Abstract
Hypothesis
We hypothesize that vestibular schwannomas (VS) exhibit up-regulation of estrogen receptor (ER) at the protein level compared to control Great auricular nerve (GAN).
Background
It has been reported in the literature that VS occur more commonly in women, tend to be larger and more vascular in women, and growth rate can accelerate during pregnancy. The literature contains widely divergent results on ER expression in VS, however, varying from no detectable levels to detection of ER in all samples.
Methods
Sixteen sporadic VS specimens were immediately snap-frozen following microsurgical excision, and analyzed for phosphorylated and total levels of ERα with Western blot analysis. ERα expression levels were normalized to actin, then relative expression to GAN was determined.
Results
All VS specimens exhibited expression of both phosphorylated and total ERα. Total ERα expression in VS is equivalent to or slightly up-regulated compared to GAN. VS specimens exhibited more pronounced up-regulation of phosphorylated (ie. activated) levels of ERα compared to GAN.
Conclusion
We have demonstrated that ERα expression in VS is equivalent to GAN. The phosphorylated form of the receptor is up-regulated compared to GAN, however, indicating a higher level of ERα activation in sporadic VS compared to normal nerve. Further investigation into antiestrogen therapy for VS is warranted.
Introduction
Vestibular schwannomas (VS) account for 8–10% of all intracranial tumors, with an annual incidence of 1 per 80,000.1 VS can arise on either branch of the vestibular nerve within the internal auditory canal, where Schwann cell density is greatest. VS are typically encapsulated and histologically benign, but can cause pressure damage to nearby cranial nerves, resulting in cranial neuropathies. Hearing loss is the most common presenting symptom, due to compression of the adjacent cochlear nerve. VS can grow into the cerebellopontine angle, and in rare cases can result in obstructive hydrocephalus, cerebellar ataxia and life-threatening brainstem compression.2,3 Most VS are sporadic, with only 4% attributed to Neurofibromatosis type 2 (NF2), an autosomal dominant disorder characterized by bilateral VS, schwannomas on other CNs, intracranial meningiomas, spinal cord ependymomas and other tumors.4,5
Current treatment modalities include microsurgical excision via craniotomy, stereotactic radiosurgery (i.e. gamma knife) or fractionated stereotactic radiotherapy. When tumors are small, and no growth is documented, “watchful waiting” has been advocated.6 Treatment of large VS, especially in association with NF2, is a challenging task. Tumors larger than three centimeters have an increased risk of hearing loss and facial paralysis.7 All current therapeutic options carry significant risks, including total loss of hearing or vestibular function, facial nerve palsy or paralysis, other cranial neuropathies, cerebrospinal fluid leak, meningitis, intracranial hemorrhage, hydrocephalus, cerebellar ataxia or death.8
Many authors have investigated the expression of the estrogen receptor (ER) in VS. This is due to several observations present in the literature: (1) VS occur more commonly in women,9 (2) VS tend to be larger and more vascular in women,9 and (3) VS growth rate is increased during pregnancy.10 The literature on ER expression is conflicting, however, with reports ranging from no expression to detection of ER in the majority of samples.11–27 Utilizing polymerase-chain reaction (PCR), our laboratory previously reported that ER is up-regulated in sporadic VS at the mRNA level, but most NF2-associated VS do not exhibit this same up-regulation.11
Development of pharmacologic therapies targeted against the molecular pathways responsible for VS tumorigenesis would provide another therapeutic modality for VS patients. Pharmacologic therapies could aid other treatment modalities, decreasing tumor size or slowing growth prior to definitive treatment. Pharmacologic therapies could potentially synergize with stereotactic radiotherapy, acting as a radiosensitizer. They could also potentially be used to slow clinical progression of disease in those patients where other treatment modalities are not an option. If ER is expressed in VS, then blocking the receptor with a selective estrogen receptor modulator, or SERM, such as Tamoxifen or Raloxifene, could be a future pharmacotherapeutic option for treating VS. Tamoxifen and Raloxifene are well-tolerated SERMs that are bioavailable orally. They are FDA-approved for both treatment of ER-alpha (ERα) positive breast cancer, as well as for long-term prevention of breast cancer in high-risk patients.28
The aim of this study is to determine the expression and activation level of ERα at the protein level in human VS specimens. Activation of the estrogen receptor signifies its capability to act as a transcriptional regulator and promote tumorigenesis.
