Abstract
Helicases are motor proteins that catalyze the unwinding of duplex nucleic acids in an ATP-dependent manner. They are involved in almost all the nucleic acid transactions. In the present study, we report a comprehensive analysis of helicase gene family in human and its comparison with homologs in model organisms. The human genome encodes for 95 non-redundant helicase proteins, of which 64 are RNA helicases and 31 are DNA helicases. 57 RNA helicases are validated based on annotations and occurrence of conserved helicase signature motifs. These include 14 DExH and 37 DExD subfamily members, six other members such as U5.snRNP, ATR-X, Suv3, FANCJ, and two of superkiller viralicidic activity 2-like helicases. 31 DNA helicases are also identified, which include RecQ, MCM and RuvB-like helicases. Finding a set of helicases in human and almost similar sequences in model organisms suggests that the “core” members of helicase gene family are highly conserved throughout evolution. The present study gives an overview of members of RNA and DNA helicases encoded by the human genome along with their conserved motifs, phylogeny and homologs in model organisms. The study on comparing these homologs will spread light on the organization and complexity of helicase gene family in model organisms. The comprehensive analysis of human helicases presented in this study will further provide an invaluable resource for elaborate biological research on these helicases.
Key words: DEAD-box, DHX and DDX helicases, human helicases, MCM proteins, RecQ helicases, RNA helicases
Introduction
The separation of complementary strands of a DNA and/or RNA duplex is fundamental requirement for many cellular metabolic processes. This is mediated by DNA and/or RNA helicases that unwind nucleic-acid duplexes in an ATP-dependent manner. The energy of ATP hydrolysis is required for an active unwinding process. Generally, helicases exhibit specific polarity depending upon the strand on which they move either in 3′-5′ or 5′-3′ direction. It is interesting to note that the unwinding reaction is independent of sequence specificity. The unwinding property of helicases depends on the type and structure of nucleic acid substrate. Thus all helicases are classified as those that preferentially unwind DNA or RNA duplexes. The unwinding of duplexes provides access to the template for proteins of the transcription, recombination, replication and repair machineries.1 The proteins with which the DNA and/or RNA helicases interact, and their substrates, might be responsible for difference in functioning of DNA and/or RNA helicases.
Each helicase in vivo requires a specific structure of the substrate, interaction with specific proteins in order to participate in a very specific pathway of nucleic acid metabolism. Thus, each helicase must be meticulous in selecting those proteins and substrates with which it will interact. Helicases act in coordination with other proteins/factors and provide molecular motor function. Helicases are ubiquitous and several DNA and RNA helicases have been identified, isolated and characterized till date from a variety of organisms.2–5 The sequence analysis of these enzymes revealed that these proteins contain some well conserved helicase motifs that are involved in ATPase and helicase activities.3,6,7 Due to presence of the sequence DExD in motif II, these proteins are commonly known as DExD box proteins.3,7 Likewise, the RNA helicase families are named as DEAD (DDX), DEAH or DExH (DHX) according to the conserved motif II. These conserved helicase signature motifs of the DExD/H helicases are concentrated in a central core region that spans ∼350–400 amino acids.8,9 But the N- and C-terminal regions are highly variable in size and composition9 and are most probably required for cellular localization and/or interactions with RNAs or other proteins.10 The DExD/H-box proteins play vital role in almost all cellular events that involve DNA and/or RNA. Biochemical characterization by studying the enzymatic activity of DExD/H-box proteins is often used to study the function of these proteins. The DExD/H helicase family constitutes DEAD-box and related DEAH, DExH and DExD families which belong to SF2 family.8,9,11 The variation within their conserved motifs distinguishes the closely related helicase families. The largest of all the families is the DEAD-box family which is characterized by the presence of nine (Q, I, Ia, Ib and from II to VI) conserved helicase motifs.3,6,7 The DExD/H-box proteins can use ATP hydrolysis to promote directly or indirectly the RNA-RNA unwinding,12,13 RNA-protein dissociation,14 and protein-protein interaction.15 The unwinding of dsRNA in vitro was demonstrated by a subset of DExD/H-box proteins.16–19 The cellular functions of DExD/H-box protein are also fulfilled upon displacement of protein factors by DExD/H-box proteins from the single-stranded RNA.14
The sequence homologies among the members of DEAD-box helicases are shared beyond and between the nine conserved helicase motifs. The role of these motifs in ATP-binding, ATP-hydrolysis (ATPase), RNA binding and dsRNA unwinding/helicase activities was shown based on structural, mutational and biochemical analyses.20–22 The motifs I (AxxGxGKT) and II (VLDEAD) are characteristic of nucleotide triphosphate (NTP) hydrolyzing proteins, the motif III (SAT) is involved in coupling of ATP hydrolysis and unwinding. The motifs IV (FVNT) and V (RGxD) are involved in RNA binding and the motif VI (HRIGRxxR) is required for the nucleic acid-dependent NTP hydrolysis.7,23 Using the energy derived from NTP hydrolysis RNA helicases function as molecular motors that rearrange RNA secondary structure thus playing vital role in many cellular processes.24 All cellular organisms and many viral genomes contain RNA helicases. In yeast Saccharomyces cerevisiae, the study of DEAD-box and related proteins has provided the best overview of cellular processes involving RNA helicases.25 Extensive genetic analyses in S. cerevisiae revealed the role of RNA helicases in RNA metabolism and all RNA-related processes. This genetic analysis has shown the role of individual RNA helicases in specific cellular processes, while biochemical experiments have shown that these proteins in the presence of ATP and Mg2+ can dissociate short RNA duplexes. Apart from disrupting secondary structures or short RNA-RNA interactions of a few base pairs, RNA helicases are also involved in unwinding long stretches of double stranded RNA.26 The dynamic RNA-RNA interactions and denaturation of short and/or long RNA duplexes seems to be necessary for all cellular processes that involve RNA helicases.
The DEAD-box RNA helicases which are found in all eukaryotes and most prokaryotes constitute a large family of proteins.4,5,20,24,27 In the genomes of Caenorhabditis elegans and Drosophila melanogaster, nearly 30 genes that encode DEAD-box RNA helicases were identified.28 The Arabidopsis thaliana and rice (Oryza sativa L.) genomes encode ∼32 and ∼21 members of DEAD-box RNA helicases respectively.5,27,28 Plasmodium falciparum genome also encodes a number of DEAD/DEAH box and other helicases.4 As reported earlier, there are at least 36 and 14 members of the DEAD and DEAH families of putative RNA helicases in humans.29
In the present study, we have compiled complete set of 95 DNA and RNA helicases encoded by the human genome. We report identification of 58 DExD/H RNA helicases which includes 42 and 16 members of the DEAD (DDX) and DEAH (DHX) families of putative RNA helicases respectively. In addition, we have also identified six RNA helicases which are independent from DDX and DHX families. These are the U5.snRNP 200 kDa helicase, transcriptional regulator ATR-X helicase, RNA helicase SUPV3L1 (mitochondria), superkiller viralicidic activity 2-like 2 (SKIV2L2), helicase SKI2W (also called as DDX13) and helicase FANCJ. A total of 31 DNA helicases (like RecQ members, KU70, MCM proteins, XPB and XPD repair helicases, RuvB-like helicases, Pif1, Twinkle, BACH1, RecQ alpha, beta and gamma and RTEL1) are also identified. Further, these helicases are validated based on protein alignment studies for conserved helicase signature motifs. A phylogenetic relationship is drawn among different members of RNA and DNA helicases. A comparative bioinformatics approach is adopted to identify homologs of human RNA and DNA helicases in different model organisms like S. cerevisiae, A. thaliana, O. sativa and P. falciparum. The data described in this manuscript clearly indicate that a multitude of helicases are encoded by the human genome. The present studies ultimately lead toward a holistic view of the structure, composition and phylogeny of helicase gene family in humans. Insights derived from this study combined with the comparative in silico approach toward identification of homologs in other model organisms will further our understanding of these ubiquitous motor proteins.
Results
RNA helicases.
We have identified 64 RNA helicases from human genome. A list of these helicases with protein Id, protein name, synonym and gene name is given in Table 1. These 64 RNA helicases are categorized into DHX, DDX and independent families. The human genome contains 16 of DHX, 42 of DDX and six independent helicases.
Table 1.
Human RNA helicases
| S. no. | Swiss-Prot Id | Protein name | Synonym | Gene name |
| 1 | Q92499 | RNA helicase DDX1 | DEAD-box protein 1 | DDX1 |
| DEAD-box protein retinoblastoma | ||||
| DBP-RB | ||||
| 2 | P60842 | RNA helicase eIF4A-1 | Eukaryotic initiation factor eIF4A-I | eIF4A-I |
| DDX2A | DDX2A | |||
| 3 | Q14240 | RNA helicase eIF4A-2 | Eukaryotic initiation factor 4A-II | eIF4A-II |
| DDX2B | DDX2B | |||
| 4 | O00571 | RNA helicase DDX3X | DEAD-box protein 3, X-chromosomal | DDX3X |
| Helicase-like protein 2 | DBX, DDX3 | |||
| HLP2 | ||||
| DEAD-box, X isoform | ||||
| 5 | O15523 | RNA helicase DDX3Y | DEAD-box Y (DBY), Y-chromosomal | DDX3Y |
| 6 | Q9NQI0 | RNA helicase DDX4 | DEAD-box protein 4 | DDX4 |
| Vasa homolog | ||||
| 7 | P17844 | RNA helicase DDX5 | DEAD-box protein 5 | DDX5 |
| RNA helicase p68 | ||||
| 8 | P26196 | RNA helicase DDX6 | DEAD-box protein 6 | DDX6 |
| RNA helicase p54 | HLR2 | |||
| Oncogene RCK | RCK | |||
| 9 | Q14562 | RNA helicase DHX8 | DEAH-box protein 8 | DHX8 |
