Abstract
The epsin family of endocytic adaptors has been found to be upregulated in cancer; however the relevance of these findings to this pathological condition is unclear. We have recently demonstrated that epsins are required for cell migration. In fact, epsin overexpression promotes cancer cell invasion. Further, and in agreement with our previous findings, we also observed that overexpression of epsins led to epithelial cell migration beyond colony boundaries. Additionally, our results show that epsin-3 is the most potent paralog enhancing cell migration and invasion. Interestingly, epsin-3 expression is not widespread but highly restricted to migratory keratinocytes and aggressive carcinomas. Upon further investigation, we also identified epsin-3 as being expressed in pancreatic cancer cells. These findings suggest that upregulation of the EPN3 gene is specifically associated with invasive, aggressive cancers. We predict that investigation of these links between the endocytic machinery and mechanisms involved in tumor dissemination will contribute to the development of novel anti-metastatic and anti-cancer strategies.
Key words: endocytosis, cancer, cell invasion, cell migration, signaling
The Epsin Family of Endocytic Adaptors Promote Cancer Cell Invasion
It is widely accepted that functional abnormalities in the endocytic machinery can lead to the onset of malignant transformation. In its most straightforward interpretation, lack of function of endocytic proteins would lead to deficient endocytosis and therefore to prolonged signaling from activated receptors. Interestingly, downregulation of the expression levels of endocytic proteins such as Dab2, Numb and POB1 have been observed in several cancers including ovarian, prostate and breast cancer.1–5 Another mechanism by which abnormal endocytic protein function can lead to carcinogenesis is through the generation of aberrant fusion pro- teins.6 For example, chromosomal translocations involving the CALM (Clathrin Assembly Lymphoid Myeloid leukemia) and AF10 (ALL1 Fused 10) genes produce a fusion protein implicated in acute leukemia.7
Nevertheless, there are several examples of endocytic proteins upregulated in cancer. For example, elevated levels of epsins have been reported to be augmented in skin, breast and lung cancer.8–10 Additionally, intersectin has been shown to induce fibroblast transformation in vitro.11 Interestingly, both endocytic proteins have been directly implicated in the activation of Rho GTPase signaling pathways. Specifically, whereas the intersectin-L isoform has intrinsic Cdc42 GEF activity, epsins bind and inhibit the function of GAPs for Cdc42 and Rac1.12 Although it is not completely clear if amplified RhoGTPase signaling is sufficient to induce malignant transformation, it is predicted to enhance the dissemination of cancer. Indeed, we have demonstrated that the epsin family of endocytic adaptors is required for cell migration13 and that this function depends on the interaction of these proteins with the Cdc42/Rac1 GAP and Ral effector RalBP1.13 Further, our studies indicate that epsin-RalBP1 complex formation is required for proper Rac1 signaling.13
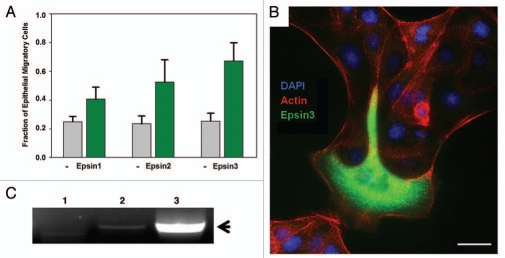
RalBP1 has been observed to be highly upregulated in several invasive cancers including bladder, lung, prostate and skin cancer14,15 and implicated in cancer cell migration, spreading and survival.16,17 It should be noted that epsins, particularly epsin-3, are upregulated in breast and skin cancer.9,10 Importantly, either epsin or RalBP1 overexpression lead to enhanced cell invasion through the basement membrane.13 This observation suggests that enhanced expression of these endocytic proteins contribute to cancer aggressiveness by promoting cell invasion. In agreement with this prediction, we have observed morphological changes in MDCK epithelial cells upon overexpression of epsin-2 and epsin-3 which indicate enhanced cell migration (Fig. 1). Specifically, epsin-transfected cells repeatedly extend lamellipodia beyond the colony boundaries in a way which closely resembles epithelial leader cell migration18 but they also can be found migrating out of epithelial colonies entirely (Fig. 1B). Interestingly, a strikingly similar transition in MDCK behavior has been observed previously upon overexpression of the Arf6 GEF ARNO.19 ARNO overexpression causes the extension of broad lammelipodia and enhanced cell migration which can be attributed to enhanced activation of both Arf6 and Rac1.19 Our previous findings show that the epsin family of adaptors is also signaling to promote Arf6 and Rac1 activation, suggesting that these independent results are obtained by the activation of similar GTPase signaling pathways.13
Figure 1.
