Abstract
Acetaminophen (APAP) overdose is the leading cause of acute liver failure in Western countries. In the last four decades, much progress has been made in our understanding of APAP-induced liver injury through rodent studies. However, some differences exist in the time course of injury between rodents and human. To study the mechanism of APAP hepatotoxicity in humans, a human-relevant in vitro system is needed. Here we present evidence that the cell line HepaRG is a useful human model for the study of APAP-induced liver injury. Exposure of HepaRG cells to APAP at several concentrations resulted in glutathione depletion, APAP-protein adduct formation, mitochondrial oxidant stress and peroxynitrite formation, mitochondrial dysfunction (assessed by JC-1 fluorescence), and lactate dehydrogenase (LDH) release. Importantly, the time course of LDH release resembled the increase in plasma aminotransferase activity seen in humans following APAP overdose. Based on propidium iodide uptake and cell morphology, the majority of the injury occurred within clusters of hepatocyte-like cells. The progression of injury in these cells involved mitochondrial reactive oxygen and reactive nitrogen formation. APAP did not increase caspase activity above untreated control values and a pancaspase inhibitor did not protect against APAP-induced cell injury. These data suggest that key mechanistic features of APAP-induced cell death are the same in human HepaRG cells, rodent in vivo models and primary cultured mouse hepatocytes. Thus, HepaRG cells are a useful model to study mechanisms of APAP hepatotoxicity in humans.
Keywords: Acetaminophen, drug-induced liver injury; oncotic necrosis; apoptosis; oxidant stress; acute liver failure; mitochondrial dysfunction; protein adducts
Introduction
Acetaminophen (APAP) is a widely used over-the-counter analgesic and antipyretic drug, and is a common component of opioid-containing prescription formulations. Though safe at therapeutic levels, overdose of APAP causes liver injury and is the foremost cause of acute liver failure in the US and the UK.1 At therapeutic doses, >90% of the drug is glucuronidated or sulfated in the liver and subsequently excreted. The remainder is metabolized by cytochromes P450 (CYP450) to the electrophilic intermediate N-acetyl-p-benzoquinoneimine (NAPQI), which can be neutralized by conjugation with glutathione.2 However, after an overdose of APAP, formation of NAPQI exceeds the detoxification capacity of glutathione, resulting in covalent binding to cellular proteins.3 Although the overall protein binding caused by an overdose of APAP or its isomer 3'-hydroxyacetanilide is similar and many adducted proteins have been identified, toxicity only occurred with APAP, which shows greater binding to mitochondrial proteins.3–6 The subsequent mitochondrial dysfunction leads to inhibition of mitochondrial respiration,7 ATP depletion,8 and formation of reactive oxygen8 and peroxynitrite9 (ROS and RNS) inside mitochondria. The oxidant stress is involved in activation of the c-jun-N-terminal kinase (JNK) pathway10 and eventually triggers the opening of the mitochondrial membrane permeability transition (MPT) pore11 resulting in collapse of the mitochondrial membrane potential.11,12 Mitochondrial matrix swelling and rupture of the outer membrane causes the release of intermembrane proteins including cytochrome c, endonuclease G and apoptosis-inducing factor (AIF).13 Only endonuclease G and AIF translocate to the nucleus and induce DNA fragmentation.14 The severe impairment of aerobic energy metabolism, massive ATP depletion and nuclear DNA damage result in necrotic cell death.15 Despite the release of cytochrome c from mitochondria, no significant activation of caspases has been detected and apoptosis contributes less than 5% to the overall injury in mice.15–17
Most of our present knowledge of APAP hepatotoxicity has been learned from rodent studies in vivo and in primary culture.2,13 However, notable differences exist in the time course of injury between rodents and humans. In particular, increased aminotransferase activity can be detected in rodent plasma within 2–6h of administration of a toxic dose of APAP, with peak activity achieved around 12h.18 In humans, increased plasma enzyme activity is rarely observed before 12–24h following ingestion and does not peak until 48–72h.19 Although such differences between humans and rodents may be mainly due to species differences in metabolic rate and body size, mechanistic dissimilarities cannot be completely ruled out.
In order to bridge this gap between rodents and humans, a human in vitro system is needed. Primary human hepatocytes as the gold standard have major drawbacks. The availability of these cells is limited, and due to significant differences in donor background they can vary considerably in drug response. Moreover, primary human hepatocytes have a limited lifespan, undergoing phenotypic changes and displaying highly variable CYP450 expression as a function of time in culture. In contrast, most hepatoma cell lines are very stable, available in large quantities, and easy to work with. Unfortunately, the majority do not express the CYP450 enzymes necessary for metabolism of drugs and are therefore not useful for studies of drug toxicity.20,21
HepaRG cells were recently isolated and cultured from a hepatoma in a female patient with cirrhosis subsequent to hepatitis C virus infection (HCV).22 HepaRG cells are bipotent progenitors. Upon differentiation, two morphologically distinct populations become apparent: hepatocyte-like cells and biliary epithelial-like cells.23,24 Several studies have demonstrated high expression and activity of xenobiotic metabolizing enzymes in this cell line, comparable to primary human hepatocytes, suggesting their use in drug studies.25,26 However, detailed investigations into the mechanisms of drug toxicities have not been performed with this cell line. Therefore, the objective of the current investigation was to assess the value of HepaRG cells as a human system to study APAP hepatotoxicity and to determine if mechanisms of cell death observed in primary mouse hepatocytes are applicable to human hepatocytes.
