Abstract
The HIV -1 nucleocapsid protein (NC) is a nucleic acid chaperone, which remodels nucleic acid structures so that the most thermodynamically stable conformations are formed. This activity is essential for virus replication and has a critical role in mediating highly specific and efficient reverse transcription. NC's function in this process depends upon three properties: (1) ability to aggregate nucleic acids; (2) moderate duplex destabilization activity; and (3) rapid on-off binding kinetics. Here, we present a detailed molecular analysis of the individual events that occur during viral DNA synthesis and show how NC's properties are important for almost every step in the pathway. Finally, we also review biological aspects of reverse transcription during infection and the interplay between NC, reverse transcriptase and human APOBEC3G, an HIV-1 restriction factor that inhibits reverse transcription and virus replication in the absence of the HIV-1 Vif protein.
Key words: nucleocapsid protein, HIV-1, nucleic acid chaperone, reverse transcription, Gag, zinc fingers, tRNALys3, APOBEC3G, retroviruses
Introduction
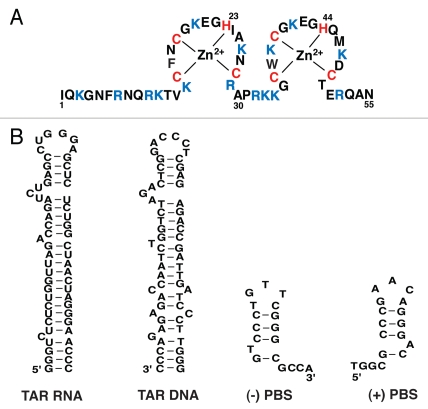
Retroviral nucleocapsid (NC) proteins are small, basic, nucleic acid binding proteins with one or two zinc-binding domains or zinc fingers, each containing the invariant metal-ion binding motif CX2CX4HX4C, more commonly referred to as a CCHC motif.1–4 The human immunodeficiency virus type 1 (HIV-1) NC has two zinc fingers, which are connected by a short flexible basic peptide linker (Fig. 1A). The amino acid composition of the two fingers is similar, though not identical. Moreover, their biological activities and biochemical properties are not equivalent and both fingers are required for virus replication.1–4 NC is derived from the multidomain 55 kDa Gag precursor protein (Pr55gag), which contains (from the N- to C-terminus) matrix (MA), capsid (CA), spacer peptide 1, NC, spacer peptide 2 and p6.5 During or shortly after virus budding from the infected cell, the HIV-1 protease (PR) is activated. This results in cleavage of Gag to the individual mature structural proteins and dramatic structural rearrangements (virus “maturation”) that lead to formation of mature, infectious HIV-1 virions containing electron-dense conical cores. To ensure proper cleavage, this series of reactions occurs in a highly ordered manner.6–8
Figure 1.
Primary sequence of HIV -1 NC and nucleic acid structures required for the strand transfer steps in reverse transcription. (A) HIV-1 NC. The sequence shown is for the NL4-3 isolate (GenBank accession no. AF324493).218 The zinc coordinating residues are shown in red and the basic residues are highlighted in blue. (B) Structures of TAR RNA, TAR DNA, (−) PBS and (+) PBS. The NL4-3 TAR RNA structure is based on the structures described in reference 254 and 255. TAR DNA is shown here as the complement of the TAR RNA structure, but it can also form alternative conformations (reviewed in ref. 146). The PBS structures are based on the structures given in reference 220 and 222.
Like other retroviral NCs, HIV-1 NC is a multifunctional protein. It binds nucleic acids non-specifically through electrostatic interactions of the basic residues with the phosphodiester backbone of nucleic acids and also exhibits sequence-specific binding to runs of Gs or T/UGs through interactions that involve the zinc fingers (reviewed in ref. 3). Importantly, NC is a nucleic acid chaperone, which means that it facilitates remodeling of nucleic acid structures to form the most thermodynamically stable conformations.2,3,9,10 This activity is independent of ATP hydrolysis (reviewed in ref. 3).
Mature NC plays a critical role in assuring the specificity and efficiency of reverse transcription and maturation of the genomic RNA dimer.1–4 In addition, NC may be important for integration of viral DNA (vDNA) into the host chromosome.11,12 The NC domain in Gag also binds nucleic acids and has nucleic acid chaperone activity, which is required for genomic RNA packaging and primer placement. i.e., annealing of the tRNALys3 primer to the primer binding site (PBS) in genomic RNA.4,13,14 Moreover, the NC domain functions in genomic RNA dimerization15–18 and interacts with the Bro 1 domain of the host protein Alix during HIV-1 assembly.19,20 Thus, NC or the NC domain in Gag has a major role in almost every step in virus replication.
In this review, we focus on HIV-1 NC function in HIV-1 reverse transcription and show how NC's nucleic acid chaperone activity facilitates this process. We begin by providing the biological context for reverse transcription to give some perspective on postentry events that lead to vDNA synthesis in the infected cell. The sections that follow are a detailed description of individual steps in the reverse transcription pathway and wherever possible, we discuss the molecular mechanisms that are involved. In view of major advances in identifying various host factors that limit HIV-1 infection, we also include a section on the human APOBEC3G (A3G) protein, a cellular cytidine deaminase that has a major effect on reverse transcription and inhibits virus replication in the absence of the HIV-1 virus infectivity factor (Vif) protein.21 Studies with A3G and NC are highlighted in this section.
