Abstract
The objective of the present investigation was to determine whether the bacterium Dietzia subsp. C79793-74, previously shown to inhibit growth of Mycobacterium subsp. paratuberculosis under in vitro culture conditions, has therapeutic value as a probiotic for adult cattle with paratuberculosis. Animals were obtained from several herds with evidence of disease based on seropositivity and/or fecal shedding. Sixty-eight cows with initial evidence of Stage II or III paratuberculosis and two with an initial Stage IV disease were evaluated longitudinally. Animals were either treated daily with variable, disease-dependent doses of Dietzia (n = 48) or left untreated (n = 22). Clinical aspects of disease (diarrhea, emaciated, cachectic and appetite) were recorded until the animal recovered or required euthanasia due to advanced clinical paratuberculosis or other severe conditions. Paratuberculosis parameters—antibody serology (ELISA, AGID) and fecal culture—were longitudinally monitored over the lifetime of each animal. The results indicated that daily treatment with Dietzia was therapeutic for paratuberculosis cows based on: (a) longitudinal decline in ELISA values only occurred in animals that were treated; (b) prolonged survival was dependant upon treatment—the length being directly associated with low initial ELISA values; and (c) treated animals were the only ones cured of disease. Further investigations are envisaged to determine optimal, long-term dosages that may result in even better therapeutic outcomes as well as to evaluate potential application for therapy of the Johne disease, human-counterpart, Crohn disease.
Key words: Johne disease, dietzia probiotic, therapy, MAP, Crohn disease
Introduction
A naturally occurring, inflammatory bowel disease found predominantly in ruminants and caused by Mycobacterium avium, subspecies paratuberculosis (MAP) is referred to as Johne disease.1–3 For paratuberculosis to be manifested in cattle, both infection with MAP and intestinal inflammation are required. MAP exposure, which leads to disease, occurs primarily in utero and/or in newborns.2,4–12 As Johne disease has become an increasingly worldwide problem, due in part to the absence of preventive vaccine/drug or curative treatments,13 including the partially protective, MAP subunit vaccine,14 awareness as to what role, if any, animals play in the transmission of MAP to humans is being raised.15–21 This concern arises from the apparent specific association of MAP with Crohn disease, even though a role (etiology agent?) remains to be established.22–24
Presently, whole herd testing for risk-based management is considered by many as the best means of curtailing the spread of MAP from animal to animal, as well as farm to farm.10,25–29 However, since such practices have yet to, and most likely will not, eliminate the spread of MAP, other preventive and/or curative measures are needed. Dietzia subspecies C79793-74 was reported to inhibit growth of MAP in culture30 similarly to a number of antimicrobioal drugs;13 however, we anticipate that Dietzia's use as a probiotic will result in less medical complications. Therefore, the research presented herein was undertaken to assess whether this bacterium would have any therapeutic benefit for paratuberculosis dairy cows.
Results
Table 1 shows parameters for 22 ELISA-positive animals that were not treated with Dietzia at any time. For presentation purposes, the animals are ordered from lowest to highest initial ELISA values. The length of survival after an ELISA was detected positive (this may or may not be the time at which it could have been first detected), ranged from 1.5 to >17 months with a median of six months (the three steers and cows 33 and O52 were excluded because they were terminated without overt disease). At some point during their life, seven were AGID positive (31.8%) and 15 (68.2%) were fecal MAP shedders. All but six (three steers and cows 33, O52 and 3056) had a single clinical episode that did not ameliorate and all 22 succumbed either with end-stage clinical Johne disease, were fecal shedders, and/or were AGID positive. Only seven survived sufficiently to obtain at least three distinct test dates and six of these seven (85.7%) had their highest ELISA values at death.
Table 1.
Parameters of non-treated paratuberculosis cows
| Cow ID | Breed1 | Initial E/A/F2 | Maximum E/A/F | Final E/A/F | BFEC3 | #CE/#CEA4 | Months survival post IE5 | Initial/Final clinical stage6 |
| 33 | J | 1.5/−/0 | 1.8/−/25 | 1.8/−/25 | 1.42 | 1/0 | 11 | II/II—foot rot |
| B9 | J | 1.5/−/0 | 2.6/−/0 | 2.6/−/0 | 1.57 | 1/0 | 11 | II/IV |
| 29 | J | 1.6/−/0 | 2.2/−/0 | 2.2/−/0 | 0.78 | 1/0 | 9 | II/IV |
| B37 | J | 2.1/−/1 | 3.0/−/1 | 3.0/−/0 | 1.73 | 1/0 | 7 | II/IV |
| 266 | J | 2.3/−/0 | 3.4/+/>300 | 2.4/+/>300 | 1.70 | 1/0 | 14 | II/IV |
| 16 | J | 2.4/−/0 | same as initial | same as initial7 | n/a7 | 1/0 | 1.5 | III/IV |
| H42 | J | 2.7/−/6 | same as initial | same as initial | n/a | 1/0 | 3 | III/IV |
| B42 | J | 2.8/−/8 | 4.3/−/>300 | 4.3/−/>300 | 2.07 | 1/0 | 9 | III/IV |
| 3056 | H | 3.0/−/0 | 3.0/−/2 | ?/?/? | ? | 0/0 | 17+ | II—alive |
| Heath | H | 3.2/+/nd | same as initial | same as initial | n/a | 1/0 | 6 | III/IV |
| B11 | J | 3.3/−/2 | 4.2/+/2 | 4.2/−/0 | 1.33 | 1/0 | 8 | II/IV |
| Caroline | J | 3.5/−/2 | same as initial | same as initial | n/a | 1/0 | 6 | II/IV |
| Apricot | J | 3.5/−/nd | same as initial | same as initial | n/a | 1/0 | 2 | II/IV |
| Jerry | J | 3.7/−/>300 | same as initial | same as initial | n/a | 1/0 | 5 | III/IV |
| 8930 | H | 3.7/−/>300 | same as initial | same as initial | n/a | 1/0 | 6 | II/IV |
| 237-M | A | 3.8/+/39 | same as initial | same as initial | n/a | 0/0 | 0 | II/II |
| 238-M | H | 4.0/−/44 | same as initial | same as initial | n/a | 0/0 | 0 | II/II |
| B12 | J | 4.0/+/100 | 4.0/+/100 | 3.2/−/nd | n/a | 1/0 | 5.5 | III/IV |
| Don4 | J | 4.1/+/nd7 | same as initial | same as initial | n/a | 1/0 | 1.5 | IV/IV |
| Ind head | H | 4.1/-/PCR+ | same as initial | same as initial | n/a | 1/0 | 6 | II/IV |
| 262-M | X | 4.2/+/0 | same as initial | same as initial | n/a | 0/0 | 0 | II/II |
| 052 | H | 4.3/-/>300 | same as initial | same as initial | n/a | 0/0 | 0.25 | II/II—R-Da8 |
Breed: J, Jersey; H, Holstein; X, Cross; A, Ayrshire.
E, ELISA value; A, AGID (positive/negative); F, Fecal cfu MAP/2gms.
