Abstract
The human pathogen Legionella pneumophila is a bacterium that infects human cells and interferes with intracellular signaling. The Legionella protein DrrA is one of the numerous effectors that the bacterium translocates into the host cytosol. DrrA binds to the Legionella containing vacuole (LCV), an organelle in which Legionella survives and replicates, and recruits and activates the vesicular trafficking regulator Rab1 to redirect vesicular trafficking between the endoplasmatic reticulum and the Golgi. After depositing Rab1 at the LCV, DrrA covalently modifies Rab1 with an AMP moiety at a specific tyrosine residue (Tyr77), which is centrally located in the functionally important switch II region. This adenylylation reaction interferes with the deactivation of Rab1 by GTPase activating proteins (GAPs), thereby presumably prolonging the active state of the protein at the LCV. Here, we summarize the versatile properties of DrrA and speculate on the effects of Rab1-adenylylation.
Key words: Legionella, Rab, vesicular trafficking, Legionella containing vacuole, adenylylation, GEF, phosphoinositide
DrrA: An Exceptional Rab1 Regulator
The bacterium Legionella pneumophila is a human pathogen that is able to infect human lung macrophages after it has been taken up via phagocytosis.1 By virtue of a complex sequence of steps interfering with the host metabolism, Legionella is able to escape the immune cell defense system and to survive and replicate intracellularly. The survival strategy is crucially dependent on a plethora of Legionella effector proteins that are secreted from the endocytosed bacterium into the cytosol of the host cell using a Dot/Icm Type IV secretion system. This leads to establishment of an organelle termed the Legionella containing vacuole (LCV).
The protein DrrA (also known as SidM) is one of these effector proteins. Its biochemistry and structural biology has been subject to intensive investigations in the past few years and has established DrrA as one of the best understood Legionella effectors. DrrA consists of three functionally different domains (Fig. 1A): A C-terminal phosphatidylinositol-4-phosphate (PI-4P) binding domain (P4M) mediating LCV localization,2 a central guanine nucleotide exchange factor (GEF) domain for Rab1 activation,3–7 and an N-terminal adenylyltransferase (ATase) domain for Rab1 modification.8 It appears that DrrA and its domains have evolved to specifically take control over the localization, activation and activity of Rab1.
Figure 1.
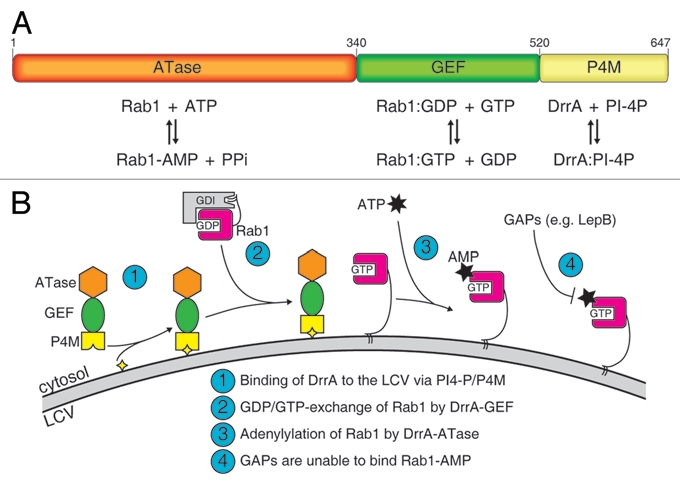
The properties of DrrA. (A) Domain architecture of DrrA showing the arrangement of the adenylyl transferase (ATase), the guanine nucleotide exchange factor (GEF) and the phosphatidylinositol-4-Phosphate (PI-4P) binding domain (P4M). The function of each domain is indicated below. (B) Schematic model of the reaction mediated by DrrA at the LCV. After association of DrrA with the LCV membrane via the P4M (step 1), DrrA recruits Rab1 form its complex with GDI and activates it by loading with GTP (step 2). Subsequently, Rab1 is adenylylated by the ATase activity of DrrA (step 3) leading to inhibition of GAP-catalyzed Rab1-deactivation. (yellow star: PI-4P, grey arc: LCV membrane, ATP: adenosine triphosphate, AMP: adenosine monophosphate, GDI: GDP dissociation inhibitor).