For detailed information published previously on the subject of this paper, the reader is referred to several earlier reviews.1–4,10
General Features of HIV-1 Reverse Transcription
Biological context.
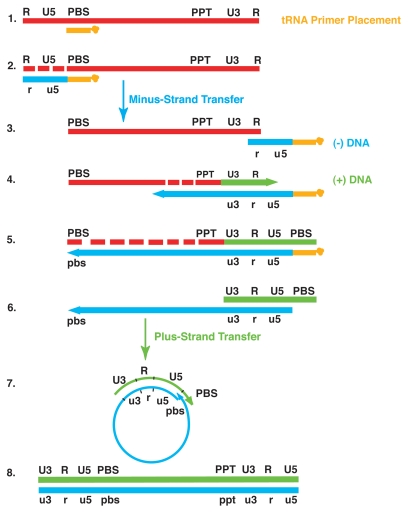
Reverse transcription consists of a complex series of biochemical reactions that culminate in synthesis of a linear double-stranded (ds) DNA copy of the single-stranded (ss) viral RNA (vRNA) genome that is ultimately integrated into the host chromosome (Fig. 2).22,23 This process, which is catalyzed by the virus-encoded reverse transcriptase (RT) enzyme, is an early postentry event that occurs in the cytoplasm of infected cells (see below). However, primer tRNALys3 extended by two bases13 or even small amounts of longer minus-strand DNA have been found in HIV-1 mature virions prior to virus entry.24,25
Figure 2.
Schematic diagram of events in reverse transcription. Step 1. Reverse transcription is initiated by the cellular tRNA primer (tRNALys3). The tRNA primer is represented by a short orange line attached to a “clover-leaf” (remaining tRNA bases). The 3′ 18 nt of the primer are annealed to an 18 nt sequence in vRNA (red solid line; upper case lettering) known as the PBS, which is located at the 5′ end of the genome. This reaction is termed “tRNA primer placement”. Step 2. RT catalyzes extension of the primer to form (−) SSDNA (blue solid line; lower case lettering), which contains copies of the unique 5′ genomic sequence (U5) and R regions. As the primer is extended, the RNase H activity of RT degrades the vRNA sequences (fragments represented by short red solid lines) that have already been reverse transcribed. Step 3. To elongate the minus-strand, (−) SSDNA is transferred to the 3′-end of the genome, which is facilitated by annealing of the complementary R sequences. This step is termed minus-strand transfer. Step 4. Following minus-strand transfer, elongation of minus-strand DNA and RNase H degradation continue. Plus-strand DNA synthesis is initiated by the 15 nt 3′ PPT, located immediately upstream of the unique 3′ genomic sequence (U3). A second 15 nt PPT (cPPT), with the same sequence as the 3′ PPT, is located at the center of the genome in the IN coding region (not shown here). The role of the cPPT in plus-strand synthesis is illustrated in Figure 5. The PPT duplexes are resistant to RNase H cleavage. Step 5. RT copies the u3, r and u5 regions in minus-strand DNA as well as the 3′ 18 nt of the tRNA primer, thereby reconstituting the PBS. The product formed is referred to as (+) SSDNA. Step 6. The tRNA and PPT primers are eventually removed by RNase H from minus- and plus-strand DNAs, respectively. Step 7. Plus-strand transfer is facilitated by annealing of the complementary PBS sequences at the 3′ ends of (+) SSDNA and minus-strand DNA. This is followed by circularization of the two DNA strands and displacement synthesis to generate two LTR sequences. Step 8. Minus- and plus-strand DNAs are elongated, resulting in a linear, dsDNA with an LTR at each end. This DNA is integration-competent. Adapted from reference 3 and 249 with permission from Elsevier and Oxford Journals (Oxford University Press), respectively.