BFEC, best-linear-fit ELISA change/year.
#CE, # of clinical episodes, #CEA, # of clinical episodes ameliorated.
Survival time after initial ELISA (IE) test shown in column 3.
Initial/final clinical stage of disease—all stage IV animals were euthanized.
n/a or nd, not applicable due to <2 data points or not done.
R-Da, Died during surgical repair of right side displaced abomasums.
Shown in Table 2 are parameters for 48 Dietzia-treated, paratuberculosis cows that were at Stage II or III (one was Stage IV) prior to initiation of treatment. Seven, shown in bold print, that were clinically asymptomatic throughout its lifetime—cows 13, 228, 229, B70, 36, 1734 and N52—were negative for ELISA, AGID and fecal shedding (they were at Stage I) when they succumbed or were terminated. All were also initially AGID negative and only one (cow 229) was shedding MAP at the initiation of treatment. This cow and three others (1734, 36, N52) shed MAP during treatment, whereas, no fecal shedding was detected for B70, 228 and 13. At autopsy of cows 13 and N52, no pathological or culture evidence for paratuberculosis was found.
Table 2.
Parameters of dietzia treated paratuberculosis cows
| Cow ID | Breed1 | Initial E/A/F2 | Maximum E/A/F | Final E/A/F | BFEC3 | #CE/#CEA4 | Months dietzia treatment | Post-dietzia survival time (mo.) | Initial/Final clinical stage5 |
| 212 | J | 1.5/−/2 | 2.0/+/100 | 1.2/−/100 | 1.29 | 1/0 | 28 | 0 | II/IV |
| R1 | X | 1.5/−/0 | 3.5/+/39 | 3.4/+/39 | 1.69 | 1/0 | 12 | 0 | II/IV |
| 232 | H | 1.6/−/0 | 4.9/+/>300 | 3.1/+/>300 | 1.61 | 3/2 | 34 | 0.1+ | II/III—mastitis |
| 231 | X | 1.6/−/0 | 2.7/−/8 | 1.9/−/8 | 0.48 | 0/0 | 14 | 13+ | II/II—mastitis |
| 36 | H | 1.7/−/0 | 1.7/−/5 | 1.1/−/0 | −0.20 | 0/0 | 11 | 39+ | II/I |
| 65 | J | 1.8/−/0 | 3.7/+/30 | 3.7/+/19 | 1.69 | 1/0 | 16 | 0 | II/IV |
| 9030 | H | 1.9/−/0 | 3.1/−/44 | 3.1/−/44 | 4.74 | 0/0 | 6 | 0.1+ | II/III—mastitis |
| 228 | J | 2.0/−/0 | 2.0/−/0 | 0.52/−/0 | −1.36 | 0/0 | 12 | 44+ | II/I |
| B70 | H | 2.0/−/0 | 2.3/−/0 | 0.53/−/0 | −0.32 | 0/0 | 20 | 14 | II/I |
| 234 | J | 2.1/−/0 | 4.0/+/22 | 4.0/+/22 | 2.19 | 1/0 | 12 | 0 | II/IV |
| 13 | J | 2.2/−/0 | 2.2/−/0 | 1.4/−/0 | −0.42 | 0/0 | 15 | 3 | II/I |
| 1734 | H | 2.2/−/0 | 3.1/−/1 | 0.52/−/0 | −0.44 | 0/0 | 17 | 33+ | II/I |
| 229 | J | 2.2/−/1 | 2.2/−/4 | 0.60/−/0 | −1.17 | 0/0 | 12 | 61+ | II/I |
| R38 | H | 2.3/−/0 | 4.0/+/>300 | 3.8/−/>300 | 0.39 | 1/0 | 31 | 0 | II/IV |
| 227 | H | 2.4/−/0 | 3.5/−/0 | 3.5/−/0 | 2.67 | 0/0 | 6 | 0 | II/II—heart failure |
| 21 | H | 2.4/−/1 | 4.3/+/>300 | 2.7/−/78 | 0.37 | 2/1 | 33 | 0 | II/IV |
| 198 | J | 2.6/−/0 | 3.8/−/60 | 3.8/−/60 | Bp6 | 2/1 | 45 | 0 | II/IV |
| Green-8 | H | 2.7/−/213 | 4.1/+/>300 | 3.4/+/19 | 1.42 | 1/0 | 7 | 0 | II/IV |
| Green-9 | H | 2.7/−/0 | 3.8/+/225 | 3.8/+/225 | 0.65 | 3/2 | 26 | 0 | II/IV |
| 1826 | H | 2.9/+/20 | 4.3/+/>300 | 3.8/+/80 | 3.34 | 4/3 | 26 | 0 | III/IV |
| R23 | H | 3.0/−/4 | 3.9/−/42 | 3.9/−/42 | n/a7 | 1/0 | 2.5 | 0 | III/IV |
| N52 | H | 3.0/−/0 | 3.0/−/8 | 1.2/−/0 | −0.38 | 0/0 | 26 | 8+ | II/I |
| Green-3 | H | 3.0/−/6 | 3.9/+/>300 | 3.4/−/117 | 0.07 | 3/2 | 34 | 0 | II/IV |
| 9238 | H | 3.0/+/26 | 3.6/+/37 | 3.6/+/0 | 0.88 | 3/2 | 14 | 0 | II/IV |
| R100 | H | 3.1/−/131 | 3.8/+/>300 | 3.2/−/212 | −0.03 | 2/1 | 10 | 0 | II/IV |
| R14 | A | 3.2/+/72 | 3.6/+/>300 | 3.3/−/67 | 0.19 | 1/0 | 6 | 0 | II/IV |
| Guern-1 | G | 3.4/−/16 | same as initial | same as initial | n/a | 1/0 | 4 | 0 | II/IV |
| 105 | H | 3.4/+/>300 | 4.6/+/>300 | 4.6/+/>300 | 2.91 | 1/0 | 6 | 0 | II/IV |
| 76 | J | 3.5/−/1 | 4.6/+/>300 | 3.8/−/>300 | 0.94 | 4/3 | 35 | 0 | II/IV |
| Guern-2 | G | 3.5/−/28 | 3.5/−/46 | 2.1/−/46 | −1.55 | 1/1 | 10 | 0.1+ | II/II—peritonitis |
| 1620 | H | 3.5/−/>300 | 4.6/+/>300 | 3.2/+/>300 | −0.25 | 4/3 | 21 | 0 | III/IV |
| Green-1 | H | 3.6/+/186 | 4.2/+/>300 | 3.8/+/>300 | 0.14 | 1/0 | 16 | 0 | II/IV |
| Green-4 | H | 3.6/−/>300 | 4.3/+/>300 | 3.6/+/>300 | −0.02 | 3/2 | 13 | 0 | IV/IV |
| 244 | H | 3.7/−/57 | 3.7/+/120 | 2.9/+/120 | n/a | 1/0 | 3 | 0 | III/IV |
| 73 | H | 3.7/−/nd | 3.7/−/0 | 3.1/−/0 | n/a | 1/0 | 3 | 0 | III/IV |
| R327 | X | 3.8/−/12 | 4.2/+/>300 | 2.7/−/136 | −1.27 | 2/1 | 10 | 0 | II/IV |
| Green-5 | H | 3.9/−/0 | 4.7/+/12 | 3.7/+/0 | −0.23 | 2/1 | 13 | 0 | II/IV |
| Green-6 | H | 4.0/−/22 | 4.7/+/>300 | 3.6/+/>300 | −0.70 | 2/1 | 10 | 0 | II/IV |
| R18 | H | 4.0/+/>300 | 4.0/+/>300 | nd/nd/nd | n/a | 1/0 | 2 | 0 | II/IV |
| Y14 | H | 4.0/−/0 | 4.0/−/0 | 1.8/−/0 | −5.24 | 0/0 | 7 | 0.1+ | II/II—mastitis |
| B71 | H | 4.1/+/0 | 4.1/+/0 | 1.5/−/0 | −3.13 | 0/0 | 9 | 0 | II/II—hairy wart |
