Abstract
Ample evidence supports a role of brain-derived neurotrophic factor (BDNF) in the survival and differentiation of selective populations of neurons in the peripheral and central nervous systems. In addition to its trophic actions, BDNF exerts acute effects on synaptic transmission and plasticity. In particular, BDNF enhances excitatory synaptic transmission through pre- and postsynaptic mechanisms. In this regard, BDNF enhances glutamate release, the frequency of miniature excitatory postsynaptic currents (mEPSCs), NMDA receptor activity and the phosphorylation of NMDA receptor subunits. Our recent studies revealed a novel cooperative interaction between BDNF and glutamate in the regulation of dendritic development. Indeed, we found that the effects of BDNF on dendritic growth of cortical neurons require both the stimulation of cAMP response element-binding protein (CREB) phosphorylation by BDNF and the activation of the CREB-regulated transcription coactivator 1 (CRTC1) by glutamate. Together, these studies highlight the importance of the cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development.
Key words: BDNF, CREB, CRTC, dendrite, glutamate, neuronal development, NMDA receptors, TORC, synaptic transmission
BDNF belongs to a family of closely related neurotrophic factors termed neurotrophins and is widely expressed in the developing and mature central nervous system.1–3 During postnatal development, BDNF levels are dynamically regulated, in part by activitydependent mechanisms.4 In this context, glutamate, the major excitatory neurotransmitter in the mammalian brain, increases the transcription and release of BDNF.5–7 The biological functions of BDNF are mediated by binding to TrkB receptor tyrosine kinase, that leads to the activation of three major intracellular signaling pathways, including MAPK, PI3K and PLCγ1.8,9 TrkB-mediated signaling can propagate to the nucleus to regulate gene transcription through the activation of several transcription factors including CREB.10 Compelling evidence supports an important role of BDNF in the survival and differentiation of selective populations of neurons in the peripheral and central nervous systems.9,11–13
BDNF and Synaptic Transmission
In addition to its trophic effects during brain development, BDNF has been shown to exert acute effects on synaptic transmission and plasticity.4,7,14,15 In particular, BDNF increases excitatory synaptic transmission in the cerebral cortex and hippocampus through pre- and postsynaptic mechanisms.15,16 Presynaptically, BDNF enhances glutamate release and increases the frequency of mEPSCs in hippocampal neurons.16–18 On the postsynaptic side, BDNF increases NMDA single-channel open probability,19,20 presumably through tyrosine phosphorylation of the NMDA receptor subunits NR1 and NR2B.21,22 In addition, BDNF was recently shown to regulate the expression of NMDA receptor subunits in hippocampal neurons by transcription-dependent mechanisms.23,24 Together, these studies highlight the cooperative actions of BDNF and glutamate in the regulation of excitatory synaptic transmission.
BDNF and Dendritic Development
In addition to its effects on the regulation of synaptic transmission, considerable evidence indicates that BDNF regulates dendritic growth during brain development.25,26 In particular, BDNF plays an important role in controlling the dendritic growth of pyramidal neurons in the developing visual cortex.27,28 Other studies have revealed that overexpression of BDNF in pyramidal neurons induces sprouting of basal dendrites,29 and release of BDNF from single cells elicits local dendritic growth in nearby neurons.30 Because dendritic morphology determines the number, pattern and types of synapses received by a neuron, regulation of cortical dendritic growth by BDNF is likely to play a major role for the proper functioning of the brain. Despite these observations, the cellular and molecular mechanisms underlying the effects of BDNF on dendritic development remain largely unknown. In a recent study aimed at identifying the signaling pathways and downstream effectors necessary for BDNF to regulate dendritic development of cortical neurons, we found that activation of MAPK signaling pathway and phosphorylation of the transcription factor CREB mediate BDNF-induced dendritic growth.31 However, our data also revealed that phosphorylation of CREB is not sufficient to regulate dendritic development in response to BDNF. Recently, a new family of CREB coactivators called CRTCs, also known as transducers of regulated CREB activity (TORCs), has been shown to dramatically increase CREB-mediated transcriptional activity.32,33 CRTCs are latent cytoplasmic coactivators that shuttle to the nucleus in response to increased levels of calcium and cAMP.34,35 The nuclear transport of CRTCs in response to increased intracellular calcium is mediated by dephosphorylation of CRTCs via the Ca2+/calmodulin-dependent protein phosphatase calcineurin.34,35 After translocation into the nucleus, CRTCs associate with the basicleucine zipper domain of CREB independently of its phosphorylation status and increase CREB transcriptional activity.32,33 Among CRTC family members, CRTC1 is primarily expressed in the brain and is involved in activity-dependent transcription of BDNF and in late-phase long-term potentiation.36,37 By using a mutant form of CREB unable to bind CRTC1, we demonstrated that BDNF-induced dendritic development also requires a functional interaction between CREB and CRTC1. Consistent with this observation, inhibition of CRTC1 by RNA interference suppressed BDNF-induced dendritic growth. In addition, we found that the translocation of CRTC1 from the cytoplasm to the nucleus of cortical neurons, which is a necessary step for the interaction between CREB and CRTC1, resulted from the activation of NMDA receptors by glutamate. Finally, nuclear translocation of CRTC1 by glutamate, via stimulation of NMDA receptors and calcineurin, was shown to be essential for the effects of BDNF on dendritic development.31
Together, these results indicate that regulation of dendritic growth by BDNF requires both the stimulation of CREB phosphorylation by BDNF and the induction of CRTC1 nuclear translocation by glutamate through NMDA receptor activation (Fig. 1). These data provide evidence for a novel cooperative interaction between BDNF- and glutamate-mediated signaling that converges on CREB to regulate the expression of target genes involved in dendritic development.
