Abstract
The Phylum Cnidaria diverged from the line leading to the Bilateria approximately 630 million years ago, making them well positioned to provide insights into the diversification of eumetazoan body plans and the molecular mechanisms by which body patterning is controlled.1,2 Our recent paper3 focused on Wnt-mediated axis formation during both metamorphosis and regeneration in the cnidarian Hydractinia echinata. We showed functionally that Wnt promotes oral and inhibits aboral development, as well as repressing the formation of additional Wnt-mediated oral organisers. It is possible to relate the role of Wnt in axial patterning to the broader question of how such a wide variety of body plans evolved from the eumetazoan ancestor, given the remarkably conserved genetic toolkit among metazoans. Our results demonstrate how even a slight initial change in a single gene's expression (temporal or spatial) could provide a radical body plan alteration on which natural selection may act and could eventually lead to the establishment of a new species.
Key words: axial patterning, anterior, Tcf, β-catenin, beta-catenin, axis formation, posterior patterning, hydractinia, cnidaria, body plan development
In our recent paper we examined the role of Wnt signaling in the axial patterning of a basal metazoan. In addition to providing insights into the conservation of axial patterning, our results can be used to look at the broader role of body plan development in speciation.
Canonical Wnt signaling is known to function in the establishment of the anterior-posterior (AP) axis throughout the Metazoa, and Wnt pathway perturbations have been found to cause dramatic axial consequences. The spatial orientation of Wnt signaling, active in posterior development and often involving anterior Wnt inhibition, is found in nearly all bilaterian animals examined, suggesting that this orientation is representative of the ancestral condition for the Bilateria.4–10
Wnt signaling is also active during axis specification in planarian regeneration and homeostasis. Wnts and their antagonists are expressed along the AP axis of planarians, and β-catenin has been shown to regulate head versus tail identity, with tails forming instead of heads when a β-catenin antagonist was knocked down. Conversely, when β-catenin itself was depleted, tails transformed into heads.11–13
Posterior expression of Wnt has now been detected in Porifera (sponges). At the stage when sponge embryos begin to display AP polarity, Wnt expression becomes localized to the posterior pole and this polar expression continues until the swimming larva stage.14 While no functional data yet exist, this expression data suggests that the Wnt signaling pathway contributed to establishing axial polarity in the very first metazoans. The use of posterior Wnt signaling and anterior Wnt inhibition might be a unifying principle of body plan development in most animals, having been conserved from an ancestral form.4,10
In cnidarians, as in bilaterians, components of the canonical Wnt signaling pathway are thought to be involved in the establishment of the AP axis.15–21 It has been shown that misexpression of Wnt3, frizzled22,23 and β-catenin,24 as well as blocking GSK3β,16,25 affect AP axis formation, suggesting that a Wnt-mediated organizer also acts to specify the position of the cnidarian oral pole.17 Nuclear β-catenin was reported in the future oral pole of the Nematostella embryo where it specifies entoderm, indicating an evolutionarily ancient role in early pattern formation.26 Both Wnt3 and Tcf mRNA have been shown to be maternally deposited at the future posterior pole of Hydractinia eggs.19 Hydractinia larvae are organized along an AP axis which later gives rise to the oral-aboral (OA) axis of the polyp. The posterior pole of the larva gives rise to the oral pole of the primary polyp. The expression and function of Wnt/β-catenin signaling in the heads of cnidarian polyps corresponds to its functional requirement for ‘posterior’ in planarians, with the polyp head being equivalent to the posterior terminal structure.27 In Hydra the polyp head has been compared to the vertebrate organizer based on both its ability to induce a secondary axis and conserved patterns of gene expression.10,27,28 This means that the cnidarian larval posterior and the polyp oral poles are likely to correspond to the bilaterian posterior, at least in terms of Wnt expression.4
Our recent work focused on axis formation, during both a regular patterning event (metamorphosis), as well as in an induced one (regeneration), in the cnidarian Hydractinia echinata. We sought to gain deeper insights into the cnidarian axial patterning mechanism. Our paper expanded on the known role of canonical Wnt signaling in cnidarian axial patterning, providing functional evidence that Wnt promotes oral formation (head) but represses the formation of aboral structures (stolons). Conversely, downregulation of Wnt promotes aboral (stolons) and represses oral (head) structures. In addition, we demonstrated that the Hydractinia oral Wnt signaling center inhibits the formation of other such centres. The removal of the polyp head and Wnt signaling component knock down by RNAi resulted in the temporary absence of a Wnt signaling center, and its inhibitory influence. This allowed the subsequent formation of multiple Wnt-controlled head organizers leading to multi-headed polyps once Wnt signaling was re-established. In addition, the absence of a Wnt signaling center allowed the development of ectopic stolons. Thus, it appears, given the results outlined above, that Wnt specification of posterior and inhibition of anterior tissue has been conserved throughout the metazoans. This begs the question how such a variety of metazoan body plans have been achieved, given the conserved nature of the patterning system of the main body axis.