| G11 | H | 4.2/+/>300 | 4.4/+/>300 | 3.6/+/215 | −0.33 | 3/2 | 26 | 0 | II/IV |
| B47 | H | 4.2/−/0 | 4.2/+/>300 | 3.9/−/182 | −0.62 | 2/1 | 15 | 0 | II/IV |
| Green-10 | H | 4.3/−/0 | 4.3/−/16 | 3.4/−/16 | −2.20 | 0/0 | 5 | 0 | II/II—peritonitis |
| 54 | H | 4.4/+/0 | same as initial | same as initial | n/a | 1/0 | 2 | 0 | III/IV |
| 1010 | H | 4.4/−/15 | 4.4/+/>300 | 3.5/+/>300 | −0.79 | 1/0 | 13 | 0 | II/IV |
| F16 | X | 4.6/−/0 | 4.6/+/>300 | 4.4/+/>300 | −0.02 | 3/2 | 21 | 3 | II/IV |
| 207 | J | 5.3/+/3 | 5.3/+/133 | 3.9/−/133 | −4.92 | 1/0 | 9 | 0 | II/IV |
J, Jersey; H, Holstein; X, Cross; A, Ayrshire; G, Guernsey.
E, ELISA value (≤1.4 considered negative); A, AGID (positive/negative); F, Fecal cfu MAP/2 gms.
BFEc, best-linear-fit ELISA change/year.
#CE, # of clinical episodes; #CEA, # of clinical episodes ameliorated.
Initial/final clinical stage of disease—all Stage IV animals were euthanized.
BP, Biphasic curve (Initial decline of −1.2 followed by incline of 1.26).
n/a, not applicable due <2 time points.
Of the remaining 41 animals, 13 with an ELISA value <2.8 were initially AGID negative. Nine of these 13 eventually tested AGID positive, three of which (212, R38, 21) became negative by the end of their lives. Only three were fecal shedders at initiation of treatment; however, all but cow 227 eventually shed. Furthermore, nine of the 12 shedders had their maximum fecal shedding values on the last test-date prior to being euthanized. Nine of the 13 were euthanized with end-stage clinical Johne disease, one died of heart failure and three (9030, 231, 227) for medical conditions other than paratuberculosis. Of this group, only 212 had a final “negative” ELISA of 1.2. This animal was ELISA, AGID and fecal positive multiple times and at autopsy, gross pathology and MAP-positive tissue was consistent with paratuberculosis. Thus, of the 19 treated animals with ELISA values <2.8, eight (42.1%) never had a clinical episode (see column 7) and each of these eight succumbed asymptomatic for Johne disease. Of those that clinically relapsed, four had at least one episode successfully ameliorated and two more than once.
Only one cow, N52, with an initial ELISA value greater than 2.8, was terminated with negative findings for all paratuberculosis parameters. Ten of these 29 were AGID positive and 20 of the 28 tested were fecal shedders at the onset of treatment. Only seven (R23, N52, Guernsey-1, Guernsey-2, 73, Y14, Green-10) remained AGID negative and four (73, Y14, B71, 54) remained fecal test-negative throughout their life (B71 was found to have one MAP culture positive lymph node at autopsy). Of 22 AGID positive animals, eight were negative at termination. Twenty-four animals (82.8%) succumbed with end-stage clinical disease; only N52, Guernsey-2, Y14, B71 and Green-10 (17.2%) remained clinically asymptomatic. Interestingly, out of the ten animals with the highest initial ELISA values (starting with cow R18), nine had their highest ELISA value at the time treatment was initiated. This differed from the majority of animals with lower ELISA values; the highest values were detected during treatment. Three of the 26 tested (11.5%), succumbed with their final ELISA value also being the maximum value; this contrasts to that of six of eight non-treated animals (75%). Four cows, Guernsey-2, Y-14, B71 and N52, were also the only ones with ELISA values dramatically lower at termination than present at the outset of treatment. Thirteen animals clinically recovered at least once; eight more than once. Four did not have a single clinical episode and succumbed of causes other than Johne disease.
It was predetermined that if a clinical relapse occurred at a specific dose of Dietzia, the dose would be increased until clinical manifestations were corrected or the animal succumbed. Since the majority had clinical episodes during treatment, the dose of Dietzia was changed multiple times. To assess whether these changes influenced MAP shedding and/or ELISA values, each was monitored longitudinally. ELISA changes were assessed by best linear fit determinations, whereas such a fit was not possible for fecal shedding. Figures 1–4 are examples of how these parameters varied with changes in the dose of Dietzia over the course of treatment.
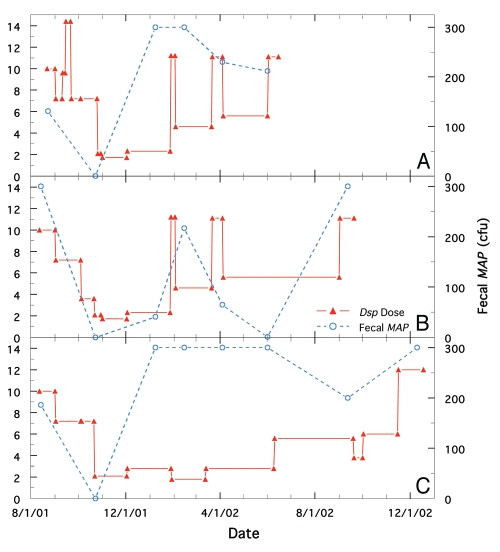
Figure 1.
Comparison of longitudinal changes in fecal MAP at different doses of Dietzia for cows, R100, Green-4 and Green-1, all initially positive for AGID and all possessing initially high ELISA and MAP values. Solid red line is Dietzia dose and dashed blue line is fecal MAP. (A) Cow, R100. (B) Cow, Green-4. (C) Cow, Green-1.
