Abstract
An important part of the innate immune response of the nematode C. elegans to fungal infection is the rapid induction of antimicrobial peptide gene expression. One of these genes, nlp-29, is expressed at a low level in adults under normal conditions. Its expression is upregulated in the epidermis by infection with Drechmeria coniospora, but also by physical injury and by osmotic stress. For infection and wounding, the induction is dependent on a p38 MAP kinase cascade, but for osmotic stress, this pathway is not required. To characterize further the pathways that control the expression of nlp-29, we carried out a genetic screen for negative regulatory genes. We isolated a number of Peni (peptide expression no infection) mutants and cloned one. It corresponds to fasn-1, the nematode ortholog of vertebrate fatty acid synthase. We show here that a pathway involving fatty acid synthesis and the evolutionary conserved wnk-1 and gck-3/Ste20/GCK-VI kinases modulates nlp-29 expression in the C. elegans epidermis, independently of p38 MAPK signaling. The control of the antimicrobial peptide gene nlp-29 thus links different physiological processes, including fatty acid metabolism, osmoregulation, maintenance of epidermal integrity and the innate immune response to infection.
Key words: innate immunity, homeostasis, signalling, model organism, genetics
Introduction
The nematode Caenorhabditis elegans can come into contact with pathogens either at the surface of its intestinal epithelium following their ingestion, or when bacteria or fungi adhere to its cuticle.1,2 Examples of the latter include Yersinia spp. and Xenorhabdus nematophilum, two Gram-negative species that can form a biofilm on head of worms,3,4 the Gram-positive bacterium Microbacterium nematophilum, which adheres to a specific region in the tail,5 and Drechmeria coniospora, a fungus with spores that attach to the worm and then penetrate the cuticle and epidermis.6 Infection by D. coniospora provokes the rapid induction of genes encoding antimicrobial peptides (AMPs), including members of the nlp family.7,8 When its epidermis is damaged, C. elegans also expresses AMPs of the nlp family, such as nlp-29, as part of a wound healing response, which concomitantly involves cellular repair.9 The expression of nlp-29 following injury or infection depends on a PKC-p38 MAPK pathway, which acts cell automonously in the epidermis.10
Mutants in some cuticular or epidermal proteins, including dpy-9 and osm-11, also exhibit increased expression of nlp-29.8 These two mutants have elevated levels of gpdh-1, which encodes the rate-limiting enzyme for the biosynthesis of the osmoprotectant glycerol and are resistant to osmotic stress.11,12 Surprisingly, it has recently been reported that this resistance can be decoupled from gpdh-1 expression. Thus, when the nematode Wnk or Ste20/GCK kinase genes (wnk-1 and gck-3, respectively) are inactivated in a dpy-9 or osm-11 background, osmotic stress resistance returns to normal, while gpdh-1 expression remains high.13 Further, both gpdh-1 and nlp-29 expression are upregulated upon exposure to high salt.8,11 While the upregulation of nlp-29 under these conditions is independent of the PKC-p38 MAPK pathway, abrogation of the function of the p38 MAPK pmk-1 in an osm-11 mutant blocks the elevated nlp-29 expression but does not affect acute osmotic resistance.8 Finally, death-associated protein kinase (dapk-1) mutants display both epidermal abnormalities and a high constitutive expression of nlp-29. The high level of nlp-29 expression is independent of the morphological defects, but is dependent upon the p38 MAPK pathway.14
The nlp-29 AMP gene is thus subject to a complex regulation. It links different physiological processes, osmoregulation, osmoresistance, epidermal integrity and the innate immune responses to infection and injury. In the present study, we used a genetic approach to try to tease apart these processes, and provide evidence that a pathway involving fatty acid synthesis and the evolutionary conserved wnk-1/WNK and gck-3/Ste20/GCK-VI kinases modulates AMP gene expression in the C. elegans epidermis.
Results
A screen for aberrant regulation of AMP expression.
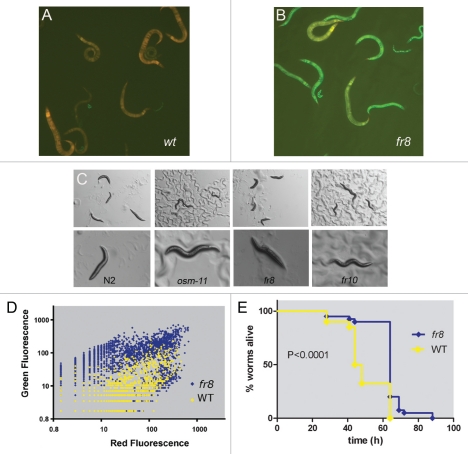
The majority of the nlp AMP genes, including nlp-29, are expressed at a low level under standard culture conditions.8 Upon infection, their expression is upregulated, and this induction can be visualized in vivo using transgenic worms carrying suitable reporter constructs. With the aim of identifying negative regulators of the signaling pathway controlling AMP gene expression, we used a strain of C. elegans carrying an integrated pnlp-29::GFP reporter gene9 to screen for mutants that showed abnormally high constitutive GFP expression. From a pilot screen of 10,000 mutagenized haploid genomes, seven Peni (peptide expression no infection) mutant alleles, fr7-fr13, were obtained (Fig. 1A and B, results not shown).
Figure 1.