Materials and Methods
Cell culture
HepaRG cells were obtained from Biopredic International (Rennes, France). The cells were seeded at 1 × 105 undifferentiated cells/cm2 in hepatocyte wash medium (Invitrogen Corporation, Carlsbad, CA) containing additives for growth (Biopredic). The cells were cultured at 37°C with 21% O2 and 5% CO2 for 14 days before differentiation. Medium was renewed every 3 days. Cell differentiation was induced as described.22 The cells were maintained up to 4 weeks after differentiation for use. HepG2 cells were grown to 90% confluence in DMSO-free Williams’ E Medium containing penicillin/streptomycin, insulin, and 10% FBS. For APAP treatment, HepaRG or HepG2 cells were washed with phosphate buffered saline (PBS) and changed to DMSO-free medium containing the desired concentration of APAP. For caspase inhibition, some cells were pretreated for 1h with medium containing 20 µM Z-VD-fmk (generous gift from Dr. S. X. Cai, Epicept Corp., San Diego, CA), then changed to medium containing 20 µM Z-VD-fmk and 20 mM APAP. As a positive control for caspase activation, some cells were treated for 16.5h with 5 mM galactosamine and 100 ng/mL recombinant human TNF (Genzyme, Cambridge, MA). HepaRG cells were used at passages 18, 19, and 20. Within this range, no variation in GSH depletion or in the kinetics of injury was observed after APAP exposure suggesting no relevant change in CYP expression or activity between these passages.
Analysis of APAP protein adducts. After protease digestion, APAP-cysteine (APAP-CYS) adducts were measured in cells and in the culture medium by LC-MS/MS as described in detail in the supplemental material.
Biochemical Analyses
Cell death was assessed by lactate dehydrogenase (LDH) release, as described in detail.12 LDH release is a more sensitive parameter of cell death because HepaRG cells contain only low levels of alanine aminotransferase activity. The JC-1 Mitochondrial Membrane Potential Kit (Cell Technology, Mountain View, CA) was used according to the manufacturer’s instructions.12 Cellular glutathione was measured using a modified Tietze assay, as described.27 For measurement of mitochondrial ROS generation, HepaRG cells were seeded on glass bottom dishes and ROS and peroxynitrite generation was measured using Mitosox Red® and dihydrorhodamine, respectively, as described.28 Caspase activity based on Z-VAD-fmk inhibitable Ac-DEVD-AMC fluorescence was measured as described.29
Propidium iodide staining
Cells were seeded on glass bottom dishes and treated with APAP and 30 µM propidium iodide in DMSO-free, phenol red-free Williams’ E Medium with penicillin/streptomycin and 10% FBS. At various time points, the live cells were imaged on a Zeiss Axiovert inverted fluorescence microscope through a Texas Red filter to assess PI uptake. All fluorescence images were taken at the same exposure and later superimposed on phase contrast images of the same fields using Image J software.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA followed by a post hoc Bonferroni test. If the data were not normally distributed, we used the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test; P<0.05 was considered significant.
