Older people experience enhanced susceptibility to viral infections and subsequent superimposed bacterial infections. Based on both experimental and clinical studies, this susceptibility is thought to be due to declining immune responses. However, our work indicates that older people may succumb to viral infection due to exaggerated immune responses as aged mice produce higher serum levels of the inflammatory mediator IL-17 than younger mice upon herpes viral infection. These age-elevated IL-17 responses induce a lethal immune pathology during viral infection. Early during the course of infection natural killer T-cells (NKT-cells) are major contributors to the elevated IL-17 response in aged mice. These responses synergize with defective viral clearance with aging noted by impaired IFN-α responses by plasmacytoid DCs. Our results indicate that novel anti-inflammatory drugs may resolve imbalanced inflammation and improve outcomes in older people infected with viruses.
Introduction: The Problem of Age Alterations to Immune Function
Within the next forty years the number of people older than sixty five years of age in the world will exceed the number of young people aged for the first time in history.1 As the number of older people grows, their health care needs will pose an ever-increasing burden on our health care system. Altered immune responses in the elderly are responsible for many diseases, as well as for increased susceptibility to infections and cancer. For example, influenza viral lung infections are a growing public health concern in older people because of their predisposition for community-acquired pneumonia and their ability to exacerbate chronic heart and lung diseases.2 These infections not only induce higher morbidity and mortality in older than younger people,3 but also appear to be increasing in number, as exemplified in a study reporting a 20% increase in hospitalization rates for community acquired pneumonia from 1988 to 2002 for patients aged 65 to 84 years.4
Efforts to protect older people from influenza viral infection have only been partially successful. Currently, the trivalent seasonal influenza vaccine reduces hospital rates for pneumonia by 48–57% and mortality by 36–54% in community dwelling older people.5,6 However, these vaccines fail to provide as high a level of protection for older people as for younger people, who typically respond to influenza viral infection with greater than 90% efficacy.6,7 Parallel to the increased susceptibility to microbial infection and cancer in older people, reduced efficacy of vaccines are likely caused by age-induced alterations in the immune system. Hence, discerning how aging modifies the immune system will be critical for development of novel therapies and treatment options that protect older people from microbial infection and cancer.
Although the current paradigm in the field indicates that declining immune responses are the dominant reason for increased susceptibility to viral infections and for reduced efficacy of vaccines with aging,8 our recent report challenges this paradigm as we find that exaggerated inflammatory responses are the principal reason why aged hosts succumb to viral infection, whereas young mice survive viral infection without disease.
Current Paradigm Concerning How Aging Impacts Immune Function
Both experimental and clinical studies have indicated that older people are more susceptible to microbial infection due to declining immune responses.9 The generation of a primary immune response is the result of a carefully orchestrated interaction of the innate and adaptive immune systems.10,11 The majority of studies on aging and immune function have focused on adaptive T cell function, with several reports showing that aging impairs T cell IL-2, IFN-γ production, and Th1 immunity,12–14 one of the critical immune defenses to infection from intracellular pathogens such as viruses. Aging also impairs T-cell CD28 signaling15 and immune synapse formation.16 Furthermore, aging reduces T cell thymic output, leading to reduced numbers of naïve T cells, cells that are essential for eradicating infections from new pathogens. In addition, aging leads to an accumulation of memory T cells,17 which are critical for prevention of re-infection and are the basis for vaccination therapies. In addition, aging leads to defective CD4+ and CD8+ memory T cell function.18,19 Other components of the adaptive immune system, including antibody formation by B cells, may also be altered with aging.20,21
Less is known about the role of aging in innate immunity, which is the first line of host defense against infections.22–25 Some studies in this area have found no alteration in the innate system with aging14 and one study found that cytokine responses were elevated in aged human dendritic cells (DCs).26 Other experimental and clinical reports have documented that aging impairs the innate immune system, including inflammatory cytokine production by human DCs.27,28 In sum, the field of aging and immune function is dominated by the paradigm that aging reduces immune function, thus providing an explanation for why older people are more susceptible to microbial infection.
Aging and Pro-inflammatory Cytokines
Several prior reports indicate that aging is associated with dysregulated cytokine production in both humans and mice.29–31 Although elevated levels of several cytokines, including IL-1, IL-6 and TNFα, have been associated with aging, the strongest evidence is for IL-6, a cytokine that has recently been shown to be important for Th17 CD4+ T-cell helper differentiation.32,33 However, it is not clear if altered inflammatory responses with aging are a result of impaired host defense to viral infection, and it is unknown whether any age-induced alteration in the inflammatory responses would impact the outcome of viral infection with aging.
A Novel Pathway by which Aging Induces Susceptibility to Viral Infection
Our recently published study challenges the paradigm that reduced immunity with aging is the dominant reason why aged hosts are susceptible to viral infections. Our work shows that aged, but not young, mice succumb to systemic herpes viral infection due to exaggerated inflammation.34 We documented that aged mice that were infected with either herpes simplex 2 virus (HSV-2) or with murine cytomegalovirus (mCMV) exhibited a rapid rise in the proinflammatory cytokines, IL-6, IL-17 and TNFα. These results were coupled to findings of increased liver injury and necrosis with neutrophil activation in aged mice than young counterparts. The increased inflammation is driven by heightened interleukin IL-17 responses. IL-17 is an inflammatory mediator that has been implicated in a variety of pathological disorders including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, psoriasis, and certain fungal infections. In our study, we found that treating aged mice with an anti-IL-17 drug prevents death due to neutrophil-dependent liver necrosis whenever aged mice are infected systemically with the human pathogen herpes simplex virus or with a murine cytomegalovirus.34 IL-17 neutralization also reduced induction of other proinflammatory cytokines, IL-6 and TNFα, indicating that IL-17 acts as a proximal orchestrator of inflammation during viral infection with aging in our experimental models. Young mice, in contrast, survive viral infection with lower IL-17 levels and no evidence of disease. The cells responsible for the elevated IL-17 in aged mice are liver NKT-cells. NKT-cells are immune cells that secrete inflammatory mediators such as IL-17 during specific immune responses. The discovery that NKT-cells produce IL-17 is relatively recent35,36 and was not known to occur during viral infection until our study was completed.34 In our study, we found that NKT-cells from aged mice express more of the IL-17 promoting transcription factor, RORγT, than young cells both at rest and after stimulation with herpes viruses. We also found that the elevated IL-17 response with aging induces neutrophil recruitment into the liver and subsequent lethal liver injury in aged mice.