Characterization of Peni mutants. Uninfected transgenic worms carrying a pnlp-29::GFP reporter do not express high levels of GFP in the wild-type background (A) while in homozygous fr8 worms there is a strong constitutive expression (B). The transgenic worms also carry a pcol-12::dsRed reporter gene, which is expressed from the L2 stage onwards. Green and red fluorescence are visualized simultaneously. (C) The acute osmotic stress resistance (>15 minutes on NGM agar plates containing 500 mM NaCl) of wild type N2 worms is compared to osm-11(n1604), fr8 and fr10 mutants (left to right). Upper and lower panel shows low and high magnification of the worms. (D) Quantification of fluorescence in the wild-type (yellow) and fr8 (blue) mutant background of a mixed stage population with the COPAS Biosort. Each dot represents an individual worm (n = 2536 and 3349 for WT and fr8, respectively). Red and green fluorescence are shown on an arbitrary logarithmic scale. (E) Survival of fr8 versus WT control worms (both containing the integrated frIs7 reporter transgene) after infection with D. coniospora at 25°C. Data are representative of three independent experiments [n > 100; p < 0.0001, log-rank (Mantel-Cox) test].
As mentioned above, as well as being induced by infection, pnlp-29::GFP can also be upregulated by hypertonic stress, such as upon exposure to concentrations of NaCl above 150 mM, and also in some mutant strains with defects in osmo-regulation, including osm-11.8 We therefore tested whether any of our newly isolated mutants were severely defective in osmo-regulation. Upon exposure to 500 mM NaCl, wild-type worms shrink due to water loss and are rapidly immobilized, whereas osmotic stress resistant (Osr) mutants like osm-11 show no body fluid loss and move normally.12 Two of the Peni mutants, fr10 and fr13, displayed an Osr phenotype; fr13 also displayed a Dpy phenotype. The remaining mutants behaved and looked wild-type (Fig. 1C and results not shown). Osmotic stress is normally associated with an increased expression of the rate-limiting enzyme for glycerol production, gpdh-1, and this can be followed using a GFP reporter under the control of the gpdh-1 promoter. Certain Osr mutants, including osm-11, show a high constitutive expression of a pgpdh-1::GFP reporter, even in conditions of normal osmolarity. When this reporter gene was transferred into the different Peni mutants, a markedly increased fluorescence was observed in the strains homozygous for fr10 and fr13, but not for fr7, fr8, fr9 or fr12 (results not shown). Taken together, these results suggest that fr10 and fr13 have a severe defect in osmo-regulation, which leads to increased pnlp-29::GFP expression; they were not characterized further.
The fr8 mutant displays other phenotypes.
To investigate whether the effect on nlp-29 regulation seen in the Peni mutants extended to a second class of antimicrobial peptide genes, we looked at the expression of a Caenacin (cnc) gene. We chose the gene cnc-2 as it is well characterized and its expression is only mildly influenced by changes in osmolarity.15 We therefore transferred by mating a pcnc-2::GFP reporter transgene into the different mutant backgrounds. An increased constitutive expression of pcnc-2::GFP was only seen in the fr8 mutant background (Suppl. Fig. 1, and results not shown); fr8 was therefore chosen for detailed analysis.
Although roughly 40% of eggs from fr8 homozygous mutants failed to hatch (Suppl. Fig. 2A), there were no obvious developmental defects in the hatching larvae and adults exhibited no gross morphological defects. The arrest in embryonic development was correlated with an abnormal permeability of the eggshell for the lipophilic dye Nile red (Suppl. Fig. 2B and C). As this is used as a criterion for solute permeability, and can reflect a problem in the eggshell or cell membrane, this suggests that although the fr8 mutant does not have a pronounced osmo-regulatory defect as an adult, the structural integrity in a fraction of eggs is compromised.
We then characterized the age-dependent change in pnlp-29::GFP expression in wild-type and mutant animals, using the COPAS Biosort. This quantitative analysis demonstrated a clear difference in green fluorescence between the two strains, which was most evident from L2 to young adult stages, when it was 10–40-fold higher in the mutants. In older mutant worms, the green fluorescence declined (Fig. 1D, Suppl. Fig. 3A and B). The reporter strains used also carry a gene for the red fluorescent protein dsRed, under the control of a constitutive epidermal promoter (pcol-12::dsRed). In the fr8 background, in L2 and L3 larvae there was an increase in the value of red fluorescence measured in the mutant worms (<5-fold compared to wild-type), but this difference disappeared in older worms, indicating that the observed increases in pnlp-29::GFP and pcnc-2::GFP expression were likely not due to a general mis-regulation of transgene expression (results not shown). Despite the constitutively high level of expression of pnlp-29::GFP, infection of fr8 mutant worms provoked a clear induction of reporter gene expression (Suppl. Fig. 4), showing that the signal transduction cascade triggered by infection was intact. Importantly, the fr8 mutant worms also showed a significantly higher resistance to infection with D. coniospora compared to wildtype (Fig. 1E).
fr8 is a mutant allele of the fatty acid synthase gene fasn-1.