The HIV-1 core is a fullerene cone, which is composed of CA molecules.26 The interior consists of a ribonucleoprotein complex containing the viral proteins RT, integrase (IN), NC, Nef and Vpr, as well as two copies of genomic RNA (coated by NC) and primer tRNALys3 (reviewed in ref. 4 and 7). Proper assembly of cores as well as uncoating or disassembly (i.e., removal of the CA shell and release of the ribonucleoprotein complex) are both critical for formation of a functional reverse transcription complex (RTC).4,27,28 Optimal core stability is also essential and virions containing cores that are hyperstable or unstable cannot synthesize vDNA, leading to a loss of infectivity (reviewed in ref. 27).29–33
As reverse transcription progresses, movement of RTCs toward the nuclear pore is facilitated by interactions with the microtubule network in the cell.34–36 The RTCs are eventually remodeled into preintegration complexes (PICs), which contain full-length linear ds vDNA, several host and viral proteins, e.g., RT and IN, but not NC.4,28,37 Since HIV-1 infects non-dividing cells, the size of the PICs precludes entry to the nucleus by passive diffusion. Instead, these complexes must be actively transported into the nucleus (reviewed in ref. 37).38
The temporal course of HIV-1 core disassembly is extremely important for successful vDNA synthesis and productive virus infection, but there is still no consensus on exactly when uncoating occurs. Previously it was thought that uncoating occurs shortly after fusion of the viral envelope protein with cell surface receptors, since intact cores could not be detected by transmission electron microscopy.39 Many groups have since shown that immediate disassembly following fusion is not compatible with newer data (see below). Moreover, when HIV-1 CA is targeted by TRIM5α, a host restriction factor found in Old World Monkey cells, premature disassembly and loss of infectivity result; this implies that proper disassembly must occur at later times postentry.40
Results from a study of isolated RTCs41 suggested that uncoating occurs several hours after entry of the core into the cytoplasm. A similar conclusion was reached in two studies using fluorescent imaging in live cells to track HIV-1 virions tagged with Vpr-GFP.35,42 In other work, it was reported that HIV-1 cores remain intact until the RTCs traffic to the nuclear pore, where following completion of reverse transcription, CA is released from the complexes and PICs are formed.43 In addition, two groups observed that for nuclear import to occur, the terminal linear vDNA product must contain a discontinuous triplestranded structure known as the central DNA flap44,45 (also see below). Although a recent paper showed that this structure has a moderate role in nuclear import,46 other investigators have questioned its importance for virus replication and more specifically, for nuclear import.47–50 Further investigation will be needed to resolve this question.
HIV-1 RT enzyme.
HIV-1 RT is an asymmetric heterodimer that is generated by cleavage of the Gag-Pol precursor and consists of two subunits, p66 and p51. The p51 subunit is derived from p66 by further proteolytic cleavage.51,52 The larger p66 subunit has two catalytic sites with the following functions: N-terminal domain (NTD), RNA- and DNA-dependent DNA polymerase activity; and C-terminal domain (CTD), RNase H activity, which degrades the RNA strand in an RNA-DNA hybrid. The p51 subunit lacks catalytic activity and has a structural role. X-ray crystallographic analysis of the HIV-1 RT structure indicates that the p66 polymerase domain can be described in relation to a right hand with fingers, palm and thumb subdomains. A fourth element is termed the “connection subdomain”, since it is localized to a region between the two active sites.51,52 (For recent reviews on the structure and function of RT, see ref. 22 and 23).
The Reverse Transcription Pathway: An Overview
In the following sections, we will describe the individual reactions that occur during reverse transcription (Fig. 2). This process is unique among the more usual host DNA synthetic pathways. Thus, in addition to a plus-strand RNA template, the integration-competent, linear, ds vDNA product has a long terminal repeat (LTR) present at each end. The strategy used by HIV-1 and other retroviruses to synthesize such a DNA involves obligatory minus- and plus-strand transfer reactions.53
Initiation of Reverse Transcription
RNA packaging.
HIV-1 genomic RNA is packaged into budding virions through extensive contacts between NC protein sequences in Gag and RNA secondary structural features within the cis-acting packaging signal (Ψ) near the 5′ end of the vRNA (Fig. 3).54,55 The zinc fingers are critical for these interactions3,56 and deletion of NC negates the specific packaging of vRNA altogether.57 In the absence of Ψ, larger amounts of non-specific cellular RNAs are packaged to compensate for the absence of vRNA.58–61
Figure 3.
Secondary structure model generated by SHAPE-constrained folding of the ex virio state55 of the first 578 nt of the HIV-1 vRNA genome (adapted from ref. 55). Nucleotides proposed to form intermolecular bp with a second monomer are enclosed within a black box; the AUG start codon for the Gag polyprotein (nt 336) is highlighted in bold. Nucleotides are colored according to their SHAPE reactivity; bars indicating base pairing are colored by their “pairing persistence”, as defined in reference 55. Effects of pretreatment of viral particles with AT-2, a zinc ejector that disrupts NC-RNA interactions (see text), are indicated with filled (strong reactivity) and clear (weak reactivity) arrowheads. Clustered sites that show a strong increase in reactivity following treatment with AT-2 and report specific NC binding sites are highlighted in cyan; proposed primary and secondary sites are identified with double (**) and single asterisks (*), respectively. Sites within the first 185 nt that show very strong reductions in SHAPE reactivity following AT-2 treatment, thus reporting on the destabilizing activity of NC, are indicated by solid gray arrows. Additional sites at well-spaced intervals in the vRNA that also display enhanced SHAPE reactivity upon compromising the zinc-binding motif are indicated by dashed gray arrows. The primer is tRNALys3 shown starting at nt 33 bound to the vRNA via the anticodon domain and variable arm.