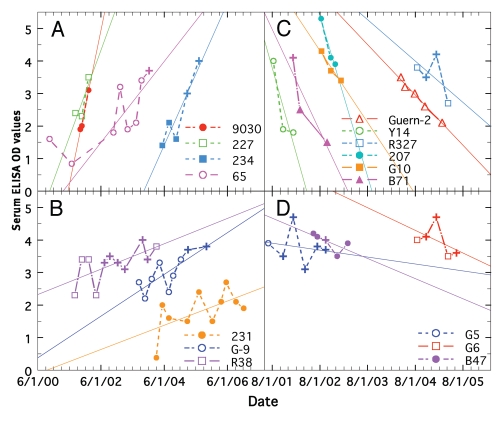
Figure 4.
Comparison of longitudinal changes in ELISA values for paratuberculosis cows treated with Dietzia. (A) Rapid increases. (B) Moderate increases. (C) Rapid decreases. (D) Moderate decreases. Data for each cow is shown by a specific color.
Fecal MAP levels for cows R100, Green-4 and Green-1 (Holsteins, all with high ELISA values and positive for AGID prior to treatment) at different doses of Dietzia are shown in Figure 1A–C, respectively. Green-4 was at Stage IV prior to treatment and the other two were at Stage II. All three had stable longitudinal ELISA values of −0.03, −0.02 and 0.14 (see Fig. 1 in preceding report for Green-1), but were treated differently based on their clinical course of disease. An initial decline in fecal MAP was found for all three when high concentrations of Dietzia (1012 cfu) were initially administered. Moreover, the stage IV disease status of Green-4 reverted to Stage II 70 days after initiation of treatment. Thereafter, fecal MAP shedding of each animal differed; at low doses (<4 × 1011 cfu for Holsteins) they increased and at high doses they declined. All three succumbed with end-stage clinical disease and all were confirmed positive at autopsy.
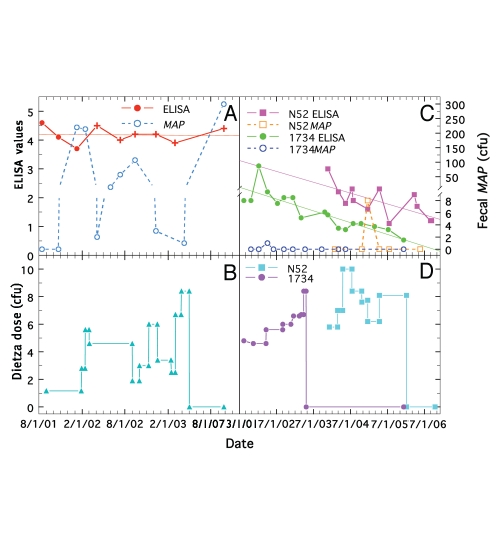
A comparison of ELISA values and fecal changes at different doses of Dietzia for three clinically asymptomatic cows at disease stage II, initially negative for AGID, but fecal shedding, is shown in Figure 2. Figure 2A and B show results for cow, F16, a Holstein x Jersey cross that had the second highest initial ELISA value (Table 2), and Figure 2C and D for cows 1734 and N52 with initial, more moderate, ELISA values of 2.2 and 3.0, respectively. Again, fecal shedding for F16 was low when the dose of Dietzia was high and high when the dose was low or after termination of treatment. The high ELISA values and AGID positivity were unchanged over the course of treatment, similar to that observed for many animals with initially high ELISA values. Treatment was stopped at 5/1/03 after which she succumbed with end-stage clinical disease. In contrast, the ELISA values shown for N52 and 1734 steadily declined to values considered negative. In addition, even though both were fecal positive during treatment, both were fecal negative at termination and neither developed any sign of clinical disease. Neither tissue MAP nor granulomatous enteritis was present at autopsy of N52 supporting the conclusion that she was negative post-treatment. It is highly unlikely that either N52 or 1734 were passive shedders since in our experience any animal considered a passive shedder was ELISA negative multiple times before and after a single shedding incidence; a situation not observed for these two animals. A similar argument can be made for all other animals herein, including those with low levels of shedding.
Figure 2.
Longitudinal changes in ELISA values and fecal MAP at different doses of Dietzia for paratuberculosis cows, F16, 1734 and N52, each initially negative for AGID and fecal shedding. (A and B) cow F16. (A) Solid line is ELISA values (best-fit is thin solid line) and dashed line is fecal MAP. Symbol (+) signifies ELISA value was also positive for AGID. (C and D) cows N52 and 1734. Solid lines are ELISA values (best-fit is thin solid line) and dashed lines are fecal MAP. (B and D) Dietzia dose in cfu.
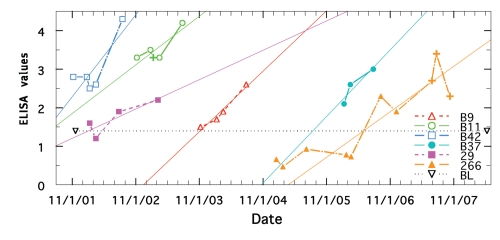
As shown in Figure 3, once ELISA values began to change for non-treated paratuberculosis animals, the longitudinal trend was to increase, never decrease. The mean yearly change (Table 1) was +1.51 ± 0.15 SE, which differed significantly (p <0.0001 and <0.0001) from that of the ELISA negative, treated (−0.05) and non-treated (−0.08) animals (data in the accompanying report).
Figure 3.
ELISA values of each animal are shown by a specific color (best-fit is thin solid line). Symbol (+) signifies ELISA was also positive for AGID.
Figure 4 shows examples of different longitudinal best-fit ELISAs of treated animals (Table 2); half of which increased and the other half decreased. There appear to be different slope patterns as shown by different colors in column 6 of Table 2. Examples of animals with minimal changes were shown in Figure 2 and in Figure 2 of the accompanying report. The mean slope of treated animals in the group with rapid increases (Fig. 2A) was 2.36 ± 0.34 SE, a value similar to that (1.51 ± 0.15 SE) of untreated ELISA positive animals (Table 1, Fig. 3). The mean slope of treated animals with moderate increases (Fig. 2B) was 0.62 ± 0.10 SE, slopes with rapid decreases (Fig. 2C) of −3.05 ± 0.69 SE, and with moderate decreases (Fig. 2D) was −0.49 ± 0.10 SE. The mean slope of the latter group was similar to that of the treated group terminated with all paratuberculosis parameters negative (−0.61 ± 0.17 SE). No animal in Figure 2C or D ever achieved negative ELISA values ≤1.4 before succumbing. Most, however, started with higher initial ELISAs than those that became negative. There are three interesting points for animals shown in Figure 2C. First, only 207 and R327 of the six, succumbed with end-stage clinical disease. Second, Guernsey-2 and B71 were initially treated with a dose of 1 × 1012 cfu and 1.25 × 1012 cfu, respectively, while the other four were started with the normal dose of 3.7 × 1011 cfu. Third, although B71 was initially AGID positive and 207 and R327 became positive; all were AGID negative at termination. At autopsy, B71 was found to have one MAP culture positive lymph node, but no evidence of granulomatous enteritis was present in any intestinal sections.