| RNA helicase HRH1 | ||||
| 10 | Q08211 | RNA helicase A | Nuclear DNA helicase II | DHX9 |
| NDH II | ||||
| DEAH-box protein 9 | ||||
| 11 | Q13206 | RNA helicase DDX10 | DEAD-box protein 10 | DDX10 |
| 12 | Q96FC9 | RNA helicase DDX11 | DEAD/H-box protein 11 | DDX11 |
| CHL1-related protein 1 (hCHLR1) | ||||
| Keratinocyte growth factor-regulated gene | ||||
| KRG-2 | ||||
| 13 | Q92771 | RNA helicase DDX12 | DEAD/H-box protein 12 | DDX12 |
| CHL1-related protein 2 (hCHLR2) | ||||
| 14 | Q15477 | Helicase SKI2W | Helicase-like protein | SKIV2L |
| HLP | DDX13 | |||
| 15 | O43143 | RNA helicase DHX15 | DEAH-box protein 15 | DHX15 |
| ATP-dependent RNA helicase #46 | ||||
| 16 | O60231 | RNA helicase DHX16 | DEAH-box protein 16 | DHX16 |
| ATP-dependent RNA helicase #3 | ||||
| 17 | Q92841 | RNA helicase DDX17 | DEAD-box protein 17 | DDX17 |
| RNA-dependent helicase p72 | ||||
| DEAD-box protein p72 | ||||
| 18 | Q9NVP1 | RNA helicase DDX18 | DEAD box protein 18 | DDX18 |
| Myc-regulated DEAD-box protein | ||||
| MrDb | ||||
| 19 | Q9NUU7 | RNA helicase DDX19A | DEAD-box protein 19A | DDX19A |
| DDX19-like protein | ||||
| 20 | Q9UMR2 | RNA helicase DDX19B | DEAD box protein 19B | DDX19B |
| DEAD box RNA helicase DEAD5 | ||||
| 21 | Q9UHI6 | RNA helicase DDX20 | DEAD-box protein 20 | DDX20 |
| DEAD-box protein DP 103 | ||||
| Component of gems 3 | ||||
| Gemin-3 | ||||
| 22 | Q9NR30 | Nucleolar RNA helicase 2 | Nucleolar RNA helicase II | DDX21 |
| Nucleolar RNA helicase Gu | ||||
| RH II/Gu | ||||
| Gu-alpha | ||||
| DEAD-box protein 21 | ||||
| 23 | Q9BUQ8 | RNA helicase DDX23 | DEAD-box protein 23 | DDX23 |
| 100 kDa U5 snRNP-specific protein (U5-100 kD) | ||||
| PRP 28 homolog | ||||
| 24 | Q9GZR7 | RNA helicase DDX24 | DEAD-box protein 24 | DDX24 |
| 25 | Q9UHL0 | RNA helicase DDX25 | DEAD-box protein 25 | DDX25 |
| Gonadotropin-regulated testicular RNA helicase | ||||
| 26 | Q96GQ7 | RNA helicase DDX27 | DEAD-box protein 27 | DDX27 |
| 27 | Q9NUL7 | RNA helicase DDX28 | Mitochondrial DEAD-box protein 28 | DDX28 |
| 28 | Q7Z478 | RNA helicase DHX29 | DEAH-box protein 29 | DHX29 |
| Nucleic acid helicase DDXx | ||||
| 29 | Q7L2E3 | RNA helicase DHX30 | DEAH-box protein 30 | DHX30 |
| 30 | Q9H8H2 | RNA helicase DDX31 | DEAD-box protein 31 | DDX31 |
| 31 | Q7L7V1 | RNA helicase DHX32 | DEAH-box protein 32 | DHX32 |
| DEAD/H helicase-like protein 1 (DHLP1) | ||||
| DEAD/H box 32 | ||||
| HuDDX32 | ||||
| 32 | Q9H6R0 | RNA helicase DHX33 | DEAH-box protein 33 | DHX33 |
| 33 | Q14147 | RNA helicase DHX34 | DEAH-box protein 34 | DHX34 |
| 34 | Q9H5Z1 | RNA helicase DHX35 | DEAH-box protein 35 | DHX35 |
| 35 | Q9H2U1 | RNA helicase DHX36 | DEAH-box protein 36 | DHX36 |
| MLE-like protein 1 | ||||
| RNA helicase associated with AU-rich element ARE | ||||
| 36 | Q8IY37 | RNA helicase DHX37 | DEAH-box protein 37 | DHX37 |
| 37 | Q92620 | RNA helicase PRP 16 | RNA helicase DHX38 | DHX38 |
| DEAH-box protein 38 | ||||
| 38 | O00148 | RNA helicase DDX39 | DEAD-box protein 39 | DDX39 |
| Nuclear RNA helicase URH49 | ||||
| DEAD-box protein UAP56 | ||||
| Spliceosome RNA helicase BAT 1 | ||||
| RNA helicase p47 | ||||
| 39 | Q8IX18 | RNA helicase DHX40 | DEAH-box protein 40 | DHX40 |
| Protein PAD | ||||
| 40 | Q9UJV9 | RNA helicase DDX41 | DEAD-box protein 41 | DDX41 |
| 41 | Q86XP3 | RNA helicase DDX42 | DEAD-box protein 42 | DDX42 |
| Splicing factor 3B-associated 125 kDa protein | ||||
| SF3b DEAD-box protein | ||||
| 42 | Q9NXZ2 | RNA helicase DDX43 | DEAD-box protein 43 | DDX43 |
| DEAD box protein HAGE | ||||
| Helical antigen | ||||
| Cancer/testis antigen 13 | ||||
| CT13 | ||||
| 43 | Q7L014 | RNA helicase DDX46 | DEAD-box protein 46 | DDX46 |
| PRP 5 homolog | ||||
| 44 | Q9H0S4 | RNA helicase DDX47 | DEAD-box protein 47 | DDX47 |
| 45 | P38919 | RNA helicase eIF4A-3 | Eukaryotic initiation factor 4A-III | eIF4A-III |
| RNA helicase DDX48 | DDX48 | |||
| DEAD box protein 48 | ||||
| Nuclear matrix protein 265 (hNMP 265) | ||||
| 46 | Q9Y6V7 | RNA helicase DDX49 | DEAD-box protein 49 | DDX49 |
| 47 | Q9BQ39 | RNA helicase DDX50 | DEAD-box protein 50 | DDX50 |
| Nucleolar protein Gu2 | ||||
| Gu-beta | ||||
| 48 | Q8N8A6 | RNA helicase DDX51 | DEAD-box protein 51 | DDX51 |
| 49 | Q9Y2R4 | RNA helicase DDX52 | DEAD-box protein 52 | DDX52 |
| RNA helicase RO K1-like | ||||
| 50 | Q86TM3 | RNA helicase DDX53 | DEAD box protein 53 | DDX53 |
| DEAD box protein CAGE | ||||
| Cancer-associated gene protein | ||||
| Cancer/testis antigen 26/CT26 | ||||
| 51 | Q8TDD1 | RNA helicase DDX54 | DEAD-box protein 54 | DDX54 |
| RNA helicase DP97 | ||||
| DEAD-box RNA helicase 97 kDa | ||||
| 52 | Q8NHQ9 | RNA helicase DDX55 | DEAD box protein 55 | DDX55 |
| 53 | Q9NY93 | RNA helicase DDX56 | DEAD-box protein 56 | DDX56 |
| ATP-dependent 61 kDa nucleolar RNA helicase | ||||
| DEAD-box protein 21 | ||||
| 54 | Q6P158 | RNA helicase DHX57 | DEAH-box protein 57 | DHX57 |
| 55 | O95786 | RNA helicase DDX58 | DEAD-box protein 58 | DDX58 |
| Retinoic acid-inducible gene 1 protein RIG-1 | ||||
| 56 | Q96C10 | RNA helicase DHX58 | DEAH-box protein 58 | DHX58 |
| helicase LGP2 | ||||
| Protein D11Lgp2 homolog | ||||
| RIG-I-like receptor LGP2 | ||||
| 57 | Q5T1V6 | RNA helicase DDX59 | DEAD-box protein 59 | DDX59 |
| Zinc finger HIT domain-containing protein 5 | ||||
| 58 | Q8IY21 | RNA helicase DDX60 | DEAD-box protein 60 | DDX60 |
| 59 | Q13838 | Spliceosome RNA helicase BAT1 | DEAD box protein UAP 56 | BAT1 |
| 56 kDa U2AF65-associated protein | UAP56 | |||
| ATP-dependent RNA helicase p47 | ||||
| HLA-B-associated transcript 1 protein | ||||
| 60 | O75643 | U5.snRNP 200 kDa helicase | U5 snRNP-specific 200 kDa protein | ASCC3L1 |
| U5-200KD | ||||
| Activating signal cointegrator 1 complex subunit 3-like 1 | ||||
| BRR 2 homolog | ||||
| 61 | P46100 | Transcriptional regulator ATRX helicase | ATP-dependent helicase ATRX | ATRX |
| X-linked helicase II | ||||
| X-linked nuclear protein (XNP) | ||||
| Znf-HX | ||||
| 62 | Q8IYB8 | RNA helicase SUPV3L1, mitochondrial | SUV3-like protein 1 | SUPV3L1 |
| Suppressor of var1 3-like protein 1 | SUV3 | |||
| 63 | P42285 | Superkiller viralicidic activity 2-like 2 | ATP-dependent helicase SKIV2L2 | SKIV2L2 |
| KIAA0052 | ||||
| 64 | Q9BX63 | Fanconi anemia group J protein | RNA helicase BRIP1 | FANCJ |
| Protein FACJ | BRCA1-interacting protein 1 |
DExH (DHX) helicases.
The identified 16 DHX members of RNA helicases are as follows: DHX8, DHX9, DHX15, DHX16, DHX29, DHX30, DHX32, DHX33, DHX34, DHX35, DHX36, DHX37, DHX38, DHX40, DHX57 and DHX58 (Table 1). The protein alignment studies indicated the presence of 8 conserved helicase motifs (except for DHX32 and DHX58) in all 14 of the DHX members (Fig. 1A). These conserved helicase motifs are as follows, GxxGxGKT, TQPRRV, TDGML, DExH, SAT, FLTG, TNIAET and QRxGRAGR (Fig. 1A). The protein residues for helicase motifs in DHX32 and DHX58 showed major alterations from the canonical sequences that are typical for helicase motifs of DHX members of RNA helicases (Fig. 1B). DHX32 showed absence of typical helicase motifs like SAT, FLTG, TNIAET and QRxGRAGR while changes in the sequence for helicase motifs like GxxKxGKS, TQVHKQ, TDDML and DDIH are observed (Fig. 1B). Similarly, DHX58 has motif DECH but showed change in motifs, LxxGxGKT, TAS and QARGRAR (Fig. 1B).
Figure 1.
Protein alignment of 14 DHX family members of RNA helicases. (A) Entire protein sequence is aligned with ClustalX software. The eight conserved helicase signature motifs are identified on top of the alignment. The names of DHX helicases are identified on the left and sequence positions are given on right. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown. (B) Schematic representations for DHX32 and DHX58 helicase motifs are shown. Note the presence of DExH motif in DDX members like DDX11, DDX12, DDX58 and DDX60. The typical motifs for DHX-type family members are represented on top.
Supplemental Figure 1 shows phylogenetic analysis performed on complete protein sequences for the 14 DHX members of RNA helicases. Most of these members showed high degree of sequence conservation as indicated from higher bootstrap values (Sup. Fig. 1). The members DHX36 and DHX57 emerged as a single clade with striking sequence identity (Sup. Fig. 1). A low bootstrap value is obtained when phylogenetic analysis was made on complete protein sequence for members DHX16 and DHX35 (Sup. Fig. 1). However, the regions corresponding to helicase motifs in DHX16 and DHX35 are well conserved (see Fig. 1A).
DExD (DDX) helicases.
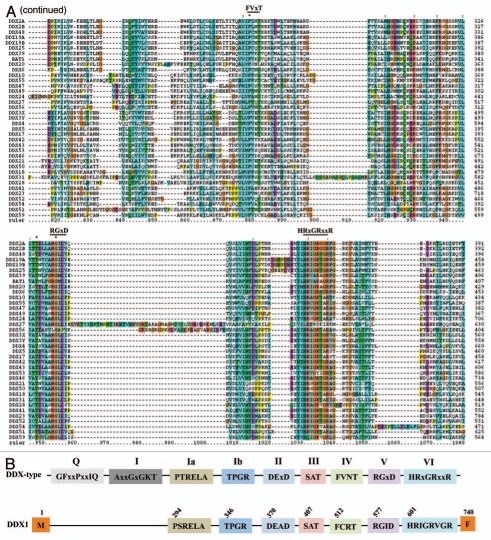
The present analysis has identified 42 DDX members of RNA helicases (Table 1), of which 37 DDX members are validated based on conserved helicase motif signatures (Fig. 2A). These 37 members are, DDX2A, DDX2B, DDX3X, DDX3Y, DDX4, DDX5, DDX6, DDX10, DDX17, DDX18, DDX19A, DDX19B, DDX20, DDX21, DDX23, DDX24, DDX25, DDX27, DDX28, DDX31, DDX39, DDX41, DDX42, DDX43, DDX46, DDX47, DDX48, DDX49, DDX50, DDX51, DDX52, DDX53, DDX54, DDX55, DDX56, DDX59 and BAT1 (Table 1). The protein alignment for the above 37 DDX members of RNA helicases is shown in Figure 2A. In these members, the 9 conserved helicase motifs which are identified are as follows, GFxxPxxIQ, AxxGxGKT, PTRELA, TPGR, DExD, SAT, FVxT, RGxD and HRxGRxxR (Fig. 2A).
Figure 2A and B.

Protein alignment of 37 DDX family members of RNA helicases. (A) Alignment is made on entire protein sequences with ClustalX software. The nine conserved helicase motifs are highlighted on top of the alignment. The names of DDX helicases are identified on the left and sequence positions are given on right. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown. (B) Diagrammatic representation of helicase motifs for typical DDX-type helicases and DDX1 are shown.
Some of these helicase motifs are either completely absent or showed changes in amino acid composition in 5 DDX members, DDX1, DDX11, DDX12, DDX58 and DDX60. In case of DDX1, the motifs GFxxPxxIQ, AxxGxGKT are absent while, motifs PSRELA (instead of PTRELA), TPGR, DEAD, SAT, FCRT (instead of FVNT), RGID and HRIGRVGR are all present (Fig. 2B). The diagrammatic representation of helicase motifs found in DDX11, DDX12, DDX58 and DDX60 is shown in Figure 1B. These helicases, although classified as DDX members, contain a DExH motif (Fig. 1B).
