Abstract
Synapse-associated protein 102 (SAP102) and postsynaptic density 95 (PSD-95) are two major cytoskeleton proteins in the postsynaptic density (PSD). Both of them belong to the membrane-associated guanylate kinase (MAGUK) family, which clusters and anchors glutamate receptors and other proteins at synapses. In our previous study, we found that SAP102 and PSD-95 have different distributions, using combined light/electron microscopy (LM/EM) methods.1 Here, we double labeled endogenous SAP102 and PSD-95 in mature hippocampal neurons, and then took images by two different kinds of super resolution microscopy—Stimulated Emission Depletion microscopy (STED) and DeltaVision OMX 3D super resolution microscopy. We found that our 2D and 3D super resolution data were consistent with our previous LM/EM data, showing significant differences in the localization of SAP102 and PSD-95 in spines: SAP102 is distributed in both the PSD and cytoplasm of spines, while PSD-95 is concentrated only in the PSD area. These results indicate functional differences between SAP102 and PSD-95 in synaptic organization and plasticity.
Key words: PSD, postsynaptic membrane, scaffolding proteins, glutamate receptors, MAGUK
The postsynaptic density (PSD) is an electron-dense region of synapses in the CNS. The PSD plays critical roles in synaptic plasticity as it is enriched with hundreds of synaptic proteins, including receptors, cytoskeleton proteins, adhesion molecules and signal proteins. SAP102 and PSD-95 are two major scaffolding proteins in the PSD.2,3 Both of them belong to the membrane-associated guanylate kinase (MAGUK) family, which cluster receptors using three PDZ binding domains and anchor to the other cytoskeleton proteins using SH3/GK domains.4 Our recent study reveals different mobility and localization between SAP102 and PSD-95 in spines.1 Here, we further compare the localization of SAP102 and PSD-95 in spines using regular confocal microscopy, two kinds of super resolution microscopy and EM. Consistent with our earlier finding, these new data confirm that PSD-95 is concentrated closer to the postsynaptic membrane than SAP102.
To study the localization of endogenous SAP102 and PSD-95 in spines of cultured hippocampal neurons, we first double stained PSD-95 and SAP102 and then observed their localization with a Zeiss 510 confocal microscope. As described in our previous study, both SAP102 and PSD-95 were enriched in spines. However, SAP102 also was observed throughout the dendritic shaft while PSD-95 was barely expressed in the dendritic shaft (Fig. 1A). The resolution of a regular light microscope is no better than about 200 nm, which makes it hard to further identify the localization of SAP102 and PSD-95 in spines.
Figure 1.
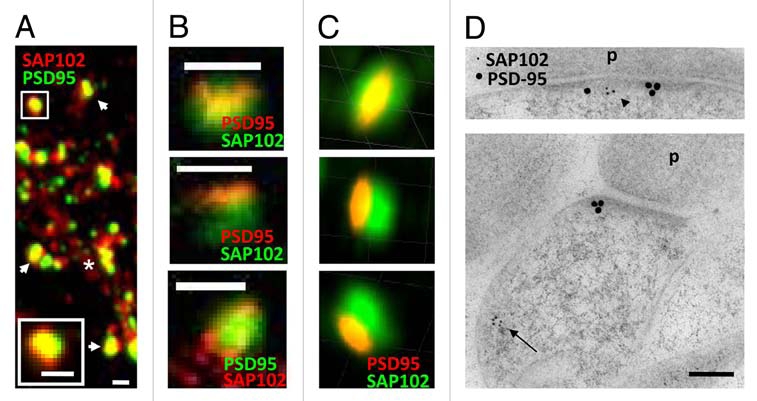
Distribution of SAP102 and PSD-95 in spines. (A–C) Double labeling of endogenous SAP102 and PSD-95 in hippocampal neurons (21 DIV). (A) Image was taken with a conventional confocal microscope. The spine in the upper left is enlarged and shown in the bottom left. The asterisk indicates the dendrite region and the arrows indicate spines. Scale bars, 500 nm. (B) Images were taken with a Leica STED microscope. For the spines in the top and the middle parts, PSD-95 (red) was imaged in the STED channel and SAP102 (green) was imaged in the regular channel. For the spine in the bottom part, SAP102 (red) was imaged in the STED channel and PSD-95 (green) was imaged in the regular channel. Scale bars, 500 nm. (C) Images were taken with a DeltaVision 3D super resolution microscope. The top, middle and bottom parts are images of the same spine viewed in 3D at three different angles. The grid squares are 500 nm. (D) Immunogold labeling of SAP102 (5 nm gold) and PSD-95 (15 nm gold) in synapses from cultured hippocampal neurons (21 DIV). SAP102 is distributed in both the PSD (arrowhead) and cytoplasm (arrow), while PSD-95 is concentrated in the PSD area. P, presynaptic terminal. Scale bar, 100 nm.