In addition to vRNA, three cellular tRNALys isoacceptor populations are selectively incorporated into virions during assembly62–64 along with the tRNALys-binding protein, lysyl-tRNA synthetase (LysRS).65 This selective packaging is achieved, in part, through specific LysRS-Gag interactions mapped to the dimerization motif (motif 1) of LysRS and the C-terminal dimerization domain of CA.66–68 Interactions between the RT thumb domain in Gag-Pol and tRNALys have also been shown to stabilize the packaging complex.69 Knockdown of newly synthesized LysRS using siRNA resulted in reduced levels of tRNALys packaging, annealing and viral infectivity, suggesting that Gag diverts newly synthesized LysRS towards viral packaging before it enters cellular compartments.70,71
Although three human tRNALys isoacceptors, tRNALys1,2 and tRNALys3 are packaged into virions, the 3′ portion of tRNALys3 is perfectly complementary to the 18-nt PBS in the 5′ region of the vRNA (Fig. 3) and only tRNALys3 serves as the primer for reverse transcription in HIV-1.63,72 Different host tRNAs are used by other retroviral genera. For example, tRNATrp and tRNAPro serve as primers for avian myeloblastosis virus (alpharetrovirus genera)73 and murine leukemia virus (MLV; gammaretrovirus genera),74 respectively. A role for tRNALys1,2 isoacceptors in the import of PICs into the nucleus of an HIV-1 infected cell has recently been proposed.75
NC effects on primer structure.
The effect of NC on tRNA primer structure both in the absence and presence of vRNA has been examined. Based on circular dichroism studies, NC was proposed to unwind tRNA in the absence of Mg2+, but the extent of unwinding was significantly reduced in the presence of divalent cation.76 Consistent with the latter result, only modest NC-induced changes were observed in 1H NMR studies of model tRNAPhe, with the major perturbations seen in the acceptor and TΨC stems.77 Förster resonance energy transfer (FRET) assays involving fluorescently 5′- and 3′-end labeled tRNALys3 molecules were used to measure intra-tRNA distance changes induced by NC in the absence and presence of vRNA.78 While only minor changes in steady-state and time-resolved fluorescence are observed when NC binds free tRNA, a 41 Å increase in probe separation was detected when tRNA was heat- or NC-annealed to vRNA.78 This suggests that NC alone induces only subtle structural changes, whereas global acceptor-TΨC stem unwinding requires the presence of the complementary vRNA.78
Using terbium to probe tRNA conformational changes in the absence of vRNA, Hargittai et al. showed that the D-TΨC tertiary interaction was disrupted at low NC concentrations, followed by slight destabilization of the acceptor-TΨC minihelix at saturating amounts of NC (8 nt/NC).79 Heteronuclear NMR studies using 15N-labeled human tRNALys3 expressed in vivo reached similar conclusions and localized preferred NC binding sites to the lower part of the acceptor stem, the D stem, the T-stem and the T-loop.80 The NMR data were consistent with NC-induced melting from the center of the molecule and identi- fied the G6:U67 base pair (bp) at the bottom of the acceptor stem and the T54:A58 pair in the TΨC loop as potential hot spots from which melting is initiated.
NC effects on vRNA structure.
RNA remodeling by NC occurs at initiation and likely also facilitates the reverse transcription process. For example, NC was found to destabilize hairpins and other secondary structures in the 5′ untranslated region (5′ UTR) to prevent pausing or stalling of RT in vitro81 (also see below). A strategy to rapidly measure RNA backbone flexibility using selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) provided a secondary structure model of the first 10% (976 nt) of the vRNA genome under a variety of conditions.55 This technique involves exposing RNA to an electrophile such as N-methylisatoic anhydride, which reacts selectively with unpaired or flexible RNA nt at the ribose 2′-hydroxyl group to form an adduct. These adducts can then be detected by primer extension assays. In this study, a zinc-ejecting agent, aldrithiol-2 (AT-2),82,83 which is known to compromise NC-RNA interactions inside virions by promoting AT-2-NC and NC-NC disulfide crosslinking, was used to map sites of interaction between NC and vRNA55 (Fig. 3). Virions were treated with AT-2 and the structure of the resulting vRNA was probed using SHAPE. By identifying nt where the 2′-hydroxyl was more reactive upon AT-2 treatment, it was established that NC specifically bound to seven sites between positions 224 and 334 (Fig. 3). Binding was observed at five ss internal loop motifs, a Ψ element site and a splice donor (SD) site. The Ψ and SD sites are stable hairpin structures. Interestingly, while the five ss sites and the Ψ element share a consensus 3- to 4-nt purine-rich motif, which lies adjacent to a helix, the most important determinant for all seven sites is a ss G residue followed by a 3′ ds region.
SHAPE was also used to locate vRNA regions affected by NC's chaperone activity by identifying sites that were less reactive upon AT-2 treatment. Surprisingly, the helix destabilizing activity of NC was not observed uniformly throughout the genomic RNA, but was detected in the first 185 nt at six compact sites between the 5′ end of the vRNA and the PBS.55 This region includes the site of tRNA annealing as well as the initial sequence copied by RT during (−) strong-stop DNA [(−) SSDNA] synthesis (also see below). Therefore, this localized destabilization activity is consistent with NC's role in facilitating both tRNA primer annealing and the reverse transcription process.
Primer placement and extension.
The interactions between NC and vRNA during packaging of the RNA genome place the NC domain of Gag at a key position for its role in the formation of the initiation complex prior to reverse transcription (Fig. 2, step 1). Whether annealing of tRNALys3 to the vRNA occurs before or after packaging is unclear, but it is known that annealing is facilitated by the nucleic acid chaperone activity of the NC domain in Gag.3,4,10,84 The basic amino acids of NC bring the complementary tRNA and PBS sequences together, while the two NC zinc finger domains are responsible for destabilizing structured RNA regions.3 Extension of the primer leads to synthesis of (−) SSDNA, a short DNA consisting of sequences complementary to the R-U5 region of vRNA (Fig. 2, step 2).