RESULTS
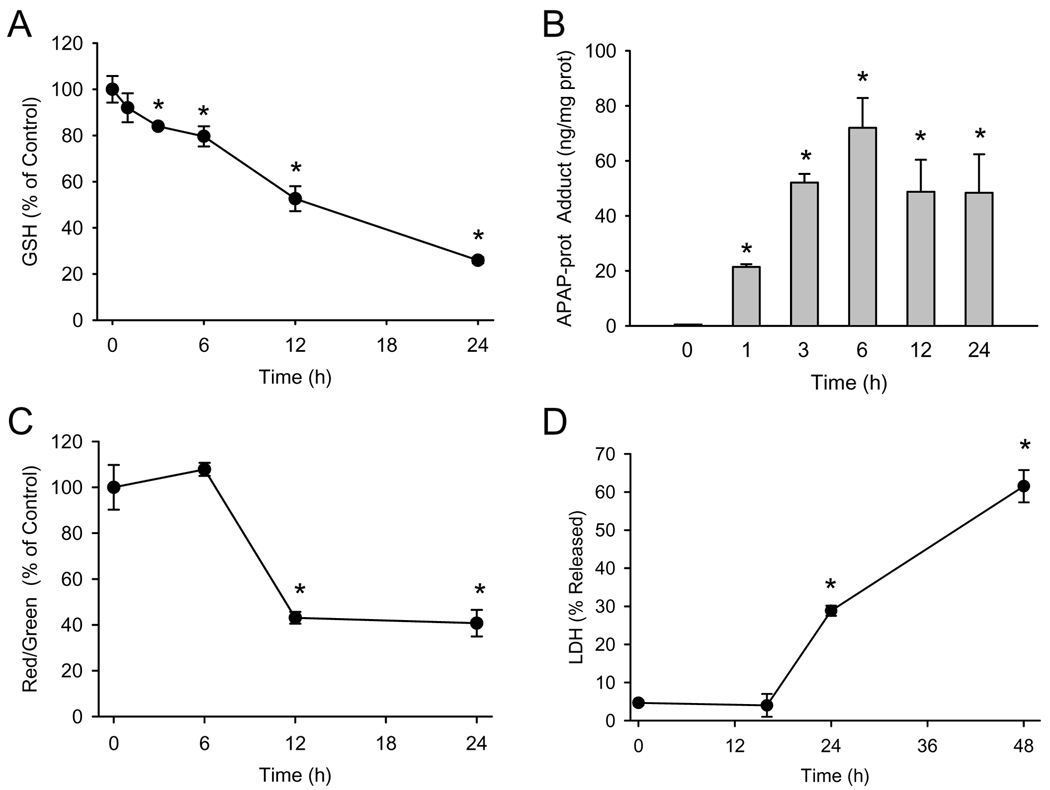
The first event in the pathogenesis of APAP hepatotoxicity in rodents is metabolism of the drug to the reactive intermediate NAPQI, which can bind to and deplete glutathione. To verify metabolism of APAP in this model, differentiated HepaRG cells were treated with 20 mM APAP and the GSH content of the cells was measured at various time points. Significant glutathione depletion (16% decrease from baseline) could be detected as early as 3h, with only 26% of control GSH levels remaining at 24h (Figure 1A). Measurement of APAP-protein adducts in these cells showed a significant increase as early as 1h, peak levels at 6h and a gradual decline during the subsequent 18h (Figure 1B). Protein adducts in culture medium were only detected at 12h (4.38±0.20 ng/ml) and 24h (24.38±1.05 ng/ml), which correlated with the decline in cellular adduct levels at these time points. These results indicate that protein adducts were formed well before cellular GSH levels were exhausted.
Figure 1. Time course of APAP toxicity in HepaRG cells.
Confluent cells were treated with 20 mM APAP and GSH, APAP-protein adducts, JC-1 fluorescence, and LDH release were measured as described in the methods section. (A) Glutathione content before and 1–24h after treatment with APAP. (B) APAP-protein adducts before and 1–24h after APAP treatment. (C) Ratio of red to green fluorescence of the JC-1 dye before and 6–24 h after APAP treatment. (D) Percentage of total LDH activity found in culture medium before and 15–48h after APAP treatment. Data are expressed as mean ± SE of 3–6 independent cell preparations. *P<0.05 (compared to time 0)
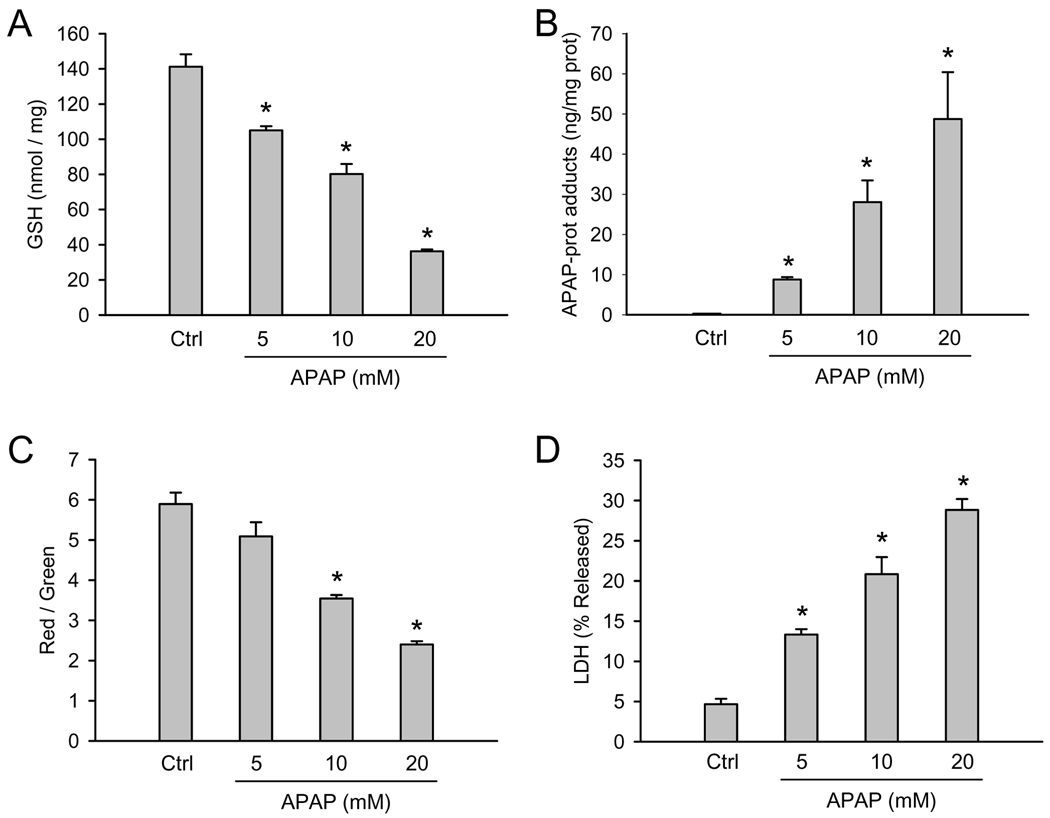
To explore the role of mitochondrial dysfunction after APAP exposure in HepaRG cells, we examined mitochondrial integrity using the JC-1 assay. In healthy cells, the dye preferentially localizes to mitochondria, where it forms aggregates which fluoresce red. When the mitochondrial membrane potential collapses (e.g. after the membrane permeability transition), the dye can diffuse into the cytosol in monomeric form which fluoresces green. Thus, the ratio of red to green fluorescence reflects mitochondrial membrane integrity. HepaRG cells showed a significant decrease in red/green fluorescence by 12h in the presence of 20 mM APAP, which persisted to at least 24h (Figure 1C). As an indicator of cell death, lactate dehydrogenase (LDH) activity was measured in cell lysate and in culture medium. LDH release into the culture medium was not observed up to 15h with 20 mM APAP (Figure 1D). However, a significant increase was found at 24h (29%), and this continued to rise until at least 48 hours, reaching 62% (Figure 1D). Notably, all four parameters discussed (GSH levels, protein adducts, JC-1 fluorescence, and LDH release) exhibited a clear concentration-response (Figure 2).