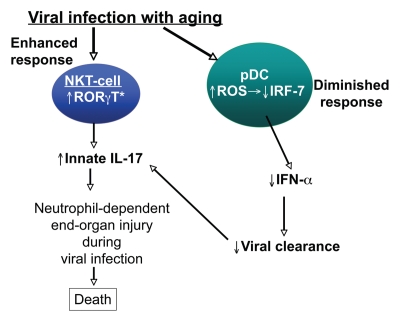
The elevated IL-17 response that occurs with age in our studies synergizes with an impaired ability to contain the virus, thus inducing mortality (Fig. 1).34 This impairment is seen in reduced levels of IFNα by plasmacytoid DCs (pDCs). A prior study by our laboratory discerned in detail the molecular defect within pDCs with aging. Specifically, we found that aging leads to the defective upregulation of interferon regulatory factor (IRF)-7, a critical signal transducer downstream of TLR9 and the IFNαβ receptor.37 Taken together, our findings indicate that elevated IL-17 produced by NKT-cells coupled with impaired IFNα responses from pDCs induces mortality during systemic herpes viral infection in aged mice.
Figure 1.
Proposed pathway of elevated IL-17 responses to viral infection with aging Aging induces increased RORγT, an IL-17 promoting transcription factor, within NKT-cells, innate immune lymphocytes. During herpes viral infection, these aged cells produce exaggerated IL-17 levels. Subsequently, IL-17 induces neutrophil-dependent liver injury, leading to worse outcomes in aged hosts than young hosts. This production is coupled with lower IFNα levels from plasmacytoid DCs, impairing viral clearance leading to further NKT-cell activation and more IL-17 production.
Conclusion
Our study has uncovered an unappreciated role of exaggerated inflammatory responses in aged hosts during viral infection with aging. Our study may indicate that aging induces an imbalanced immunological response to microbial infection and our study lays the foundation for exciting possibilities in the development of novel anti-inflammatory therapies for older people infected with viruses and possibly other pathogens. Such therapies could include novel therapeutics to treat disease after viral infection. Before such possibilities, our results need to be verified in other diseases models of localized viral infections and also translated to humans.
Acknowledgements
The study described in this addendum was supported by National Institute on Aging grants AG028082 and AG033049. DRG is also supported by American Heart Association Grant 0940006N.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/12009
References
- 1.United Nations Population Division, author. World Population Ageing 195--2050 Executive summary. 2010. www.un.org/esa/population/pulibcations /worldageing19502050/pdf/62executivesummary_english.pdf.
- 2.Kaplan V, Angus DC. Community-acquired pneumonia in the elderly. Critical Care Clinics. 2003;19:729–748. doi: 10.1016/s0749-0704(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 3.Gorina Y, Kelly T, Lubitz J, Hines Z. Trends in influenza and pneumonia among older persons in the United States. Aging Trends. 2008;8:1–11. [PubMed] [Google Scholar]
- 4.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 5.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons Living in the Community. N Engl J Med. 1994;331:778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 6.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. Ann Int Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Burns EA, LL, L'Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J Gerontol. 1993;48:B231–B236. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- 8.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Current Opinion in Immunology. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiskopf D, B W, Grubeck-Loebenstein B. The aging of the immune system. Trans Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 12.Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common {gamma} chain binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoman ML, Weigle WO. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982;128:2358–2361. [PubMed] [Google Scholar]
- 14.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, et al. Murine myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 15.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33:267–282. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 16.Garcia GG, Miller RA. Single-Cell Analyses Reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 17.Linton P-J, Dorschkind K. Age related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8+ cells. J Exp Med. 2002;195:283–293. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. PNAS. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 22.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1998;64:703–712. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 23.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 24.Saurwein-Teissl M, Romani N, Grubeck-Loebenstein B. Dendritic cells in old age-neglected by gerontology. Mech Ageing Dev. 2000;121:123–130. doi: 10.1016/s0047-6374(00)00203-7. [DOI] [PubMed] [Google Scholar]
- 25.Clayberger C, Krensky AM. Granulysin. Curr Op Immunol. 2003;15:560–565. doi: 10.1016/s0952-7915(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A, Agrawal S, Cao J-N, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 27.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: Impaired toll-like receptor cxpression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 28.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 30.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinf lammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruunsgard H, Klarlund Pedersen B. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;11:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 33.Ershler WB. Biological Interactions of aging and anemia: A focus on cytokines. J Am Geriatr Soc. 2003;51:18–21. doi: 10.1046/j.1532-5415.51.3s.2.x. [DOI] [PubMed] [Google Scholar]
- 34.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6:446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel M-L, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and ROR{gamma}t and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 upregulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]