The protein sequence based phylogenetic analysis revealed high level identity features among various DDX family members of RNA helicases (Sup. Fig. 2). For instance, close relationships are established between members like DDX2A-DDX2B, DDX19A-DDX19B, DDX39-BAT1, DDX21-DDX50, DDX3X-DDX3Y, DDX5-DDX17, DDX43-DDX53, DDX10-DDX18 and DDX47-DDX49 which emerged as independent clades with high bootstrap values (Sup. Fig. 2). For the remaining DDX members, a moderately high to low bootstrap support is obtained (Sup. Fig. 2) although these members show a high degree of conservation in the regions composed of helicase motifs (see Fig. 2A).
Independent category of RNA helicases.
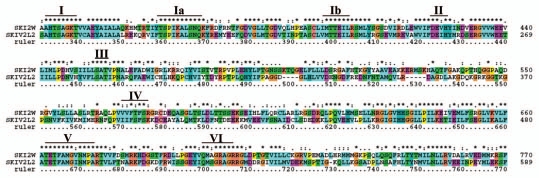
This category includes helicases which neither belong to DHX nor to DDX members of RNA helicases. We classified six members under this category like the U5.snRNP 200 kDa helicase, transcriptional regulator ATR-X [X-linked alpha-thalassemia/mental retardation syndrome (ATR-X syndrome)], RNA helicase SUPV3L1 (mitochondria), superkiller viralicidic activity 2-like 2 (SKIV2L2), helicase SKI2W and helicase FANCJ (Table 1). In humans, the superkiller helicase family contains only 2 members, SKIV2L2 and SKI2W. The protein alignment of these superkiller helicases showed 8 conserved helicase motifs (Fig. 3). The motif I (AHTSAGKT), motif Ia (TSPIKALSNQ), motif Ib (MTTEIL), motif II (DExH), motif III (SAT), motif IV (xxFTFSx), motif V (TETFAMGxNMPA) and motif VI (QMxGRAGR) are identified in SKIV2L2 and SKI2W helicases (Fig. 3).
Figure 3.
Protein alignment of superkiller helicase group members, SKI2W and SKIV2L2. The consensus for eight conserved motifs, motif I (AHTSAGKT), motif Ia (TSPIKALSNQ), motif Ib (MTTE IL), motif II (DExH), motif III (SAT), motif IV (xxFTFSx), motif V (TET FAMGxNMPA) and motif VI (QMxGRA GR) are identified on top of the alignment made with ClustalX alignment program. The names of superkiller helicases are identified on the left and sequence positions are given on right. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown.
DNA helicases.
A list of identified DNA helicases from human genome is given in Table 2. Altogether, 31 DNA helicases have been identified which includes 5 RecQ members, KU70, 9 MCM (minichromosome maintenance) proteins, nucleolin, 2 chromodomain helicases, 2 DNA repair helicases, lymphoid-specific helicases, DNA helicase INO80 complex homolog 1 and 2 RuvB-like helicases, Pif1, Twinkle, BACH1 and three RecQ5 isoforms alpha, beta and gamma, and regulator of telomere elongation helicase (RTEL1) (Table 2).
Table 2.
Human DNA helicases
| S. no. | Swiss-Prot Id | Protein name | Synonym | Gene name |
| 1 | P46063 | DNA helicase Q1 | RECQ1 | RECQ1 |
| DNA-dependent ATPase Q1 | ||||
| 2 | P54132 | Bloom syndrome protein | RECQ2; RECQL2; RECQL3 | BLM |
| RecQ-like type 2 | ||||
| RecQ protein-like 3 | ||||
| 3 | Q14191 | Werner syndrome protein | RECQ3; RECQL2; RECQL3 | WRN |
| 4 | O94761 | DNA helicase Q4 | RecQ protein-like 4 | RECQ4 |
| RECQ4; RECQL4 | ||||
| 5 | O94762 | DNA helicase Q5 | RecQ protein-like 5 | RECQ5 |
| RECQ5; RECQL5 | ||||
| 6 | P12956 | DNA helicase 2 subunit 1 | DNA helicase II 70 kDa subunit | KU70 |
| Lupus Ku autoantigen protein p70 (ku70) | ||||
| 70 kDa subunit of Ku antigen | ||||
| Thyroid-lupus autoantigen (TLAA) | ||||
| CTC box-binding factor 75 kDa subunit (CTCBF) | ||||
| DNA repair protein XRCC6 | ||||
| 7 | P49736 | MCM2 | DNA replication licensing factor MCM2 | MCM2 |
| Nuclear protein BM28 | ||||
| 8 | P25205 | MCM3 | DNA replication licensing factor MCM3 | MCM3 |
| RLF subunit beta | ||||
| P1-MCM3 | ||||
| 9 | P33991 | MCM4 | DNA replication licensing factor MCM4 | MCM4 |
| CDC21 homolog | ||||
| P1-CDC21 | ||||
| 10 | P33992 | MCM5 | DNA replication licensing factor MCM5 | MCM5 |
| CDC46 homolog | ||||
| P1-CDC46 | ||||
| 11 | Q14566 | MCM6 | DNA replication licensing factor MCM6 | MCM6 |
| p105MCM | ||||
| 12 | P33993 | MCM7 | DNA replication licensing factor MCM7 | MCM7 |
| CDC47 homolog | ||||
| P1.1-MCM3 | ||||
| 13 | Q9UJA3 | MCM8 | DNA replication licensing factor MCM8 | MCM8 |
| Minichromosome maintenance 8 | ||||
| 14 | Q9NXL9 | MCM9 | DNA replication licensing factor MCM9 | MCM9 |
| 15 | Q7L590 | MCM10 | Protein MCM10 homolog | MCM10 |
| 16 | P19338 | Nucleolin | Protein C23 | NCL |
| 17 | O14647 | CHD2 | Chromodomain-helicase-DNA-binding protein 2 | CHD2 |
| 18 | Q9P2D1 | CHD7 | ATP-dependent helicase CHD7 | CHD7 |
| CHD-7 | ||||
| 19 | P19447 | XPB | TFIIH basal transcription factor complex 89 kDa subunit | ERCC3 |
| 20 | P18074 | XPD | TFIIH basal transcription factor complex 80 kDa subunit | ERCC2 |
| 21 | Q9NRZ9 | Lymphoid-specific helicase | SWI/SNF2-related matrix-associated actin-dependent- | HELLS |
| regulator of chromatin subfamily A member 6 | ||||
| Proliferation-associated SNF2-like protein | PASG, SMARCA6 | |||
| 22 | Q9ULG1 | hINO | 80 DNA helicase INO 80 complex homolog 1 | INOC1 |
| 23 | Q9Y265 | RuvB-like 1 | INO 80H, NMP238, TIP49, TIP49A | RUVBL1 |
| 24 | Q9Y230 | RuvB-like 2 | INO 80J, TIP48, TIP49B | RUVBL2 |
| 25 | Q9H611 | PIF1 | RRM3 DNA helicase | Pif1 |
| 26 | Q96RR1 | Twinkle | T7 gp4-like protein | PEO1 |
| Progressive external ophthalmoplegia 1 protein | ||||
| T7-like mitochondrial DNA helicase | ||||
| 27 | AAI01475* | BACH1 | BRCA1 interacting protein C-teminal helicase | BRIP1; BACH1 |
| 28 | BAA95952* | RecQ5 alpha | DNA helicase recQ5 alpha | RecQ5a |
| 29 | BAA95953* | RecQ5 beta | DNA helicase recQ5 beta | RecQ5b |
| 30 | BAA95954* | RecQ5 gamma | DNA helicase recQ5 gamma | RecQ5c |
| 31 | Q9NZ71 | RTEL1 | Novel helicase-like | RTEL1 |
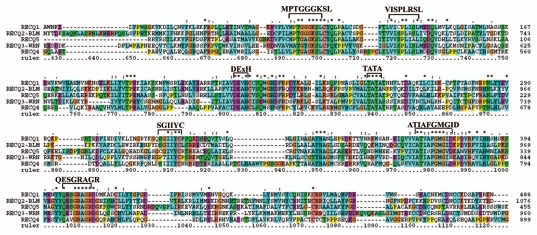
The 5 RecQ members which are identified are, RecQ1 (DNA helicase Q1), RecQ2 [Bloom (BLM) syndrome], RecQ3 [Werner (WRN) syndrome], RecQ4 [DNA helicase Q4 or Rothmund-Thomson syndrome (RTS)] and RecQ5 (DNA helicase Q5) (Table 2). Of the 5 RecQ members that are identified, two are smaller (RecQ1 and RecQ5 of 649 and 991 amino acids, respectively) and three larger (BLM, WRN and RecQ4 of 1,417, 1,432 and 1,208 amino acids, respectively). A common structural feature in this family of enzymes is the presence of central domain with ∼400–450 amino acids containing the 7 helicase signature motifs (Fig. 4). These motifs are MPTGGGKSL, VISPLRSL, DExH, TATA, SGIIYC, ATIAFGMGID and QESGRAGR (Fig. 4). It is interesting to observe a high degree of sequence conservation within these 7 helicase motifs among all the members of the RecQ family (Fig. 4). The phylogenetic analysis of the 5 RecQ family members is shown in Supplemental Figure 3. The RecQ1-RecQ2 and RecQ3-RecQ4 form separate clades with 97 and 58% of bootstrap support respectively (Sup. Fig. 3).
Figure 4.
Protein alignment of five RecQ helicases in human. The five RecQ members are identified on left and sequence position of amino acid residues are shown on right. The seven conserved RecQ motifs are labeled on top of the alignment. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown.
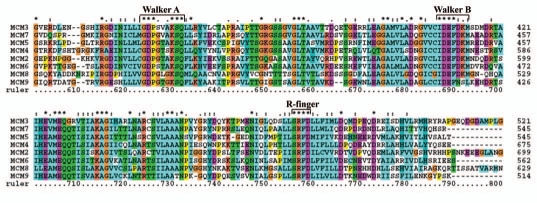
The MCM complex in humans is composed of subunits MCM2-7 (Table 2). Other MCMs like MCM8-10 are also identified (Table 2). The MCM2-7, MCM8 and MCM9 are characterized by the presence of the MCM-box of ∼200 residues (Fig. 5). The MCM-box showed two ATPase consensus motifs, the Walker A and Walker B motifs (Fig. 5). An invariant lysine (K) residue which is found in all ATP-binding proteins also occurs in the Walker A motif (Fig. 5). The MCM-specific consensus, GDPxx(A/S)KSQ, is present in Walker A consensus motif wherein a glycine (G) occurs in place of alanine (A) or serine (S) in MCM8 and MCM9 along with several additional conserved residues (Fig. 5). The Walker B element consensus, IDEFDKM, is present in all MCMs (MCM2-7 and MCM8) except MCM9 wherein the amino acids residues in this motif are only partially conserved (IDEFNSL) (Fig. 5). All MCM proteins contain a short motif SRFD known as the “arginine finger“ approximately 60 residues after the Walker B element (Fig. 5). MCM10 has no obvious sequence similarity with MCM2-7. The protein sequences of MCM2-7, MCM8 and MCM9 are analyzed for their phylogenetic relationship (Sup. Fig. 4). The study revealed a close relationship between MCM3 and 7, MCM4 and 5 and MCM8 and 9 (Sup. Fig. 4).
Figure 5.
Protein alignment of MCM proteins in human. Amino acid alignment of canonical MCM2-7, and the unusual MCM8 and MCM9 proteins. The figure is constructed with ClustalX program, names of MCM proteins are given on left, and sequence positions on right. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown. Note the presence of a glycine (G) instead of alanine (A) or serine (S) in the Walker A motif of MCM8 and MCM9 proteins.