Figure 2. Dose-response for APAP toxicity in HepaRG cells.
Confluent cells were treated with various concentrations of APAP and GSH, APAP-protein adducts, JC-1 fluorescence, and LDH release were measured before and 24h after APAP treatment (0–20 mM) as described in the methods section. (A) Cellular glutathione content (B) APAP-protein adducts (C) Ratio of red to green fluorescence of the JC-1 assay (D) Percentage of total LDH activity found in culture medium. Data are expressed as mean ± SE of 3–4 independent cell preparations. *P<0.05 (compared to controls)
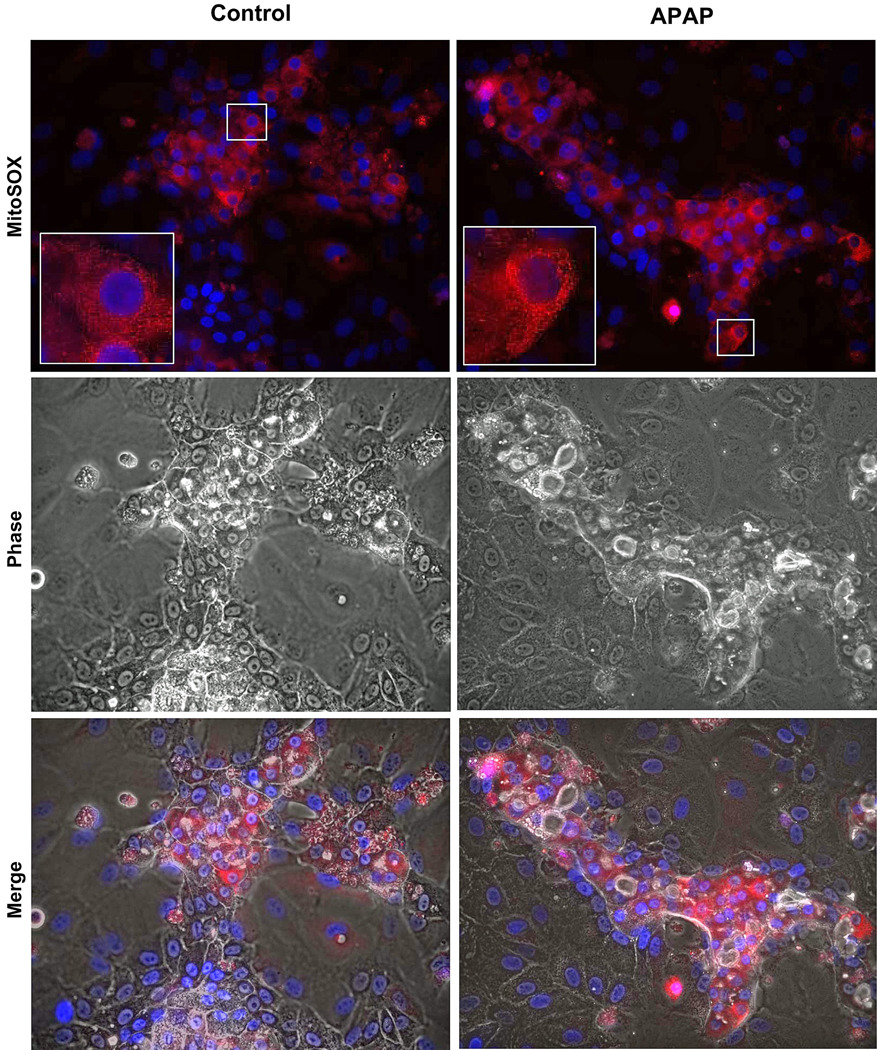
To test for mitochondrial ROS in HepaRG cells, cultures were treated with 20 mM APAP and Mitosox Red® fluorescence was evaluated. It has been suggested that Mitosox Red® detects mainly mitochondrial superoxide.30 Compared to control cells there was a clear increase in Mitosox Red® fluorescence 6h after APAP (Figure 3), which was the time point with the highest fluorescence (data not shown). Higher magnification (inserts) shows the punctate fluorescence characteristic of mitochondrial staining (Figure 3). Merging the Mitosox Red® fluorescence with phase contrast images demonstrates that the oxidant stress occurred only in hepatocytes (Figure 3).
