MF, Michniewicz J, Cunningham AM, Wu Y, et al. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds and characterization of nanomole amounts by 600 mhz (1)h and (13)c NMR analysis. J Biol Chem. 2000;275:3896–3906. doi: 10.1074/jbc.275.6.3896. [DOI] [PubMed] [Google Scholar]
- 71.Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 72.Tummuru MK, Blaser MJ. Characterization of the Campylobacter fetus sapA promoter: Evidence that the sapA promoter is deleted in spontaneous mutant strains. J Bacteriol. 1992;174:5916–5922. doi: 10.1128/jb.174.18.5916-5922.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.