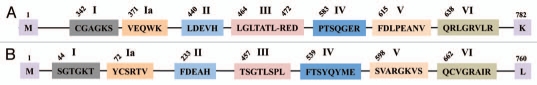
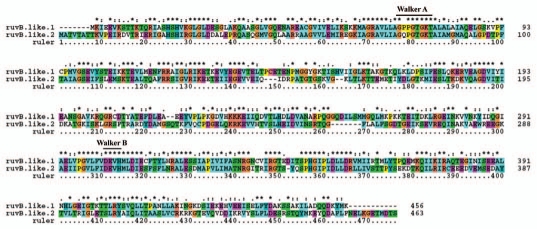
The DNA repair helicases, XPB and XPD, have also been identified in human genome (Table 2). Each of these helicases shows the presence of 7 consensus motifs indicated as I, Ia, II, III, IV, V and VI (Fig. 6A and B). However, the amino acid residues of the 7 consensus motifs differ between these two helicases (Fig. 6A and B). In the XPB helicase, the amino acid composition of these motifs is, CGAGKS (motif I), VEQWK (motif Ia), LDEVH (motif II), LGLTATLxRED (motif III), PTSQGER (motif IV), FDLPEANV (motif V) and QRLGRVLR (motif VI) (Fig. 6A). These 7 consensus motifs in XPD helicase are, SGTGKT (motif I), YCSRTV (motif Ia), FDEAH (motif II), TSGTLSPL (motif III), FTSYQYME (motif IV), SVARGKVS (motif V) and QCVGRAIR (motif VI) (Fig. 6B). The protein alignment of DNA helicases RuvB-like 1 and RuvB-like 2 is shown in Figure 7. The protein length is 456 and 463 amino acids in RuvB-like 1 and RuvB-like 2 respectively. The protein sequence of these helicases is relatively well conserved (Fig. 7). The characteristic Walker A and Walker B motifs are present (Fig. 7). The Walker A consensus, G(x)4GKT and Walker B consensus DExH are well conserved in these helicases (Fig. 7).
Figure 6.
Schematic representation of seven conserved helicase motifs found in DNA repair helicases, XPB and XPD. The numbers given on top of the diagram indicate amino acid position of the first residue of a conserved motif for the entire protein sequence. (A) Amino acid consensus for motifs of XPB helicase. The sequences representing CGAGKS (motif I), VEQWK (motif Ia), LDEVH (motif II), LGLTATLxRED (motif III), PTSQGER (motif IV), FDLPEANV (motif V) and QRLGRVLR (motif VI) are shown. The first and last protein residues are methionine (M) and lysine (K) respectively are indicated. (B) Amino acid consensus for motifs of XPD helicase. The consensus sequence for these motifs is shown, SGTGKT (motif I), YCSRTV (motif Ia), FDEAH (motif II), TSGTLSPL (motif III), FTSYQYME (motif IV), SVARGKVS (motif V) and QCVGRAIR (motif VI). The first and last protein residues are methionine (M) and leucine (L) are shown.
Figure 7.
Protein alignment of members of human ‘RuvB-like’ family of helicases. The Walker A (G(x)4GKT) and Walker B consensus (DExH) are indicated on top of the alignment. The sequences are aligned using ClustalX program. Names of RuvB-like proteins are given on left, and sequence positions on right. Asterisks and dots drawn on top of sequence indicate identical residues and conservative amino acid changes, respectively. Gaps in the amino acid sequences are introduced to improve the alignment. Only a part of protein alignment with conserved helicase motifs is shown.
Homology search in model organisms.
We identified the homologs of human RNA and DNA helicases in model organisms like S. cerevisae, A. thaliana, O. sativa and P. falciparum. The Table 3 shows homologs of human RNA helicases in these organisms. Several human homologs for DDX and DHX members in rice have been annotated as DEAD-box, or DEAD/DEAH-box proteins respectively (Table 3). The homologs of human DNA helicases in S. cerevisae, A. thaliana, O. sativa and P. falciparum are given in Table 4. We have also identified human homologs of RNA and DNA helicases in P. falciparum and reconfirmed their names/annotations based on the BLAST searches (Sup. Tables 1 and 2).
Table 3.
Homologs for human RNA helicases in different model organisms
| S. no. | Human | Rice* | Arabidopsis | Yeast | P. falciparum‡ |
| 1 | ASCC3L1 | U5.snRNP (LOC_Os02g01740) | RH13 (Q93Y39) | BRR 2 (P32639) | U5.snRNP (PFD1060w) |
| 2 | ATRX | SNF2 (LOC_Os10g31970) | PICKLE (Q9S775) | RDH54 (P38086) | PfSNF2L (PF11_0053) |
| 3 | DDX1 | DEAD-box (LOC_Os01g07080) | RH37 (Q84W89) | DBP2 (P24783) | DEAD-box (PFE1085w) |
| 4 | DDX2A | DEAD-box (LOC_Os06g48750) | eIF4A-3 (Q9CAI7) | eIF4A (P10081) | helicase 45 (PF14_0655) |
| 5 | DDX2B | DEAD-box (LOC_Os02g05330) | eIF4A-2 (P41377) | eIF4A (P10081) | helicase 45 (PF14_0655) |
| 6 | DDX3X | DEAD-box 52B (LOC_Os07g10250) | RH52 (Q9M2F9) | DBP1 (P24784) | RNA helicase (PF08_0096) |
| 7 | DDX3Y | DEAD-box (LOC_Os11g38670) | RH52 (Q9M2F9) | DBP1 (P24784) | helicase (PF14_0437) |
| 8 | DDX4 | rhlE (LOC_Os01g08930) | RH39 (Q56X76) | DBP2 (P24783) | helicase (PF14_0437) |
| 9 | DDX5 | DEAD-box (LOC_Os01g10050) | RH20 (Q9C718) | DBP2 (P24783) | helicase (PF14_0437) |
| 10 | DDX6 | dhh1 (LOC_Os02g42860) | RH8 (Q8RXK6) | DHH1 (P39517) | RNA helicase (PFC0915w) |
| 11 | DDX10 | DBP4 (LOC_Os02g57980) | RH32 (Q9FFT9) | DBP4 (P20448) | DEAD/DEAH-box (PFF1500c) |
| 12 | DDX11 | helicase (LOC_Os05g13300) | UVH6 (Q8W4M7) | CHL1 (P22516) | DNA-repair helicase (PF14_0081) |
| 13 | DDX12 | helicase (LOC_Os05g13300) | UVH6 (Q8W4M7) | CHL1 (P22516) | DEAD-box (MAL13P1.134) |
| 14 | DDX13 | DSHCT (LOC_Os02g06500) | RH29 (O49289) | SKI2 (P35207) | helicase (PFI0480w) |
| 15 | DDX17 | recG (LOC_Os02g48100) | RH2 (Q94A52) | DBP5 (P20449) | helicase (PF14_0437) |
| 16 | DDX18 | DDX18 (LOC_Os06g34420) | RH51 (Q9LIH9) | HAS1 (Q03532) | DEAD/DEAH-box (PFF1500c) |
| 17 | DDX19A | DBP5 (LOC_Os03g06220) | RH38 (Q93ZG7) | DBP5 (P20449) | helicase 45 (PF14_0655) |
| 18 | DDX19B | DEAD-box (LOC_Os03g06220) | RH38 (Q93ZG7) | DBP5 (P20449) | helicase 45 (PF14_0655) |
| 19 | DDX20 | PICKLE (LOC_Os01g65850) | eIF4A-1 (P41376) | DHH1 (P39517) | helicase 45 (PF14_0655) |
| 20 | DDX21 | NRH2 (LOC_Os03g61220) | RH3 (Q8L7S8) | DBP1 (P24784) | helicase (PFE0215w) |
| 21 | DDX23 | DDX23 (LOC_Os03g50090) | RH21 (P93008) | PRP 28 (P23394) | snrnp protein (PFE0925c) |
| 22 | DDX24 | MAK5 (LOC_Os04g43140) | RH13 (Q93Y39) | MAK5 (P38112) | DEAD/DEAH-box (PFB0860c) |
| 23 | DDX25 | DEAD-box (LOC_Os03g06220) | RH38 (Q93ZG7) | DBP5 (P20449) | helicase 45 (PF14_0655) |
| 24 | DDX27 | DRS1 (LOC_Os12g29660) | RH28 (Q9ZRZ8) | DRS1 (P32892) | DEAD/DEAH-box (PFL2475w) |
| 25 | DDX28 | DEAD-box (LOC_Os01g08930) | RH39 (Q56X76) | DBP8 (P38719) | snrnp protein (PFE0925c) |
| 26 | DDX31 | DEAD-box (LOC_Os03g58810) | RH17 (Q7XJN0) | DBP7 (P36120) | DEAD-box (MAL7P1.113) |
| 27 | DDX39 | DEAD-box (LOC_Os01g36920) | RH56 (Q9LFN6) | SUB2 (Q07478) | UAP 56 (PFB0445c) |
| 28 | DDX41 | DDX41 (LOC_Os06g48210) | RH35 (Q9LU46) | DBP2 (P24783) | RNA-helicase 1 (PFE1390w) |
| 29 | DDX42 | DEAD-box (LOC_Os03g19530) | RH24 (O22907) | DBP2 (P24783) | helicase (PF14_0437) |
| 30 | DDX43 | DEAD-box (LOC_Os01g10050) | RH20 (Q9C718) | DBP2 (P24783) | helicase (PF14_0437) |
| 31 | DDX46 | DDX46 (LOC_Os08g05810) | RH42 (Q8H0U8) | PRP 5 (P21372) | RNA helicase (PFE0430w) |
| 32 | DDX47 | DDX47 (LOC_Os03g46610) | RH10 (Q8GY84) | RRP3 (P38712) | DEAD/DEAH-box (PFB0860c) |
| 33 | DDX48 | DEAD-box (LOC_Os03g36930) | RH2 (Q94A52) | FAL1 (Q12099) | eIF (PFD1070w) |
| 34 | DDX49 | DDX49 (LOC_Os07g43980) | RH36 (Q9SA27) | DBP8 (P38719) | DEAD/DEAH-box (PFB0860c) |
| 35 | DDX50 | DEAD-box 7 (LOC_Os09g34910) | RH7 (Q39189) | DBP1 (P24784) | helicase (PFE0215w) |
| 36 | DDX51 | DDX51 (LOC_Os02g55260) | RH1 (Q7FGZ2) | DBP6 (P53734) | DEAD/DEAH-box (PFB0860c) |
| 37 | DDX52 | DDX52 (LOC_Os07g45360) | RH57 (Q84TG1) | ROK1 (P45818) | helicase (PF14_0437) |
| 38 | DDX53 | DEAD-box 30 (LOC_Os01g68320) | RH30 (Q8W4R3) | DBP2 (P24783) | helicase (PF14_0437) |
| 39 | DDX54 | DDX54 (LOC_Os08g32090) | RH29 (O49289) | DBP10 (Q12389) | RNA helicase (MAL8P1.19) |
| 40 | DDX55 | DEAD-box (LOC_Os01g07080) | RH18 (Q9FLB0) | SPB4 (P25808) | DEAD/DEAH-box (PFF1500c) |
| 41 | DDX56 | DDX56 (LOC_Os03g51900) | RH16 (Q9SW44) | DBP9 (Q06218) | DEAD/DEAH-box (PFB0860c) |
| 42 | DDX58 | Dicer (LOC_Os03g02970) | Dicer (Q9SP32) | MPH1 (P40562) | helicase (PF14_0437) |
| 43 | DDX59 | DEAD-box 41 (LOC_Os02g10770) | RH41 (Q3EBD3) | DBP2 (P24783) | helicase (PF14_0437) |
| 44 | DDX60 | DSHCT (LOC_Os02g06500) | RH57 (Q84TG1) | DOB1 (P47047) | DEAD/DEAH-box (PFI0165c) |
| 45 | DHX8 | DHX8 (LOC_Os06g09280) | sf (O22899) | PRP22 (P24384) | RNA helicase (PF10_0294) |
| 46 | DHX9 | DEAD/DEAH-box (LOC_Os10g33275) | DHX15 (O22899) | YLR419W (Q06698) | RNA helicase (PFI0860c) |
| 47 | DHX15 | pre-mRNA sf (LOC_Os03g19960) | DHX15 (O22899) | PRP43 (P53131) | RNA helicase (PFI0860c) |
| 48 | DHX16 | DHX16 (LOC_Os03g17432) | DHX8 (Q38953) | PRP22 (P24384) | RNA helicase (PF10_0294) |
| 49 | DHX29 | DEAD/DEAH-box (LOC_Os04g35260) | DHX8 (Q38953) | YLR419W (Q06698) | RNA helicase (PFI0860c) |
| 50 | DHX30 | DEAD/DEAH-box (LOC_Os10g33275) | DHX15 (O22899) | YLR419W (Q06698) | RNA helicase (PF10_0294) |
| 51 | DHX32 | pre-mRNA sf (LOC_Os03g19960) | DHX15 (O22899) | PRP43 (P53131) | RNA helicase (PFI0860c) |
| 52 | DHX33 | DHX8 (LOC_Os06g09280) | DHX8 (Q38953) | PRP22 (P24384) | RNA helicase (PF10_0294) |
| 53 | DHX34 | DEAD/DEAH-box (LOC_Os10g33275) | DHX8 (Q38953) | PRP2 (P20095) | RNA helicase (PF10_0294) |
| 54 | DHX35 | DHX35 (LOC_Os01g11370) | DHX8 (Q38953) | PRP22 (P24384) | RNA helicase (PFI0860c) |
| 55 | DHX36 | DHX36 (LOC_Os01g02884) | sf (O22899) | YLR419W (Q06698) | RNA helicase (PF10_0294) |
| 56 | DHX37 | helicase (LOC_Os02g50370) | DHX8 (Q38953) | DHR1 (Q04217) | RNA helicase (PF10_0294) |
| 57 | DHX38 | PRP16 (LOC_Os07g32430) | DHX8 (Q38953) | PRP16 (P15938) | splicing factor (MAL13P1.322) |
| 58 | DHX40 | PRP16 (LOC_Os07g32430) | DHX8 (Q38953) | PRP22 (P24384) | RNA helicase (PFI0860c) |
| 59 | DHX57 | DEAD/DEAH-box (LOC_Os10g33275) | DHX8 (Q38953) | YLR419W (Q06698) | RNA helicase (PF10_0294) |
| 60 | DHX58 | Dicer (LOC_Os03g02970) | Dicer (Q9SP32) | MPH1 (P40562) | DEAD/DEAH-box (PFL2475w) |
| 61 | BAT1 | DEAD-box (LOC_Os01g36920) | RH56 (Q9LFN6) | SUB2 (Q07478) | UAP56 (PFB0445c) |
| 62 | SUPV3L1 | SUV3 (LOC_Os03g53500) | RH53 (Q9LUW5) | SUV3 (P32580) | DEAD-box (PFF1140c) |
| 63 | SKIV2L2 | SKIV2L2 (LOC_Os12g18140) | ISE2 (B9DFG3) | DOB1 (P47047) | RNA helicase (PFF0100w) |
| 64 | FANCJ | helicase (LOC_Os09g37920) | UVH6 (Q8W4M7) | CHL1 (P22516) | DNA-repair helicase (PF14_0081) |
Table 4.