Our results show that even though metazoans possess conserved mechanisms to establish the primary axis, slight changes to the components of the patterning mechanism can give rise to a variety of body plans. Such alteration in body plans are a potential driver of speciation. They provide a starting point upon which evolution can act. The phenotypic results we obtained effectively highlight how changes in the expression of a single gene can result in major phenotypic alterations and helps to explain how the complex array of body plans present in extant metazoans can be generated using a common genetic tool kit.
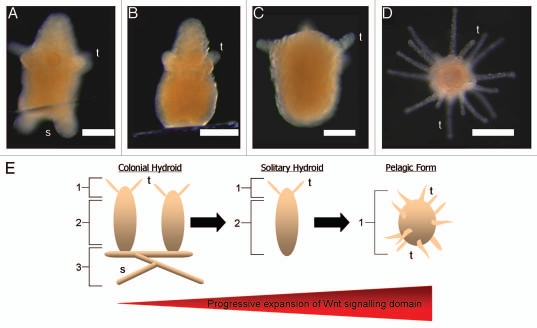
It is interesting to postulate how minor changes in expression of Wnt signaling components could result in the foundation of changes in body plan, and accompanying life-style, which eventually lead to speciation. For example, within the Hydrozoa the solitary polyp Hydra is thought to have been derived from a colonial species, such as Hydractinia. All that might be required to begin this evolutionary transition is the specification of less stolon tissue. As we have shown, this could be achieved either by a slight expansion of the Wnt signaling domain or by a decrease in the stolon inducing signal. When one looks at the oral-tissue-only phenotype induced by ectopically activating Wnt signaling it is not hard to imagine a milder alteration in Wnt expression resulting in the patterning of only the head and body column with stolons being absent, as was the case for some treated animals (Fig. 1B and C). Alternatively, a more severe Wnt upregulation produced a floating ball of tentacles (Fig. 1E). While not overly similar to the usual hydrozoan free living medusa (jellyfish) stage, a similar life-style could be envisaged for such a phenotype (body plan), given the correct environmental conditions. It is already free to float on ocean currents and possesses more than sufficient tentacles with which to capture prey.
Figure 1.
From Hydractinia to Hydra? (A) Normally developing Hydractinia primary polyp. Both developing tentacle and stolon buds are visible. (B and C) Examples of the primary-polyp-only phenotype produced by deregulating Wnt signaling with a combination of azakenpaullone and Tcf RNAi. Heads (top) and body columns were patterned normally, but stolons failed to develop. This phenotype resembles the body plan of the solitary polyp Hydra, providing a hypothetical insight into the evolutionary origin of Hydra from a colonial ancestor, such as Hydractinia. (D) Ectopic activation of Wnt signaling during metamorphosis, using azakenpaullone results in a completely oralised phenotype consisting of a free floating ball of tentacles in which body column and stolons fail to develop. (E) Schematic representation of how a change in the expression of a Wnt signaling component during development could result in a dramatically altered body plan. 1, Wnt3 expressing head tissue; 2, body column tissue; 3, stolon tissue. Scale bars: 200 µm. t, tentacles; s, stolons.
Our recently reported findings provide further evidence for the involvement of Wnt signaling in axial patterning from early in eumetazoan evolution. In addition, the varied phenotypes we obtained by altering the expression of Wnt components provide insights into how even given a conserved AP patterning system it would not be difficult to evolve the broad variety of body plans that exist within the Eumetazoa today. Most intriguingly, our results hint at how it may only require a slight alteration in the expression of a single Wnt component to generate radically different bodies, which may be co-opted as new life stages or even lead to speciation.
Acknowledgements
Thanks to Uri Frank and Jenny Whilde for advice and discussions. Funding was provided by Science Foundation Ireland (SFI).
