Abstract
Understanding the diversity of animal signals requires knowledge of factors which may influence the different stages of communication, from the production of a signal by the sender up to the detection, identification and final decision-making in the receiver. We studied a Neotropical katydid (Docidocercus gigliotosi) which uses airborne sound for long distance communication, but also an alternative form of private signalling through substrate vibration. Males spend more time with private signalling under full moon conditions, when the nocturnal rainforest favours predation by visually hunting predators. For either type of signal we measured the energetic costs of producing it, its active space, and the background noise levels in both transmission channels. Signal perception was studied using neurophysiological methods under outdoor conditions, which is more reliable for the private mode of communication. Our results demonstrate the complex effects of ecological conditions, such as predation, nocturnal ambient light levels and masking noise on the performance of receivers in detecting mating signals, affecting the net advantage or disadvantage of either mode of communication.
Key words: communication, predation, transmission channel, sensory coding, habitat noise
Airborne sound signals are well studied in birds, frogs and insects,1,2 some represent the most conspicuous signals ever found in intraspecific communication. However, due to their conspicuousness, they are subject to eavesdropping by unintended receivers, with potentially dramatic consequences for the signallers' survival if the eavesdropper is a parasitoid or a predator.3,4 This strong selection pressure may result in evolutionary adaptations that reduce the conspicuousness to predators. However, Endler6 emphasized the fact that conspicuousness of a signal is not a fixed property, but varies with environmental conditions. We would therefore expect selection to act on the individual to adjust its signalling behaviour in response to these varying conditions.
By combining methods from ecology, behaviour, physiology, neurophysiology and biophysics we extended previous studies on the neotropical katydid Docidocercus gigliotosi demonstrating anti-predator defences to escape predation by foliage-gleaning bats which are attracted by the prey's calling songs.7–9 One such adaptation in the prey is the use of substrate-borne tremulation signals, which are undetectable by the predator. As predicted by the “Sensory Drive Model”6,10,11 (Fig. 1), we found changes in the use of the two modes of communication with environmental conditions, such as the lunar cycle, and resulting consequences for signal detection in receivers.12 Our results demonstrate that each adaptive response in signalling of a prey species may be followed by a cascade of consequences for the cooperative communication system, including changes in the costs for signal production, in the active range of a signal, the accuracy of signal detection or discrimination by receivers.
Figure 1.
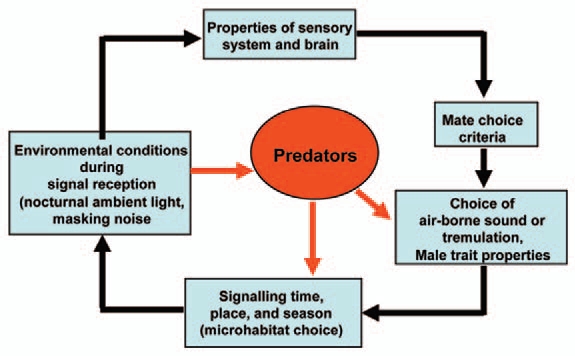
The “Sensory Drive Model” (modified from ref. 10 and 11) adapted for D. gigliotosi using air-borne sound and tremulation for communication. Sensory systems, signals, signaling behavior, predator avoidance, signal modes and habitat choice are all evolutionary coupled. As a shift in one of these traits would have significant consequences for the other traits, they should also coevolve in predictable directions.
Yet, in order to fully understand the evolution of such a system, further knowledge about the influence of the ecology of a species for each step between signal generation and perception is badly needed. This is in particular true for signal perception and discrimination in receivers under realistic ecological conditions. For example, what are the consequences of the extreme low duty cycle of the katydid call (rate 1/10 seconds; duration 23 ms) for female receivers? In the nocturnal rainforest perception and discrimination is complicated by high noise levels in the air-borne sound channel13,14 and the fact that a receiver has to extract information about sound signals based entirely on the analysis of ongoing afferent spiking activity in the auditory pathway. Using a “biological microphone-approach”,15,16 which allows studying sensory coding of sound signals directly in the natural environment of an organism, we have shown that sensory bursts in response to background noise may be falsely misinterpreted by the CNS of a receiver as resulting from conspecific signals (Fig. 2A). This type of error rarely appeared in the private mode of communication based on substrate vibrations.
Figure 2.