Earlier studies found that tRNA placement onto the PBS occurs even in virions lacking HIV-1 PR activity, suggesting that Gag chaperones the annealing process in vivo.85 Indeed, GagΔp6, which lacks the C-terminal p6 domain and is not myristoylated at its N-terminus,86 can facilitate tRNA annealing in vitro.16 More recent studies showed that Gag-annealed tRNA is not as stable as NC-annealed tRNA,87,88 suggesting that there is a two-step annealing process; initial tRNA annealing is promoted by Gag with final primer/template remodeling promoted by mature NC. Interestingly, Roldan et al. found that NC annealing was more effective when NC was combined with the C-terminal domain of CA.89 The latter study used an elongation assay where protein, genomic RNA, RT and tRNALys3 were preincubated to allow for annealing to take place prior to the addition of Mg2+ to initiate reverse transcription. This suggests that the other protein domains in the context of Gag or Gag-Pol may have an effect on vRNA-tRNA interactions.
Recently, additional in vitro studies have been carried out to probe the contribution of NC and non-NC domains in Gagmediated tRNA annealing activity. These studies were all performed in the context of GagΔp6. Gag was shown to be a more efficient chaperone on a molar basis than mature NC, as less Gag was required to achieve maximal tRNA annealing relative to NC, although the rate of tRNA annealing by saturating levels of Gag is reduced relative to that of NC.256 Results of annealing studies with Gag deletion variants showed that the NC domain was absolutely required for tRNA annealing. Interestingly, deletion of the MA domain or addition of inositol phosphates (IPs) increased the rate of Gag-facilitated tRNA annealing. Since IPs bind to MA and were shown to effectively compete for MA/nucleic acid binding, these data support the notion that Gag binds to RNA either by interactions with both the NC and MA domains or with the NC domain alone. Interactions of MA with IPs trigger the switch between these two binding modes and stimulate Gag's chaperone function, which may be important for regulation of events such as tRNA primer annealing.256 Recent studies by Wu et al.90 using a minus-strand transfer assay to monitor chaperone function are consistent with the conclusion that the NC domain is the major determinant of Gag chaperone function. However, in this system, comparison of the activities of Gag and a processed intermediate such as CANC showed that the absence of MA had a relatively small effect on annealing and overall minus-strand transfer activity (also, see review by A. Rein in this issue).90
Numerous studies have shown that NC's tRNA annealing activity is optimal under saturating NC binding conditions. Li et al. used reverse transcription reactions involving a tRNALys3 primer and a 483-nt vRNA template and determined a maximal effect on tRNA/PBS annealing at a ratio of one NC molecule per 6 RNA nt.91 Levels of tRNALys3-primed synthesis of (−) SSDNA were stimulated ∼50-fold in the presence of saturating NC.91 In reverse transcription assays with mutant vRNA templates, a 54-nt deletion immediately downstream of the PBS (i.e., 3′ of the PBS in vRNA) significantly reduced (−) SSDNA synthesis.91 Furthermore, when various concentrations of NC were preincubated with primer tRNA and mutant vRNA templates and then fractionated on a 1.5% agarose gel, reduced levels of the tRNAvRNA complex formation were observed for the mutant vRNA with the 54-nt downstream deletion.91 As discussed below, the data suggest that regions of the tRNA outside the 3′ 18 nt drive the equilibrium in favor of formation of a stable tRNA/vRNA initiation complex by NC.
A similar conclusion was reached by Iwatani et al. who identified an additional interaction outside the PBS region92 (also see below). In the absence of NC, at least 24 bases immediately downstream of the PBS are required for efficient (−) SSDNA synthesis primed by 18-nt anti-PBS RNA, but not with an anti-PBS DNA primer. The stem structure adopted by the downstream bases may stabilize the initiation complex or enhance binding to RT in the absence of NC.92 Interestingly, NC abolishes the need for these additional bases in the presence of native tRNALys3 , but not with the 18-nt RNA primer. This result with the RNA primer is probably due to the absence of necessary additional interactions between tRNALys3 and vRNA proximal to the PBS-tRNALys3 complex.92 Indeed, mutagenesis data were consistent with an interaction between nt 143–149 of the vRNA and nt 40–46 in the anticodon stem/variable arm of tRNALys3 that was facilitated by NC. This interaction had previously been suggested to promote RT binding93 and is in accord with recent SHAPE analysis of the binary complex extracted from virions55 (Fig. 3).
