Abstract
Cell migration is a highly complex process that requires the extension of cell membrane in the direction of travel. This membrane is continuously remodeled to expand the leading edge and alter its membrane properties. For a long time it has been known that there is a continual flow of polarized membrane traffic towards the leading edge during migration and that this trafficking is essential for cell migration. However, there is little information on how the cell coordinates exocytosis at the leading edge. It is also unclear whether these internal membranes are incorporated into the leading edge or are just delivering the necessary proteins for migration to occur. We have shown that recycling endosome membrane is incorporated into the plasma membrane at the leading edge to expand the membrane and at the same time delivers receptors to the leading edge to mediate migration. In order for this to happen the surface Q-SNARE complex Stx4/SNAP23 translocates to the leading edge where it binds to the R-SNARE VAMP3 on the recycling endosome allowing incorporation into the plasma membrane. Loss of any one of the components of this complex reduces efficient lamellipodia formation and restrains cell migration.
Key words: cell migration, membrane fusion, lamellipodia formation, SNAREs, recycling endosome, integrin
Cell migration is a highly dynamic and fundamental component of many physiological and pathological processes, including mounting an effective immune response, organogenesis, wound healing and tumor metastasis. In many cases migration is targeted, such as in development, and some sort of chemotactic gradient may well attract cells to their destination. It is a remarkable process in that cells may have to pass over many different surfaces, squeeze themselves through small spaces and sometimes through other cells to reach their destinations. There are a number of distinct steps that contribute to cell migration including protrusion of the leading edge, adhesion at the leading edge and de-adhesion of the rear of the cell, and cytoskeletal contraction to pull the cell body forward as the cell moves.1 Actin polymerization just under the plasma membrane at the front of the cell and altered membrane dynamics help drive protrusion of the dynamic lamellipodia during migration (Fig. 1). In comparison to our knowledge of actin dynamics and its regulation during migration exactly how a cell controls its plasma membrane at this leading edge is poorly understood.
Figure 1.
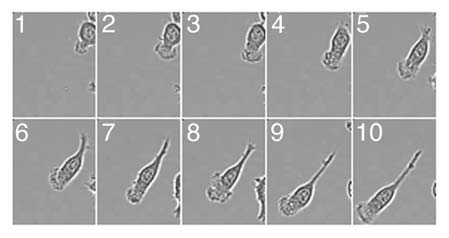
Dynamics of the lamellipodia formation in migrating macrophages. Macrophages plated on fibronectin coated glass bottom dishes (MatTek) were stimulated with 100 nM fMLP and imaged at 37°C in CO2 independent media. Movie frame rates were captured every 5 min over 3 h. Images were captured using Metamorph V67.1 software (Molecular Devices, Sunnyvale, CA) and edited using ImageJ. Selected frames are shown.
Insertion of Recycling Endosome Membrane in the Plasma Membrane is Required for Efficient Lamellipodia Expansion
Data suggests that there is an inverse relationship between the rate of lamellipodia extension and plasma membrane tension.2,3 Actin polymerization at the leading edge pushes against the plasma membrane and while this membrane is reasonably flexible and fluid it is relatively inextensible and does not stretch leading to increases in membrane tension.4,5 Expansion of the plasma membrane with amphiphilic compounds or fluorescent lipids decreases plasma membrane tension and augments lamellipodia extension.3 Conversely, increases in membrane tension by means of osomotic swelling were found to decrease lamellipodia extension rate.3 Accordingly, this inverse correlation between lamellipodia expansion rate and membrane tension suggests that a lowering of membrane tension could aid lamellipodia extension. This loss of membrane tension may well result in sufficient displacement of the plasma membrane to allow addition of actin monomers to the actin filaments that drive lamellipodia formation and migration.6 The lowering of membrane tension could arise through the flattening of existing membrane folds, which effectively act as an initial membrane reservoir to buffer plasma membrane tension.7 Stationary macrophages have many surface invaginations that may serve this purpose.8 However, depletion of this membrane reservoir would lead to a rapid rise in membrane tension7 and data suggest that the addition of internal pools of membrane to the plasma membrane may act to counteract this tension.9–11 Whilst migrating cells have been shown to undergo polarized, microtubule-dependent exocytosis towards the leading edge,9–11 the source of extra membrane and the molecular mechanisms involved in its incorporation during lamellipodia expansion are poorly understood. Integrins are heterodimeric transmembrane adhesion receptors by which cells attach to the extracellular matrix and are key components in cell migration.12 It has been proposed that one source of membrane for lamellipodia expansion may be from the internal pools of membrane that deliver these receptors to the front of the cell for adhesion during migration.9–11