Materials and Methods
All participants provided written, informed consent. The study protocol was approved by the institutional review boards of the University of California, San Diego and Kaiser Permanente, Southern California. VS specimens were collected following microsurgical excision and snap-frozen in liquid nitrogen within ten minutes of excision to prevent protein degradation. Great auricular nerve (GAN) was collected from a patient undergoing planned neck dissection.
Tissue specimens were pulverized over liquid nitrogen, then protein lysate was extracted with the addition of Cell Lysis Buffer (Cell Signaling, Danvers, MA), 0.1% phosphatase inhibitor cocktail (Roche, Indianapolis, IN) and 0.01% protease inhibitor cocktail (Roche), using sonication, per manufacturer’s instructions. Protein concentration was determined with the bichinchoninic acid (BCA) protein assay (Thermo Fischer, Rockford, IL) according to manufacturer’s instructions. Commercially available whole cell lysate from the immortalized breast cancer cell line, MCF-7, served as positive control (Cell Signaling). 20 μg of protein was separated by electrophoresis using sodium dodecyl sulfate (SDS) on a 7.5% Tris-HCL minigel (Bio-Rad, Hercules, CA). Gels were then transferred onto polyvinylidene fluoride (PVDF) membranes (Sigma Aldrich, St. Louis, MO). Membranes were blocked with 5% non-fat milk (Cell Signaling) in TBST (200 mM Tris-HCl, 1.37 M NaCl, 0.1% Tween-20, pH 7.6) for one hour, then incubated with primary antibody diluted in either 5% bovine serum albumin (BSA; Sigma) or 5% non-fat milk in TBST, overnight at 4 degrees Celsius. Membranes were rinsed in TBST and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for one hour, rinsed again, and incubated with enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) reagent or SuperSignal® West Dura substrate (Thermo Fischer). Blots were then exposed to film (Genesee Scientific, San Diego, CA) and developed using an SRX-101A film processor (Konica Minolta Medical and Graphic Inc, NJ). Membranes were incubated first with phosphorylated antibody, then stripped with Restore™ Western Blot Stripping Buffer (Thermo Fischer), and re-blocked in 5% non-fat milk, diluted in TBST, for one hour at room temperature. Membranes were then incubated with total antibody. Antibodies used were: mouse monoclonal anti-phosphoERα Serine 118 (1:500, Cell Signaling), rabbit monoclonal anti-ERα (1:400, Lab Vision, Fremont, CA), mouse anti-actin (1:5000, BD Biosciences, San Jose, CA), goat anti-mouse (1:10,000, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) and donkey anti-rabbit (1:10,000, Jackson ImmunoResearch Laboratories).
Analysis of images was performed using the public domain Image J program (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/ij/). Signal intensities were corrected for protein loading by normalization to actin intensity. Quantified phosphorylated and total ER intensity in GAN was arbitrarily designated as “one.” Relative intensities of VS specimens to GAN were then graphed using Microsoft Excel software.
Results
Sporadic vestibular schwannoma specimens from sixteen patients who underwent microsurgical excision from 2008 through 2010 were analyzed. Characteristics of the patients are listed in Table 1. Patient age ranged from sixteen to seventy-two. Five patients were male and eleven were female, with a male to female ratio of 1:2.2.
TABLE 1. Characteristics of study patients.
Vestibular schwannoma (VS) specimens from twenty-one patients were analyzed. When possible, tumor dimensions were recorded in two axes from magnetic resonance images (MRI).