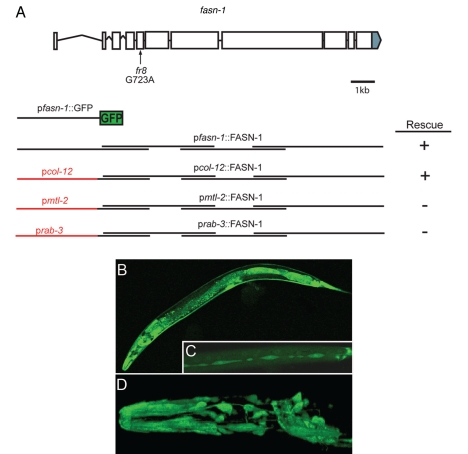
To identify the molecular lesion in the fr8 mutant, we first performed conventional SNP mapping, and placed fr8 in a genomic region of 0.2 cM on the right arm of chromosome I. Microinjection and transformation rescue were used to narrow down the location of fr8 to one gene, fasn-1. fasn-1 encodes a putative fatty acid synthase, orthologous to human FASN. Sequencing revealed a single nucleotide change in fr8 (G723A) (Fig. 2A), predicted to change a methionine to an isoleucine (M241I) in a highly conserved region of the protein (Suppl. Fig. 5). fasn-1(RNAi) provoked increased expression of both pnlp-29::GFP and pcnc-2::GFP reporter genes (Suppl. Fig. 6 and results not shown), further confirming the identity of the gene. As previously reported,16–18 we found that fasn-1 is an essential gene; abrogation of its function by RNAi led to embryonic and larval lethality. In addition, blocking fasn-1 function by RNAi in a transgenic strain carrying a pgpdh-1::GFP reporter gene, provoked a high level of GFP fluorescence. While consistent with prior studies,11 the disparity between the results of RNAi and the observed phenotype of the fasn-1 mutant indicates that fasn-1(fr8) is a mild loss of function allele.
Figure 2.
fr8 is a mutation in the fatty acid synthase gene fasn-1. (A) Structure of the fasn-1 genomic locus. The location of the fr8 mutation is indicated with an arrow. Exons are shown as boxes, introns are represented as lines, the grey region shows the 3′UTR of fasn-1. Several constructs are shown below the gene structure, from top to bottom, respectively: the promoter region used for the GFP reporter construct (pfasn-1::GFP), the overlapping rescuing fragments, the rescuing fragments under the control of the tissue specific promoters of col-12, mtl-2 and rab-3. The length of these promoters is not to scale. (B–D) Confocal fluorescence images of pfasn-1::GFP transgenic worms illustrating expression in multiple neurons in the head and tail region and socket cells (B and D), intestine, spermatheca and epidermis (B) and seam cells (C). (E–G) Expression of fasn-1 in the epidermis is sufficient to rescue the Peni phenotype. Micrographs of fasn-1(fr8) worms carrying the frIs7 transgene with a second transgene driving expression of fasn-1 under the control of the epidermal col-12 promoter (E), of the intestinal mtl-2 promoter (F) and of neuronal rab-3 promoter (G).
The gene F32H2.6, which is 12 kb downstream of fasn-1, encodes a protein with high sequence similarity to the N-terminal region of fasn-1 (65% identity over 167 amino acids). The similarity extends to the nucleic acid level, with more than 75% identity over 500 nucleotides for the predicted transcripts, and stretches of up to 27 contiguous identical nucleotides. RNAi of F32H2.6 provokes the same pleiotropic phenotypes as fasn-1(RNAi), e.g., constitutive pgpdh-1::GFP expression, and embryonic and larval arrest.17 RNAi of F32H2.6 also provoked a marked increase in pnlp-29::GFP expression, i.e., a Peni phenotype (Suppl. Fig. 7). On the other hand, we found that an available deletion allele of F32H2.6, tm3581, predicted to be a molecular null, did not display a Peni phenotype. In all likelihood, therefore, the Peni phenotype observed with F32H2.6(RNAi) is a consequence of the concomitant interference of fasn-1 expression.
fasn-1 functions cell-autonomously in the epidermis of C. elegans.
To investigate which tissues and cells express fasn-1, we generated transgenic animals expressing a transcriptional reporter construct including more than 2 kb of upstream sequence and with the start of the second exon of fasn-1 fused to GFP (Fig. 2A). Expression was visible in the intestine, spermatheca, many head and tail neurons, in the main epidermal syncytium hyp7 and the seam cells (Fig. 2B–D). Specific expression of fasn-1 in the epidermis, driven by the col-12 promoter completely rescued the Peni phenotype of fasn-1(fr8), whereas expression of fasn-1 in the intestine and neurons, under the control of the mtl-2 and rab-3 promoters, respectively, gave no rescue (Fig. 2E–G). Thus, fasn-1 can act cell-autonomously in the epidermis to influence nlp-29 expression.
fasn-1(fr8) has a subtly altered fatty acid composition.
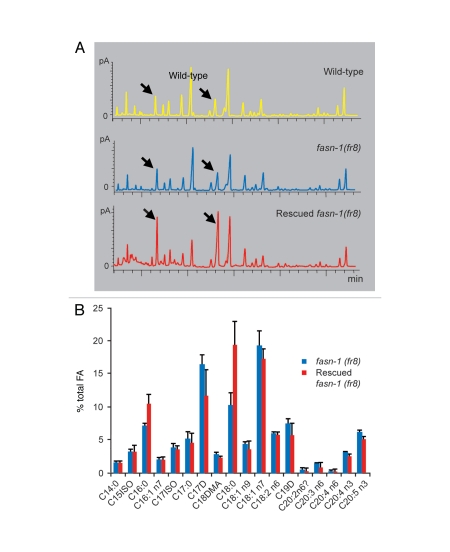
FASN-1 is a key enzyme involved in the de novo synthesis of fatty acids, elongating malonyl-CoA in a step wise fashion to generate unsaturated fatty acids (FA), mainly C16:0 (palmitic acid). We therefore wished to address the question of whether the fat content in fasn-1(fr8) mutants was altered, and so used gas chromatography as a precise assay. We looked specifically at the stages between L2 and young adult stage, when differences in pnlp-29::GFP expression are most marked between wild-type and mutant worms. When we compared wild-type and fasn-1(fr8) mutants animals, we observed little difference (Fig. 3A). We then compared the FA content in worms from a transgenic rescued strain with their age-matched non-transgenic fasn-1(fr8) siblings. There was a marginal, but not significant difference in C17D levels (p > 0.05, paired student's t test). This cyclo-FA comes mainly from the bacterial diet. Variations in C17D levels can reflect differences in the amount of bacteria inside the worms' intestines or in the efficiency of washing worms from plates. Compared to the fasn-1(fr8) worms, the rescued siblings did show significantly increased levels of C16:0 (palmitic acid), the main product of FASN-1, and its elongated form C18:0 (Fig. 3A and B), which is directly synthesized from C16:0 by the elongation enzyme ELO-2.19 This presumably reflects the overexpression of fasn-1 in the transgenic animals and the associated increase in fatty acid synthase activity.