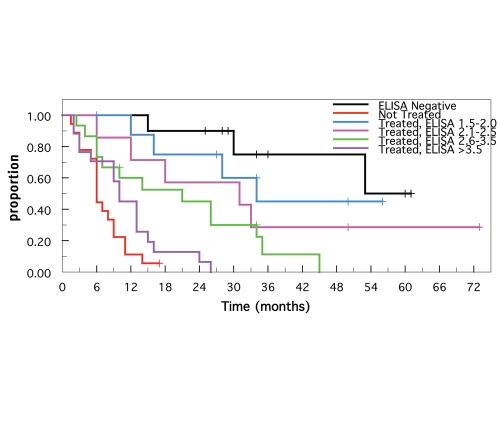
The Kaplan-Meier survival estimates are shown in Figure 5. The estimated survival times were highest for ELISA-negative animals and lowest for untreated paratuberculosis animals (steers and cows 33 and 052 excluded because of nonparatuberculosis demise). The survival estimates for the treated animals decreased incrementally as the initial ELISA values increased (arbitrarily grouped in 0.5 OD increments), i.e., survival was inversely associated with initial ELISA values. The survival of the ELISA-negative group was not significantly different from that of groups with ELISA values 1.5–2.0 (p = 0.35) or with ELISA values 2.1–2.5 (p = 0.14). In addition, the survival estimates of the 1.5–2.0 and 2.1–2.5 groups were not significantly different from each other (p = 0.45). Similarly, the survival of the untreated paratuberculosis group was not significantly different from that of the treated >3.5 group (p = 0.098). In contrast, survival of the 2.6–3.5 group was significantly greater than survival of the >3.5 group (p = 0.0337). There was no significant difference in the survival of the 2.1–2.5 and 2.6–3.5 groups (p = 0.26).
Figure 5.
Kaplan-Meier survival estimates of ELISA-negative and paratuberculosis positive animals. Except for paratuberculosis-free and non-treated paratuberculosis positive animals, survival of treated animals were divided into subgroups (OD intervals of 0.5) based on their initial ELISA values.
Discussion
The validity of conclusions presented herein depends upon the reliability and accuracy of assays used to define Johne disease. As presented in the accompanying report, various parameters were assessed as to their value to monitor changes in disease status of individual animals; they were not examined as a means to define herd status or management decisions. Findings relevant for the present report include:
of the 75 animals with an initial positive ELISA, 68 were considered paratuberculosis positive based on the following ELISA-independent parameters, AGID, MAP present in feces or necropsied tissues, postmortem pathology and end-stage clinical disease; the other 7 (5 of which were ELISA positive multiple times) were also considered paratuberculosis positive as discussed;
11 of 14 animals that would have been in Allied's ELISA “suspect” category based on initial ELISAs, were considered paratuberculosis positive, again based on the longitudinal determination of ELISA-independent parameters;
36 of 38 animals (mostly treated) with serum positive for AGID eventually developed end-stage disease and/or were fecal shedders (the only exceptions were B71 (was treated aggressively) and a steer terminated at 24–25 months of age); thus AGID was predictive of paratuberculosis, which confirms the results of Sherman et al.31,32 for untreated animals;
longitudinal increasing or decreasing ELISA values were the most informative parameter for monitoring change in disease status; and
the first positive ELISA detected for each animal was not due to Dietzia exposure and ELISA positivity detected during treatment was also most likely not due to Dietzia exposure.
Therapeutic benefit of Dietzia.
For any treatment to be effective for Johne disease, two processes must be curtailed: (1) inflammation of the intestine, presumed to be due to a specific immune response to MAP, and (2) eradication of the agent causing and perpetuating inflammation, i.e., MAP. Since our goal was to define a treatment protocol that could be successfully applied on site to ameliorate Johne disease, controlled laboratory experiments were deemed unsuitable. Therefore, we chose to undertake a field-trial approach in which all animals were under our ownership and management.
Although clearly not 100% accurate, we presumed for presentation purposes that the degree of infection and/or extent of Johne disease were associated with both ELISA magnitude (the higher the more advanced) and AGID status; both detecting serum antibodies specific for MAP. As reported for treated animals in the accompanying report, ELISA values less than 2.5 had no predictive value for detectable fecal shedding, whereas animals with higher values were more likely to be detectable shedders. AGID positive animals were assumed to be more heavily infected than AGID negative animals since (a) AGID positive sera was always detected at the same time or subsequent to a positive ELISA; no AGID positive animal was simultaneously ELISA negative,31–34 (b) the appearance of AGID positive serum and detected fecal shedding closely paralleled one another,31,32 and (c) loss of detectable AGID always occurred prior to any reduction in ELISA values.
Animals in the present report possessed a broad spectrum of ELISA values prior to initiation of treatment; 56 were at clinical Stage II, 12 at Stage III, and 2 at Stage IV.35 Seventy-four percent of Stage II, 50% of Stage III and one of the two stage IV animals were treated. The non-treated Stage IV animal (Don4) succumbed at 1.5 months post ELISA positive, whereas the treated Stage IV animal (Green 4) regressed to a healthy, clinical Stage II status 70 days after initiation of treatment. However, she eventually succumbed after 13 months of treatment. Clinical disease of two of the six treated Stage III animals (cows 1620 and 1826) regressed three different times to Stage II, whereas disease of the six non-treated Stage III animals never clinically regressed. Of the 28 treated Stage II animals that relapsed, amelioration of disease occurred in 14 (50%) at least once, whereas of the nine non-treated Stage II animals that relapsed, none ever recovered. Further, of the treated Stage II animals, 13 (31.7%) never relapsed, whereas of the non-treated animals, only one (3056) did not relapse (9.1%). The absence of regression in 16 non-treated Stage II or III animals would support, “a few unusual cases will regress,”2,35 whereas the 42.9% regression found for treated Stage II, III and IV animals is unlikely a consequence of unusual spontaneous regression. Treatment required to achieve and maintain remission appeared to depend upon the stage of disease and the dose of Dietzia as shown in Figure 5. As the ELISA values increased, treatment was less effective and survival declined.
ELISA values were either relatively stable (Fig. 2), progressively increased, or progressively declined over the treatment period; only the former two changes agree with that reported for non-treated animals.36–41 As a means to partially accommodate biological variability observed with the increases and decreases of antibodies, linear best-fit analysis was utilized. MAP shedding was so variable that best-fit analysis was not possible, which differed from non-treated paratuberculosis cows; i.e., fecal shedding increased in a linear manner.42 This unpredictability of fecal shedding appeared to be dependent upon the dose of Dietzia. Treatment with high doses of Dietzia was associated with a decline in fecal shedding by Green-4, Green-1, R100 and F16, but had no detectable effect on antibody serology parameters. One interpretation of this is that the load of MAP (detected as fecal shedding) must be systemically reduced prior to any curtailment of antibody formation and inflammation. This MAP reduction was observed only at high doses of Dietzia. Whether a high dose, given continuously over an extended time interval, would reduce this antigenic load sufficiently to impact antibody synthesis is an interesting and testable possibility. An obvious obstacle, however, is whether survival would be sufficiently lengthened prior to recovery of the integrity of damaged intestinal tissue.