| VS Specimen Number | Age (years) | Gender | Size (cm) | NF2 status |
|---|---|---|---|---|
| 5 | 62 | Male | 1.2 | - |
| 6 | 51 | Male | 2.6 | - |
| 10 | 48 | Female | 1.9 × 2.0 | - |
| 13 | 60 | Female | 2.8 × 3.3 | - |
| 15 | 59 | Female | 1.2 × 0.6 | - |
| 17 | 54 | Female | 2.0 × 2.0 | - |
| 21 | 34 | Male | 3.4 × 3.5 | - |
| 22 | 63 | Female | 2.1 | - |
| 23 | 49 | Female | 2.5 | - |
| 25 | 72 | Female | 2.3 × 1.7 | - |
| 27 | 56 | Male | 2.0 × 1.4 | - |
| 28 | 28 | Male | 3.3 × 3.3 | - |
| 40 | 52 | Female | 2.2 × 1.5 | - |
| 41 | 43 | Female | 2.5 × 3.5 | - |
| 42 | 40 | Female | 1.5 × 2.0 | - |
| 43 | 16 | Female | 4.5 × 5.5 | - |
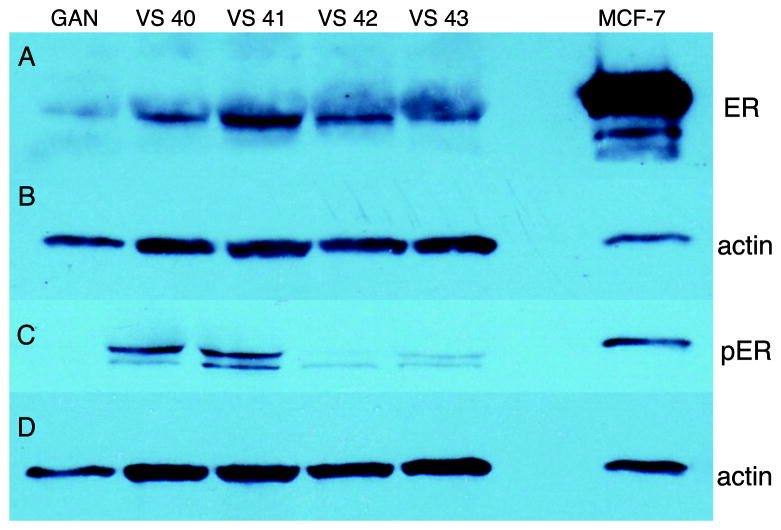
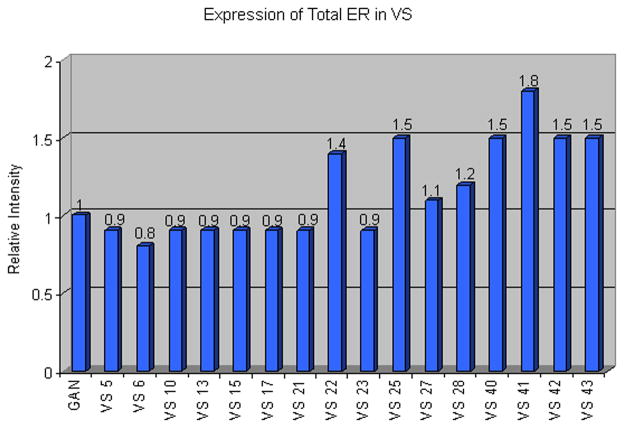
All sixteen VS specimens exhibited expression of both phosphorylated (Serine 118) and total ERα. Figure 1A depicts four VS specimens that demonstrate strong up-regulation of total ERα. However, most specimens analyzed had equivalent or slightly up-regulated ERα expression compared to control GAN. Band intensities, normalized to actin and expressed as relative intensity to GAN, are presented in Figure 2.
Figure 1.
Expression of (A) total ERα and (C) phosphorylated (Ser118) ERα. (B) and (D) depict actin for total and phosphorylated ERα, respectively. From left to right, lanes are control nerve (GAN), VS specimen 40, 41, 42 and 43, followed by an empty lane. MCF-7, an immortalized breast cancer cell line, serves as positive control in the far right lane.
Figure 2.
Band intensities for total ERα were quantified using the public domain Image J program (developed at the NIH). To correct for protein loading, band intensities were normalized to actin, and then expressed as Relative Intensity to control nerve (GAN). GAN is arbitrarily designated as “one.”
