Abstract
Intravenous vasopressin at 0.01 to 0.04 units/kg/h increased median mean blood pressure from 26 mm Hg (range 18-44) to 41 mm Hg (range 17-90) by 12 hours of infusion (P = .002) and allowed weaning of catecholamines in a group of extremely low birth weight infants with refractory hypotension.
Among extremely low birth weight infants (ELBW infants < 1000 g birth weight), sepsis remains an important cause of illness and death. Septic shock may become unresponsive to conventional management with catecholamines and hydrocortisone. Treatment with vasopressin effectively restored blood pressure while allowing for a reduction of doses of catecholamines in patient s with septic shock.1-4 Reports of vasopressin use in infants with hypotension are scarce.5,6
Methods
We identified all ELBW infants born at the Duke University between January 2005 and July 2007 treated for ≥1 hour with vasopressin. This study was approved by the Duke International Review Board. Acceptable mean blood pressure (MBP) was defined as >10th percentile for gestational and postnatal age.7 Hydrocortisone (2 to 4 mg/kg/day) was used for hypotension unresponsive to catecholamines. Refractory hypotension was defined as the inability to maintain acceptable MBP despite treatment with dopamine, epinephrine, and hydrocortisone. Presumed sepsis was defined as circulatory compromise with negative cultures and concurrent antimicrobial treatment. The diagnosis of necrotizing enterocolitis (NEC) was made if the infant was ≥ stage IIA of the modified Bell's criteria.8
Data Collection
Hemodynamic variables and doses of catecholamines were recorded at baseline (before vasopressin infusion) and at 1, 6, and 12 hours after initiation of vasopressin. Early deaths were defined as those occurring during or within 72 hours of vasopressin infusion and late deaths were those occurring 72 hours after the end of the infusion.
Vasopressin (Abraxis Pharmaceutical, Schaumburg, Illinois) dosing was administered at 0.01 to 0.04 units/kg/h (0.00017 to 0.0007 units/kg/min).1,5,9 Vasopressin was administered through central or midline venous catheters, at the discretion of the bedside physician for refractory hypotension.
Clinical variables at baseline were compared with values at 12 hours after initiation of vasopressin infusion. Nonparametric tests were used and intragroup comparisons were performed with the Wilcoxon signed rank test. A 2-sided P value <.05 was considered statistically significant. Stata 9.0 (Stata Corp., College Station, Texas) was used for statistical analyses.
Results
We identified 33 separate vasopressin infusions in 20 infants with a median gestational age at birth of 25 weeks (range 23-27) and a median birth weight of 680 g (400-980). Treatment with vasopressin began at a median age of 10 postnatal days and lasted a median of 20 hours. The vasopressin dose used was 0.01 to 0.04 units/kg/h, except for one infant who received 0.08 units/kg/h for 1 hour. Diagnoses at the start of vasopressin infusions were culture confirmed sepsis (n = 14), presumed sepsis (n = 14), and NEC (n = 5) (Table). All infants were treated with dopamine and hydrocortisone before beginning the vasopressin infusions. One vasopressin infusion started before epinephrine use.
Table. Clinical details.