Figure 3. Mitochondrial reactive oxygen species generation after APAP exposure in HepaRG cells.
Confluent HepaRG cells were treated with 20 mM APAP as described in the methods section. Six hours following APAP, cells were loaded with Mitosox Red® and live cell imaging carried out. The top panels show MitoSOX fluorescence in control and APAP treated cells (400×), with the boxed areas digitally magnified in the inserts to demonstrate mitochondrial localization of the signal. The bottom panel is a merge of fluorescence and phase contrast images to demonstrate that the majority of MitoSOX signal is from hepatocytes.
In rodents, RNS such as peroxynitrite are critically involved in the injury mechanism after APAP overdose.18,31 Dihydrorhodamine (DHR) fluorescence can serve as a marker of peroxynitrite in biological systems.32 The compound is taken up into cells, where it can react with intracellular RNS resulting in formation of the fluorescent rhodamine. To investigate RNS formation in HepaRG cells, DHR fluorescence was measured at several time points during exposure to APAP. Rhodamine fluorescence was much more intense than untreated controls at 6 and 12h post-APAP (Figure 4A–C). Similar to rodent hepatocytes,28 rhodamine fluorescence peaked in HepaRG cells (12h) after the peak of Mitosox Red® fluorescence (Figure 4A–C).
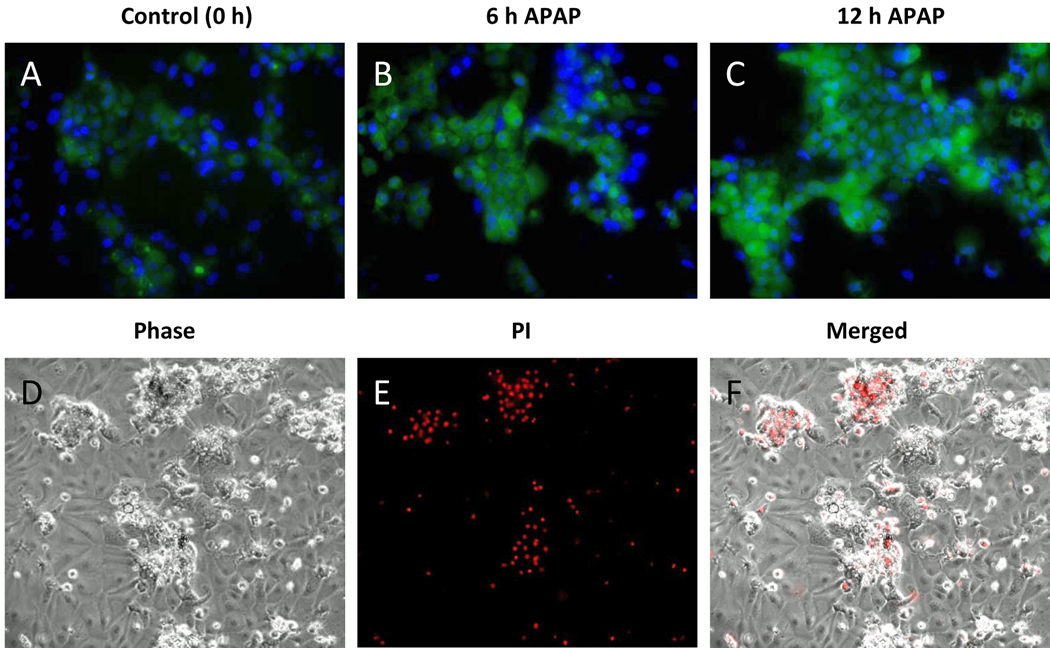
Figure 4. Reactive oxygen and reactive nitrogen species and cell type-specific death after APAP.
(A–C) HepaRG cells were treated with 20 mM APAP. At various time points the cells were washed and loaded with 10 µM dihydrorhodamine (DHR) and 300 nM DAPI for imaging. Merged images showing DAPI (blue) and DHR (green) fluorescence at 0, 6, and 12 h. (D–F) HepaRG cells were co-treated with 20 mM APAP and 30 µM propidium iodide in phenol red-free medium and imaged at 24 h. (D) Phase contrast, (E) PI fluorescence, and (F) merged images of cells 24h after APAP treatment. All images 200×.
HepaRG cells are bipotent progenitors which differentiate into two morphologically distinct cell populations.23,24 The hepatocyte-like cells have a characteristic granular appearance and grow in small clusters or “hepatocyte islands” (Figure 3, 4D). Surrounding these islands are flatter, clearer biliary epithelial-like cells (Figure 3, 4D). To assess the contribution of each cell type to our data showing APAP toxicity, APAP-treated cells were exposed to propidium iodide (PI), which stains nuclei of necrotic cells red (Figure 4E,F). At 24h, the majority of the PI staining was seen in the hepatocyte-like cells, with very little among the biliary epithelial-like cells (Fig. 4E,F). The distribution was similar at 48h (data not shown). This suggests that APAP mainly affects the hepatocytes. Together, these data indicate that - similar to rodent hepatocytes - cell death in human HepaRG cells is preceded by GSH depletion, protein binding, formation of reactive oxygen and peroxynitrite and mitochondrial dysfunction.