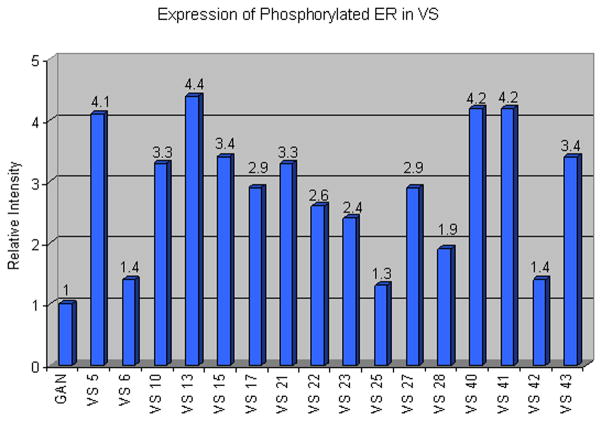
Specimens were also analyzed for expression levels of ERα phosphorylated at Serine 118, which is the major residue phosphorylated in response to both estradiol and mitogen-activated protein kinase (MAPK).29 GAN exhibited very faint expression of phosphorylated ERα with prolonged exposure times. VS samples, however, demonstrated variable but strong up-regulation of phosphorylated ERα compared to GAN. Figure 1C exemplifies the varied expression levels of phosphorylated ERα we found in VS specimens, with some specimens demonstrating stronger expression than others. Figure 3 represents band intensities for phosphorylated ERα normalized to actin, and expressed as relative intensity to GAN. Most VS specimens demonstrated relative intensities greater than 2 for phosphorylated ERα. No obvious correlations between total or phosphorylated ERα expression levels and patient age, gender, or tumor size was evident.
Figure 3.
Band intensities of phosphorylated ERα, normalized to actin and expressed as Relative Intensity to control GAN. GAN is arbitrarily designated as “one.”
Discussion
In 1917, Harvey Cushing first published the observation that growth of vestibular schwannomas may be accelerated during pregnancy.30 Kasantikul and Brown were the first authors to investigate the expression of ER quantitatively in VS.12 Using a fluorescent immunohistochemical technique, they analyzed eight VS specimens. They found strong staining in five specimens from female patients, and weaker staining in three male specimens. Since the publication of this data, numerous authors have investigated ER expression in VS. The methods employed in the literature are varied, including immunohistochemistry (IHC), radioimmunoassays, sucrose gradient methods and polymerase chain reaction (PCR).11–27 Many subsequent studies have reported negative results for ER expression.13–22
Recently, there has been renewed interest in ER expression in VS, with four published studies in the past two years. One of the studies was from our laboratory, in which we reported up-regulation of ER mRNA in 52% of sporadic VS, but only 25% of NF2-associated VS.11 The three remaining authors, utilizing IHC, reported no ER expression at the protein level in any specimens.13–15 Two of these authors examined greater than 50 VS specimens.13,14 All three studies also investigated the expression of progesterone receptor (PR) in VS, but had conflicting results. Two studies found no PR expression in any samples,14,15 while another study detected PR expression in all specimens.13
Receptors, which are present in the cell at very low levels, are often difficult to detect with IHC. In the lung cancer literature, multiple studies using IHC were unable to detect ER expression,31,32 until a study utilizing Western blot analysis reported ER-beta expression in several types of human lung cancer tissue.33 We thus chose to use Western blot analysis for detection of ERα in VS specimens. However, western blot analysis is a delicate, technically-challenging, and labor intensive technique. Each step in the procedure must be optimized to successfully identify the protein of interest, including appropriate use of controls to assure specificity. Western blot analysis, quantified with Image J, is a semi-quantitative method. It is more specific than other methods of protein identification, such as enzyme-linked immunosorbent assays (ELISA), but is not as sensitive. It is often necessary to optimize and evaluate multiple antibodies in order to successfully detect the protein of interest. Both positive and negative protein controls may be necessary to assure adequate sensitivity and specificity.
Using Western blot analysis, we have demonstrated that ERα is expressed in VS. Total receptor levels are roughly equivalent to levels found in GAN, while phosphorylated receptor levels exhibit more robust up-regulation. This implies greater activation of ERα in VS compared to normal nerve. There is one report in the literature of statistically significant growth of VS explants in response to estradiol in vivo.34 This increase in growth was abrogated with the antiestrogen Tamoxifen. Side effects of Tamoxifen include thromboembolic events, cataracts and endometrial hyperplasia. However, Tamoxifen is orally-bioavailable, well-tolerated for the most part, and is FDA-approved for long-term prevention of ERα positive breast cancer in high-risk patients.28 Based on our results that ERα is expressed in its activated form in the majority of sporadic VS, we believe that further investigation into antiestrogen therapy in VS is warranted.