In order for membrane from internal organelles to be incorporated into the cell surface the two membranes must first fuse. All fusion events between internal organelles/tubules/vesicles and the plasma membrane rely on the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) family of proteins.13,14 SNARE proteins regulate membrane fusion by bringing two membranes into close proximity to facilitate fusion.13,14 This is achieved by an R-SNARE located on the donor membrane binding to its cognate Q-SNAREs on the target membrane to form a trans-SNARE complex (Fig. 2). Inhibiting the formation of a specific trans-SNARE complex either by expression of mutant proteins or by reducing protein levels using siRNA results in loss of fusion mediated by this complex. There are 38 known mammalian SNARE proteins that are selectively distributed on different membranes within the cell and by defining their location and functional partners they can be used to map trafficking pathways and fusion events.8,15–17 Using SNARE proteins our study revealed the R-SNARE VAMP3-positive recycling endosome membrane to be a source of internal membrane that is incorporated into the plasma membrane at the leading edge to facilitate lamellipodia expansion (Fig. 2A). Live imaging confirmed the insertion of VAMP3-positive membrane into the lamellipodia as it is expanding. VAMP3 on the recycling endosome was found to pair with Stx4/SNAP23 on the cell surface. Reducing fusion using siRNA specific to any one of the components of the Stx4/SNAP23/VAMP3 complex restrained lamellipodia expansion and altered macrophage migration on fibronectin. Our study shows that along with the incorporation of its membrane the recycling endosome also delivers its cargo and machinery proteins including the integin α5β1 (the fibronectin receptor) that directs migration of cells on fibronectin. Thus, insertion of the recycling endosome into the leading edge not only regulates the delivery of the recycling endosome cargo integrin required for cell migration, but it is also a major source of extra membrane necessary for efficient expansion of the lamellipodium.
Figure 2.
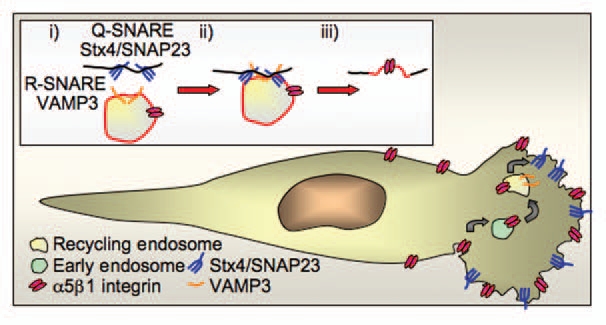
The SNARE complex Stx4/SNAP23/VAMP 3 regulates incorporation of recycling endosome membrane into lamellipodia. (A) The R-SNARE VAMP3 on the recycling endosome forms a complex with its cognate partner Q-SNARE complex Stx4/SNAP23 on the cell surface to mediate incorporation of the recycling endosome membrane in the leading edge. (B) Integrin α5β1 (the fibronectin receptor) is endocytosed from the cell surface and trafficked through the early endosome to the VAMP3-positive recycling endosome. Integrin α5β1 is then recycled to the leading edge through the incorporation of the recycling endosome membrane at the leading edge, this not only delivers the cargo integrin to the leading edge but also adds extra membrane for expansion of the lamellipodia. Stx4/SNAP23 translocates to the leading edge in migrating cells where it acts as the surface Q-SNARE that partners VAMP 3 to regulate incorporation of recycling endosome membrane.
The Stx4/SNAP23/VAMP3 SNARE Complex Regulates Cell Morphology and Lamellipodia Formation in Macrophages
Our study showed that migrating macrophages with reduced levels of the recycling endosome R-SNARE VAMP3, which regulates it fusion with the plasma membrane, had an altered morphology. Cells were mainly spindly and elongated with one major leading edge that was reduced in actin as compared to control cells that were generally more spread with well-formed lamellipodia and strong actin-rich dorsal ruffling. Thus, VAMP3 regulates cell morphology and lamellipodia formation. Surprisingly, our study showed that cells lacking Stx4 had multiple actin-rich spindly protrusions and were unable to exocytose VAMP3-positive recycling endosomes. The multiple sites of protrusion in cells that lack Stx4 may indicate a defect in cell polarity in these cells.