Using gel-shift assays to follow annealing of 32P-labeled tRNALys 3 to a 105-nt PBS-containing vRNA fragment, annealing was shown to be enhanced 105-fold by NC.94 Detailed kinetic studies revealed that the annealing reaction is second order, consistent with a rate-limiting nucleation step. Two alternative locations for initial tRNA/PBS interaction have been proposed. Based on kinetic studies using mutant unmodified tRNA constructs, the reaction was proposed to initiate via hybridization of 4–5 bases at the 3′ end of the tRNA to the complementary PBS bases.94 In contrast, based on NMR studies using 15N-labeled tRNA, annealing was proposed to initiate at the bottom of the acceptor-TΨC stem.95,96 The NMR studies used human tRNA expressed in E. coli, which therefore contains a subset of base modifications found in native human tRNALys3 .97 A more recent NMR study using an 15N-labeled PBS-containing template provided new insights into these conflicting mechanisms. This study, which again used partially modified human tRNALys3 , revealed that complex formation initiates both from the CCA-3′ end and from the bases in the TΨC stem.98 This study also showed that NC binds specifically to an RNA hairpin that mimics the D-arm of the tRNA and suggested that this interaction may also facilitate the overall annealing reaction.98
While the tRNA annealing process is rate-limited by the nucleation step, which involves PBS stem melting, zippering of the remaining complementary bp occurs at a much faster rate. Consistent with this idea, deletions/mutations in tRNA resulting in altered secondary or tertiary structure and stability appear to have a minimal effect on annealing.94 As mentioned earlier, NC facilitates annealing by destabilizing the RNA secondary structure via preferential binding of its zinc fingers to ssRNA and by inducing short-ranged attraction between nucleic acid molecules. Interestingly, the second order rate constant for tRNA annealing by SSHS NC,99 a mutant that lacks the zinc finger structures, is three-fold higher than for wild-type NC at all temperatures examined.94 This is most likely due to the fact that while SSHS NC is less effective at destabilizing helices, it is even more effective at nucleating duplex formation than wild-type NC.
Interactions between primer tRNA and vRNA.
The 5′ UTR of the HIV-1 vRNA is the most conserved part of the genome and is rich in complex structural motifs with known functions in enhancing transcription and translation, primer annealing, splice site recognition and genomic RNA dimerization and packaging (reviewed in ref. 100). Interactions between primer tRNALys3 and vRNA outside of the PBS sequence, as well as vRNA intramolecular interactions within the 5′ UTR appear to be dynamic and biologically significant to the viral life cycle. While there is agreement that interactions outside the PBS help to stabilize the primer/tempate complex, the nature of these alternate interactions has been a matter of significant debate. This topic has been recently reviewed in reference 14 and will only be discussed briefly here.
In addition to the 3′ 18 nt and anticodon stem/variable arm interactions described above, interaction between the U-rich 33Umcm5s2 UUU36 sequence in the anticodon loop of tRNALys3 and an A-rich 164AAAA167 element just upstream of the PBS in vRNA has been proposed101 and changes in the A-rich sequence modulate reverse transcription efficiency.92,102–105 The TΨC arm of the tRNA and the U5-leader stem have also been proposed to interact. This interaction involves the 8 to 10 nt primer activation signal (PAS) sequence immediately upstream of the PBS and nt 148–155 of the tRNA.106 A similar interaction between a U5 motif in Rous sarcoma virus (RSV) vRNA and the TΨC arm of the corresponding primer tRNATrp has also been identified.107 Formation of the PAS:tRNALys3 hybrid would require further unwinding of the U5 leader stem facilitated by the duplex destabilizing activity of NC. The accessibility of this activation signal repressed by base pairing has been proposed to constitute a unique mechanism for positive and negative regulation of reverse transcription.106,108–110 The contribution of the PAS to differences between Gag- and NC-facilitated tRNALys3 annealing in HIV-1 have also been examined.88 In PR-minus (PR(−)) virions, the amount of tRNA annealed by Gag is 35% less than that annealed by mature NC in PR(+) virions. The Gag-annealed tRNA has a reduced ability to initiate reverse transcription and the primer appears to be less stably bound. PR(−) virions containing a constitutively expressed ss PAS do not have more tRNA annealed and the tRNA remains weakly bound. Initiation assays performed with total vRNA show that levels of initiation with the mutant are increased relative to otherwise wild-type PR(−) virions. However, the mutant PR(−) virus does not achieve levels of initiation as high as those reached in assays with PR(+) virions with or without a ss PAS.88 Taken together, these data support a possible role for the PAS in initiation, but confirm that mature NC is the critical determinant for achieving optimal tRNA annealing and initiation.
Given the structural complexity of the 5′-UTR and the large number of intra- and intermolecular interactions proposed to occur within this region of the genome, it is not surprising that additional factors may play a role in tRNA primer annealing. In addition to the chaperone activity of the NC domain of Gag, tRNA primer placement may be facilitated by cellular helicases such as RNA helicase A.111 The HIV-1 Vif protein has also been demonstrated to have chaperone activity and to promote annealing of tRNALys3 to the PBS in vitro.112 In addition, a role for human LysRS in this process has recently been proposed. In particular, in vitro and cell-based studies suggest that LysRS targets the tRNA to the PBS region of the genome by binding to a tRNALys3-like element (TLE) in the 5′ UTR proximal to the PBS (C.P. Jones, J. Saadatmand, L. Kleiman, K. Musier-Forsyth, submitted). The TLE mimics the U-rich anticodon loop of tRNALys3 and mutation of the U-rich sequence greatly attenuates binding of LysRS in vitro. In addition, initiation assays performed using total vRNA showed that TLE mutation significantly reduces the amount of initiation, as well as reducing viral infectivity. Thus, the TLE/LysRS interaction may promote tRNALys3 annealing by both directing an annealing complex containing tRNALys3/LysRS to vRNA sequences proximal to the PBS and by stimulating subsequent release of tRNALys3 from human LysRS.