Homologs for DNA helicases in different model organisms
| S. no. | Human | Rice* | Arabidopsis | Yeast | P. falciparum‡ |
| 1 | RECQ1 | RECQ4A (LOC_Os11g48090) | RH20 (Q9C718) | SGS1 (P35187) | DNA helicase (PF14_0278) |
| 2 | BLM | RECQ4B (LOC_Os04g35420) | RH20 (Q9C718) | SGS1 (P35187) | DNA helicase (PFI0910w) |
| 3 | WRN | RecQSim (LOC_Os05g05810) | RH41 (Q3EBD3) | SGS1 (P35187) | DNA helicase (PF14_0278) |
| 4 | RECQ4 | RH40 (LOC_Os04g40970) | RH30 (Q8W4R3) | SGS1 (P35187) | DNA helicase (PF14_0278) |
| 5 | RECQ5 | RecQl3 (LOC_Os02g54020) | RH14 (Q8H136) | SGS1 (P35187) | DNA helicase (PFI0910w) |
| 6 | KU70 | ku80 (LOC_Os07g08729) | ARP (P45951) | KU70 (P32807) | n/f |
| 7 | MCM2 | MCM2 (LOC_Os11g29380) | MCM3 (Q9FL33) | MCM2 (P29469) | MCM2 (PF14_0177) |
| 8 | MCM3 | MCM3 (LOC_Os05g39850) | MCM3 (Q9FL33) | MCM3 (P24279) | MCM3 (PFE1345c) |
| 9 | MCM4 | MCM4 (LOC_Os01g36390) | PROL (P43299) | CDC54 (P30665) | MCM4-related (PF13_0095) |
| 10 | MCM5 | MCM5 (LOC_Os02g55410) | MCM3 (Q9FL33) | MCM5 (P29496) | MCM5 (PFL0580w) |
| 11 | MCM6 | MCM6 (LOC_Os05g14590) | PROL (P43299) | MCM6 (P53091) | Replication licensing factor (PF13_0291) |
| 12 | MCM7 | MCM7 (LOC_Os12g37400) | PROL (P43299) | CDC47 (P38132) | MCM7 (PF07_0023) |
| 13 | MCM8 | MCM8 (LOC_Os05g38850) | PROL (P43299) | MCM6 (P53091) | MCM protein (PFL0560c) |
| 14 | MCM9 | MCM9 (LOC_Os06g11500) | MCM3 (Q9FL33) | CDC54 (P30665) | Replication licensing factor (PF13_0291) |
| 15 | MCM10 | MCM10 (LOC_Os09g36820) | MCM10 (Q5XVE2) | MCM10 (P32354) | conserved Plasmodium protein (PF11_0207) |
| 16 | NCL | NCL (LOC_Os04g52960) | AT1G48920# | PUB1 (P32588) | RNA binding protein (PFI1175c) |
| 17 | CHD2 | SNF2 (LOC_Os07g46590) | PKL (Q9S775) | CHD1 (P32657) | CHD1 (PF10_0232) |
| 18 | ERCC3 | XPB2 (LOC_Os01g49680) | XPB2 (Q9FUG4) | RAD25 (Q00578) | DNA repair helicase rad25 (PF10_0369) |
| 19 | ERCC2 | UVH6 (LOC_Os05g05260) | UVH6 (Q8W4M7) | RAD3 (P06839) | DNA excision-repair helicase (PFI1650w) |
| 20 | CHD7 | CHD3 (LOC_Os06g08480) | ISW2 (Q8RWY3) | ISW2 (Q08773) | CHD1 (PF10_0232) |
| 21 | HELLS | SNF2 (LOC_Os03g22900) | BRM (Q6EVK6) | P43610 | DEAD/DEAH box helicase (PFB0730w) |
| 22 | INOC1 | SNF2 (LOC_Os03g22900) | INO80 (Q8RXS6) | INO80 (P53115) | ATP-dependent helicase (PF08_0048) |
| 23 | RuvB-like 1 | ruvB-like (LOC_Os07g08170) | AT5G67630# | RUVBL1 (Q03940) | RuvB DNA helicase (PF11_0071) |
| 24 | RuvB-like 2 | ruvB-like 2 (LOC_Os06g08770) | AT3G49830# | RUVBL2 (Q12464) | DNA helicase (PF13_0330) |
| 25 | PIF1 | n/f | PIF1 (AT 3G51690#) | PIF1 (P07271) | n/f |
| 26 | Twinkle | n/f | n/f | n/f | n/f |
| 27 | BACH1 | helicase (LOC_Os09g37920) | UVH6 (Q8W4M7) | CHL1 (P22516) | DNA repair helicase (PF14_0081) |
| 28 | RecQ5 alpha | DEAD-box (LOC_Os02g54020) | AT4G35740# | SGS1 (P35187) | DNA helicase (PFI0910w) |
| 29 | RecQ5 beta | DEAD-box (LOC_Os02g54020) | AT4G35740# | SGS1 (P35187) | DNA helicase (PFI0910w) |
| 30 | RecQ5 gamma | DEAD-box (LOC_Os02g54020) | AT4G35740# | SGS1 (P35187) | DNA helicase (PFI0910w) |
| 31 | RTEL1 | helicase (LOC_Os01g40980) | UVH6 (Q8W4M7) | RAD3 (P06839) | DNA repair helicase (PF14_0081) |
Swiss-Prot Ids are given in brackets. n/f, not found.
Locus Ids are indicated in brackets for rice homologs (Rice Genome Annotation Project; rice.plantbiology.msu.edu).
Gene Ids are indicated in brackets for P. falciparum homologs (PlasmoDB: Malaria Parasite Genome Project; plasmodb.org/plasmo). #TA IR Locus Ids (www.arabidopsis.org).
Discussion
Helicases are DNA and RNA unwinding enzymes found in various organisms ranging from prokaryotes to mammals, cellular pathogens and many viruses. With the availability of genome sequences several new helicases have been identified in various organisms. Many of these helicases are ‘putative,’ which are partially or completely uncharacterized in the databases. Nevertheless, defining the complete set of genome-encoded helicases is a prerequisite for detailed characterization of each helicase type. There has been tremendous interest in studying helicases as crucial regulators of several key aspects of cellular metabolism including DNA replication, DNA repair, RNA transcription, translation, and also as potential drug targets.4 In addition, the importance of helicases can be reemphasized from our present findings that a human cell possesses a large number of helicases, up to 31 DNA and 64 RNA helicases, respectively. The present study has identified and validated sets of both RNA and DNA helicases from human. We have unequivocally validated 14 DHX and 37 DDX family members of RNA helicase based on protein alignment studies followed by identification for the presence or absence of helicase consensus motifs. It is worth mentioning here that some of the putative RNA helicase members like DHX32, DHX58, DDX1, DDX11, DDX12, DDX58 and DDX60 wherein the helicase motifs are partially conserved, or absent, need to be characterized biochemically before classifying them as bona fide helicases.