The Surface Q-SNARE Stx4/SNAP23 is a Key Regulator of Focal Exocytosis at the Plasma Membrane
Membrane fusion must be coordinated at the leading edge in order to expand membrane in the lamellipodium. The surface Q-SNARE Stx4/SNAP23 is emerging as a key regulator in the focal exocytosis of recycling endosomes.8,17–19 We showed that Stx4/SNAP23, normally found over the entire macrophage cell surface in non-activated stationary cells, translocates and accumulates at the leading edge in migrating macrophages (Fig. 2). In the lamellipodia Stx4/SNAP23 regulates the focal exocytosis of membrane from recycling endosomes by forming a complex with its R-SNARE VAMP3 to expand the lamellipodia and deliver receptors essential for efficient cell migration (Fig. 2). Loss of this complex leads to a decrease in integrin delivery to the cell surface and reduction in migration, signifying the importance of this complex in migration. The ability of a macrophage to migrate in tissues is critical for it to perform many of it immune functions, however many other cell types are also capable of migration. Thus, we tested whether this same complex is also located at the leading edge in fibroblasts and epithelial cells in response to wounding and a similar redistribution of Stx4/SNAP23 to lamellipodia was seen. These results suggest that the relocation of Stx4/SNAP23 to lamellipodia is also required by other cell types for migration to occur.
The capacity of macrophages to hastily expand their cell surface is also a requirement for other specific immune functions and the relocation of Stx4/SNAP23 at the cell surface is key to this expansion. During phagocytosis membrane protrusions or pseudopods extend around the microorganism to engulf it. The site-specific exocytosis of recycling endosome membrane at the nascent phagocytic cup contributes membrane for this elongation of pseudopods during phagocytosis.8,17 Again, the plasma membrane Q-SNARE Stx4/SNAP23 translocates to the site of expansion where, in combination with VAMP3 on the recycling endosome, it regulates the focal exocytosis of membrane required for expansion of the phagocytic cup during engulfment of a microbe.8,17 In neuronal cells Stx4 has recently been shown to define a site of exocytosis for AMPA receptor-containing recycling compartments at the tips of dendritic spines that direct membrane fusion and regulate post-synaptic plasticity.19 The location of Stx4 to the basolateral membrane in polarized epithelial cells is thought to regulate delivery of cargo specifically located on the basolateral membrane.20,21 In all the cases mentioned above Stx4 is key to the polarized delivery of vesicles to specific locations on the plasma membrane suggesting Stx4 acts to define sites of focal exocytosis in the plasma membrane. These results together suggest Stx4/SNAP23 is a key regulator in focal exocytosis of recycling compartments at the plasma membrane both in macrophages and in other cell types.
Acknowledgements
This work was supported by a grant and a fellowship to R.Z.M. from the National Health and Medical Research Council of Australia and support from the Children's Hospital Burns Research Institute and the Burns Trust.
Abbreviations
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- Stx
syntaxin
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Ji L, Lim J, Danuser G. Fluctuations of intracellular forces during cell protrusion. Nat Cell Biol. 2008;10:1393–1400. doi: 10.1038/ncb1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- 5.Kozlov MM, Mogilner A. Model of polarization and bistability of cell fragments. Biophys J. 2007;93:3811–3819. doi: 10.1529/biophysj.107.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–89. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- 7.Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77:1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 9.Schmoranzer J, Kreitzer G, Simon SM. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci. 2003;116:4513–4519. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- 10.Pierini LM, Lawson MA, Eddy RJ, Hendey B, Maxfield FR. Oriented endocytic recycling of alpha-5beta1 in motile neutrophils. Blood. 2000;95:2471–2480. [PubMed] [Google Scholar]
- 11.Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998;10:537–541. doi: 10.1016/s0955-0674(98)80070-7. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- 14.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 15.Murray RZ, Wylie FG, Khromykh T, Hume DA, Stow JL. Syntaxin 6 and Vti1b form a novel SNARE complex, which is upregulated in activated macrophages to facilitate exocytosis of tumor necrosis Factor-alpha. J Biol Chem. 2005;280:10478–10483. doi: 10.1074/jbc.M414420200. [DOI] [PubMed] [Google Scholar]
- 16.Pagan JK, Wylie FG, Joseph S, Widberg C, Bryant NJ, James DE, et al. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr Biol. 2003;13:156–160. doi: 10.1016/s0960-9822(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 17.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 18.Veale KJ, Offenhauser C, Whittaker SP, Estrella RP, Murray RZ. Recycling endosome membrane incorporation into the leading edge regulates lamellipodia formation and macrophage migration. Traffic. 2010;11:1370–1379. doi: 10.1111/j.1600-0854.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, et al. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ter Beest MB, Chapin SJ, Avrahami D, Mostov KE. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol Biol Cell. 2005;16:5784–5792. doi: 10.1091/mbc.E05-07-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]