To compare HepaRG cells to other hepatoma cell lines, APAP toxicity was evaluated in HepG2 cells. HepG2 cells treated with 20 mM APAP for 24h showed no evidence of GSH depletion, mitochondrial dysfunction (JC-1 assay) or cell injury (LDH release) in response to the toxic dose of APAP (Table 1). However, low levels of protein adducts were identified despite the absence of toxicity (Table 1). Thus, the near absence of drug-metabolizing enzymes drastically reduced the metabolic activation of APAP and prevented any toxicity in HepG2 cells.
Table 1.
APAP Toxicity in HepG2 Cells
| Treatment | GSH + GSSG | JC-1 | LDH Released | APAP-Prot. Adducts |
|---|---|---|---|---|
| (nmol/mg protein) | Red/Green | (% of total) | (ng/mg protein) | |
| Controls | 98.5 ± 12.0 | 4.38 ± 1.0 | 5.9 ± 2.0 | ND |
| 20 mM APAP | 90.8 ± 11.0 | 4.43 ± 0.1 | 10.3 ± 3.0 | 3.9 ± 0.8 |
HepG2 cells were grown to 90% confluence and treated with 20 mM APAP for 24 h. Total glutathione (GSH+GSSG) content, mitochondrial function (JC-1 assay), LDH release, and APAP-protein adducts were measured as described in the methods section. Values represent mean ± SE (n=3 experiments). (ND = not detectable)
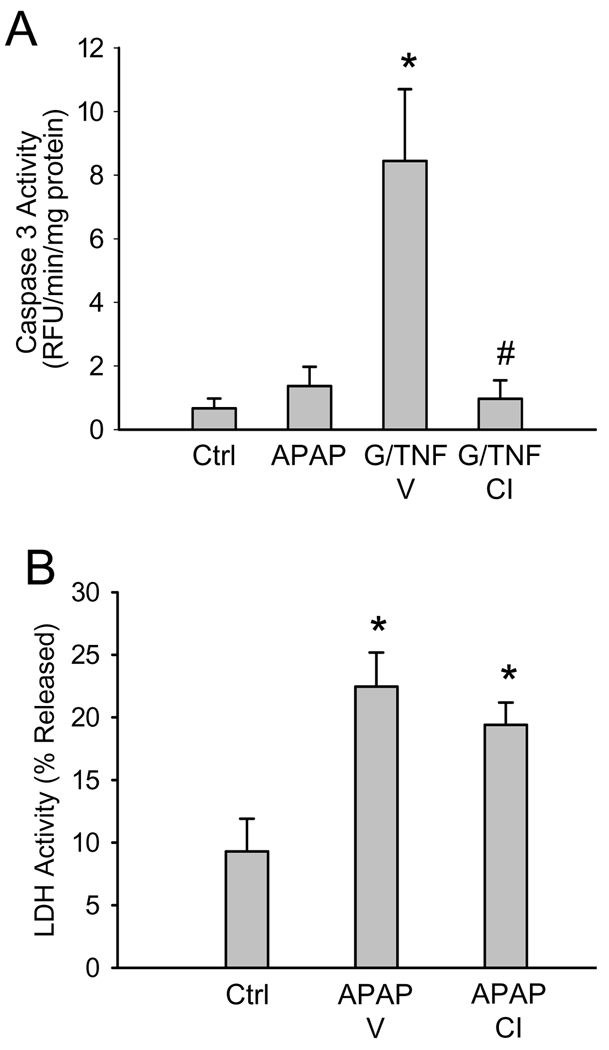
Loss of mitochondrial membrane integrity can result in the release of pro-apoptotic proteins, including the caspase activator cytochrome c, into the cytosol. To determine whether or not APAP toxicity in HepaRG cells involves apoptosis, caspase-3 activity was measured in lysates of cells treated for 24h with APAP. There was no significant increase in caspase activity over control with 20 (Figure 5A), 5 or 10 mM APAP (data not shown). In addition, the potent pan-caspase inhibitor Z-VD-fmk had no effect on APAP-induced LDH release at 24h (Figure 5B), suggesting that APAP did not cause apoptosis in HepaRG cells. In contrast, caspase activity was significantly increased when cells were exposed to 100 ng/mL human rTNF-α and 5 mM galactosamine for 16.5h as a positive control (Figure 5A). The caspase inhibitor prevented the increase in caspase activity after G/TNF. This indicates that HepaRG cells do have the capacity to undergo apoptotic cell death in response to an appropriate stimulus.
Figure 5. Effect of a pan-caspase inhibitor on APAP toxicity in HepaRG cells.