Based on in vitro experiments the contribution of several DExD/H box proteins in the accomplishment of crucial cellular functions has been revealed. DDX and DHX members of RNA helicases have been the focus of intensive research with several important findings associated with these multifunctional enzymes. It is well established that the members of DExD/H-box protein family also act as splicing factors that are involved in many steps of spliceosome assembly and require ATP hydrolysis.30 For instance, it has been shown that DHX16 is required for human pre-mRNA splicing after formation of a pre-catalytic spliceosome.31 The cells expressing a mutant DHX16 with mutation in the helicase domain accumulated unspliced, intron-containing minigene transcripts.31 Studies were also conducted on other DHX members of RNA helicases. The helicase DHX29 promotes efficient NTPase-dependent 48S complex formation on mRNAs that have highly structured 5′ untranslated regions.32 It has been shown that the preferential unwinding of RNA-containing substrates by Werner DNA helicase is stimulated by DHX9 in vitro.33 The DHX9 translocation from nucleolus to the nucleus studied using inosine monophosphate dehydrogenase (IMPDH) inhibition does not cause the arrest of rRNA synthesis.34 It has been reported that DHX32 is localized in the nucleus and mitochondria.35 Chen et al.36 have reported that the constitutive expression of DHX32 leads to an altered subcellular localization of heat shock protein 60 (Hsp60). The expression of DHX36 mRNA in human organs is highest in testis and this protein is being utilized in cells with high rates of proliferation.37 DHX36 has been reported to be responsible for major G4 DNA resolvase activity because proliferating cells contain G4 DNA structures.38 DDX47 has been identified as a binding partner of GABA(A) receptor-associated protein (GABARAP) in cortical neurons and the co-transfection of GABARAP and DDX47 cDNA into a tumor cell line induced apoptosis.39 Human DEAD box protein DDX42 possesses RNA helicase, protein displacement and RNA annealing activities.40 The importance of DDX3 in anti-viral immunity, and that viruses target DDX3 for immune evasion was proposed.41 Further, a method to study the role of DDX3 in HIV-1 replication was also studied.42 It has been shown that DDX3 has a conserved general function mediated via its interaction with eIF3 in promoting translation.43
The X- and Y-chromosome encoded DEAD-box helicases are DDX3X and DDX3Y respectively. The DEAD-box helicases are mainly RNA helicases but DDX3X can also bind to DNA.44 The DDX3X has been reported to directly interact with transcription factors and promote transcription.45 The orthologs of DDX3 have previously been shown to play role in diverse cellular processes like nuclear export of RNA, splicing and translation regulation.46 This helicase has also been implicated in cancer biogenesis.47 It has been reported that DDX3 undergoes nucleocytoplasmic shuttling and interestingly this property of DDX3 is exploited by the HIV for exporting its mRNA out of the nucleus.48 DDX3Y is also called as DEAD-box RNA helicase Y (DBY) and DBY plays an important role in human spermatogenesis.49 In a recent study it has been reported that the human DBY RNA helicase gene is the major azoospermia factor a (AZFa) gene.50 Men with its deletion display no somatic pathologies, but suffer from complete absence of germ cells.50 Accordingly, DDX3Y protein is expressed only in the germline in spermatogonia, although the transcripts were found in many tissues.50
Suv3 is a nuclear-gene encoded mitochondrial RNA helicase and its gene product localizes to mitochondria.51,52 The yeast Suv3 shows NTP-dependent RNA helicase activity in vitro.51 The multifunctional complex formed by the Suv3 together with a 3′-5′ exoribonuclease (Dss1) is involved in mitochondrial RNA degradation.52,53 In yeast, genetic ablation of Suv3 causes respiratory negative phenotype, while it also shows an effect on stability and processing of mitochondrial RNAs, a total block of mitochondrial translation and accumulation of RNA by-products.54 The human Suv3 shows an ATP-dependent unwinding activity in vitro and localizes to the mitochondrial matrix.55 The Suv3 knockout mice die in utero.56 Depletion of Suv3 leads to the accumulation of shortened mitochondrial RNA species, which is likely due to impaired mitochondrial RNA degradation.54 A similar accumulation of shortened mitochondrial RNA like omega RNA and truncated mitochondrial RNA that cannot be degraded was observed in yeast Suv3 null cells.57
The present in silico studies have identified 31 DNA helicases which includes several prominent members like RecQ helicases, MCM proteins and DNA repair helicases, XPB and XPD. The RecQ members are DNA helicases named after the RecQ protein in Escherichia coli, which was identified during a search for mutants sensitive to thymine starvation.58 The RecQ family helicases found in prokaryotes to eukaryotes are involved in cellular processes such as recombination and DNA replication.58 All RecQ enzymes possess 3′ to 5′ helicase activity.58 WRN also encodes a 3′-5′ DNA exonuclease activity.59 Within RecQ family, there are members with short sequences like RecQ1 or RecQL,60 and members with extremely long sequences like FFA from Xenopus laevis61 and QDE3 from Neurospora crassa,62 which show considerable variation in length and sequence motifs beyond the conserved helicase motifs.60 Only 1 RecQ homolog is present in the genome of E. coli (RecQ), budding yeast (Sgs1) and fission yeast (Rqh1), whereas, 5 different homologs are found in the human genome.58 The genomes of A. thaliana and O. sativa each encode 6 different RecQ family helicases.2,5 The RecQ enzymes can unwind various DNA substrates such as standard B-form DNA duplexes, triple helices, D-loops, 3- or 4-way junctions and G-quadruplex DNA.63
RecQ helicases are also known as guardians of the genome because these DNA helicases have diverse roles in various DNA metabolic pathways which include DNA recombination, replication, repair, transcription and telomere maintenance. The high levels of genomic instability leading to premature aging and a high incidence of cancer result from mutations in 3 of the human RecQ genes.58 The mutations in WRN, BLM and RECQ4 are linked to three recessive genetic disorders: Werner's, Bloom and Rothmund-Thomson syndromes.58 To the best of our knowledge up to now there are no reports of association of any disease with RecQ1 and RecQ5. The human RecQ4 can unwind forked duplexes and the unwinding is independent of strand annealing.64 It was also demonstrated that a point mutation of conserved lysine in Walker A abolished human RecQ4 helicase activity.64 Distinct roles for human RecQ1 and RecQ4 were proposed.65 Followed with origin recognition complex (ORC) and MCM complex assembly, the human RecQ4 is recruited to origins at late G1 phase while, RecQ1 and additional RecQ4 are loaded at origins at the onset of S phase during origin firing.65 Depletion of RecQ1 from human cells by RNA interference resulted in elevated sister chromatid exchange and DNA damage sensitivity.66 The RecQ5 gene in Drosophila and human undergoes alternative splicing and exists in different isoforms.67 A proteomic approach has revealed the interaction of RecQ5 helicase with RNA polymerase II.68 The recruitment of RecQ5 at the sites of DNA damage is mediated by the MRE11-RAD50-NBS1 (MRN) complex.69 The RecQ5 physically interacts with the RAD51 recombinase and can disrupt RAD51 presynaptic filaments.70 RecQ5 is involved in stabilization of replication fork wherein overexpressed RecQ5 promotes survival of human cells during thymidine-induced slowing of replication forks.71 In other species, mutants in the RecQ genes lead to unstable chromosomes.58 Collectively, these findings indicate that RecQ helicases play a central role in the maintenance of genomic stability.
Sgs1 is a yeast homolog of the human BLM helicase. The loss of Sgs1 also causes elevated recombination, increased chromosome instability and DNA-damage sensitivity.72 Some of the associated phenotypes in sgs1 deficient cell are suppressed by the expression of the human BLM or WRN gene.58 By the process of unwinding the D-loops and/or by dissolving double Holliday junctions (dHJs) the Sgs1 is thought to disassemble joint molecules (JMs) as well.73 The RecQ helicases from both yeast and human can interact with DNA topoisomerase III.74,75 The proteins BLM and Rad51 can also interact suggesting a possible link between RecQ helicases and homologous recombination in human cells.76 The transcription analysis in a large array of human tumor types showed RECQL4 to be the sixth most highly expressed gene.77
MCM family genes have been found in all eukaryotes. The MCM-related proteins are present in archaea but MCM proteins do not exist in prokaryotes.78 Our search criterion has identified 9 MCMs (MCM2-7, MCM8, MCM9 and MCM10) in human genome. Only 6 proteins, MCM2 through MCM7, are members of the MCM family. These six essential subunits are each AAA+ ATPases and form a heterohexamer complex.79 The MCM2-7 complex acts as replicative helicase in vivo.80 Both the replication initiation and fork regression requires MCM2-7 complex.81 In Drosophila, MCM4 is called disc proliferation abnormal,82 while A. thaliana MCM7 is also called as PROLIFERA.83
Similar to MCM2-7, the MCM8 and MCM9 proteins also occur only in eukaryotes.84 The MCM8 has been identified in humans and it contains the consensus IDEFDKM and arginine finger motifs.85,86 The occurrence of GKS motif in Walker A sequence is unique to MCM8. In addition to proliferating cells, MCM8 is also expressed in a variety of tissues and throughout the cell cycle.85,86 Recombinant MCM8 displays both DNA helicase and ATPase activities in vitro.87 The results also indicated involvement of human MCM8 in G1 phase progression.88 The human MCM8 has been proposed to be a crucial component of the pre-RC and its interaction in vivo with two components of the pre-RC, namely, cdc6 and ORC2 has been identified.88 Depletion of human MCM8 protein leads to reduced loading of cdc6 and the MCM2-7 complex.88 Human MCM9 protein also contains Walker A motif, a partially conserved Walker B motif, and an arginine finger and the phylogenetic analysis shows that MCM9 is more closely related to MCM8. The full length human MCM9 was described.89 It was demonstrated that MCM9 binds to chromatin and is required for recruitment of MCM2-7 helicase onto chromatin.90 The MCM9 has been suggested to be an essential linker between licensing factor Cdt1 and the MCM2-7 complex.90
In spite of its name, the MCM10 protein is not a member of the canonical MCM2-7 family. The MCM2-7 complex and other replication factors are known to interact with MCM10 protein.91 MCM10 was isolated in a screen for replication mutants and also in the same S. cerevisiae screen as the true MCM proteins.92 Experiments with fission yeast, Xenopus extracts and human cells have indicated that MCM10 acts after association of canonical MCM2-7 with the pre-RC.93 Direct interaction of human MCM10 with RecQ4 regulating its DNA unwinding activity was demonstrated.94 It was shown that degradation of the 26 S proteasome caused due to UV-specific damage leads to MCM10 downregulation in mammalian cells.95
The BLAST search has identified DNA repair helicases, XPB and XPD, in the human genome. XPB and XPD play crucial roles in NER and in transcription initiation.96 The core TFIIH a basal transcription factor, contains these two helicases which are implicated in human autosomal recessive disorders such as xeroderma pigmentosum (XP), Cockayne syndrome (CS) and trichothiodystrophy (TTD).97 These syndromes are caused due to defects in TFIIH which in turn might affect not only nucleotide excision repair (NER), but also transcription.98 The DEAD 2 domain is also present in XPD and mutations in this domain lead to TTD disease.99 Similarly, the mutations in human ATR-X gene result in X-linked alpha-thalassaemia with mental retardation (ATR-X) syndrome and correlate with changes in methylation of repetitive DNA sequences.100
Our analysis has also identified DNA helicases like Pif1, RecQ5 beta, Twinkle, BACH1, nucleolin and RTEL1 encoded by the human genome. The Pif1 is a member of SF1 helicase conserved from yeast to human involved in several aspects of DNA replication and repair.11,101 RecQ5 beta, one among the 3 different isoforms of RecQ5, is a 3′-5′ DNA helicase which can promote migration of Holliday junctions.102 Twinkle is a mitochondrial DNA helicase with 5′ to 3′ helicase activity, and possesses specified substrate requirements.103 The autosomal dominant progressive opthalmoplegia caused due to multiple deletions in the human mitochondrial DNA results from mutations in Twinkle.103 Twinkle colocalizes with mtDNA in mitochondrial nucleoids.104 The BACH1 (for BRCA1-associated C-terminal helicase), a member of SF2 helicase superfamily, is a 5′ to 3′ helicase which directly interacts with the BRCA1 BRCT motifs and is implicated in double strand break repair.105,106 The mutant BACH1 participates in breast cancer development.107 The helicase nucleolin can unwind DNA-DNA duplexes, as well as RNA-RNA, and DNA-RNA duplexes, and is involved in several metabolic processes.108,109 The helicase RTEL1 play role in telomere homeostasis and was shown to disrupt D-loops in vitro.110 The human cells with siRNA-mediated knockdown of RETL1 show signs of elevated recombination.111
Materials and Methods
Database search and enlistment of RNA and DNA helicases.
The following methodology is adopted for identification of almost all the RNA and DNA helicases that are encoded by the human genome. The Homo sapiens RefSeq protein database available as a link to NCBI/BLAST home (http://www.ncbi.nlm.nih.gov; http://blast.ncbi.nlm.nih.gov) is searched using the program BLASTP-Compare Protein Sequences against ‘BLAST human sequences’ resource. As an input sequence, the human prototype for the DEAD-box proteins, the translation initiation factor eIF4A-1 (Swiss-Prot Id: P60842) is used. A tentative list of putative candidates for human helicases, DDX and/or DDH RNA helicases is compiled. Specific protein sequences that belong to the category of DExD/H RNA helicases are further used with the progress of the search. The search is further refined with enlisting all the helicase proteins which belong to DExD/H RNA helicases. A comprehensive list is prepared with the Swiss-Prot Ids, name of the protein, synonyms and gene name for these RNA helicases.
A dataset representing DNA helicases from rice (http://rice.plantbiology.msu.edu)5 is used to identify DNA helicases encoded by the human genome. A BLAST against human RefSeq protein database on NCBI with individual sequences of rice DNA helicases is performed. The best hit is enlisted with its Swiss-Prot Ids, name of the protein, synonyms and gene name for these DNA helicases.
Validation of helicases.
Protein sequences of human RNA and DNA helicases are validated based on the occurrence of conserved helicase motif signatures.3,5,7
Protein alignment and phylogenetic studies.
Protein sequences for RNA and DNA helicases are retrieved from NCBI database. These sequences are aligned with ClustalX using default parameters. The helicase motif signatures are verified based on alignment comparison and manual assignment of the motifs. Phylogenetic analysis is performed based on entire protein sequences with MEGA4 software. Phylogenies are built using the Neighbor-Joining (NJ) method.
Identification of homologs for helicase proteins in model organisms.
To identify the homologs of human RNA and DNA helicases in yeast and Arabidopsis, the protein sequences are used for the BLAST search on NCBI against the Swiss-Prot protein sequences database with blastp (protein-protein BLAST) algorithm. The homologs of human helicases in rice are traced in the Rice Genome Annotation Project (RAP) database (http://rice.plantbiology.msu.edu). Similarly, homologs for these helicases in P. falciparum are identified in PlasmoDB: Malaria Parasite Genome Project (http://plasmodb.org/plasmo). A list of homologs is prepared with Swiss-Prot Ids for yeast and A. thaliana, Locus Ids for O. sativa and Gene Ids for P. falciparum.