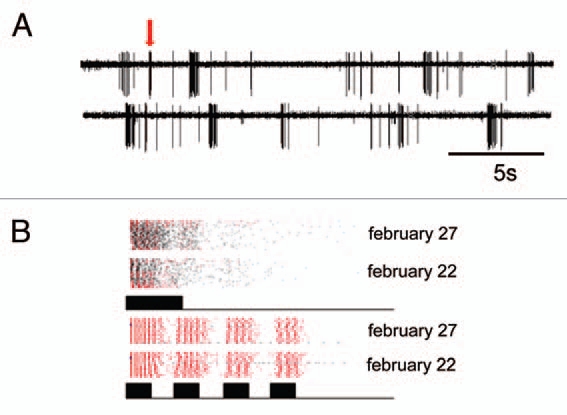
(A) Recording of single cell activity in the tropical rainforest, made at about 45 minutes after sunset, a time when the background noise level had increased from 40 dB SPL to 65 dB SPL. Note the bursting activity of this auditory cell in response to the background, but only one of the bursts (arrow) was elicited by a short, 20 ms stimulus. (B) Clusters of sensory bursts from two recordings in the natural habitat (February 22 and 27, 2004). Bursts elicited by artificial stimuli (either a simple, 20 ms stimulus or series of 4 times 10 ms pulses) are plotted red, bursts associated with noise are plotted in black. Note the similarity of bursts in the two preparations in different nights, and that bursts in response to the simple stimulus clustered together with bursts from noise.
We explored a recently developed unsupervised machine learning algorithm based on probabilistic inference17,18 and asked how much information the CNS can extract from such bursts without ever being told by a postulated “supervisor,” which type and which variants of bursts are characteristic for particular stimuli. One result was that stimuli with more complex temporal structure elicited reliable burst patterns in the afferent neuron which very rarely cluster together with bursts induced by noise, in contrast to “simple” stimuli (Fig. 2B). However, males of the katydid D. gigliotosi use exactly such “simple” stimuli for intraspecific communication, as a result of natural selection exerted by unintended receivers (bats). Although we are currently unable to explain how female receivers solve the detection problem of low duty cycle signals, our results nevertheless would argue for more research on receiver mechanisms in integrated approaches on animal communication.
Acknowledgements
The project was funded by the Austrian Science Foundation, (FWF-P14257 and P17986-B06). We are grateful to the Smithsonian Tropical Research Institute (STRI) and the National Authority for the Environment (ANAM) for research permits and logistical support, which ensured that all work was conducted in conformity with current Panamanian laws.
References
- 1.Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. Chicago and London: University of Chicago Press; 2002. [Google Scholar]
- 2.Greenfield MD. Mechanisms and evolution of arthropod communication. New York: Oxford University Press; 2002. Signalers and receivers. [Google Scholar]
- 3.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- 4.Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Quarter Rev Biol. 1998;73:415–438. [Google Scholar]
- 5.Endler JA. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- 6.Endler JA. Signals, signal conditions and the direction of evolution. Am Nat. 1992;139:125–153. [Google Scholar]
- 7.Belwood JJ, Morris GK. Bat predation and its influence on calling behaviour in Neotropical katydids. Science. 1987;238:64–67. doi: 10.1126/science.238.4823.64. [DOI] [PubMed] [Google Scholar]
- 8.Belwood JJ. Anti-predator defences and ecology of Neotropical forest katydids, especially the Pseudophyllinae. In: Bailey W, Renz DCF, editors. The Tettigoniidae: Behaviour, Systematics, Evolution. Bathurst: Crawford House Press; 1990. pp. 6–26. [Google Scholar]
- 9.Morris GK, Mason AC, Wall P, Belwood JJ. High ultrasonic and tremulation signals in Neotropical katydids (Orthoptera: Tettigoniidae) J Zool. 1994;233:129–163. [Google Scholar]
- 10.Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- 11.Endler J. Some general comments on the evolution and design of animal communication systems. Phil Trans R Soc Lond B. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- 12.Römer H, Lang A, Hartbauer M. The signaller's dilemma: a cost—benefit analysis of public and private communication. PLoS ONE. 2010;5:13325. doi: 10.1371/journal.pone.0013325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang A, Teppner I, Hartbauer M, Römer H. Predation and noise in communication networks of neotropical katydids. In: McGregor P, editor. Animal Communication Networks. Cambridge: Cambridge University Press; 2005. pp. 152–169. [Google Scholar]
- 14.Brumm H, Slabberkoorn H. Acoustic Communication in Noise. Adv Study Behav. 2005;35:151–209. [Google Scholar]
- 15.Rheinlaender J, Römer H. Insect hearing in the field—the use of identified nerve cells as biological microphones. J Comp Physiol A. 1986;158:647–651. [Google Scholar]
- 16.Römer H, Lewald J. High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol. 1992;157:631–642. [Google Scholar]
- 17.Pfeiffer M, Hartbauer M, Lang A, Maass W, Römer H. Probing real sensory worlds of receivers with unsupervised clustering. PLoS Comp Sci. doi: 10.1371/journal.pone.0037354. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey BJ, Dueck D. Clustering by passing messages between data points. Science. 2007;315:972–976. doi: 10.1126/science.1136800. [DOI] [PubMed] [Google Scholar]