Not all cows benefited from the Dietzia treatment. Possible reasons for this include: (a) different resistance/susceptibility genetics of the cows,43–46 (b) genetics of different subtypes of MAP present on the seven different donor farms, (c) use of insufficient amounts of Dietzia, and/or (d) extent of disease and intestinal damage present at the start of treatment. Because of limited numbers of animals, these alternatives were not analyzable, although the linear-best-fit analysis sheds some insight. The most intriguing result is that about half of the treated animals had declining longitudinal ELISA values (suggesting that antibodies specific for MAP were declining), whereas all of the non-treated animals had longitudinal increasing values. We were unable to assess if all animals, especially those with rapid declines, were on a path in which disease was being ameliorated, because (a) survival was too short to allow recovery of lumen integrity, or (b) other diseases shortened their lives. In fact, four of the six with rapid declines (exceptions were cows R327 and 207) died of non-paratuberculosis circumstances. In contrast, Dietzia treated animals that had initially low ELISAs, were AGID negative, and had moderate, longitudinal declines eventually became negative for all parameters and none succumbed with end-stage clinical disease.
In conclusion, unlike ineffective antimicrobial drugs,13 daily treatments with Dietzia resulted in long-term therapeutic value for adult paratuberculosis cows based on the following:
longitudinal decline in ELISA values only occurred in animals that were treated;
prolonged survival was associated with animals that were treated—longest survivals were associated with low initial ELISA values; and
regression of clinical disease was dependent upon treatment (treated vs. non-treated was 42.9 vs. 0%).
These present findings raise three intriguing questions: Were animals that regressed from Stage II to Stage I cured? Would a high dose of Dietzia given over a prolonged interval result in better therapeutic value for animals with advanced disease? And what is the mechanism by which Dietzia was therapeutic?
Materials and Methods
Experimental design.
Our primary goal was to define a treatment protocol that could successfully be implemented on the farm to ameliorate Johne disease. To this end, a controlled laboratory undertaking was deemed unsuitable. Therefore, a roughly 4:1 ratio of paratuberculosis-free to -positive animals (see below for definition of each), under our ownership and management, were housed in a tie-stall facility as a single dairy herd under conditions designed to mimic those on a “typical” small dairy farm. At any given time, the herd was comprised of 50–60 females. Once an animal tested ELISA positive, treatment was, or was not, started and then it was monitored for various paratuberculosis parameters over its remaining natural lifetime. It was predetermined that the dose of Dietzia would be adjusted for each animal based on any change in clinical status and not on changes in any paratuberculosis-specific test parameter. Since most animals clinically relapsed, the dose was empirically varied based on body weight and severity of clinical disease throughout the study; i.e., essentially no two animals were treated the same. Emaciated animals were defined as having end-stage clinical Johne disease based on the presence of both “pipestream” diarrhea and reduced feed intake. Local veterinarians humanely euthanized recumbent, emaciated and/or cachectic end-stage animals when they no longer could get up and stand on their own by intravenous injection of a sodium pentobarbital solution (Fatal Plus, 6 gm/ml) at 1 ml/4.5 kg body weight. Animals that showed potential life-threatening non-Johne disease ramifications were either euthanized or terminated.
Animals.
Most animals in the study were adult dairy cows in their second through fourth lactation (see pp. 134–144 of this issue). Over the eight years of investigation, more than 200 paratuberculosis-free adult cows were obtained from a single local low prevalence herd. Those continuing to test negative for all parameters at every test-date for the remainder of their lifetimes were considered negative. Stage II or III clinically diseased35 dairy cows (or their offspring), detected positive for specific paratuberculosis MAP antibodies at dry-off by ELISA (determined by the owners and veterinarians), were purchased (two Stage IV animals were donated) from seven local, well managed, moderately high-prevalence herds over a five year period. Independent substantiation that an ELISA-positive animal was positive for Johne disease depended upon whether she also (a) tested positive for a different parameter (fecal shedding or serum AGID), (b) developed end-stage (Stage IV) clinical disease, (c) had characteristic paratuberculosis lesions and tissue pathology at autopsy, and/or (d) as a last resort, tested ELISA positive multiple times. As reported in the companion report, of 75 ELISA-positive animals used to test the reliability of ante-mortem assays to monitor progression/regression of disease, all but seven, ELISA-positive animals were also positive for either parameter (a), (b) or (c). Five of these seven, however, tested ELISA positive multiple times (maximum values ranged from 2.2 to 4.0).
Five animals in the accompanying report were not used for the present examination of the efficacy of Dietzia; i.e., those that (a) had potentially false positive ELISAs (cow 270), (b) survived less than four months and had no alternative positive paratuberculosis parameter (cow 3036), and (c) were treated with other reagents in addition to Dietzia (cows 255, April, Trixie-results with these three are reported elsewhere). As the purpose of the present report was to assess the effect of intervention rather than confirm the findings of others that non-treated paratuberculosis animals eventually succumb with clinical disease, more animals were in the treated group (n = 48) than in the non-treated group (n = 22).
Dietzia.
Dietzia, partially characterized and originally classified as Mycobacterium gardonae, was isolated from fecal material of a paratuberculosis sero- and fecal-positive cow by Richards.30 It was reclassified as Dietzia based on its 16S rRNA sequence (performed by MIDI Labs, Inc., Newark, DE), which is considered the gold standard for bacterial identification.47 Growth requirements were defined in agar plates. Ultimately, Dietzia was grown under contract in 75-liter fermenters by Encore, LLC (Minneapolis, MN) or by the University of Minnesota Biotechnology Institute (St. Paul, MN) for 3–4 days at 29°C in fructose supplemented tryptic soy broth. Batches from each source were used interchangeably with no obvious differences in effectiveness. Large batches prepared by Encore were stored at −20°C in one liter lots, whereas those from the University were centrifuged, washed and concentrated 20-fold prior to storage in 45 ml aliquots at −80°C (long term) or −20°C (short term). New lots were prepared as needed, approximately every 2–3 months. The number of colony forming units/ml was determined prior to use. Viability of the University batches was found to steadily decline at −20°C with a loss of 50% at six months. Once thawed, excess material was maintained at 4°C for up to 10 days, at which time a new sample was thawed. Dietzia treatment was always initiated after an animal was detected with a positive ELISA value (see below for positive/negative ELISA definition). Based on preliminary dosage experiments, small cows (Jerseys, Ayrshires, Jersey x Holstein crosses), and large cows (Holsteins and Guernseys) were treated by supplementing their morning feed of corn with Dietzia at a minimal effective daily dose of 2–3 × 1011 and 4–5 × 1011 cfu, respectively. Some genetically valuable animals were treated with doses 3–4 times higher. If an animal refused to eat, Dietzia was directly deposited into its mouth with a syringe. The dose was increased if an animal showed clinical signs of disease and then lowered if remission was achieved. Based on the ineffectiveness of X-irradiated and antibiotic killed Dietzia (unpublished), non-treated animals were not given any placebo-type Dietzia growth medium or inert materials.
Serum and fecal protocols.