| Infant | Weight (g) | GA | Diagnosis | Age at VP infusions (days) | Duration of VP infusions (hours) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 580 | 26 | Presumed sepsis | 2 | 5 | Survived |
| 2 | 540 | 23 | Presumed sepsis | 1 | 9 | Survived |
| 3 | 610 | 24 | Presumed sepsis | 1 | 13 | |
| E Coli sepsis | 32 | 26 | Late death | |||
| 4 | 400 | 24 | NEC | 10 | 37 | Early death |
| 5 | 600 | 26 | Presumed sepsis | 12 | 12 | Survived |
| 6 | 840 | 26 | NEC | 14 | 10 | Survived |
| 7 | 820 | 25 | NEC | 43 | 42 | |
| NEC | 51 | 11 | ||||
| Enterococcus sepsis | 240 | 10 | Early death | |||
| 8 | 507 | 26 | CoNS sepsis | 13 | 3 | Survived |
| 9 | 656 | 27 | Presumed sepsis | 1 | 70 | |
| Presumed sepsis | 6 | 26 | Early death | |||
| 10 | 700 | 23 | Presumed sepsis | 2 | 20 | |
| Presumed sepsis | 3 | 27 | Early death | |||
| 11 | 980 | 26 | NEC | 26 | 62 | Late death |
| 12 | 710 | 27 | Presumed sepsis | 2 | 126 | |
| CoNS sepsis | 29 | 171 | ||||
| Presumed sepsis | 44 | 14 | ||||
| Presumed sepsis | 45 | 33 | Early death | |||
| 13 | 840 | 26 | NEC | 12 | 13 | Early death |
| 14 | 750 | 25 | E. coli sepsis | 1 | 2 | Early death |
| 15 | 660 | 24 | Enterobacter sepsis | 9 | 3 | |
| Enterobacter sepsis | 10 | 7 | Early death | |||
| 16 | 770 | 27 | Presumed sepsis | 22 | 22 | |
| Presumed sepsis | 24 | 2 | Survived | |||
| 17 | 710 | 24 | GBS** | 1 | 25 | |
| GBS/Candida sepsis | 3 | 1 | ||||
| GBS/Candida sepsis | 3 | 3 | Late death | |||
| 18 | 860 | 24 | MRSA*** sepsis | 1 | 35 | |
| 3 | 49 | Early death | ||||
| 19 | 630 | 23 | CoNS sepsis | 12 | 6 | Survived |
| 20 | 550 | 24 | CoNS sepsis | 15 | 11 | Early death |
GA, Gestational age; VP, vasopressin; CoNS, coagulase-negative Staphylococcus; GBS, group B Streptococcus; MRSA, methicillin-resistant Staphylococcus aureus.
Median MBP increased from 26 mm Hg (range 18-44) at baseline to 41 mm Hg (17-90) by 12 hours of vasopressin infusion (P = .002) (Figure). Mean dopamine dose decreased from 20 μg/kg/min (SD ± 2.6) at baseline to 13 μg/kg/min (±8.6) after 12 hours of vasopressin infusion (P = .006). Mean epinephrine dose decreased from 0.10 μg/kg/min (±0.07) at baseline to 0.05 μg/kg/min (±0.07) after 12 hours of vasopressin infusion (P = .04). Median heart rate was 174 beats/min (range 120–207) at baseline versus 168 beats/min (145-216) after 12 hours of vasopressin infusion (P = .45).
Figure.
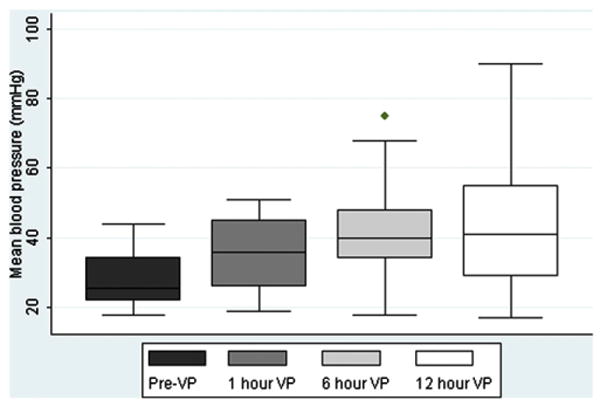
Median MBP increased from 26 mm Hg (range 18-44) at baseline to 41 mm Hg (range 17-90) at 12 hours of vasopressin infusion (P = .002). Surviving infants included at each vasopressin infusion time (n): Pre VP (baseline) (n = 20), 6 hours (n = 18), 12 hours (n = 16).
Median urine output was 3.7 mL/kg/h at baseline and 3.0 mL/kg/h after 12 hours of vasopressin infusion (P = .36). Median serum pH was 7.24 at baseline and 7.33 after 12 hours of vasopressin infusion (P = .58). Median serum lactate was 4.2 mmol/L at baseline and 4.1 mmol/L during vasopressin infusion (P = .76). Median serum sodium concentrations remained stable, 133 mmol/L at baseline versus 131 mmol/L during the infusion (P = .54).
There were 10 (50%) early deaths and 3 (15%) late deaths. Causes of death in the early death group included NEC (Table, infants 4 and 13), presumed sepsis (n = 3), and confirmed sepsis (n = 5). In the late death group, 2 infants died of NEC (Table, infants 3 and 11) and another of Escherichia coli meningitis (Table, infant 17).