(A) Caspase-3 activity was measured in cell lysates of untreated cells or 24h after APAP treatment (20 mM). As positive control, cells were treated with galactosamine and recombinant human TNF for 16.5h in the presence or absence of vehicle (Tris-buffer) or the pan-caspase inhibitor (CI) Z-VD-fmk (20 µM). (B) LDH release in untreated cells or 24h after APAP treatment. Some cells were pretreated for 1h with vehicle or CI before addition of 20 mM APAP. Data are expressed as mean ± SE for 4 independent cell preparations. *P<0.05 (compared to Ctrl) #P<0.05 (compared to APAP or G/TNF)
DISCUSSION
HepaRG cells as tool to study hepatotoxicity
The objective of this study was to assess the value of HepaRG cells as a model to investigate mechanisms of APAP toxicity in a human system and to determine if certain key features of toxicity observed in rodents apply to this cell line. Our data suggest that APAP treatment leads to GSH depletion, protein adduct formation, mitochondrial dysfunction and oxidant stress and eventually oncotic necrosis in HepaRG cells, similar to what has been observed in primary mouse hepatocytes but not in typical human hepatoma cells. The basis for this behavior is that HepaRG cells are capable of differentiating into two subpopulations: one with hepatocyte-like morphology and gene expression and one with the appearance of biliary epithelial cells.22,24 The hepatocyte-like cells express a nearly complete complement of drug-metabolizing enzymes, including most of the cytochrome P450 enzymes.25,26 HepaRG cells also possess many other characteristics unique to adult differentiated hepatocytes, including hepatocyte-specific transporter expression,25,33 iron-loading capacity,34 and inducibility of CYPs and other proteins.33,35 Thus, these cells have the potential to be a useful tool to study mechanisms of drug hepatotoxicity in a human system. The distinct advantage of the HepaRG cell line over primary human hepatocytes is the unlimited availability of identical cells. Nevertheless, they are hepatoma-derived and there is the possibility that certain intracellular signaling mechanisms might be different. It is therefore important to study mechanisms of cell death induced by known hepatotoxicants in these cells.
Mechanisms of acetaminophen-induced liver injury
Acetaminophen hepatotoxicity in rodents depends on the formation of the reactive metabolite NAPQI, which can be detoxified by glutathione. However, after depletion of GSH in the cell, NAPQI binds to cellular proteins, which is considered the initiating event in the toxicity.2,36 Our experiments with HepaRG cells identified depletion of cellular GSH and the formation of protein adducts as the earliest detectable events. This is consistent with mouse studies of APAP hepatotoxicity.18,37 Evidence for increased GSH turnover and detection of APAP-protein adducts in human plasma after APAP exposure suggests that these events also occur in humans.38,39 Although our data agree with the general hypothesis of reactive metabolite formation, GSH depletion and protein adduct formation as early response to APAP exposure, the sequence of events is not as previously assumed. Our data clearly show that protein adduct formation occurs parallel to GSH consumption and does not require extensive GSH depletion. In fact, protein adducts were detected in HepaRG cells and in HepG2 cells before significant effects on GSH levels and well before any evidence of mitochondrial dysfunction and cell death. This suggests that small amounts of protein binding per se does not initiate toxicity and probably a certain level needs to be reached to trigger the early mitochondrial effects.
More recently, mitochondrial dysfunction and the MPT have emerged as central to the mechanism of APAP-induced cell death in cultured rodent hepatocytes10–12 and in vivo.8,9,18 Consistent with this, APAP triggered mitochondrial dysfunction in HepaRG cells (indicated by JC-1 fluorescence) before cell death (LDH release). In addition, mitochondrial oxidant stress with peroxynitrite formation is a hallmark of the mechanism of APAP-induced injury in rodents8,9,31 and is critically involved in the MPT pore opening and cell death.40 Similar evidence for mitochondrial oxidant stress (MitoSox Red®) and peroxynitrite (DHR) was detected in the HepaRG cells before massive mitochondrial dysfunction and cell death. Although the specificity of fluorescence dyes is sometimes questioned, DHR can be directly oxidized by peroxynitrite but not by reactive oxygen without a catalyst41 and DHR fluorescence has been used as an indicator for peroxynitrite in cell culture.32 Consistent with these findings, we showed the correlation between nitrotyrosine protein adducts and DHR fluorescence as indicators for peroxynitrite formation in mouse hepatocytes.28 Thus, the mechanisms of APAP-induced cell death in human HepaRG cells is similar to rodent hepatocytes, involving reactive metabolite formation with GSH depletion, protein adduct formation, mitochondrial oxidant stress and peroxynitrite formation, and loss of the mitochondrial membrane potential (MPT) before cell death (LDH release, PI uptake). Interestingly, however, the time course of cell death resembles more closely what is observed in humans. The discussed events appear to occur almost exclusively in the hepatocyte-like cells as markers of oxidant stress and cell death (PI staining) were only observed in hepatocytes but not in the biliary epithelial-like cells. The fact that none of the events (except very minor protein adduct formation) including cell death are observed in HepG2 cells, which lack relevant P450 activity, indicates that HepaRG cells are a suitable human model to study drug hepatotoxicity that is dependent on metabolic activation.