The ErbB family consists of epidermal growth factor receptor 1 (EGFR), ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4). Up-regulation of ErbB family members in VS has been reported by multiple authors. Sturgis et al. reported positive immunohistochemical staining for EGFR in sporadic VS.35 Hansen et al. found up-regulation of activated ErbB2 and ErbB3, as well as neuregulin (Nrg1β), the preferred ligand of ErbB3.36 Doherty et al. demonstrated up-regulation of EGFR, ErbB2 and ErbB3 in both sporadic and NF2-related VS. EGFR, ErbB2 and EGF were more commonly upregulated in NF2-related VS, whereas ErbB2 and Nrg1β were upregulated more frequently in sporadic VS.37 Moreover, Hansen et al. have demonstrated that monoclonal antibodies against ErbB2 decrease proliferation of VS in vitro and in vivo.36,38 A small molecule inhibitor targeting EGFR slowed VS proliferation in vivo as well as accelerated apoptosis.38 Some downstream signaling pathways that have been implicated in VS tumorigenesis through ErbB signaling include the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways.36,39
It has been demonstrated in human lung and breast cancer that bi-directional signaling between the ER and ErbB pathways exist.40 The estrogen receptor is primarily cytosolic, and becomes phosphorylated with estradiol binding, resulting in receptor activation and translocation to the nucleus, where it functions as a transcription factor. 29 Two authors have provided evidence for non-nuclear transactivation of ERα via the ErbB pathway. In the absence of estradiol, EGF induces MAPK to directly phosphorylate ERα at Serine 118 in the cytosol, ultimately resulting in increased transcriptional activity.41,42 More evidence for cross-talk between the ER and ErbB family is demonstrated by the antiestrogen resistance that develops in breast cancer due to EGFR and ErbB2 overexpression.43 ERα has been demonstrated to sensitize breast cancer cells to the effects of various growth factors by increasing the expression of their receptors, such as EGFR.44 Estradiol has also been shown to induce EGF secretion from breast cancer cells.45
Based on the bi-directional signaling between the ER and ErbB family members, there have been several studies performed on combination antiestrogen therapy and ErbB inhibition in human breast and lung cancer. They have demonstrated a synergistic effect of antiestrogen therapy and ErbB inhibitors, resulting in decreased proliferation and increased apoptosis in vitro and in vivo.33,46
Relationships between ERα and ErbB signaling in VS need further elucidation. We are currently conducting studies to investigate the effects of combination Tamoxifen and dual EGFR/ErbB2 inhibition on primary VS cultures. Further studies would investigate the effects of combination inhibition on VS in vivo. Tamoxifen is orally bioavailable, and is FDA-approved for long-term prevention in patients at risk of invasive breast cancer. Second-generation SERMs, such as Raloxifene, also have FDA approval for long term preventive therapy. They carry lower risks of thromboembolic events and endometrial hyperplasia. Antiestrogen therapy could prove an effective therapeutic modality for VS in the future, alone or in combination with ErbB inhibition. Future clinical applications of pharmacotherapies for VS include decreasing the size of VS prior to definitive treatment, acting as a radiosensitizer to synergize with stereotactic radiotherapy, or slowing clinical progression of disease in those patients where other treatment modalities are not an option.
Conclusion
Our results indicate that ERα is expressed in its activated form at the protein level in sporadic VS. Evidence of bi-directional signaling between the ER and ErbB pathways in human breast and lung cancer exists, and studies in these fields have demonstrated a synergistic effect of antiestrogen therapy and ErbB inhibitors. The literature has shown that inhibition of ErbB family members in VS results in decreased proliferation and increased apoptosis. Based on our findings, we feel that further investigation into combined antiestrogen therapy and ErbB inhibition in VS is warranted.
Acknowledgments
We would like to thank Dr. Roberto A. Cueva, for his invaluable support, and for providing the majority of vestibular schwannoma specimens.
Support: This research is supported by NIH/NIDCD 5 R01 DC00129-24 (A.F.R.), the NIH Ruth L. Kirschstein National Research Service Award (T32) Training Grant (DC000028-13) awarded to the Division of Otolaryngology - Head & Neck Surgery in support of the first author (C.M.B.), and a VA Merit Review grant to A.F.R.