Conclusions
The collections of RNA and DNA helicases identified during the present study will provide an invaluable resource for elaborate biological research on these helicases. We have also shown that in combination with protein alignment and phylogenetic studies, the identification of homologs using comparative genomic approach in model organisms can be used effectively to carry out rapid characterization of these helicases as well. The phylogenetic analysis of different helicase categories could also represent an evolutionary upgrade in humans. In addition, the identified homologs in sequenced genomes such as A. thaliana, O. sativa, S. cerevisae and P. falciparum will provide valuable insights on the evolutionary history of these helicases. It is interesting to note that no clear homologs for some of the putative human RNA and/or DNA helicases could be found in yeast suggesting that these proteins might perform distinct functions that are not present in this lower eukaryote. The study on comparing these homologs will spread light on the organization and complexity of helicase gene family in model organisms.
Acknowledgements
This work is partially supported by Department of Science and Technology, Department of Biotechnology and Defence Research and Development Organization grants. Infrastructural support from the Department of Biotechnology, Government of India is gratefully acknowledged.
Abbreviations
- ATP
adenosine triphosphate
- BLM
Bloom
- MCM
minichromosome maintenance
- RTS
Rothmund-Thomson syndrome
- SKIV2L2
superkiller viralicidic activity 2-like 2
- WRN
Werner
Footnotes
Dedicated to the memory of Professor Arturo Falaschi
Supplementary Material
References
- 1.Compton SA, Tolun G, Kamath-Loeb AS, Loeb LA, Griffith JD. The Werner syndrome protein binds replication fork and holliday junction DNAs as an oligomer. J Biol Chem. 2008;283:24478–24483. doi: 10.1074/jbc.M803370200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartung F, Plchova H, Puchta H. Molecular characterization of RecQ homologues in Arabidopsis thaliana. Nucleic Acids Res. 2000;28:4275–4282. doi: 10.1093/nar/28.21.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuteja N, Tuteja R. Prokaryotic and eukaryotic DNA helicases. Essential molecular motor proteins for cellular machinery. Eur J Biochem. 2004;271:1835–1848. doi: 10.1111/j.1432-1033.2004.04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuteja R. Genome wide identification of Plasmodium falciparum helicases: a comparison with human host. Cell Cycle. 2010;9:104–120. doi: 10.4161/cc.9.1.10241. [DOI] [PubMed] [Google Scholar]
- 5.Umate P, Tuteja R, Tuteja N. Genome-wide analysis of helicase gene family from rice and Arabidopsis: a comparison with yeast and human. Plant Mol Biol. 2010;73:449–465. doi: 10.1007/s11103-010-9632-5. [DOI] [PubMed] [Google Scholar]
- 6.Tanner NK, Cordin O, Banroques J, Doere M, Linder P. The Q motif: a newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol Cell. 2003;11:127–138. doi: 10.1016/s1097-2765(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 7.Tuteja N, Tuteja R. Unravelling DNA helicases. Motif, structure, mechanism and function. Eur J Biochem. 2004;271:1849–1863. doi: 10.1111/j.1432-1033.2004.04094.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanner NK, Linder P. DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell. 2001;8:251–262. doi: 10.1016/s1097-2765(01)00329-x. [DOI] [PubMed] [Google Scholar]
- 9.Caruthers JM, McKay DB. Helicase structure and mechanism. Curr Opin Struct Biol. 2002;12:123–133. doi: 10.1016/s0959-440x(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 10.Hogbom M, Collins R, van den Berg S, Jenvert RM, Karlberg T, Kotenyova T, et al. Crystal structure of conserved domains 1 and 2 of the human DEAD-box helicase DDX3X in complex with the mononucleotide AMP. J Mol Biol. 2007;372:150–159. doi: 10.1016/j.jmb.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 12.Laggerbauer B, Achsel T, Luhrmann R. The human U5-200 kD DEXH-box protein unwinds U4/U6 RNA duplices in vitro. Proc Natl Acad Sci USA. 1998;95:4188–4192. doi: 10.1073/pnas.95.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghunathan PL, Guthrie C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr Biol. 1998;8:847–855. doi: 10.1016/s0960-9822(07)00345-4. [DOI] [PubMed] [Google Scholar]
- 14.Fairman ME, Maroney PA, Wang W, Bowers HA, Gollnick P, Nilsen TW, et al. Protein displacement by DExH/D “RNA helicases” without duplex unwinding. Science. 2004;304:730–734. doi: 10.1126/science.1095596. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Zheng X, Shen J, Zhang L, Zhao R, Green MR. Distinct activities of the DExD/H-box splicing factor hUAP56 facilitate stepwise assembly of the splicesome. Genes Dev. 2008;22:1796–1803. doi: 10.1101/gad.1657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozen F, Edery I, Meerovitch K, Dever TE, Merrick WC, Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990;10:1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas AA, van Aalzum L, Voorma HO. RNA unwinding by eukaryotic initiation factor 4A and nucleotide modification. Biochem Int. 1992;27:17–23. [PubMed] [Google Scholar]
- 18.Wang Y, Wagner JD, Guthrie C. The DEAH-box splicing factor Prp16 unwinds RNA duplexes in vitro. Curr Biol. 1998;8:441–451. doi: 10.1016/s0960-9822(98)70178-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Jankowsky E. ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry. 2005;44:13591–1360. doi: 10.1021/bi0508946. [DOI] [PubMed] [Google Scholar]
- 20.Rocak S, Linder P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat Rev Mol Cell Biol. 2004;5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 21.Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Linder P. Dead-box proteins: A family affair-active and passive players in RNP-remodeling. Nucleic Acids Res. 2006;34:4168–4180. doi: 10.1093/nar/gkl468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuteja N, Tuteja R. DNA helicases as molecular motors: an insight. Physica Acta. 2006;372:70–83. doi: 10.1016/j.physa.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–198. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- 25.Daugeron MC, Linder P. Are DEAD-box proteins becoming respectable helicases? Nat Struct Biol. 2000;7:97–99. doi: 10.1038/72464. [DOI] [PubMed] [Google Scholar]
- 26.Jankowsky E, Gross CH, Shumann S, Pyle AM. The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature. 2000;403:447–451. doi: 10.1038/35000239. [DOI] [PubMed] [Google Scholar]
- 27.Aubourg S, Kreis M, Lecharny A. The DEAD-box RNA helicase family in Arabidopsis thaliana. Nucleic Acids Res. 1999;27:628–636. doi: 10.1093/nar/27.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudet N, Aubourg S, Toffano-Nioche C, Kreis M, Lecharny A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis and Drosophila. Genome Res. 2001;11:2101–2114. doi: 10.1101/gr.200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelhaleem M, Maltais L, Wain H. The human DDX and DHX gene families of putative RNA helicases. Genomics. 2003;81:618–622. doi: 10.1016/s0888-7543(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 30.Staley JP, Guthrie C. Mechanical devices of the spliceosome: Motors, clocks, springs and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 31.Gencheva M, Kato M, Newo AN, Lin RJ. Contribution of DEAH-box protein DHX16/hPRP2 in human prem-RNA splicing. Biochem J. 2010;429:25–32. doi: 10.1042/BJ20100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5′ UTRs requires DExH-box protein DHX29. Cell. 2008;135:1237–1250. doi: 10.1016/j.cell.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty P, Grosse F. WRN helicase unwinds Okazaki fragment-like hybrids in a reaction stimulated by the human DHX9 helicase. Nucleic Acids Res. 2010;38:4722–4730. doi: 10.1093/nar/gkq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang M, Mitchell BS. Guanine nucleotide depletion mediates translocation of nucleolar proteins, including RNA helicase A (DHX-9) Nucleosides Nucleotides Nucleic Acids. 2008;27:704–711. doi: 10.1080/15257770802145132. [DOI] [PubMed] [Google Scholar]
- 35.Alli Z, Ackerley C, Chen Y, Al-Saud B, Abdelhaleem M. Nuclear and mitochondrial localization of the putative RNA helicase DHX32. Exp Mol Pathol. 2006;81:245–248. doi: 10.1016/j.yexmp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Alli Z, Ackerley C, Al-Saud B, Abdelhaleem M. Altered distribution of heat shock protein 60 (Hsp60) with dysregulated expression of DHX32. Exp Mol Pathol. 2007;82:256–261. doi: 10.1016/j.yexmp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Fu JJ, Li LY, Lu GX. Molecular cloning and characterization of human DDX36 and mouse Ddx36 genes, New members of the DEAD/H box superfamily. Acta Biochim Biophys Sinica. 2002;34:655–661. [PubMed] [Google Scholar]
- 38.Vaughn JP, Creacy SD, Routh ED, Joyner-Butt C, Jenkins GS, Pauli S, et al. The DEXH protein product of the DHX36 gene is the major source of tetramolecular quadruplex G4-DNA resolving activity in HeLa cell lysates. J Biol Chem. 2005;280:38117–38120. doi: 10.1074/jbc.C500348200. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Rho SB, Chun T. GABAA receptor-associated protein (GABARAP) induces apoptosis by interacting with DEAD (Asp-Glu-Ala-Asp/His) box polypeptide 47 (DDX 47) Biotechnol Lett. 2005;27:623–628. doi: 10.1007/s10529-005-3628-2. [DOI] [PubMed] [Google Scholar]
- 40.Uhlmann-Schiffler H, Jalal C, Stahl H. Ddx42p—a human DEAD box protein with RNA chaperone activities. Nucleic Acids Res. 2006;34:10–22. doi: 10.1093/nar/gkj403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulhern O, Bowie AG. Unexpected roles for DEAD-box protein 3 in viral RNA sensing pathways. Eur J Immunol. 2010;40:933–935. doi: 10.1002/eji.201040447. [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Yedavalli VR, Jeang KT. A method to study the role of DDX3 RNA helicase in HIV-1. Methods Mol Biol. 2010;587:281–289. doi: 10.1007/978-1-60327-355-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CS, Dias AP, Jedrychowski M, Patel AH, Hsu JL. Reed R, Human DDX3 functions in translation and interacts with the translation initiation factor eIF3. Nucleic Acids Res. 2008;36:4708–4718. doi: 10.1093/nar/gkn454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franca R, Belfiore A, Spadari S, Maga G. Human DEAD-box ATPase DDX3 shows a relaxed nucleoside substrate specificity. Proteins. 2007;67:1128–1137. doi: 10.1002/prot.21433. [DOI] [PubMed] [Google Scholar]
- 45.Chao CH, Chen CM, Cheng PL, Shih JW, Tsou AP, Lee YH. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 2006;66:6579–6588. doi: 10.1158/0008-5472.CAN-05-2415. [DOI] [PubMed] [Google Scholar]
- 46.Rosner A, Rinkevich B. The DDX3 subfamily of the DEAD box helicases: divergent roles as unveiled by studying different organisms and in vitro assays. Curr Med Chem. 2007;14:2517–2525. doi: 10.2174/092986707782023677. [DOI] [PubMed] [Google Scholar]
- 47.Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P, Jr, et al. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene. 2008;27:3912. doi: 10.1038/onc.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Abdelhaleem M. RNA helicases: regulators of differentiation. Clin Biochem. 2005;38:499–503. doi: 10.1016/j.clinbiochem.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Rauschendorf MA, Zimmer J, Hanstein R, Dickemann C, Vogt PH. Complex transcriptional control of the AZFa gene DDX3Y in human testis. Int J Androl. 2011;34:84–96. doi: 10.1111/j.1365-2605.2010.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margossian SP, Li H, Zassenhaus HP, Butow RA. The DExH box protein Suv3p is a component of yeast mitochondrial 3′-to-5′ exoribonuclease that suppresses group I intron toxicity. Cell. 1996;84:199–209. doi: 10.1016/s0092-8674(00)80975-7. [DOI] [PubMed] [Google Scholar]
- 52.Minczuk M, Piwowarski J, Papworth MA, Awiszus K, Schalinski S, Dziembowski A, et al. Localisation of the human Suv3p helicase in the mitochondrial matrix and its preferential unwinding of dsDNA. Nucleic Acids Res. 2002;30:5074–5086. doi: 10.1093/nar/gkf647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dziembowski A, Piwowarski J, Hoser R, Minczuk M, Dmochowska A, Siep M, et al. The yeast mitochondrial degradosome. Its composition, interplay between RNA helicase and RNase activities and the role in mitochondrial RNA metabolism. J Biol Chem. 2003;278:1603–1611. doi: 10.1074/jbc.M208287200. [DOI] [PubMed] [Google Scholar]