Figure 3.
fasn-1 mutants carrying a rescuing extrachromosomal array have an altered FA composition. (A) Gas chromatograms showing the FA composition in wild type worms, as well as rescued fasn-1(fr8);frEx288 worms and their fasn-1(fr8) mutant siblings. Arrows point to the peaks corresponding to C16:0 and C18:0. The FA compositions in wild-type and fasn-1 mutants are comparable suggesting that a decrease in FASN-1 activity does not preferentially affect a specific type of FA. Expression, and presumably overexpresion, of fasn-1 from an extrachromosomal array resulted in an imbalance of FA composition, towards saturated C16 and C18. (B) The graph shows the average levels (with standard deviation) of individual FA species normalized to total FA from analysis of three biological replicates. The difference for C16:0 and C18:0 levels are significant (p = 0.05 and <0.05, respectively, paired Student's t test).
Loss of acetyl-CoA carboxylase function also provokes a Peni phenotype.
The key rate-limiting enzyme in endogenous synthesis of fatty acids is the acetyl-CoA carboxylase (ACC). This enzyme carboxylates acetyl-CoA to malonyl-CoA, providing the substrate for FASN-1. Knockdown of the unique ACC gene in C. elegans, pod-2, by RNAi resulted in embryonic and larval lethality, consistent with previous results,16 and also increased expression of pnlp-29::GFP (Suppl. Fig. 8). This result suggests that abnormalities in fatty acid metabolism, in particular the de novo synthesis of saturated FAs, can lead to a Peni phenotype.
Loss of function of downstream components of the fatty acid synthesis pathway do not alter pnlp-29::GFP expression.
In mammals, saturated FA, formed through the action of FASN, is transformed into mono-unsaturated FA by Δ-9-stearoyl-CoA desaturase (SCD). Monounsaturated FAs are the most abundant group of FAs found in phospholipids. These serve as the main component of the lipid membrane and are responsible for sustaining optimal membrane fluidity, as well as being mediators of signal transduction.20 The SCDs are essential and ubiquitous in eukaryotes. In C. elegans there are 2 such enzymes, FAT-6 and 7 and a third enzyme, the palmitoyl-CoA desaturase FAT-5, which has high sequence similarity to the SCDs.21 Inhibition of fat-5, fat-6 or fat-7 leads to altered FA composition,22 causes changes in body fat, and reduces fertility and body size.23 We tested whether these SCD genes are also involved in controlling the expression of nlp-29. In contrast to the consequences of loss of pod-2 function, no upregulation of pnlp-29::GFP was observed when fat-5, fat-6 or fat-7 expression was abrogated by RNAi (Suppl. Fig. 9A). This suggests that monounsaturated FAs may not be directly involved in the regulation of the expression of nlp-29.
The elo genes encode another group of FA modifying enzymes that elongate the carbon backbone of FAs. Some are also involved in the biosynthesis of certain monomethyl branched-chain FA.17,19 RNAi of the elo genes (elo-1-7) did not provoke an increased expression of pnlp-29::GFP expression (Suppl. Fig. 9B). Although this result could possibly be a result of inefficient RNAi, redundant gene function, or compensatory FA synthesis, it does suggest that only direct products of FASN-1 activity, and/or the FASN-1-specific contribution to the FA composition in a particular tissue are involved in the regulation of nlp-29 expression.
fasn-1 acts in parallel to, or downstream of, the p38 MAPK cascade.
Induction of nlp AMP gene expression in C. elegans following D. coniospora infection is known to depend on a PKC-p38 MAPK pathway.10 To test whether fasn-1 acts in this pathway, we tried to make a double mutant between fasn-1(fr8) and pmk-1(km25). These attempts were unsuccessful, apparently due to a synthetic lethality between the two mutants during development. We therefore used RNAi to knock down expression of different target genes in the fasn-1(fr8) background.
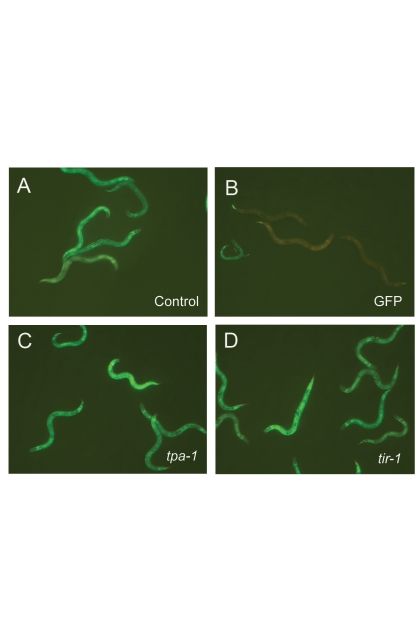
When the activity of tpa-1, tir-1, nsy-1, sek-1 or pmk-1 was abrogated by RNAi, there was no suppression of the Peni phenotype in the fasn-1(fr8) mutant background (Fig. 4A–D, Suppl. Fig. 10A). Interestingly, a tir-1;fasn-1 double mutant was viable and allowed us to confirm that loss of tir-1 function did not diminish expression of pnlp-29::GFP in the fasn-1(fr8) background (Suppl. Fig. 10B). Taken together, these results suggest that fasn-1 acts downstream or in parallel to the p38 MAPK pathway to control pnlp-29::GFP expression.