Minus-strand Transfer
Initial studies.
Following synthesis of (−) SSDNA (Fig. 2, step 2), minus-strand transfer is required to extend this short DNA so that a full-length minus-strand DNA copy of the vRNA genome can be synthesized (Fig. 2, step 3). During this step, (−) SSDNA is transferred to the 3′ end of the genome in a reaction facilitated by annealing of the complementary repeat (R) regions at the 3′ ends of the RNA and DNA substrates. The R region consists of the highly structured transactivation response element (TAR) (Fig. 1B) and a portion of the poly A-signal hairpin. Since retrovirus particles contain two identical copies of the vRNA genome, strand transfer can occur in an intra- or intermolecular manner.113–116 In instances where the virus particles contain two nonidentical RNA genomes, intermolecular strand transfer leads to recombination, which in turn generates viral diversity and provides a mechanism to resist antiviral host factors and the action of antiviral agents.117–121
The first reconstituted systems that were developed to investigate minus-strand transfer contained weakly structured donor and acceptor templates with sequences representing the 5′ and 3′ ends of the vRNA genome, respectively, but no NC (reviewed in ref. 3). These early studies established that RNase H degradation of the donor RNA is required to allow translocation of (−) SSDNA to the acceptor RNA, consistent with in vivo studies performed with mutant virions lacking RNase H (reviewed in ref. 3). It was also shown that during virus infection, minus-strand transfer is efficient and occurs rapidly.122,123 More recently, many investigators have demonstrated NC's crucial role in retroviral minus-strand transfer, using reconstituted systems based on HIV-1, MLV, RSV and feline immunodeficiency virus nucleic acid templates (reviewed in ref. 3).
NC's role in facilitating efficient and specific minus-strand transfer.
NC function in minus-strand transfer is associated with its ability to act as a nucleic acid chaperone. NC has the following properties: (1) ability to facilitate annealing, a reaction that is dependent on NC-promoted nucleic acid aggregation and is associated with the basic residues in the NTD of the protein; (2) moderate helix destabilizing activity, associated with the zinc fingers; and (3) rapid nucleic acid binding/dissociation kinetics.
NC plays a major role in promoting the annealing of the complementary R sequences in (−) SSDNA and acceptor RNA and dramatically accelerates the rate of annealing99,124–128 by as much as 3,000-fold.124 The reaction was originally shown to follow pseudo-first-order kinetics (rather than the expected second-order biomolecular association), with a conformational change in the 81-nt R-containing complementary TAR RNA and TAR DNA stem-loops (Fig. 1B) as the proposed rate-limiting step.124 However, more recently, using shorter 55 to 59 nt TAR hairpins or 27-nt mini-TAR constructs (derived from the top of the 59 nt TAR RNA/DNA structures), the kinetics were shown to be second order.127–129 In addition, it was demonstrated that NC's aggregation activity is a major component of NC chaperone function in annealing.127,128,130,131 Moreover, high concentrations of Mg2+ and Na+ concentrations were found to inhibit annealing, since these cations compete with NC for non-specific binding to the negatively charged phosphate groups in the nucleic acid reactants.132,133
The mechanism of annealing has been investigated using model nucleic acid substrates, both in the absence and presence of NC. One proposed mechanism involves initial “kissing” loop interaction between the apical loops in TAR RNA and TAR DNA.127,129,134,135 Evidence in support of an alternate “zipper” mechanism involving nucleation through the 3′/5′ lower stems and bulges has also been obtained128,129,132,136 (also, see review by J. Godet and Y. Mély in this issue).
It appears that NC-assisted annealing may occur through multiple pathways, depending on the solution conditions and substrates used. For example, single-molecule (SM) FRET studies using a DNA version of TAR RNA and complementary DNA oligonucleotides are consistent with both a more rapid zipper pathway and a slower kissing-mediated reaction in the presence of NC.136 Moreover, using the DNA version of TAR RNA and the complementary TAR DNA (sometimes referred to as cTAR DNA), the major NC(11–55)-mediated annealing pathway involves nucleation of residues in the central ds segment of both DNA stems, with the kissing interaction detected as a minor pathway.129 Loop-loop kissing is observed in the case of annealing of 27-nt mini-TAR RNA and DNA hairpins in the absence of NC, whereas multiple pathways (kissing and zipper) are observed in the presence of NC.127 Interestingly, when full-length 59 nt TAR RNA/DNA were annealed, the presence of NC was shown to switch the pathway completely from loop-loop kissing to zipper.128
Since the TAR hairpins are stable structures, the helix destabilizing activity of NC is also required for annealing. Several studies using fluorescence spectroscopy (e.g., ensemble or SM FRET and/or fluorescence correlation spectroscopy) showed that when NC is bound to TAR DNA, the equilibrium is shifted from a fully “closed” conformation to a “partially open” conformation, representing “fraying” of the DNA ends.137–141 It was also found that in the presence of TAR RNA, an “open” (i.e., unfolded) conformation of TAR DNA predominates.140 (For a more detailed discussion, see the review by J. Godet and Y. Mély in this issue.)
