Abstract
Cycads are among the most ancient of extant Spermatophytes, and are known for their numerous pharmacologically active compounds. One compound in particular, β-methylamino-L-alanine (BMAA), has been implicated as the cause of amyotrophic lateral sclerosis/Parkinson dementia complex (ALS/PDC) on Guam. Previous studies allege that BMAA is produced exclusively by cyanobacteria, and is transferred to cycads through the symbiotic relationship between these cyanobacteria and the roots of cycads. We recently published data showing that Cycas micronesica seedlings grown without endophytic cyanobacteria do in fact increase in BMAA, invalidating the foundation of the BMAA hypothesis. We use this example to suggest that the frenzy centered on BMAA and other single putative toxins has hindered progress. The long list of cycad-specific compounds may have important roles in signaling or communication, but these possibilities have been neglected during decades of attempts to force single metabolites into a supposed anti-herbivory function. We propose that an unbiased, comprehensive approach may be a more appropriate means of proceeding with cycad biochemistry research.
Key words: BMAA, chromatography, Cycadaceae, Cycas micronesica, mass spectrometry, metabolomics
Introduction
Cycads have been known for their toxic properties for centuries, and reports dating back to the 1770s described severe illnesses and gastrointestinal disturbances that can result from ingestion. Nevertheless, cycads were a documented food source among certain native populations including Australia, Fiji and Guam.1,2
In the 1950s, a unique combination of neurodegenerative diseases was discovered among the native Guamanian population. This condition was referred to as amyotrophic lateral sclerosis/Parkinson dementia complex (ALS/PDC) and the intense research that followed showed the disease rates to be consistent with some unknown combination of genetic and environmental factors.3–6 Ingestion of foods prepared from cycads was singled out early on as a possible cause of ALS/PDC since cycads produce a variety of toxic substances. This causal link was confirmed decades later.7
Among the studied toxins are cycasin,8 steryl glucosides9 and β-methylamino-L-alanine (BMAA), a non-protein amino acid.10 Moderate doses of BMAA produce no neurological or behavioral effects on animals, and high doses cause acute toxicity; neither of these scenarios mimic the gradual onset of ALS/PDC in humans.11 These inconsistent results and the lack of a BMAA animal model for ALS/PDC have been ignored to sustain BMAA as the environmental trigger for ALS/PDC and other neurological conditions.12–14 BMAA has been quantified in several samples types,15–18 yet none of these studies has attempted to fully examine the origin of BMAA, its role in cycad biology, or the precise role of cyanobacteria in the symbiotic relationship with cycads. Furthermore, attempts to reproduce the detection of BMAA in human tissues have not been successful, despite the use of validated methods.19,20
Cyanobacteria cultured from cycad roots have been found to contain BMAA, but we do not regard this as evidence that BMAA was produced by the endosymbiont. An equally plausible explanation was that BMAA was synthesized by cycad tissue then transferred to the endosymbiont. In that light, we recently published the first study that directly determines if endophytic cyanobacteria are required for biosynthesis of BMAA in cycads,21 and the results clearly verified an increase in BMAA occurred without cyanobacteria symbiosis.
We offer several lines of reasoning for re-thinking the approach to studying cycad toxins. First, a plethora of secondary compounds are present in cycads,22 and little is known about the biological significance of these molecules. The function of each plant metabolite is never expressed in isolation, but instead acts in tandem with other molecules present within the cornucopia of the “metabolome.” We view the “tunnel vision” approach of ignoring the many co-occurring metabolites as an example of Ockham's broom.23 Second, the primary role of putative or proven toxins may be physiological or signaling,24 with mammalian toxicity being an incidental trait. Indeed, our results indicated BMAA was heavily concentrated in cycad roots,21 which does not support the notion that the primary role of BMAA is an anti-herbivore compound as proclaimed by advocates of the BMAA hypothesis. Third, more recent work has indicated that uncharacterized toxins, excluding BMAA, may play a role in the development of cycad-induced disease.25,26
Comprehensive Analysis
“Metabolomics” is the comprehensive analysis of small molecules present in biological specimens that reflects a particular condition or phenotype. This type of analysis is mainly concerned with the identification of small organic compounds such as amino acids, sugars and fatty acids, and has already been used to characterize a variety of biological specimens.27–30
While several techniques can be used to assess the metabolome of a sample, a single analytical technique that enables the analysis of all metabolites present would be ideal. For example, multi-dimensional chromatography methods, in particular comprehensive two-dimensional gas chromatography coupled with time-of-flight-mass-spectrometry analysis (GC x GC-TOFMS), have been developed to offer a more complete picture of an organism's metabolome. A two dimensional chromatographic separation is attained by employing complementary stationary phases,31–33 and compounds are identified using two unique retention times as well as mass spectral data. The advantages of this recent technology are increased sensitivity and greater utilization of separation space, which helps alleviate the problem of co-elution in complex samples. A representative separation using this technique for a Cycas micronesica leaf sample reveals the plethora of compounds produced by this species (Fig. 1).