Figure 4.
Regulation of pnlp-29::GFP by fasn-1 is independent of the PKC-p38 MAPK pathway. RNAi of tpa-1 (C) and tir-1 (D) in fasn-1(fr8) background does not reduce the constitutive expression of pnlp-29::GFP seen in control worms raised on bacteria containing an empty RNAi vector (A), unlike GFP RNAi (B).
Supernumerary copies of fasn-1 specifically block induction of pnlp-29::GFP expression after osmotic shock.
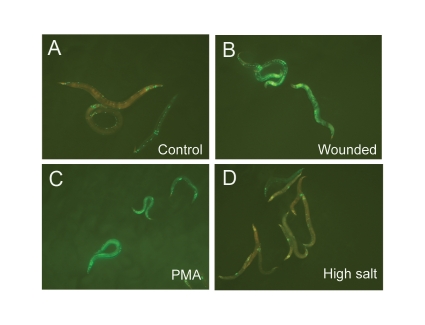
We then transferred by mating the fasn-1-containing rescuing transgenic array into the wild-type background. When we assayed this strain's response to different stimuli, we observed the expected increase in pnlp-29::GFP expression after infection with D. coniospora and exposure to PMA (Suppl. Fig. 11). Unexpectedly, the induction of pnlp-29::GFP after osmotic shock was clearly blocked (Fig. 5), suggesting that fasn-1 may in fact be involved in controlling the osmotic stress-mediated induction of nlp gene expression.
Figure 5.
Extra copies of fasn-1 block the osmotic shock-induced expression of pnlp-29::GFP. (A–D) Compared to untreated controls (A), wt;frIs7;frEx288 worms exhibit a high level of pnlp-29::GFP expression after injury (B) or treatment with PMA (C), but not after osmotic stress (D).
fasn-1 acts upstream of wnk-1/WnK and gck-3/Ste20/GCK-VI kinases.
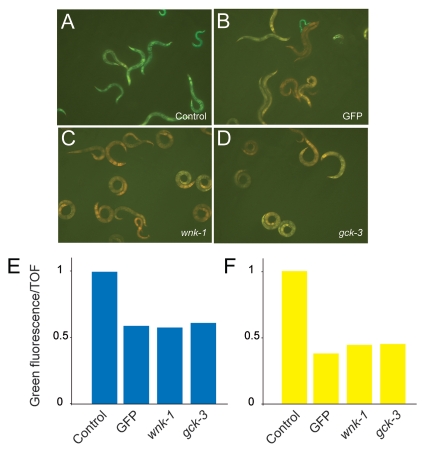
In C. elegans, the response to hypertonic shrinkage is believed to involve components of the cuticle and secreted proteins, such as DPY-10 and OSM-11, respectively. These proteins have been proposed to be part of a systemic mechanosensory apparatus that detects hypertonic shrinkage. Interestingly, dpy-10 and osm-11 mutants display a Peni phenotype, like fasn-1(fr8).8 The evolutionary conserved wnk-1/WNK and gck-3/Ste20/GCK-VI are required for the regulation of the systemic volume and survival after hypertonic shrinkage.24 Knockdown of the gene function of wnk-1 or gck-3 by RNAi in osm-11 or dpy-10 mutants leads to the suppression of the Osr phenotype, but does not suppress glycerol accumulation or alter systemic volume.13 When we abrogated wnk-1 and gck-3 expression by RNAi, fasn-1(fr8) worms showed a marked decrease in their level of pnlp-29::GFP expression. Additionally, wnk-1(RNAi) and gck-3(RNAi) reduced the expression of pnlp-29::GFP in wild-type worms exposed to conditions of high osmolarity (Fig. 6). These results suggest that wnk-1 and gck-3 act downstream of fasn-1 and that part of the response of C. elegans to osmotic stress requires these two kinases.
Figure 6.
Abrogation of gck-3 or wnk-1 block the elevated level of pnlp-29::GFP expression seen in fasn-1(fr8). Treatment of fasn-1(fr8);frIs7 animals with RNAi for gck-3 (C) and wnk-1 (D) lead to the downregulation of pnlp-29::GFP expression similar to that seen upon worms treated with GFP RNAi (B), compared to an empty RNAi vector control (A). (E) Quantification of the effect of gck-3 and wnk-1 RNAi on pnlp-29::GFP expression in fasn-1(fr8);frIs7 animals with the COPAS Biosort. The normalized average ratio of green fluorescence to time of flight (TOF) is shown. The analysis was restricted to worms with a TOF between 200 and 400. In each sample, data is from, from left to right, 100, 60, 87 and 67 worms. For reasons given elsewhere,9 in this and the subsequent graphs, error bars are not shown. Data are representative of three independent experiments. (F) Treatment of wt;frIs7 animals with RNAi for gck-3 and wnk-1 also lead to the downregulation of pnlp-29::GFP expression after 6 h of hyperosmotic stress, compared to the empty vector control. The number of worms used in each sample was, from left to right, 142, 122, 360 and 309 worms. Data are representative of two independent experiments.
wnk-1 and gck-3 regulate nlp-29 expression in parallel to the p38 MAPK pathway.