Shortly after translocation via a Type IV secretion system into the host cell cytosol, DrrA becomes localized to the LCV membrane,9 mediated by binding to PI-4P.2 The affinity of the interaction with the lipid is exceptionally tight (Kd = 18 nM) and as yet unprecedented compared to other phosphatidylinositol binding proteins,10 thereby probably ensuring the localization of DrrA at the LCV3,4 and preventing cell-wide (unspecific) redistribution of the protein as long as the LCV is positive for PI-4P.
Once localized to the LCV, DrrA recruits the vesicular trafficking regulator Rab1.3,4 Like all proteins belonging to the family of small GTPases, Rab1 can exist in the active guanosine triphosphate (GTP) bound and inactive guanosine diphosphate (GDP) bound states, whose relative concentrations are regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating protein (GAPs) (reviewed for Rab proteins in ref. 11 and 12). In the inactive state, Rab-proteins are bound to the GDP dissociation inhibitor (GDI), which acts a molecular chaperone by solubilizing the C-terminal geranylgeranyl moieties of Rab-proteins, resulting in cytosolic localization of Rabs. The affinity of GDI to prenylated Rabs is only high when the GTPase is in the GDP state, but low when the protein is activated and bound to GTP.13 GEFs like DrrA can exploit this phenomenon to effectively displace GDI from Rab:GDP:GDI complexes by loading the Rab protein with GTP. DrrA possesses high catalytic GEF-activity towards Rab1,5 and due to its LCV localization recruits Rab1 from Rab1:GDP:GDI complexes to this organelle. After dissociation of GDI followed by nucleotide exchange to the GTP-state, the C-terminal geranylgeranyl moieties of Rab1 are free and attach to the membrane of the LCV. This results in the accumulation of activated Rab1 on the LCV, and this is presumably involved in recruitment of ER-derived vesicles in an as yet unclarified mechanism.
Until recently, the function of the N-terminal domain of DrrA was unknown. Only a cytotoxic effect was attributed to the first 300 amino acids when transfected into mammalian cells.4 We have now shown that this domain has adenylylation activity towards Rab1b resulting in the posttranslational modification of the GTPase on tyrosine 77 with an adenosine monophosphate (AMP) moiety.8 The modification occurred on Rab1b in both nucleotide bound states, with the GTP-state apparently being the far better substrate.8 Preliminary catalytic activity determination indicated that the specific adenylylation activity of DrrA towards Rab1b:GTP is approximately a factor of 270 higher than for Rab1b:GDP. Pathogen-mediated interference of signaling by adenylylation has also been observed for the Rho-family of small GTPases, although in this case the reaction is catalyzed by a different family of enzymes.14,15
The well established biochemistry of DrrA now allows delineation of the probable sequence of events mediated by DrrA (Fig. 1B). Due to its high affinity for PI-4P,10 the P4M of DrrA localizes the protein to the closest PI-4P-positive membrane after translocation into the host cytosol, which is the LCV. The ATase and GEF activities of DrrA will now compete for adenylylation and nucleotide exchange on Rab1. However, because the activity of the ATase for GDP-bound Rab1 is low, the GEF domain (which has high catalytic activity irrespective of the GDP/GTP-state) will be the first to act, resulting in active Rab1:GTP on the LCV. Rab1 in the GTP-state is now a much better substrate for DrrA and undergoes adenylylation.