Figure 1.
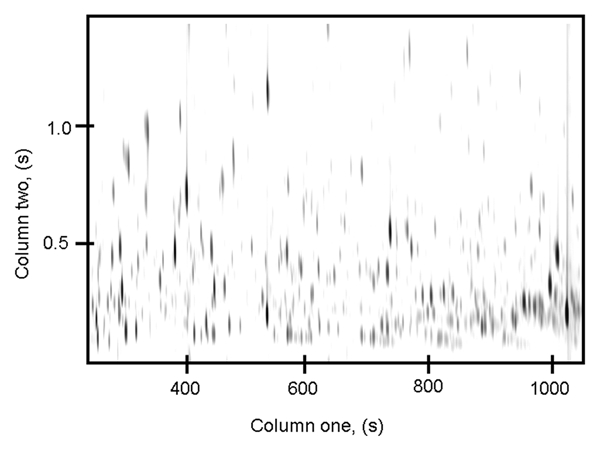
A representative chromatographic separation of a Cycas micronesica leaf sample displaying a large number of peaks. Each of these peaks corresponds to a compound which illustrates the chemical complexity of the cycad metabolome.
Conclusions
Chasing single metabolites such as BMAA has been the traditional and expensive approach of cycad toxicity research for decades; copious funds and many years have been wasted as a result. This approach has led to several dead-ends and engendered the fabrication of elaborate biomagnification proposals involving several trophic levels. Our simple study,21 which could have been conducted early on, invalidated the foundation of the BMAA hypothesis after years of research devoted to prove its legitimacy.
These attempts to force single metabolites into an anti-herbivory function have hindered progress in uncovering more realistic functions of the collective cycad metabolome, such as signaling.24 Indeed, the high concentration of BMAA in coralloid roots18 is consistent with a communication role where it may initiate or sustain the symbiotic relations of cycad roots and endosymbionts. Unfortunately, this type of thinking has not yet been applied to cycad chemistry research. Considering that cycads are the most threatened group of plant species on Earth, and some cycad chemicals are not found in any other plant group,34 time may be running out. Perhaps it is time to retire the use of Okham's broom that is used to ignore the metabolome while chasing single metabolites. We opine that the study of cycad chemistry should at least temporarily change course and adopt a comprehensive metabolomics approach.
References
- 1.Whiting MG. Toxicity of cycads. Econ Bot. 1963;17:270–302. [Google Scholar]
- 2.Whiting MG. Food practices in ALS foci in Japan, the Marianas and New Guinea. Fed Proc. 1964;23:1343–1345. [PubMed] [Google Scholar]
- 3.Plato CC, Galasko D, Garruto RM, Plato M, Gamst A, Craig UK, et al. ALS and PDC of Guam: Forty year follow-up. Neurology. 2002;58:765–773. doi: 10.1212/wnl.58.5.765. [DOI] [PubMed] [Google Scholar]
- 4.Plato CC, Garruto RM, Galasko D, Craig UK, Plato M, Gamst A, et al. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol. 2003;157:149–157. doi: 10.1093/aje/kwf175. [DOI] [PubMed] [Google Scholar]
- 5.Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70:1984–1990. doi: 10.1212/01.wnl.0000312571.81091.26. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman HM. Monthly report to Medical Officer in Command; US Naval Medical Research Unit No. 2. Guam: Micronesian Area Research Center; 1945. [Google Scholar]
- 7.Borenstein AR, Mortimer JA, Schofield E, Wu Y, Salmon DP, Gamst A, et al. Cycad exposure and risk of dementia, MCI and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–1771. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- 8.Schneider D, Wink M, Sporer F, Lounibos P. Cycads: their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften. 2002;89:281–294. doi: 10.1007/s00114-002-0330-2. [DOI] [PubMed] [Google Scholar]
- 9.Shaw CA, Wilson JMB, Cruz-Aguado R, Singh S, Hawkes EL, Lee V, et al. Cycad-induced neurodegeneration in a mouse model of ALS-PDC: Is the culprit really BMAA or is a novel toxin to blame? Mem New York Bot Gard. 2007;97:286–307. [Google Scholar]
- 10.Vega A, Bell EA. alpha-Amino-beta-methylaminopropionic acid, a new amino acid from the seeds of Cycas circinalis. Phytochemistry. 1967;6:759–762. [Google Scholar]
- 11.Karamyan VT, Speth RC. Animal models of BMAA neurotoxicity: a critical review. Life Sci. 2008;82:233–246. doi: 10.1016/j.lfs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Banack SA, Murch SJ, Cox PA. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J Ethnopharmacol. 2006;106:97–104. doi: 10.1016/j.jep.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes and ALS-PDC disease in Guam. Neurology. 2002;58:956–959. doi: 10.1212/wnl.58.6.956. [DOI] [PubMed] [Google Scholar]
- 14.Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci USA. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam. Neurology. 2003;61:387–389. doi: 10.1212/01.wnl.0000078320.18564.9f. [DOI] [PubMed] [Google Scholar]
- 16.Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, et al. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand. 2009;120:216–225. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 17.Banack SA, Johnson HE, Cheng R, Cox PA. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar Drugs. 2007;5:180–196. doi: 10.3390/md504180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banack SA, Cox PA. Distribution of the neurotoxic nonprotein amino acid BMAA in Cycas micronesica. Botanical J Linnean Soc. 2003;143:165–168. [Google Scholar]