Overexpression of sek-1 leads to a constitutively high level of pnlp-29::GFP expression.9 In contrast to pmk-1(RNAi), which as expected reduced pnlp-29::GFP expression, neither wnk-1 nor gck-3 RNAi provoked a change in the constitutively high reporter gene expression in the sek-1 overexpressing strain. This suggests that wnk-1 and gck-3 do not act downstream of sek-1 and so may regulate nlp-29 via a pathway which is parallel to the p38 MAPK pathway (Fig. 7).
Figure 7.
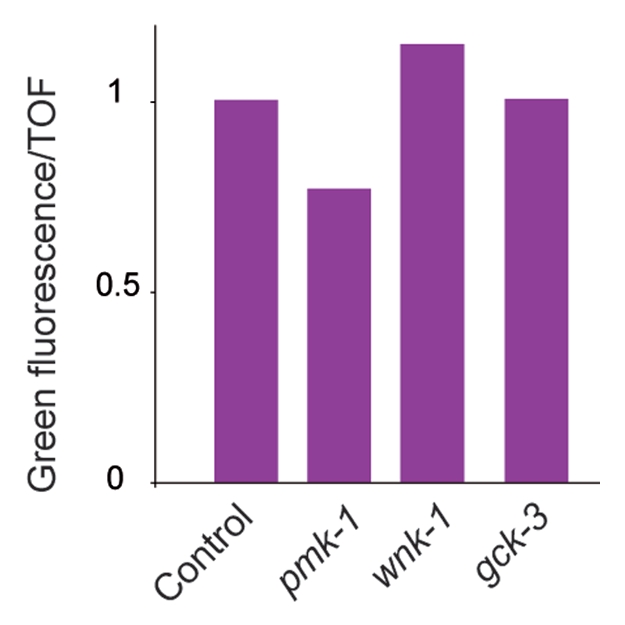
wnk-1 and gck-3 act up-stream or in parallel to the MAP2K sek-1. Overexpression of sek-1 provokes constitutively high pnlp-29::GFP expression, which is reduced by pmk-1(RNAi) but not by wnk-1 nor gck-3 RNAi. The normalized average ratio of green fluorescence to TOF is shown. The analysis was restricted to worms with a TOF between 200 and 400. The number of worms used in each sample was, from left to right, 1381, 754, 371 and 367 worms. Data are representative of two independent experiments.
Discussion
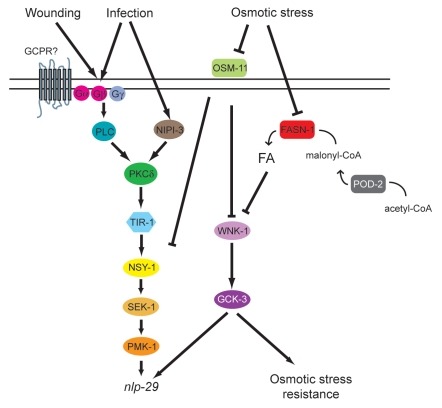
Our previous studies have shown that regulation of AMP genes such as nlp-29 is an important part of C. elegans' anti-fungal defenses. Activation of the expression of nlp-29 after infection or wounding involves a PKC-p38MAPK pathway. In the current study, we identified a mutation in fasn-1 that causes upregulation of the AMP gene nlp-29. In C. elegans, polyunsaturated FAs (PUFAs) gamma-linolenic and stearidonic acid play a role in resistance against Pseudomonas aeruginosa infection.25 No alteration was evident, however, for PUFA's in the fasn-1 mutant. Indeed, loss of fasn-1 activity did not differentially affect FA content in C. elegans, but rather decreased the levels of all FA proportionally. It should be mentioned, however, that because of technical constraints, the GC analysis of FA is necessarily performed on whole animal extracts. There could conceivably be a significant imbalance of FA composition, or a more dramatic drop in total FA level, in the epidermis. In plants, fatty acid synthesis has also been implicated in the control of AMP expression. This was suggested potentially to be the consequence of alterations in lipid signaling.26 While we cannot exclude such a possibility for fasn-1, we favor an explanation based on changes in FAs altering properties of the cuticle or affecting epidermal cell membrane integrity. Support for such an idea comes from the fact that overexpression of fasn-1 diminishes the induction of nlp-29 expression upon osmotic stress, and the observation that the control of nlp-29 expression by fasn-1 required wnk-1 and gck-3, but was independent of the PKC∂/TIR-1/p38MAPK pathway. WNK-1 and GCK-3 act downstream of the putative systemic osmotic sensors DPY-10 and OSM-11.13 Interestingly, the increased expression of pnlp-29::GFP in an osm-11 mutant is dependent upon the p38 MAPK PMK-1.8 This suggests that multiple pathways regulate the response to hypertonic stress, and that FA levels affect one of the branches that lead to increased nlp-29 expression (Fig. 8).
Figure 8.
Model of the control of nlp-29 expression. Signals perceived upon D. coniospora infection and injury are transduced by a PKC-p38 MAPK pathway to regulate the expression of nlp-29. OSM-11 acts as a negative regulator of this pathway, intervening at the level of pmk-1, or above. It also acts in a WNK-1- and GCK-3-dependent parallel pathway to negatively regulate nlp-29 expression. The OSM-11/WNK-1/GCK-3 pathway additionally controls the organismal resistance to osmotic stress, and via a separate as yet undefined pathway, the expression of gpdh-1, and thereby glycerol levels (not shown here for the sake of simplicity). Both POD-2 and FASN-1 influence fatty acid (FA) levels, and negatively regulate nlp-29 expression, in a WNK-1- and GCK-3-dependent manner. The exact manner in which FA levels alter nlp-29 expression has yet to be determined. They may act indirectly by affecting the structure of the cuticle or epidermal cell membrane integrity.