Epsin overexpression induces migratory behavior in epithelial cells. MDCK cells were transiently transfected with GFP-Epsin1, 2 and 3. After transfection, cells were trypsinized and seeded on glass coverslips for 24 hr at low density to promote the formation of colonies containing approximately 50 cells. The coverslips were then fixed, co-stained with rhodamine-phalloidin and DAPI, and imaged by epifluorescence microscopy. (A) Fraction of cells at the colony periphery acquiring migratory behavior was quantified in three independent experiments. Results for epsin-transfectants and untransfected (−) cells are indicated as the Mean ± SEM . (B) Example of epithelial cells transfected with GFP-epsin-3 displaying migratory behavior. Scale bar: 20 microns. (C) RNA prepared from HeLa (1), Panc-1 (2) and BxPC-3 (3) cell lines was used as template for RT-PCR with human epsin-3 specific primers. Arrow points to epsin-3 cDNA specific fragment.
We consistently observed that epsin-3 was the most potent paralog for inducing enhancement of cell invasion13 and MDCK migration (Fig. 1). Interestingly, epsin-3 has a limited expression pattern, essentially restricted to migratory cells and basal carcinomas.9 In fact, epsin-3 expression is highly upregulated in breast cancer cell lines.9,10 Further, we have also identified epsin-3 as being expressed in mouse pancreatic cancer models13 and in invasive human pancreatic cell lines such as BxPC-3 (Fig. 1C). These findings suggest that upregulation of the EPN3 gene is specifically associated with invasive cancer. Our laboratory is currently engaged in further investigations required to prove or disprove this hypothesis.
Perspectives
Additional epsin-dependent mechanisms for the enhancement of cancer cell invasion.
Although our data indicate that the ability of epsin to affect cell invasion is mediated by its interaction with RalBP1 and the resulting RhoGTPase activation,13 we cannot discard additional contributions by other mechanisms. Indeed, endocytosis itself has been proposed to play an important role during cell migration. Thus, defects in the function of endocytic proteins such as Dab2, ARH, Numb, AP2 and clathrin, have also been linked to abnormal cell migration due to defective integrin endocytosis.20–23
Additionally, epsin has been directly and specifically connected to the activation of the Notch signaling pathway24,25 which is known to be involved in cell migration/invasion.26 In Drosophila, epsin is the only endocytic adaptor necessary for activation of Notch signaling in signal sending cells, likely due to its special ability to internalize ubiquitinated Notch-ligands.24,25 Further, this Notch-signaling activation function has been shown to be conserved in worms and mice.27,28 Nevertheless, this juxtacrine cell-to-cell mechanism is unlikely to be involved in the epsinmediated enhancement of fibrosarcoma cell migration and invasion.13 The epsin-3′s prevalent effects over other paralogs' cannot be explained by this mechanism. Specifically, since Notch-ligand internalization is a ubiquitin-dependent process,29 all epsin paralogs (which bear functional ubiquitin-interacting motifs) are predicted to be equally effective in promoting cell invasion enhancement. However, it is possible that an epsin-induced, Notch-dependent mechanism operates in the context of multi-cellular environments, such as pancreatic acini.
Nevertheless, the contributions of epsin-mediated enhancement of cancer cell invasion due to endocytosis in general, and of Notch-ligands in particular, still needs to be assessed.
Cell sensitivity to anti-cancer drugs.
Metastatic cells are usually associated with enhanced resistance to chemotherapy.30 Therefore, factors or pathways that contribute to migratory behavior are of high interest for therapeutic purposes. Given our recent findings, epsins rightfully join the list of potential targets for anti-metastatic and anti-cancer strategies, which already includes their interaction partner RalBP1. In fact, it is tempting to speculate that in addition to other proposed mechanisms,17 RalBP1's ability to promote cancer cell survival is related to its capability of inducing migratory behavior. Therefore, function impairment of endocytic proteins crucial to cell invasion (such as epsins and RalBP1) represents an exciting new direction for developing effective cancer therapeutics.