References
- 1.Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26:93–7. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Abaza MM, Makariou E, Armstrong M, Lalwani A. Growth rate characteristics of acoustic neuromas associated with neurofibromatosis type 2. Laryngoscope. 1996;106:694–8. doi: 10.1097/00005537-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Jeyakumar A, Seth R, Brickman TM, et al. The prevalence and clinical course of patients with ‘incidental’ acoustic neuromas. Acta oto-laryngol. 2007;127:1051–7. doi: 10.1080/00016480701200210. [DOI] [PubMed] [Google Scholar]
- 4.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Quart J Med. 1992;84:603–18. [PubMed] [Google Scholar]
- 5.Evans DG. Neurofibromatosis type 2: genetic and clinic features. ENT J. 1999;78:97–100. [PubMed] [Google Scholar]
- 6.Taschudi DC, Liinder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000;21:722–8. [PubMed] [Google Scholar]
- 7.Marouf R, Noudel R, Roche PH. Facial nerve outcome after microsurgical resection of vestibular schwannoma. Prog Neurol Surg. 2008;21:103–7. doi: 10.1159/000156714. [DOI] [PubMed] [Google Scholar]
- 8.House JW, Friedman RA. Translabyrinthe approach. In: Brackman DE, Shelton C, Arriaga MA, editors. Otologic Surgery. 2. Philadelphia: W.B. Saunders Company; 2001. pp. 534–6. [Google Scholar]
- 9.Kasantikul V, Netsky MG, Glasscock ME, Hays JW. Acoustic neurilemmoma: clinicoanatomic study of 103 patients. J Neurosurg. 1980;52:28–35. doi: 10.3171/jns.1980.52.1.0028. [DOI] [PubMed] [Google Scholar]
- 10.Gaughan R, Harner S. Acoustic neuroma in pregnancy. Am J Otol. 1993;14:88–91. [PubMed] [Google Scholar]
- 11.Patel AK, Alexander TH, Andalibi A, et al. Vestibular schwannoma quantitative polymerase chain reaction expression of estrogen and progesterone receptors. Laryngoscope. 2008;118:1458–63. doi: 10.1097/MLG.0b013e318177e20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasantikul V, Brown WJ. Estrogen receptors in acoustic neurilemmomas. Surgical Neurology. 1981;15:105–109. doi: 10.1016/0090-3019(81)90023-9. [DOI] [PubMed] [Google Scholar]
- 13.Cafer S, Bayramoglu I, Uzum N, et al. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 2008;122:125–7. doi: 10.1017/S0022215107000229. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal S, Agrawal V, Jaiswal AK, et al. Expression of estrogen and progesterone receptors in vestibular schwannomas and their clinical significance. J Negat Results Biomed. 2009;8:9–13. doi: 10.1186/1477-5751-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalgorf DM, Rowsell C, Bilbao JM, Chen JM. Immunohistochemical investigation of hormone receptors and vascular endothelial growth factor concentration in vestibular schwannoma. Skull Base. 2008;18:377–84. doi: 10.1055/s-0028-1096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll RS, Zhang JP, Black PML. Hormone receptors in vestibular schwannomas. Acta Neurochir. 1997;139:188–93. doi: 10.1007/BF01844749. [DOI] [PubMed] [Google Scholar]
- 17.Curley JWA, Ramsden RT, Howell A, et al. Oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 1990;104:865–7. doi: 10.1017/s0022215100114197. [DOI] [PubMed] [Google Scholar]
- 18.Doyle KJ, Luxford WM. Acoustic Neuroma in Pregnancy. Am J Otol. 1994;15:111–3. [PubMed] [Google Scholar]
- 19.Klinken L, Thomsen J, Rasmussen B, et al. Estrogen and progesterone receptors in acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1990;116:202–4. doi: 10.1001/archotol.1990.01870020078020. [DOI] [PubMed] [Google Scholar]
- 20.Labit-Bouvier C, Crebassa B, Bouvier C, et al. Clinicopathologic growth factors in vestibular schwannomas: a morphological and immunohistochemical study of 69 tumors. Acta Otolaryngol. 2000;120:950–4. doi: 10.1080/00016480050218681. [DOI] [PubMed] [Google Scholar]
- 21.Siglock TJ, Rosenblatt SS, Finck F, et al. Sex hormone receptors in acoustic neuromas. Am J Otol. 1990;11:237–9. [PubMed] [Google Scholar]
- 22.Whittle IR, Hawkins RA, Miller JD. Sex hormone receptors in intracranial tumours and normal brain. Eur J Surg Oncol. 1987;13:303–7. [PubMed] [Google Scholar]