One open question is why AMP expression should be part of the physiological response to hyperosmolarity, especially since it does not appear to correlate with resistance to osmotic stress.8 Notably, expression of gpdh-1, and by extension glycerol, can be decoupled from osmotic stress resistance,13 despite the clear role of glycerol as an osmoprotectant, indicating that this process is complex. It will be interesting to see to what degree osmotic stress resistance pathways are intertwined with innate immune pathways in other organisms.
Material and Methods
Strains and culture condition.
Worms were grown and maintained on nematode growth medium (NGM) and cultured with the E. coli strain OP50, as described (Stiernagle, 2006). The integrated transgene kbIs5 [pgpdh-1::GFP, rol-6] I was a kind gift from Todd Lamitina. tir-1(tm3036) and F32H2.6(tm3581) were received from S. Mitani and the Japanese National Bioresource Project. The osm-11(n1604), pod-2(ye60), dpy-5(e61) and pmk-1(km25), mutants were obtained from the Caenorhabditis Genetics Center (CGC).
Ems mutagenesis and screening.
The current screen was conducted in parallel to one previously described.9 Individual uninfected F2 worms that showed a high constitutive expression of pnlp-29::GFP were selected. Those showing severe morphological defects were discarded, and seven strains that showed a strong and penetrant phenotype were retained for further analysis and outcrossed three times.
Mapping and cloning of fasn-1.
Standard genetic27 and single nucleotide polymorphism (SNP) mapping procedures were used to map fasn-1.28 The first round of mapping placed fr8 on LGI between +2.87 and +3.58 cM. Subsequently the double mutant fr8; dpy-5 was created and utilized for further SNP mapping. This procedure narrowed down the position of fr8 to a 187 kb region containing 44 genes. To narrow down further the genomic location of fr8, different sets of seven overlapping fosmids were micro-injected into fr8 mutants. The concentration of the fosmid for microinjection was generally 30 ng/µl with 70 ng/µl of the coinjection marker pBunc-53::GFP.29 Rescue of fr8 was obtained with fosmids WRM0612bD08 and WRM062bH01, but not WRM0638dH10, narrowing down the candidates to one annotated gene, fasn-1. Sequencing of cDNA from fasn-1 from the fr8 strain revealed a G to A transition at a position 723 3′ of the initiation codon (flanking sequences with fasn-1 G/A substitution in bold: …CTT GAG ATT GGG CAT ACT CAC AGA TAA GGG A…).
Reporter gene constructs and transgenic lines.
The IG274 strain containing the frIs7 transgene and the pcol-12::sek-1 transgene is described elsewhere.9 The rescuing construct frEx288 consisted of four overlapping PCR amplicons including a promoter region that span 2.26 kb upstream region of the start ATG and the whole genomic locus of fasn-1. Each PCR fragment overlapped its neighbor by at least 900 bp. The primers for the PCR products were: A: JEP1283-JEP1105, B: JEP1104-JEP1143, C: JEP1142-JEP1115, D: JEP1114-JEP1121. The PCR products were microinjected at an estimated concentration of less than 5 ng/µl, together with the co-injection marker pBunc-53::GFP at 100 ng/µl. Tissue specific fasn-1 rescue constructs were obtained with the same method, concatenating by fusion PCR30 tissue-specific promoters (from the col-12, mtl-2 and rab-3 genes) to amplicons derived from the fasn-1 coding region, starting with the second exon, which possess an in-frame ATG, and microinjecting these individually with the overlapping PCR products from the genomic region of fasn-1. The constructs were all injected at the concentration of 3 ng/µl with the coinjection marker pBunc-53::GFP at a concentration of 100 ng/µl. Transcriptional fasn-1 green fluorescent protein (GFP) reporters were also generated by fusion PCR. The pfasn-1::GFP was produced by fusion PCR amplification with the functional promoter region of fasn-1 fused with GFP, the concentration of the construct was 100 ng/µl. The strain IG938 [fasn-1(fr8) I; frEx333 (F32H2.5, pnlp-29::GFP, pcol-12::dsRed)] which was used for gas chromatography analysis was obtained by microinjection of pnlp-29::GFP at 60 ng/µl, pcol-12::dsRed at 50 ng/µl and the four overlapping PCR amplicons able to rescue fasn-1 at 3 ng/µl. The sequences of the primers used for all the different constructs are available upon request.
RNAi.
All RNAi feeding experiments were performed as described,31 using clones from the Ahringer library, except for gck-3. A gck-3 fragment was amplified with the primers JEP1544-JEP1545 (sequence of the primers available upon request) with Pst I restriction sites. The Pst I digested gck-3 fragment was ligated into L4440-PstI dephosporylated RNAi vector. All RNAi clones were sequence verified before use. In general, when exposure of L1 larvae to one or more RNAi bacterial clones in a given experimental series caused noticeable developmental defects, or larval lethality, the experiment was started with worms cultured on OP50 until the L4 stage.
Infection, wounding and osmotic stress.
Infection, and wounding were performed as described.9 The osmotic stress resistance test with the different Peni mutants was performed as described.32
Biosorter.
The quantification of fluorescent reporter gene (GFP or DsRed) expression was performed with the COPAS Biosort (Union Biometrica), essentially as described.7,9,15 Generally, animals were analyzed for length (time of flight), optical density (extinction), green and red fluorescence.
Gas chromatography analysis.