Figure 1.
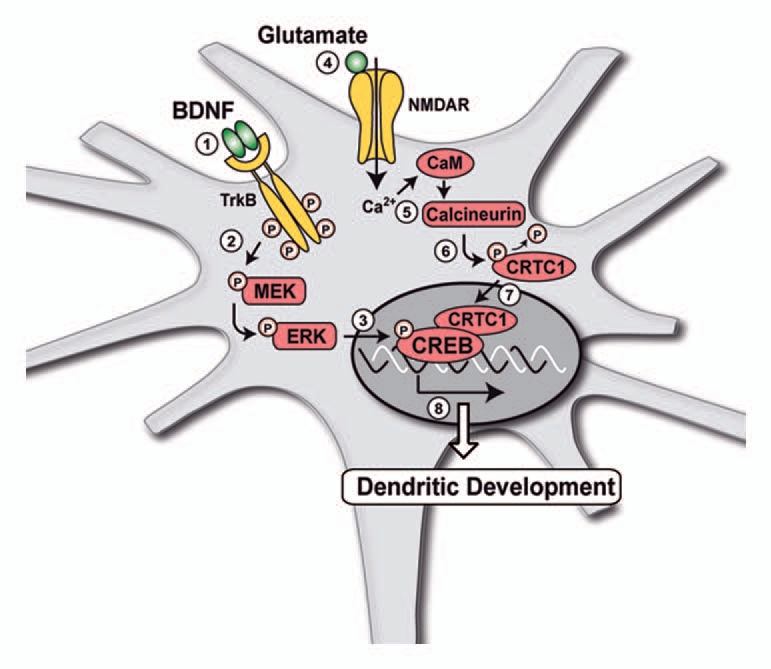
Schematic representation of the mechanisms underlying the effects of BDNF on dendritic development of cortical neurons. (1–3) Binding of BDNF to its receptor TrkB activates MAPK signalling pathway, resulting in the increased phosphorylation of the MEK and ERK kinases and the transcription factor CREB. However, phosphorylation of CREB is not sufficient for the regulation of dendritic development by BDNF, which also requires the nuclear translocation of the CREB coactivator CRTC1. (4–7) Nuclear translocation of CRTC1 is triggered by activation of NMDA receptors by glutamate, resulting in stimulation of the Ca2+/calmodulin (CaM)-dependent protein phosphatase calcineurin. Activation of calcineurin induces the dephosphorylation of CRTC1 and its translocation from the cytoplasm to the nucleus of cortical neurons. (8) Both the stimulation of CREB phosphorylation by BDNF and the induction of CRTC1 nuclear translocation by glutamate are required to increase cortical dendritic development.31
Conclusions
An increasing number of studies support the existence of functional and cooperative interactions between BDNF and glutamate. In particular, glutamate and BDNF co-regulate one another such that glutamate increases the transcription and secretion of BDNF and, conversely, BDNF enhances glutamate release. Other studies provide evidence that BDNF regulates the phosphorylation and expression of NMDA receptor subunits. These cooperative actions of BDNF and glutamate have important implications for the regulation of synaptic transmission and plasticity in the central nervous system. Our studies extend these observations by revealing a novel cooperative interaction between BDNF and glutamate that converges on CREB to increase the transcription of target genes that contribute to the development of dendritic arbor morphology. Together, these observations support the conclusion that cooperation between BDNF and glutamate plays a central role in the regulation of synaptic transmission, plasticity and neuronal development.
Acknowledgements
Work from the authors' laboratory has been supported by the Swiss National Science Foundation.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP response element-binding protein
- CRTC
CREB-regulated transcription coactivator
- mEPSCs
miniature excitatory postsynaptic currents
- TORC
transducers of regulated CREB activity
References
- 1.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, et al. NT-3, BDNF and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 3.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 5.Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 7.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 9.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 11.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 12.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 17.Lessmann V, Heumann R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience. 1998;86:399–413. doi: 10.1016/s0306-4522(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 18.Takei N, Numakawa T, Kozaki S, Sakai N, Endo Y, Takahashi M, et al. Brain-derived neurotrophic factor induces rapid and transient release of glutamate through the non-exocytotic pathway from cortical neurons. J Biol Chem. 1998;273:27620–27624. doi: 10.1074/jbc.273.42.27620. [DOI] [PubMed] [Google Scholar]
- 19.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine ES, Kolb JE. Brain-derived neurotrophic factor increases activity of NR2B-containing N-methyl-D-aspartate receptors in excised patches from hippocampal neurons. J Neurosci Res. 2000;62:357–362. doi: 10.1002/1097-4547(20001101)62:3<357::AID-JNR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, et al. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 23.Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153:310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- 26.Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- 27.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 28.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 29.Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 30.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 31.Finsterwald C, Fiumelli H, Cardinaux JR, Martin JL. Regulation of dendritic development by BDNF requires activation of CRTC1 by glutamate. J Biol Chem. 2010;285:28587–28595. doi: 10.1074/jbc.M110.125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, et al. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci USA. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittinger MA, McWhinnie E, Meltzer J, Iourgenko V, Latario B, Liu X, et al. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, et al. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Wu H, Li S, Chen Q, Cheng XW, Zheng J, et al. Requirement of TORC1 for late-phase long-term potentiation in the hippocampus. PLoS One. 2006;1:16. doi: 10.1371/journal.pone.0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]