References
- 1.Peterson KJ, Lyons JB, Nowak KS, Takacs CM, Wargo MJ, McPeek MA. Estimating metazoan divergence times with a molecular clock. Proc Natl Acad Sci USA. 2004;101:6536–6541. doi: 10.1073/pnas.0401670101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris SC. The Cambrian “explosion”: Slow-fuse or megatonnage? Proc Natl Acad Sci USA. 2000;97:4426–4429. doi: 10.1073/pnas.97.9.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy DJ, Plickert G, Kuenzel T, Tilmann W, Frank U. Wnt signaling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development. 2010;137:3057–3066. doi: 10.1242/dev.046631. [DOI] [PubMed] [Google Scholar]
- 4.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Schneider S, Steinbeisser H, Warga RM, Hausen P. beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- 6.Niehrs C. Head in the WNT: the molecular nature of Spemann's head organizer. Trends Genet. 1999;15:314–319. doi: 10.1016/s0168-9525(99)01767-9. [DOI] [PubMed] [Google Scholar]
- 7.Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, et al. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev. 2004;121:119–130. doi: 10.1016/j.mod.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.McGregor AP, Pechmann M, Schwager EE, Feitosa NM, Kruck S, Aranda M, et al. Wnt8 is required for growth-zone establishment and development of opisthosomal segments in a spider. Curr Biol. 2008;18:1619–1623. doi: 10.1016/j.cub.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 11.Gurley KA, Rink JC, Alvarado AS. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias M, Gomez-Skarmeta JL, Saló E, Adell T. Silencing of Smed-βcatenin1 generates radial-like hypercephalized planarians. Development. 2008;135:1215–1221. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- 13.Petersen CP, Reddien PW. Smed-beta catenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 14.Adamska M, Degnan SM, Green KM, Adamski M, Craigie A, Larroux C, et al. Wnt and TGFβ expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE. 2007;2:1031. doi: 10.1371/journal.pone.0001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, et al. WNT signaling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000;407:186–189. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- 16.Müller WA, Teo R, Möhrlen F. Patterning a multi-headed mutant in Hydractinia: enhancement of head formation and its phenotypic normalization. Int J Dev Biol. 2004;48:9–15. doi: 10.1387/ijdb.15005569. [DOI] [PubMed] [Google Scholar]
- 17.Broun M, Gee L, Reinhardt B, Bode HR. Formation of the head organizer in hydra involves the canonical Wnt pathway. Development. 2005;132:2907–2916. doi: 10.1242/dev.01848. [DOI] [PubMed] [Google Scholar]
- 18.Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- 19.Plickert G, Jacoby V, Frank U, Muller WA, Mokady O. Wnt signaling in hydroid development: Formation of the primary body axis in embryogenesis and its subsequent patterning. Dev Biol. 2006;298:368–378. doi: 10.1016/j.ydbio.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Lee PN, Kumburegama S, Marlow HQ, Martindale MQ, Wikramanayake AH. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev Biol. 2007;310:169–186. doi: 10.1016/j.ydbio.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Amiel A, Houliston E. Three distinct RNA localization mechanisms contribute to oocyte polarity establishment in the cnidarian Clytia hemispherica. Dev Biol. 2009;327:191–203. doi: 10.1016/j.ydbio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Momose T, Houliston E. Two oppositely localised Frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biol. 2007;5:70. doi: 10.1371/journal.pbio.0050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momose T, Derelle R, Houliston E. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development. 2008;135:2105–2113. doi: 10.1242/dev.021543. [DOI] [PubMed] [Google Scholar]
- 24.Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, et al. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassel M, Albert K, Hofheinz S. Pattern formation in Hydra vulgaris is controlled by lithium-sensitive processes. Dev Biol. 1993;156:362–371. doi: 10.1006/dbio.1993.1083. [DOI] [PubMed] [Google Scholar]
- 26.Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, et al. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- 27.Meinhardt H. Beta-catenin and axis formation in planarians. BioEssays. 2009;31:5–9. doi: 10.1002/bies.080193. [DOI] [PubMed] [Google Scholar]
- 28.Lee PN, Pang K, Matus DQ, Martindale MQ. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Semin Cell Dev Biol. 2006;17:157–167. doi: 10.1016/j.semcdb.2006.05.002. [DOI] [PubMed] [Google Scholar]