A stage matched population of the strain IG938 was washed off the plates with M9, and non-transgenic and transgenic siblings were separated with the COPAS biosorter on the basis of their red fluorescence. The two separated populations were frozen at −80°C, after aspiration of excess liquid. Lipid extraction was performed as described.33 GC was performed as described.17
Acknowledgements
We thank J. Belougne and Y. Duverger for worm sorting using the facilities of the Marseille-Nice Genopole®, M. Fallet for help with confocal microscopy, A. Millet for her participation in the screen and A. Chisholm for discussion. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR) or by the National Bioresource Project coordinated by S. Mitani. This work was funded by institutional grants from INSERM and the CNRS and a grant from the FRM. The Ewbank group is an Equipe Labellisé of the FRM.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/10974
Supplementary Material
References
- 1.Darby C. The C. elegans Research Community. WormBook:WormBook; Interactions with microbial pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell JR, Ausubel FM. Models of Caenorhabditis elegans Infection by Bacterial and Fungal Pathogens. In: Ewbank J, Vivier E, editors. Methods Mol Biol. Humana Press; 2008. pp. 403–427. [DOI] [PubMed] [Google Scholar]
- 3.Darby C, Hsu JW, Ghori N, Falkow S. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature. 2002;417:243–244. doi: 10.1038/417243a. [DOI] [PubMed] [Google Scholar]
- 4.Couillault C, Ewbank JJ. Diverse Bacteria Are Pathogens of Caenorhabditis elegans. Infect Immun. 2002;70:4705–4707. doi: 10.1128/IAI.70.8.4705-4707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgkin J, Kuwabara PE, Corneliussen B. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr Biol. 2000;10:1615–1618. doi: 10.1016/s0960-9822(00)00867-8. [DOI] [PubMed] [Google Scholar]
- 6.Dijksterhuis J, Veenhuis M, Harder W. Ultrastructural study of adhesion and initial stages of infection of the nematode by conidia of Drechmeria coniospora. Mycological research. 1990;94:1–8. [Google Scholar]
- 7.Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 8.Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H, et al. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008;4:1000105. doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujol N, Cypowyj S, Ziegler K, Millet A, Astrain A, Goncharov A, et al. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, et al. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–352. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci USA. 2006;103:12173–12178. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler JM, Thomas JH. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics. 2006;174:1327–1336. doi: 10.1534/genetics.106.059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe KP, Strange K. Systemic osmotic signaling pathways function upstream of WNK and GCK-VI kinases to regulate hypertonic stress resistance in C. elegans. FASEB J. 2008;22:933–939. [Google Scholar]
- 14.Tong A, Lynn G, Ngo V, Wong D, Moseley SL, Ewbank JJ, et al. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc Natl Acad Sci USA. 2009;106:1457–1461. doi: 10.1073/pnas.0809339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGFbeta signaling pathway in Caenorhabditis elegans epidermis. Nature immunology. 2009;10:249–256. doi: 10.1038/ni.1700. [DOI] [PubMed] [Google Scholar]
- 16.Rappleye CA, Tagawa A, Le Bot N, Ahringer J, Aroian RV. Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity. BMC Dev Biol. 2003;3:8. doi: 10.1186/1471-213X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kniazeva M, Crawford QT, Seiber M, Wang CY, Han M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004;2:257. doi: 10.1371/journal.pbio.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer ER, Perez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008;8:118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kniazeva M, Sieber M, McCauley S, Zhang K, Watts JL, Han M. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Genetics. 2003;163:159–169. doi: 10.1093/genetics/163.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- 21.Watts JL, Browse J. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem Biophys Res Commun. 2000;272:263–269. doi: 10.1006/bbrc.2000.2772. [DOI] [PubMed] [Google Scholar]
- 22.Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa M, Nomura T, Hashimoto T, Sakamoto K. Elongation and desaturation of fatty acids are critical in growth, lipid metabolism and ontogeny of Caenorhabditis elegans. J Biochem. 2008;144:149–158. doi: 10.1093/jb/mvn055. [DOI] [PubMed] [Google Scholar]
- 24.Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;293:915–927. doi: 10.1152/ajpcell.00126.2007. [DOI] [PubMed] [Google Scholar]
- 25.Nandakumar M, Tan MW. Gamma-linolenic and stearidonic acids are required for basal immunity in Caenorhabditis elegans through their effects on p38 MAP kinase activity. PLoS Genet. 2008;4:1000273. doi: 10.1371/journal.pgen.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano M, Robatzek S, Torres M, Kombrink E, Somssich IE, Robinson M, et al. Chemical interference of pathogen-associated molecular pattern-triggered immune responses in Arabidopsis reveals a potential role for fatty-acid synthase type II complex-derived lipid signals. J Biol Chem. 2007;282:6803–6811. doi: 10.1074/jbc.M608792200. [DOI] [PubMed] [Google Scholar]
- 27.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fay D, Bender A. The C. elegans Research Community. WormBook: WormBook: Chapter 4-SNPs: Introduction and two-point mapping. [DOI] [PubMed] [Google Scholar]
- 29.Stringham E, Pujol N, Vandekerckhove J, Bogaert T. unc-53 controls longitudinal migration in C. elegans. Development. 2002;129:3367–3379. doi: 10.1242/dev.129.14.3367. [DOI] [PubMed] [Google Scholar]
- 30.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- 31.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 32.Solomon A, Bandhakavi S, Jabbar S, Shah R, Beitel GJ, Morimoto RI. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics. 2004;167:161–170.. doi: 10.1534/genetics.167.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miquel M, Browse J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem. 1992;267:1502–1509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.