Next we observed the SAP102/PSD-95 double stained neurons using Leica TSC Stimulated Emission Depletion microscopy (STED), which achieves super resolution.5,6 The STED system that we used had one super resolution channel and one regular channel. We first labeled PSD-95 with a secondary antibody for STED (Atto 647) and labeled SAP102 with regular Alexa 488 secondary antibody. After overlapping the STED channel with the regular 488 channel, we found that most PSD-95 clusters co-localized with SAP102 clusters, although PSD-95 clusters were much smaller than SAP102 clusters (Fig. 1B). The SAP102 cluster often had a diameter of ∼500 nm, while the PSD-95 cluster often was often found shaped like a bar or a thin oval (∼100 nm wide and ∼500 nm length) on the top of SAP102 staining (Fig. 1B). Interestingly, another STED study showed similar structures for labeling with the presynaptic protein Bassoon.7 Next, we labeled SAP102 with the secondary antibody for STED and labeled PSD-95 with the regular Alexa 488 secondary antibody. In this preparation, small SAP102 clusters were observed throughout the dendritic shaft, whereas some SAP102 clusters colocalized with PSD-95 clusters in spines. The PSD-95 clusters also had diameters of ∼500 nm, however, SAP102 did not form the same bar shape as seen with PSD-95 in the STED channel (Fig. 1B). We also imaged SAP102/PSD-95 double stained neurons using DeltaVision 3D structured-illumination microscopy.8 Consistent with the 2D super resolution image of PSD-95, the 3D super resolution image also showed narrow PSD-95 labeling co-localized with the broader SAP102 labeling pattern (Fig. 1C).
Overall, our 2D and 3D super resolution data were consistent with our EM data in synapses from hippocampal neurons from primary culture (21 days in vitro (DIV); Fig. 1D; also see previous EM data for the adult hippocampus1), suggesting that PSD-95 and SAP102 have different expression patterns in spines and dendrites. The bar-shaped narrow labeling of PSD-95 may represent the PSD structure, since the majority (94.2%) of PSD-95 in spines was clustered at the PSD.1 The scattered expression of SAP102 suggests that SAP102 distributes in the PSD as well as in the cytoplasm of both spine and dendritic shaft.
Acknowledgements
We thank Geoff Daniels and Jochen Sieber from Leica for assisting with the STED microscopy and Peter Franklin and Jennifer Atkins from Applied Precision for assisting with the DeltaVision OMX 3D super resolution microscopy. Research was supported by the NIDCD Intramural Research Program.
References
- 1.Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30:4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, et al. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 3.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 4.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 5.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 6.Nagerl UV, Bonhoeffer T. Imaging living synapses at the nanoscale by STED microscopy. J Neurosci. 2010;30:9341–9346. doi: 10.1523/JNEUROSCI.0990-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuss M, Engelhardt J, Hell SW. Birefringent device converts a standard scanning microscope into a STED microscope that also maps molecular orientation. Opt Express. 2010;18:1049–1058. doi: 10.1364/OE.18.001049. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson MG, Shao L, Carlton PM, Wang CJ, Golubovskaya IN, Cande WZ, et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys J. 2008;94:4957–4970. doi: 10.1529/biophysj.107.120345. [DOI] [PMC free article] [PubMed] [Google Scholar]