A limitation of HepaRG cells as with other cultured cells is the absence of nonparenchymal cells. Although the majority of experimental evidence argues against direct cytotoxicity of Kupffer cells, infiltrating neutrophils and macrophages in this model, cytokines derived from nonparenchymal cells may modulate the intracellular signaling mechanisms and this limitation needs to be kept in mind when extrapolating these data to the in vivo situation.42
Mode of APAP-induced cell death
It is generally accepted that the mode of cell death in APAP hepatotoxicity in primary mouse hepatocytes and in vivo is oncotic necrosis.11,15 Our findings in HepaRG cells indicate that there is no significant caspase activation and a potent pancaspase inhibitor did not prevent APAP-induced cell injury. In addition, loss of cell viability correlated with PI uptake and LDH release, both of which are indicators of necrotic cell death. In contrast, exposure of HepaRG cells to galactosamine/hTNF-α, a well-established system of TNF-mediated apoptosis, induced substantial caspase activation, which was inhibited by the caspase inhibitor. This demonstrated that HepaRG cells have the capability to undergo apoptosis when appropriately stimulated. However, APAP exposure was not able to induce caspases and apoptotic cell death in these cells. The absence of apoptosis in human HepaRG cells is consistent with a case report on APAP overdose where no markers of apoptosis were detectable in plasma.43
In summary, our data indicate that APAP overdose causes necrotic cell death in HepaRG cells. The hepatocyte-like cells but not the biliary epithelial cell-like cells are primarily affected. The sequence of cellular events include GSH depletion, APAP-protein adduct formation, oxidant stress and peroxynitrite generation, loss of the mitochondrial membrane potential and ultimately necrotic cell death. Thus, these mechanisms of APAP-induced cell death are the same as were reported for mouse hepatocytes and mouse liver in vivo. In addition, APAP-induced cell death in HepaRG cells follows a time course similar to that in humans and all intracellular events are also consistent with the limited mechanistic observations in humans. Therefore, we conclude that HepaRG cells are a reliable and useful model to study mechanisms of APAP hepatotoxicity and possibly other drugs in a human system.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT:
This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916, and by grants P20 RR016475 and P20 RR 021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. HepaRG cells were provided by Biopredic, Intl. (Rennes, France). The analysis of APAP protein adducts was supported by a grant from McNeil Consumer Healthcare, Fort Washington, PA.
ABBREVIATIONS
- APAP
acetaminophen
- ALT
alanine aminotransferase
- CYP450
cytochrome P450
- DHR
dihydrorhodamine
- GSH
glutathione
- GSSG
glutathione disulfide
- LDH
lactate dehydrogenase
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- PI
propidium iodide
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
Contributor Information
Mitchell R. McGill, Email: mmcgill@kumc.edu.
Hui-Min Yan, Email: hyan@kumc.edu.
Anup Ramachandran, Email: aramachandran@kumc.edu.
Gordon J. Murray, Email: gordon.murray@utah.edu.
Douglas E. Rollins, Email: doug.rollins@utah.edu.
Hartmut Jaeschke, Email: hjaeschke@kumc.edu.
REFERENCES
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. HEPATOLOGY. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, et al. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 4.Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 5.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 6.Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3'-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2001;500:663–673. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- 7.Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- 9.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 10.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrotic and apoptotic cell death to cultured mouse hepatocytes. HEPATOLOGY. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 12.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 14.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 15.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 16.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen-induced liver injury: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 19.Singer AJ, Carracio TR, Mofenson HC. The temporal profile of increased transaminase levels in patients with acetaminophen-induced liver dysfunction. Ann Emerg Med. 1995;26:49–53. doi: 10.1016/s0196-0644(95)70237-7. [DOI] [PubMed] [Google Scholar]
- 20.Jover R, Bort R, Gómez-Lechón MJ, Castell JV. Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. HEPATOLOGY. 2001;33:668–675. doi: 10.1053/jhep.2001.22176. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Antona C, Donato MT, Boobis A, Edwards RJ, Watts PS, Castell JV, Gomez-Lechon MJ. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: molecular mechanisms that determine lower expression in cultured cells. Xenobiotica. 2002;32:505–520. doi: 10.1080/00498250210128675. [DOI] [PubMed] [Google Scholar]
- 22.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parent R, Marion MJ, Furio L, Trépo C, Petit MA. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology. 2004;126:1147–1156. doi: 10.1053/j.gastro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, Drenou B, Gripon P, et al. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. HEPATOLOGY. 2007;45:957–967. doi: 10.1002/hep.21536. [DOI] [PubMed] [Google Scholar]
- 25.Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- 28.Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3586. [PubMed] [Google Scholar]
- 30.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 32.Crow JP. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 33.Le Vee M, Jigorel E, Glaise D, Gripon P, Guguen-Guillouzo C, Fardel O. Functional expression of sinusoidal and canalicular hepatic drug transporters in the differentiated human hepatoma HepaRG cell line. Eur J Pharm Sci. 2006;28:109–117. doi: 10.1016/j.ejps.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Troadec MB, Glaise D, Lamirault G, Le Cunff M, Guerin E, Le Meur N, Detivaud L, et al. Hepatocyte iron loading capacity is associated with differentiation and repression of motility in the HepaRG cell line. Genomics. 2006;87:93–103. doi: 10.1016/j.ygeno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Antherieu S, Chesne C, Li RY, Camus S, Lahoz A, Picazo L, Turpeinen M, et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38:516–525. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 37.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 38.Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 1987;4:206–211. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- 39.Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran A, Lebofsky M, Baines C, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2010 Oct;13 doi: 10.3109/10715762.2010.520319. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713–716. doi: 10.1111/j.1478-3231.2007.01566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.