Monthly changes in body weights and milk weights were initially used to monitor the progression of disease; but were discontinued because changes in each paralleled one another and because fecal composition and reduced feed intake were found to better and earlier define onset of impending clinical disease. Fecal material collected directly from the rectum using individual disposable gloves and blood obtained aseptically from the tail vein were transferred to sterile containers, coded and sent chilled on the day of collection to Allied Monitor, Inc., (Fayette, MO). Allied Monitor is a USDA- and NVSL-approved laboratory that specializes in assays for Johne disease. The majority, but not all, of the fecal and serum samples were obtained concurrently. All serum ELISA and AGID assays specific for MAP antibodies and all fecal MAP cultures were performed upon receipt as described in the preceding article. Assessment of the validity, sensitivity and specificity of assays (ELISA, AGID, fecal culture) are also presented in the accompanying report. Any fecally-shed MAP (especially low cfu levels) was considered as pass-through only if ELISA values, both prior to and post MAP fecal-detection, were negative and all other test parameters also remained negative. Based on this criterion, only two cases of MAP shedding (both >300 cfu) were considered pass-through. Allied®s classification of ELISAs as Negative (≤ 1.4 OD), Suspect (1.5 to 2.0 OD) and Positive (>2.0 OD) was reinterpreted based on the fact that 11 of 14 animals with initial “suspect” serum ELISA values eventually became either fecal shedders and/or developed end-stage clinical disease. Of the other three, one had multiple ELISAs >2.0, one was Dietzia treated and became negative for all parameters, and one, not treated, was terminated early (3 months) for mastitis. Therefore, the “suspect” category was not used and all animals with serum ELISAs >1.4 were classified paratuberculosis positive.
Postmortem analysis.
As an additional means to document the Johne disease status of specific animals (see previous report), gross pathology and tissue culture for MAP were undertaken at the University of Minnesota Veterinary School Diagnostic Laboratory (St. Paul, MN). Findings from necropsy, histopathology of multiple organs, bacteriology, parasitology, serology and molecular diagnostics performed on each animal were used simply to confirm an animal as positive or negative for paratuberculosis. The postmortem findings were not used to define specific aspects or extent of disease21 nor compared to any antemortem parameters.
Statistical methods.
Animal survival was analyzed using the Kaplan-Meier method to estimate survival probabilities for groups in which animals were lost to follow-up. The survival time of individual animals was defined as the number of months from the first detected ELISA value >1.4 (or the initial ELISA test for ELISA-negative animals presented in Table 1 of the accompanying manuscript) until their termination. Cox Proportional Hazard Survival Analysis was used to assess differences in the survival estimates. Linear best-fit analysis was used to estimate the trend in ELISA values over time for each animal and expressed as change in ELISA OD/year. The Student's t-test was used to assess differences in mean ELISA values and changes in mean ELISA values. For all comparisons, p values less than 0.05 were considered to be statistically significant.
Acknowledgements
This research was funded, in part, by NIH Grant R01AI027331, by Altick Associates, River Falls, WI., and by Paralab, LLC, Eau Claire, WI. We wish to thank William D. Richards for the initial ICON 6 isolate (Dietzia).
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/10896
Conflict of interest
Both authors are members of Paralab, LLC, which is the assignee of a patent application covering the Dietzia technology presented in this paper, and R.E.C. is a partner in Altick, Associates.
References
- 1.Chiodini RJ. Crohn's disease and the mycobacterioses: A review and comparison of two disease entities. Clin Microbiol Rev. 1989;2:90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease)—the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 3.Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 4.Taylor TK, Wilks CR, McQueen DS. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Vet Rec. 1981;109:532–533. [PubMed] [Google Scholar]
- 5.Seitz SE, Heider LE, Heuston WD, Bech-Nielsen S, Riggs DM, Spangler L. Bovine fetal infection with Mycobacterium paratuberculosis. J Am Vet Med Assoc. 1989;194:1423–1426. [PubMed] [Google Scholar]
- 6.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis isolated from fetuses of infected cows not manifesting signs of the disease. Am J Vet Res. 1992;53:477–480. [PubMed] [Google Scholar]
- 7.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol. 1992;30:166–171. doi: 10.1128/jcm.30.1.166-171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streeter RN, Hoffsis GF, Cech-Nielsen S, Shulaw WP, Rings DM. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res. 1995;56:1322–1324. [PubMed] [Google Scholar]
- 9.Sweeney RW. Transmission of paratuberculosis. Vet Clin North Am Food Anim Pract. 1996;12:305–312. doi: 10.1016/s0749-0720(15)30408-4. [DOI] [PubMed] [Google Scholar]
- 10.Benedictus A, Mitchell RM, Linde-Widmann M, Sweeney R, Fyock T, Schukken YH, et al. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev Vet Med. 2008;83:215–227. doi: 10.1016/j.prevetmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Stabel JR. Pasteurization of colostrum reduces the incidence of paratuberculosis in neonatal dairy calves. J Dairy Sci. 2008;91:3600–3606. doi: 10.3168/jds.2008-1107. [DOI] [PubMed] [Google Scholar]
- 12.Whittington RJ, Windsor PA. In utero infection of cattle with Mycobacterium avium subsp. paratuberculosis: A critical review and meta-analysis. Vet J. 2009;179:60–69. doi: 10.1016/j.tvjl.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 13.St. Jean G. Treatment of clinical paratuberculosis in cattle. Vet Clin North Am Food Anim Pract. 1996;12:417–430. doi: 10.1016/s0749-0720(15)30414-x. [DOI] [PubMed] [Google Scholar]
- 14.Kathaperumal K, Park SU, McDonough S, Stehman S, Akey B, Huntley J, et al. Vaccination with recombinant Mycobacterium avium subspecies paratuberculosis proteins induces differential immune responses and protects calves against infection by oral challenge. Vaccine. 2008;26:1652–1663. doi: 10.1016/j.vaccine.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 15.McDowell RM. McElvaine MD, Long-term sequelae to foodborne disease. Rev Sci Tech. 1997;16:337–341. [PubMed] [Google Scholar]
- 16.Grant IR, Ball HJ, Rowe MT. Incidence of Mycobacterium avium subsp. paratuberculosis in bulk raw and commercially pasteurized cow's milk from approved dairy processing establishments in the United Kingdom. Appl Environ Microbiol. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayele WY, Svastova P, Roubal P, Bartos M, Pavlik I. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cows milk in the Czech Republic. Appl Environ Microbiol. 2005;71:1210–1214. doi: 10.1128/AEM.71.3.1210-1214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellingson JLE, Anderson JL, Koziczkowski JJ, Radcliff RP, Sloan SJ, Allen SE, et al. Detection of viable Mycobacterium avium subs. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J Food Protection. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. [DOI] [PubMed] [Google Scholar]