Discussion
Vasopressin at doses of 0.01 to 0.04 units/kg/h increased MBP in this group of critically ill ELBW infants and allowed for a decrease of catecholamine dosages. The increase in MBP could reflect improved vasopressin levels. In preterm infants with low blood pressure, plasma vasopressin levels may be consistent with relative vasopressin deficiency in shock conditions.10,11
Splanchnic hypoperfusion is a potential complication of vasopressin, particularly concerning in ELBW infants. Meyer et al reported an infant that received 0.36 units/kg/h of vasopressin was found at autopsy to have liver necrosis.6 In our case series, the diagnosis of NEC was made prior to the initiation of vasopressin in 3/4 (75%) infants who died of NEC (Table, infants 4, 11, and 13). However, the possibility exists that treatment with vasopressin could worsen splanchnic perfusion and negatively impact the outcome of these infants.
Intraventricular hemorrhage, possibly multifactorial in origin, was diagnosed in 5/9 (55%) infants who received vasopressin in the first 3 days of life. None of the patients had evidence of ischemic complications of the limbs.
The early mortality rate was 50% in these critically ill ELBW infants. In a review of vasopressin use in infants and children with shock, the early mortality rate was 52/109 (48%).12 The issue of timing of initiation of vasopressin relative to the onset of sepsis may be important, as suggested in a recent adult randomized trial, where the mortality rate was lower among patients with less severe septic shock who received vasopressin compared with norepinephrine (26% vs 36%, P = .05).4 Further research evaluating the use of vasopressin in ELBW infants with refractory hypotension is needed to determine the pharmacokinetics, timing of treatment, efficacy, and side effects of vasopressin.
Acknowledgments
We thank Kimberley A. Fisher, RN, PhD, and Sandra Grimes, RN, for their expert technical contributions (financial support provided by the Jean and George W. Brumley, Jr. Neonatal Perinatal Research Institute).
P.B.S. received financial support from NIH-1K23HD060040-01, and R.G. received financial support from NIH-TL1RR024126.
Glossary
- ELBW
Extremely low birth weight
- MBP
Mean blood pressure
- NEC
Necrotizing enterocolitis
Footnotes
The authors declare no conflicts of interest.
References
- 1.Landry DW, Levin HR, Gallant EM, Ashton RC, Jr, Seo S, D'Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–5. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 2.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–95. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 3.Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107:2313–9. doi: 10.1161/01.CIR.0000066692.71008.BB. [DOI] [PubMed] [Google Scholar]
- 4.Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–87. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig EB, Starc TJ, Chen JM, Cullinane S, Timchak DM, Gersony WM, et al. Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation. 1999;100:II182–6. doi: 10.1161/01.cir.100.suppl_2.ii-182. [DOI] [PubMed] [Google Scholar]
- 6.Meyer S, Gottschling S, Baghai A, Wurm D, Gortner L. Arginine-vasopressin in catecholamine-refractory septic versus non-septic shock in extremely low birth weight infants with acute renal injury. Crit Care. 2006;10:R71. doi: 10.1186/cc4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuntnarumit P, Yang W, Bada-Ellzey HS. Blood pressure measurements in the newborn. Clin Perinatol. 1999;26:981–96. x. [PubMed] [Google Scholar]
- 8.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes CL, Walley KR, Chittock DR, Lehman T, Russell JA. The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. 2001;27:1416–21. doi: 10.1007/s001340101014. [DOI] [PubMed] [Google Scholar]
- 10.Ezaki S, Suzuki K, Kurishima C, Miura M, Moriwaki K, Arakawa H, et al. Levels of catecholamines, arginine vasopressin and atrial natriuretic peptide in hypotensive extremely low birth weight infants in the first 24 hours after birth. Neonatology. 2009;95:248–55. doi: 10.1159/000166845. [DOI] [PubMed] [Google Scholar]
- 11.Landry DW, Oliver JA. Vasopressin and relativity: on the matter of deficiency and sensitivity. Crit Care Med. 2006;34:1275–7. doi: 10.1097/01.CCM.0000208105.76876.2E. [DOI] [PubMed] [Google Scholar]
- 12.Meyer S, Gortner L, McGuire W, Baghai A, Gottschling S. Vasopressin in catecholamine-refractory shock in children. Anaesthesia. 2008;63:228–34. doi: 10.1111/j.1365-2044.2007.05317.x. [DOI] [PubMed] [Google Scholar]