- 19.Snyder LR, Hoggard JC, Montine TJ, Synovec RE. Development and application of a comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry method for the analysis of L-beta-methylamino-alanine in human tissue. J Chromatogr A. 2010;1217:4639–4647. doi: 10.1016/j.chroma.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder LR, Cruz-Aguado R, Sadilek M, Galasko D, Shaw CA, Montine TJ. Parkinson-dementia complex and development of a new stable isotope dilution assay for BMAA detection in tissue. Toxicol Appl Pharmacol. 2009;240:180–188. doi: 10.1016/j.taap.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marler TE, Snyder LR, Shaw CA. Cycas micronesica (Cycadales) plants devoid of endophytic cyanobacteria increase in beta-methylamino-L-alanine. Toxicon. 2010;56:563–568. doi: 10.1016/j.toxicon.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Pan M, Mabry TJ, Cao P, Moini M. Identification of nonprotein amino acids from cycad seeds as N-ethoxycarbonyl ethyl ester derivatives by positive chemical-ionization gas chromatography-mass spectrometry. J Chromatogr A. 1997;787:288–294. doi: 10.1016/s0021-9673(97)00789-9. [DOI] [PubMed] [Google Scholar]
- 23.Brenner S. In theory. Curr Biol. 1997;7:202. doi: 10.1016/s0960-9822(97)70095-2. [DOI] [PubMed] [Google Scholar]
- 24.Brenner ED, Stevenson DW, Twigg RW. Cycads: evolutionary Innovations and the role of plant-derived neurotoxins. Trends Plant Sci. 2003;8:446–452. doi: 10.1016/S1360-1385(03)00190-0. [DOI] [PubMed] [Google Scholar]
- 25.McDowell KA, Hadjimarkou MM, Viechweg S, Rose AE, Clark SM, Yarowsky PJ, et al. Sleep alterations in an environmental neurotoxin-induced model of parkinsonism. ExpNeurol. 2010;226:84–89. doi: 10.1016/j.expneurol.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen WB, McDowell KA, Siebert AA, Clark SM, Dugger NV, Valentino KM, et al. Environmental neurotoxin-induced progressive model of parkinsonism in rats. Ann Neurol. 2010;68:70–80. doi: 10.1002/ana.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce KM, Hoggard JC, Hope JL, Rainey PM, Hoofnagle AN, Jack RM, et al. Fisher ratio method applied to third-order separation data to identify significant chemical components of metabolite extracts. Anal Chem. 2006;78:5068–5075. doi: 10.1021/ac0602625. [DOI] [PubMed] [Google Scholar]
- 28.Pierce KM, Hope JL, Hoggard JC, Synovec RE. A principal component analysis based method to discover chemical differences in comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GCxGC-TOFMS) separations of metabolites in plant samples. Talanta. 2006;70:797–804. doi: 10.1016/j.talanta.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Sadilek M, Synovec RE, Lidstrom ME. Liquid chromatography-tandem quadrupole mass spectrometry and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry measurement of targeted metabolites of Methylobacterium extorquens AM1 grown on two different carbon sources. J Chromatogr A. 2009;1216:3280–3289. doi: 10.1016/j.chroma.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hope JL, Prazen BJ, Nilsson EJ, Lidstrom ME, Synovec RE. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry detection: analysis of amino acid and organic acid trimethylsilyl derivatives, with application to the analysis of metabolites in rye grass samples. Talanta. 2005;65:380–388. doi: 10.1016/j.talanta.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Adahchour M, van Stee LL, Beens J, Vreuls RJ, Batenburg MA, Brinkman UA., III Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection for the trace analysis of flavour compounds in food. J Chromatogr A. 2003;1019:157–172. doi: 10.1016/s0021-9673(03)01131-2. [DOI] [PubMed] [Google Scholar]
- 32.Marriott PJ. Comprehensive two-dimensional gas chromatography—GC x GC. J Sep Sci. 2004;27:357. doi: 10.1002/jssc.200490016. [DOI] [PubMed] [Google Scholar]
- 33.Marriott PJ, Kinghorn RM. New operational modes for multidimensional and comprehensive gas chromatography by using cryogenic modulation. J Chromatogr A. 2000;866:203–212. doi: 10.1016/s0021-9673(99)01061-4. [DOI] [PubMed] [Google Scholar]
- 34.International Union for Conservation of Nature, author. The IUCN Red List of Threatened Species October 2010 update: Cycad Facts. http://cmsdata.iucn.org/downloads/cycad_factsheet_final.pdf.