- 23.Beatty CW, Scheithauer BW, Katzmann JA, et al. Acoustic schwannoma and pregnancy: a DNA flow cytometric, steroid hormone receptor, and proliferation marker study. Laryngoscope. 1995;105:693–700. doi: 10.1288/00005537-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Filipo R, Petrangeli E, Monini S, et al. Expression of steroid receptors in acoustic neuroma. Clin Otolaryngol. 1995;20:413–7. doi: 10.1111/j.1365-2273.1995.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 25.Markwalder T, Waelti E, Markwalder R. Estrogen and progestin receptors in acoustic and spinal neurilemmomas. Surg Neurol. 1986;26:142–8. doi: 10.1016/0090-3019(86)90366-6. [DOI] [PubMed] [Google Scholar]
- 26.Martuza RL, MacLaughlin DT, Ojemann RG. Specific estradiol binding in schwannomas, meningiomas, neurofibromas. Neurosurgery. 1980;9:665–71. doi: 10.1227/00006123-198112000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Monsell EM, Wiet RJ. Estrogen and progesterone binding by acoustic neuroma tissue. Otolaryngol Head Neck Surg. 1990;103:377–9. doi: 10.1177/019459989010300307. [DOI] [PubMed] [Google Scholar]
- 28.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 29.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 30.Cushing H. Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle. Philadelphia: W.B. Saunders, Co; 1917. p. 148. [Google Scholar]
- 31.Brown RW, Campagna LB, Dunn JK, Cagle PT. Immunohistochemical identification of tumor markers in metastatic adenocarcinoma of the colon, pancreas, and lung. Arch Pathol Lab Med. 1994;118:630–32. [Google Scholar]
- 32.Di Nunno L, Larsson LG, Rinehart JJ, Beissner RS. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med. 2000;124:1467–70. doi: 10.5858/2000-124-1467-EAPRIN. [DOI] [PubMed] [Google Scholar]
- 33.Stabile LP, Davis AG, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor α and β and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 34.Stidham KR, Roberson JB. Effects of estrogen and tamoxifen on growth of human vestibular schwannomas in the nude mouse. Otolaryngol Head Neck Surg. 1999;120:262–4. doi: 10.1016/S0194-5998(99)70416-X. [DOI] [PubMed] [Google Scholar]
- 35.Sturgis EM, Woll SS, Aydin F, Marrogi AJ, Amedee RG. Epidermal growth factor receptor expression by acoustic neuromas. Laryngoscope. 1996;106:457–62. doi: 10.1097/00005537-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 37.Doherty JK, Ongkeko W, Crawley B, et al. ErbB and nrg: potential molecular targets for vestibular schwannoma pharmacotherapy. Otol Neurotol. 2007;29:50–7. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- 38.Hansen MR, Clark JJ, Provenzano M, et al. The ErbB inhibitors traztuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: a preliminary study. Otol Neurotol. 2008;29:846–53. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann O. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–45. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 40.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2002;17:309–17. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 41.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 42.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO. 1996;15:2174–83. [PMC free article] [PubMed] [Google Scholar]
- 43.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors derive from a single transcript: studies of ERα and ERβ expressed in CHO cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 44.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7:4429–35. [PubMed] [Google Scholar]
- 45.Dickson RB, Huff KK, Spencer EM, Lippman ME. Induction of epidermal growth factor-related polypeptides by 17 β-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986;118:138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- 46.Okubo S, Kurebayashi J, Otsuki T, et al. Additive antitumour effect of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (Iressa, ZD1839) and the antioestrogen fulvestrant (Faslodex, ICI I 82,780) in breast cancer cells. Br J Cancer. 2004;90:236–44. doi: 10.1038/sj.bjc.6601504. [DOI] [PMC free article] [PubMed] [Google Scholar]