- 19.Pickup RW, Rhodes G, Bull TL, Arnott S, Sidi-Boumedine K, Hurley M, et al. Mycobacterium avium subsp. paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: diverse opportunities for environmental cycling and human exposure. Appl Environ Microbiol. 2006;72:4067–4077. doi: 10.1128/AEM.02490-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abubakar I, Myhill DJ, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 21.Antognoli MC, Garry FB, Hirst HL, Lombard JE, Dennis MM, Gould DH, et al. Characterization of Mycobacterium avium subspecies paratuberculosis disseminated infection in dairy cattle and its association with antemortem test results. Vet Micro. 2008;127:300–308. doi: 10.1016/j.vetmic.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, et al. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- 23.Behr MA, Kapur V. The evidence for Mycobacterium paratuberculosis in Crohn's disease. Curr Opin Gastroenterol. 2008;24:17–21. doi: 10.1097/MOG.0b013e3282f1dcc4. [DOI] [PubMed] [Google Scholar]
- 24.Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Pathogens. 2009;1:1–15. doi: 10.1186/1757-4749-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells SJ, Wagner BA. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J Am Vet Med Assoc. 2000;216:1450–1457. doi: 10.2460/javma.2000.216.1450. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy DJ, Benedictus G. Control of Mycobacterium avium subspecies paratuberculosis infection in agricultural species. Rev Sci Tech. 2001;20:151–179. doi: 10.20506/rst.20.1.1274. [DOI] [PubMed] [Google Scholar]
- 27.McKenna SL, Keefe GP, Tiwari A, VanLeeuwen J, Barkema HW. Johne's disease in Canada part II: disease impacts, risk factors and control programs for dairy producers. Can Vet J. 2006;47:1089–1099. [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Mitchell RM, Smith RL, Van Kessel JS, Chapagain PP, Schukken YH, et al. The importance of culling in Johne's disease control. J Theor Biol. 2008;254:135–146. doi: 10.1016/j.jtbi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Tavornpanich S, Johnson WO, Anderson RJ, Gardner IA. Herd characteristics and management practices associated with seroprevalence of Mycobacterium avium subspecies paratuberculosis infection in dairy herds. Am J Vet Res. 2008;69:904–911. doi: 10.2460/ajvr.69.7.904. [DOI] [PubMed] [Google Scholar]
- 30.Richards WD. In: Milner AR, Wood PR, editors. In vitro and in vivo inhibition of Mycobacterium paratuberculosis by iron deprivation: A hypothesis; Proc Conf Johne's Disease Australia 1988; pp. 87–94. [Google Scholar]
- 31.Sherman DM, Markham RJ, Bates F. Agar gel immunodiffusion test for diagnosis of clinical paratuberculosis in cattle. J Am Vet Med Assoc. 1984;85:179–182. [PubMed] [Google Scholar]
- 32.Sherman DM, Bray B, Gay JM, Bates F. Evaluation of the agar gel immunodiffusion test for diagnosis of subclinical paratuberculosis in cattle. Am J Vet Res. 1989;50:525–530. [PubMed] [Google Scholar]
- 33.Colgrove GS, Thoen CO, Blackburn BO, Murphy CD. Paratuberculosis in cattle: a comparison of three serologic tests with results of fecal culture. Vet Microbiol. 1989;19:183–187. doi: 10.1016/0378-1135(89)90083-7. [DOI] [PubMed] [Google Scholar]
- 34.Paolicchii FA, Zumarraga MJ, Gioffre A, Zamorano P, Morsella C, Verna A, et al. Application of different methods for the diagnosis of paratuberculosis in a dairy cattle herd in Argentina. J Vet Med B Infect Dis Vet Public Health. 2003;50:20–26. doi: 10.1046/j.1439-0450.2003.00606.x. [DOI] [PubMed] [Google Scholar]
- 35.Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology) Vet Clin North Am Food Anim Pract. 1996;12:345–356. doi: 10.1016/s0749-0720(15)30410-2. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen SS, Grohn YT, Enevoldsen C. Variation of the milk antibody response to paratuberculosis in naturally infected dairy cows. J Dairy Sci. 2002;85:2795–2802. doi: 10.3168/jds.S0022-0302(02)74366-X. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen SS. Transition in diagnostic tests used for detection of Mycobacterium avium subspecies paratuberculosis infections in cattle. Vet Microbiol. 2008;132:274–282. doi: 10.1016/j.vetmic.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 38.van Schaik G, Rossiter CR, Stehman SM, Shin SJ, Schukken YH. Longitudinal study to investigate variation in results of repeated ELISA and culture of fecal samples for Mycobacterium avium subspecies paratuberculosis in commercial dairy herds. Am J Vet Res. 2003;64:479–484. doi: 10.2460/ajvr.2003.64.479. [DOI] [PubMed] [Google Scholar]
- 39.van Schaik G, Stehman SM, Jacobson RH, Schukken YH, Shin SJ, Lein DH. Cow-level evaluation of a kinetics ELISA with multiple cutoff values to detect fecal shedding of Mycobacterium avium subspecies paratuberculosis in New York State dairy cows. Prev Vet Med. 2005;72:221–236. doi: 10.1016/j.prevetmed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Barrington GM, Gay JM, Eriks IS, Davis WC, Evermann JF, Emerson C, et al. Temporal patterns of diagnostic results in serial samples from cattle with advanced paratuberculosis infections. J Vet Diagn Invest. 2003;15:195–200. doi: 10.1177/104063870301500219. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney RW, Whitlock RH, McAdams S, Fyock T. Longitudinal study of ELISA seroreactivity to Mycobacterium avium subspecies paratuberculosis in infected cattle and culture-negative herd mates. J Vet Diagn Invest. 2006;18:2–6. doi: 10.1177/104063870601800102. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen SS, Toft N. Age-specific characteristics of ELISA and fecal culture for purpose-specific testing for paratuberculosis. J Dairy Sci. 2006;89:569–579. doi: 10.3168/jds.S0022-0302(06)72120-8. [DOI] [PubMed] [Google Scholar]
- 43.Koets AP, Adugna G, Janss LL, van Weering HJ, Kalis CH, Wentink GH, et al. Genetic variation of susceptibility to Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J Dairy Sci. 2000;83:2702–2708. doi: 10.3168/jds.S0022-0302(00)75164-2. [DOI] [PubMed] [Google Scholar]
- 44.Mortensen H, Nielsen SS, Berg P. Genetic variation and heritability of the antibody response to Mycobacterium avium subsp. paratuberculosis in Danish Holstein cows. J Dairy Sci. 2004;87:2108–2113. doi: 10.3168/jds.S0022-0302(04)70029-6. [DOI] [PubMed] [Google Scholar]
- 45.Gonda MG, Chang YM, Shook GE, Collins MT, Kirkpatrick BW. Genetic variation of Mycobacterium avium subsp. paratuberculosis infection in US Holsteins. J Dairy Sci. 2006;89:1804–1812. doi: 10.3168/jds.S0022-0302(06)72249-4. [DOI] [PubMed] [Google Scholar]
- 46.Pinedo PJ, Buergelt CD, Donovan GA, Melendez P, Morel L, Wu R, et al. Association between CARD15/NOD2 gene poly-morphisms and paratuberculosis infection in cattle. Vet Microbiol. 2009;134:346–352. doi: 10.1016/j.vetmic.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 47.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]