Effects of Rab1 Adenylylation
Adenylylation by DrrA affects the switch II region of Rab1, which is a crucial structure and sequence element for the specific binding of regulator and effector proteins. It was found that the presence of the covalently attached AMP-moiety can interfere with deactivation of Rab1 by GAP-stimulated GTP-hydrolysis, apparently locking the protein in the GTP-state (Fig. 1B). However, the exact function and physiological consequence of this modification is not yet quite clear, especially because the in vitro interaction with certain proteins is impaired (MICAL-3, LepB), whereas others seem to be able to still bind to Rab1-AMP:GTP with moderate (TBC1D20, GEF-domain of DrrA) or no apparent impairment (LidA). What is the significance of this? Obviously, Legionella has developed strategies to block or weaken host cell processes that involve Rab1 (MICAL-3, TBC1D20). On the other hand, interaction with e.g., LidA, another translocated Legionella effector, is still possible with Rab1-AMP and appears to be necessary for infection.3 In this respect it is remarkable that the deactivation of Rab1 by Legionella LepB is largely eliminated for Rab1-AMP. Why does the bacterium provide a GAP (LepB) for Rab1 while concomitantly blocking its activity by adenylylating Rab1? One possibility could be a temporary inhibition of LepB-activity, which is resolved at a later stage of the infection. Support for this hypothesis is the different rates of recruitment of DrrA, Rab1 and LepB to the LCV: Whereas DrrA and Rab1 appear on the LCV already shortly after infection, the recruitment of LepB is delayed.9 As the infection progresses, DrrA and Rab1 are removed from the LCV (4 h post infection), whereas LepB steadily increases. Since Rab1 can be removed from the vacuole only in the GDP form, LepB must have acted on Rab1:GTP at some point, and since this seems to be possible only for unadenylylated Rab1, the AMP moiety is presumably removed by an as yet unidentified deadenylylase.
Cycles of protein nucleotidylylation and denucleotidylylation as means to control the activity of proteins have been characterized in the regulation of glutamine synthetase adenylyl transferase in bacteria16–20 and might hence exist for Rab1 as well. However, the possible origin of such a deadenylylase is unclear and purely speculative. Perhaps Legionella provides an active deadenylylase at later stages of infection, thereby making Rab1 accessible to GAPs. Alternatively, there might be a host deadenylylating activity. Whether other Rab-proteins are subject to adenylylation/deadenylylation by either bacterial pathogens or by mammalian Rab regulatory proteins will be an interesting topic for further investigation.
References
- 1.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, et al. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem. 2009;284:4846–4856. doi: 10.1074/jbc.M807505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 5.Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A. RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell. 2009;36:1060–1072. doi: 10.1016/j.molcel.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Suh HY, Lee DW, Lee KH, Ku B, Choi SJ, Woo JS, et al. Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J. 2010;29:496–504. doi: 10.1038/emboj.2009.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Hu L, Zhou Y, Yao Q, Liu L, Shao F. Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc Natl Acad Sci USA. 2010;107:4699–4704. doi: 10.1073/pnas.0914231107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 9.Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- 10.Schoebel S, Blankenfeldt W, Goody RS, Itzen A. High-affinity binding of phosphatidylinositol-4-phosphate by Legionella pneumophila DrrA. EMBO Rep. 2010;11:598–604. doi: 10.1038/embor.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 12.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 13.Wu YW, Oesterlin LK, Tan KT, Waldmann H, Alexandrov K, Goody RS. Membrane targeting mechanism of Rab GTPases elucidated by semi-synthetic protein probes. Nat Chem Biol. 2010;6:534–540. doi: 10.1038/nchembio.386. [DOI] [PubMed] [Google Scholar]
- 14.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 15.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, et al. The fic domain: regulation of cell signaling by adenylylation. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadtman ER. The story of glutamine synthetase regulation. J Biol Chem. 2001;276:44357–44364. doi: 10.1074/jbc.R100055200. [DOI] [PubMed] [Google Scholar]
- 17.Wulff K, Mecke D, Holzer H. Mechanism of enzymatic inactivation of glutamine synthetase from E. coli. Biochem Biophys Res Commun. 1967;28:740–745. doi: 10.1016/0006-291x(67)90378-6. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro BM, Stadtman ER. Glutamine synthetase deadenylylating enzyme. Biochem Biophys Res Commun. 1968;30:32–37. doi: 10.1016/0006-291x(68)90708-0. [DOI] [PubMed] [Google Scholar]
- 19.Kingdon HS, Shapiro BM, Stadtman ER. Regulation of glutamine synthetase, VIII. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci USA. 1967;58:1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro BM, Kingdon HS, Stadtman ER. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci USA. 1967;58:642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]