Acknowledgements
We thank Debarati Mukherjee (Purdue University) for critical reading of the manuscript and various colleagues for insightful discussions and suggestions. This project was supported by start-up funds from the Dept. of Biological Sciences, Purdue University, an American Cancer Society Institutional Research Grant through the Purdue Center for Cancer Research and a SIRG Award to R.C.A.
References
- 1.Xu XX, Yi TL, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene. 1998;16:1561–1569. doi: 10.1038/sj.onc.1201678. [DOI] [PubMed] [Google Scholar]
- 2.Schwahn DJ, Medina D. p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene. 1998;17:1173–1178. doi: 10.1038/sj.onc.1202038. [DOI] [PubMed] [Google Scholar]
- 3.Tseng CP, Ely BD, Li YM, Pong RC, Hsieh JT. Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology. 1998;139:3542–3553. doi: 10.1210/endo.139.8.6159. [DOI] [PubMed] [Google Scholar]
- 4.Oosterhoff JK, Penninkhof F, Brinkmann AO, Grootegoed JA, Blok LJ. REPS2/POB1 is downregulated during human prostate cancer progression and inhibits growth factor signaling in prostate cancer cells. Oncogene. 2003;22:2920–2925. doi: 10.1038/sj.onc.1206397. [DOI] [PubMed] [Google Scholar]
- 5.Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 6.Crosetto N, Tikkanen R, Dikic I. Oncogenic breakdowns in endocytic adaptor proteins. FEBS Letts. 2005;579:3231–3238. doi: 10.1016/j.febslet.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Huh JY, Chung S, Oh D, Kang MS, Eom HS, Cho EH, et al. Clathrin assembly lymphoid myeloid leukemia-AF10-positive acute leukemias: a report of 2 cases with a review of the literature. Kor J Lab Med. 2010;30:117–121. doi: 10.3343/kjlm.2010.30.2.117. [DOI] [PubMed] [Google Scholar]
- 8.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276:29257–29267. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 9.Wang YH, Dai ZY, Sadee WG, Hancock WS. A pharmacoproteomics study of the cancer cell line EKVX using capillary-LC/MS/MS. Mol Pharmaceut. 2006;3:566–578. doi: 10.1021/mp060002b. [DOI] [PubMed] [Google Scholar]
- 10.Pawlowski KM, Krol M, Majewska A, Badowska-Kozakiewicz A, Mol JA, Malicka E, et al. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. J Physiol Pharmacol. 2009;60:85–94. [PubMed] [Google Scholar]
- 11.Wang JB, Wu WJ, Cerione RA. Cdc42 and Ras cooperate to mediate cellular transformation by intersectin-L. J Biol Chem. 2005;280:22883–22891. doi: 10.1074/jbc.M414375200. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar RC, Longhi SA, Shaw JD, Yeh LY, Kim S, Schon A, et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc Natl Acad Sci USA. 2006;103:4116–4121. doi: 10.1073/pnas.0510513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coon BG, Burgner J, Camonis JH, Aguilar RC. The Epsin family of endocytic adaptors promotes fibrosarcoma migration and invasion. J Biol Chem. 2010;285:33073–33081. doi: 10.1074/jbc.M110.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SC, Oxford G, Baras AS, Owens C, Havaleshko D, Brautigan DL, et al. Expression of Ral GTPases, their effectors and activators in human bladder cancer. Clin Canc Res. 2007;13:3803–3813. doi: 10.1158/1078-0432.CCR-06-2419. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, et al. RLIP76 and cancer. Clin Canc Res. 2008;14:4372–4377. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J Cell Biol. 2006;174:877–888. doi: 10.1083/jcb.200603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem Pharmacol. 2005;70:481–488. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, et al. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol. 2009;186:98–110. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. Febs Letts. 2009;583:1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang WD, Struhl G. Drosophila epsin mediates a select endocytic pathway that DSL ligands must enter to activate notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 25.Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 26.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian XL, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C-elegans. Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Ko G, Zatti A, Di Giacomo G, Liu LJ, Raiteri E, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin1 and epsin2 in mice. Proc Natl Acad Sci USA. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Canc Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]