- 54.Khidr L, Wu G, Davila A, Procaccio V, Wallace D, Lee WH. Role of SUV3 helicase in maintaining mitochondrial homeostasis in human cells. J Biol Chem. 2008;283:27064–27073. doi: 10.1074/jbc.M802991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shu Z, Vijayakumar S, Chen CF, Chen PL, Lee WH. Purified human SUV3p exhibits multiple-substrate unwinding activity upon conformational change. Biochemistry. 2004;43:4781–4790. doi: 10.1021/bi0356449. [DOI] [PubMed] [Google Scholar]
- 56.Pereira M, Mason P, Szczesny RJ, Maddukuri L, Dziwura S, Jedrzejczak R, et al. Interaction of human SUV3 RNA/DNA helicase with BLM helicase; loss of the SUV3 gene results in mouse embryonic lethality. Mech Ageing Dev. 2007;128:609–617. doi: 10.1016/j.mad.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Dziembowski A, Malewicz M, Minczuk M, Golik P, Dmochowska A, Stepien PP. The yeast nuclear gene DSS1, which codes for a putative RNase II, is necessary for the function of the mitochondrial degradosome in processing and turnover of RNA. Mol Gen Genet. 1998;260:108–114. doi: 10.1007/s004380050876. [DOI] [PubMed] [Google Scholar]
- 58.Bjergbaek L, Cobb JA, Gasser SM. RecQ helicases and genome stability: lessons from model organisms and human disease. Swiss Med Wkly. 2002;132:433–442. doi: 10.4414/smw.2002.09886. [DOI] [PubMed] [Google Scholar]
- 59.Shen JC, Gray MD, Oshima J, Kamath-Loeb AS, Fry M, Loeb LA. Werner syndrome protein. I. DNA helicase and dna exonuclease reside on the same polypeptide. J Biol Chem. 1998;273:34139–34144. doi: 10.1074/jbc.273.51.34139. [DOI] [PubMed] [Google Scholar]
- 60.Puranam KL, Blackshear PJ. Cloning and characterization of RECQL, a potential human homologue of the Escherichia coli DNA helicase RecQ. J Biol Chem. 1994;269:29838–29845. [PubMed] [Google Scholar]
- 61.Yan H, Chen CY, Kobayashi R, Newport J. Replication focus-forming activity 1 and the Werner syndrome gene product. Nat Genet. 1998;19:375–378. doi: 10.1038/1263. [DOI] [PubMed] [Google Scholar]
- 62.Cogoni C, Macino G. Posttranscriptional gene silencing in Neurospora by a RecQ DNA helicase. Science. 1999;286:2342–2344. doi: 10.1126/science.286.5448.2342. [DOI] [PubMed] [Google Scholar]
- 63.Pike ACW, Shrestha B, Popuri V, Burgess-Brown N, Muzzolini L, Costantini S, et al. Structure of the human RECQ1 helicase reveals a putative strand-separation pin. Proc Natl Acad Sci USA. 2009;106:1039–1044. doi: 10.1073/pnas.0806908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi ML, Ghosh AK, Kulikowicz T, Croteau DL, Bohr VA. Conserved helicase domain of human RecQ4 is required for strand annealing-independent DNA unwinding. DNA Repair (Amst) 2010;9:796–804. doi: 10.1016/j.dnarep.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thangavel S, Mendoza-Maldonado R, Tissino E, Sidorova JM, Yin J, Wang W, et al. Human RECQ1 and RECQ4 helicases play distinct roles in DNA replication initiation. Mol Cell Biol. 2010;30:1382–1396. doi: 10.1128/MCB.01290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bugreev DV, Brosh RM, Jr, Mazin AV. RECQ1 possesses DNA branch migration activity. J Biol Chem. 2008;283:20231–20242. doi: 10.1074/jbc.M801582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekelsky JJ, Brodsky MH, Rubin GM, Hawley RS. Drosophila and human RecQ5 exist in different isoforms generated by alternative splicing. Nucleic Acids Res. 1999;27:3762–3769. doi: 10.1093/nar/27.18.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aygün O, Svejstrup J, Liu Y. A RECQ5-RNA polymerase II association identified by targeted proteomic analysis of human chromatin. Proc Natl Acad Sci USA. 2008;105:8580–8584. doi: 10.1073/pnas.0804424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng L, Kanagaraj R, Mihaljevic B, Schwendener S, Sartori AA, Gerrits B, et al. MRE11 complex links RECQ5 helicase to sites of DNA damage. Nucleic Acids Res. 2009;37:2645–2657. doi: 10.1093/nar/gkp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwendener S, Raynard S, Paliwal S, Cheng A, Kanagaraj R, Shevelev I, et al. Physical interaction of RECQ5 helicase with RAD51 facilitates its anti-recombinase activity. J Biol Chem. 2010;285:15739–15745. doi: 10.1074/jbc.M110.110478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blundred R, Myers K, Helleday T, Goldman AS, Bryant HE. Human RECQL5 overcomes thymidine-induced replication stress. DNA Repair (Amst) 2010;9:964–975. doi: 10.1016/j.dnarep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Watt PM, Hickson ID, Borts RH, Louis EJ. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Brabant AJ, Ye T, Sanz M, German IJ, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- 74.Angloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, et al. The Bloom's syndrome gene product interacts with topoisomerase III. J Biol Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 76.Wu L, Davies SL, Levitt NC, Hickson ID. Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J Biol Chem. 2001;276:19375–19381. doi: 10.1074/jbc.M009471200. [DOI] [PubMed] [Google Scholar]
- 77.Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 78.Kearsey SE, Labib K. MCM proteins: evolution, properties and role in DNA replication. Biochim Biophys Acta. 1998;1398:113–136. doi: 10.1016/s0167-4781(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 79.Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev. 2004;68:109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 81.Labib K, Kearsey SE, Diffley JF. MCM2-7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint. Mol Biol Cell. 2001;12:3658–3667. doi: 10.1091/mbc.12.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feger G, Vaessin H, Su TT, Wolff E, Jan LY, Jan YN. dpa, a member of the MCM family, is required for mitotic DNA replication but not endoreplication in Drosophila. EMBO J. 1995;14:5387–5398. doi: 10.1002/j.1460-2075.1995.tb00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Springer PS, McCombie WR, Sundaresan V, Martienssen RA. Gene trap tagging of PROLIFERA and essential MCM2-3-5 like gene in Arabidopsis. Science. 1995;268:877–880. doi: 10.1126/science.7754372. [DOI] [PubMed] [Google Scholar]
- 84.Maiorano D, Lutzmann M, Méchali M. MCM proteins and DNA replication. Curr Opin Cell Biol. 2006;18:130–136. doi: 10.1016/j.ceb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Gozuacik D, Chami M, Lagorce D, Faivre J, Murakami Y, Poch O, et al. Identification and functional characterization of a new member of the human Mcm protein family: hMcm8. Nucleic Acids Res. 2003;31:570–579. doi: 10.1093/nar/gkg136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson EM, Kinoshita Y, Daniel DC. A new member of the MCM protein family encoded by the human MCM8 gene, located contrapodal to GCD10 at chromosome band 20p12.3–13. Nucleic Acids Res. 2003;31:2915–2925. doi: 10.1093/nar/gkg395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maiorano D, Cuvier O, Danis E, Méchali M. MCM8 is an MCM2-7-related protein that functions as a DNA helicase during replication elongation and not initiation. Cell. 2005;120:315–328. doi: 10.1016/j.cell.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Volkening M, Hoffmann I. Involvement of human MCM8 in prereplication complex assembly by recruiting hcdc6 to chromatin. Mol Cell Biol. 2005;25:1560–1568. doi: 10.1128/MCB.25.4.1560-1568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lutzmann M, Maiorano D, Méchali M. Identification of full genes and proteins of MCM9, a novel, vertebrate-specific member of the MCM2-8 protein family. Gene. 2005;362:51–56. doi: 10.1016/j.gene.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 90.Lutzmann M, Méchali M. MCM9 binds Cdt1 and is required for the assembly of prereplication complexes. Mol Cell. 2008;31:190–200. doi: 10.1016/j.molcel.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Homesley L, Lei M, Kawasaki Y, Sawyer S, Christensen T, Tye BK. Mcm10 and the MCM2-7 complex interact to initiate DNA synthesis and to release replication factors from origins. Genes Dev. 2000;14:913–926. [PMC free article] [PubMed] [Google Scholar]
- 92.Maine GT, Sinha P, Tye BK. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics. 1984;106:365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wohlschlegel JA, Dhar SK, Prokhorova TA, Dutta A, Walter JC. Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Mol Cell. 2002;9:1–20. doi: 10.1016/s1097-2765(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 94.Xu X, Rochette PJ, Feyissa EA, Su TV, Liu Y. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J. 2009;28:3005–3014. doi: 10.1038/emboj.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma A, Kaur M, Kar A, Ranade SM, Saxena S. Ultraviolet radiation stress triggers the downregulation of essential replication factor MCM10. J Biol Chem. 2010;285:8352–8362. doi: 10.1074/jbc.M109.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guzder SN, Sung P, Bailly V, Prakash L, Prakash S. RAD25 is a DNA helicase required for DNA repair and RNA polymerase II transcription. Nature. 1994;369:578–581. doi: 10.1038/369578a0. [DOI] [PubMed] [Google Scholar]
- 97.Sung P, Bailly V, Weber C, Thompson LH, Prakash L, Prakash S. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993;365:852–855. doi: 10.1038/365852a0. [DOI] [PubMed] [Google Scholar]
- 98.Zurita M, Merino C. The transcriptional complexity of the TFIIH complex. Trends Genet. 2003;19:578–584. doi: 10.1016/j.tig.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Dubaele S, Proietti De Santis L, Bienstock RJ, Keriel A, Stefanini M, Van Houten B, et al. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol Cell. 2003;11:1635–1646. doi: 10.1016/s1097-2765(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 100.Gibbons R. Alpha thalassaemia-mental retardation, X linked. Orphanet J Rare Dis. 2006;1:15. doi: 10.1186/1750-1172-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ivessa AS, Zhou JQ, Zakian VA. The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell. 2000;100:479–489. doi: 10.1016/s0092-8674(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 102.Garcia PL, Liu Y, Jiricny J, West SC, Janscak P. Human RECQ5b, a protein with DNA helicase and strand-annealing activities in a single polypeptide. EMBO J. 2004;23:2882–2891. doi: 10.1038/sj.emboj.7600301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korhonen JA, Gaspari M, Falkenberg M. TWINKLE has 5′-3′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J Biol Chem. 2003;278:48627–48632. doi: 10.1074/jbc.M306981200. [DOI] [PubMed] [Google Scholar]
- 104.Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 105.Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 106.Gupta R, Sharma S, Doherty KM, Sommers JA, Cantor SB, Brosh RM., Jr Inhibition of BACH1 (FANCJ) helicase by backbone discontinuity is overcome by increased motor ATPase or length of loading strand. Nucleic Acids Res. 2006;34:6673–6683. doi: 10.1093/nar/gkl964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantor S, Drapkin R, Zhang F, Lin Y, Han J, Pamidi S, et al. The BRCA1-associated protein BACH1 is a DNA helicase targeted by clinically relevant inactivating mutations. Proc Natl Acad Sci USA. 2004;101:2357–2362. doi: 10.1073/pnas.0308717101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuteja N, Huang NW, Skopac D, Tuteja R, Hrvatic S, Zhang J, et al. Human DNA helicase IV is nucleolin, an RNA helicase modulated by phosphorylation. Gene. 1995;160:143–148. doi: 10.1016/0378-1119(95)00207-m. [DOI] [PubMed] [Google Scholar]
- 109.Tuteja R, Tuteja N. Nucleolin: a multifunctional major nucleolar phosphoprotein. Crit Rev Biochem Mol Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- 110.Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O'Neil NJ, Petalcorin MI, et al. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135:261–271. doi: 10.1016/j.cell.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chavez A, Tsou AM, Johnson FB. Telomeres do the (un)twist: Helicase actions at chromosome termini. Biochim Biophys Acta. 2009;1792:329–340. doi: 10.1016/j.bbadis.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.