NC's helix destabilizing activity is also required to block a non-specific self-priming (SP) reaction that competes with minus-strand transfer (reviewed in ref. 3). SP results from intramolecular formation of TAR-induced fold-back structures at the 3′ end of (−) SSDNA, which are elongated by RT. SP products are therefore minus-strand DNAs with plus-strand extensions.142 NC's ability to block SP is dependent on the presence of the native zinc fingers99,143,144 and the basic linker connecting the two fingers (residues 29 to 35).144 Inhibition of SP during minus-strand transfer occurs when NC and acceptor RNA are both present in the reaction.140,142,145,146 These conditions promote the formation of a thermodynamically stable annealed product with more bp than the original TAR-containing substrates.
Since NC's helix destabilizing activity is associated with the native zinc fingers, it is not surprising that mutations that change the zinc-coordinating CCHC motifs, certain residues surrounding the CCHC residues, or the positions of the zinc finger domains severely compromise NC chaperone activity and in vitro minus-strand transfer, in particular.99,143,144,147–153 Interestingly, many studies have shown that the N-terminal zinc finger is more important than the C-terminal finger for NC function.3 For effects of zinc finger mutations on replication and reverse transcription in vivo, see reference 4 and the review by G. Mirambeau et al. in this issue.
Recent work has revealed a previously unrecognized requirement for effective nucleic acid chaperone activity, i.e., NC must have fast on-off binding kinetics to facilitate nucleic acid strand annealing.154 This behavior is partly dependent on the native zinc fingers.154 Retroviral NCs differ in the level of their nucleic acid chaperone activities and in some cases, this is due to differences in how rapidly dissociation occurs. For example, HIV-1 NC, which exhibits rapid on-off kinetics, is one of the most efficient NC chaperones that has been examined.154–156 In contrast, human T-cell leukemia virus type 1 NC, a protein with a basic NTD and anionic CTD, is a poor chaperone despite having strong duplex destabilizing activity, since it dissociates very slowly from bound nucleic acid.156–158
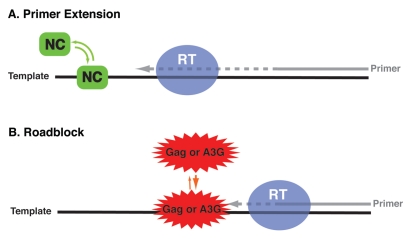
The importance of rapid dissociation kinetics has been observed in another context. Studies were performed to compare the nucleic acid chaperone activities of mature NC and NC embedded in the Gag precursor, using a reconstituted minus-strand transfer system as the readout for chaperone function.90 (The GagΔp6 construct was used for this work). At low concentrations, Gag could chaperone strand transfer and inhibit SP, indicating that it has annealing and helix destabilizing activities; however, once a certain Gag concentration was reached (∼0.23 µM), the DNA elongation step in strand transfer was drastically reduced and at 0.46 µM, it was completely inhibited. This result is consistent with “nucleic acid-driven multimerization” of Gag (facilitated by CA domain interactions) and the known slow dissociation of Gag from bound nucleic acid,155 which in turn prevent RT from traversing the ssRNA or ssDNA template. This phenomenon is referred to as the “roadblock” mechanism for inhibition of HIV-1 reverse transcription (Fig. 4) and is relevant to the inability of Gag to facilitate extension of the tRNALys3 primer following annealing to the PBS in vRNA89 (Jones CP and Musier-Forsyth K, unpublished observations). These findings illustrate how the presence of other domains in the multidomain Gag protein modulates the nature of NC's nucleic acid chaperone activity. In addition, the discovery of the roadblock mechanism raises the possibility that Gag might help to facilitate proper timing for initiation of reverse transcription. For a detailed discussion of Gag chaperone activity, see the review by A. Rein in this issue.
Figure 4.
Schematic representation of “Roadblock” mechanism to explain the inhibition of RT-mediated primer extension by A3G or Gag. (A) Rapid on-off binding kinetics displayed by NC upon binding to the ss nucleic acid template allows primer extension by RT to proceed. (B) Slow dissociation of A3G or Gag from the nucleic acid template blocks RT movement along the template during primer extension. The presence of A3G or Gag results in reduced primer extension (B) compared to extension in the presence of NC (A). The gray dashes followed by an arrow represent extension from the primer (solid gray). RT is depicted as a purple ellipse and the template is shown as a solid black line. NC and Gag or A3G are highlighted in green and red, respectively.
Nucleic acid structural requirements for NC-mediated minus-strand transfer.
The extent of complementarity between the R regions in (−) SSDNA and acceptor RNA was originally thought to be the crucial determinant for successful annealing and minus-strand transfer, presumably since a more stable product would be formed under these conditions. However, several studies with HIV-1 and other retroviruses showed that even with shorter homologies (in some instances, as small as 12 to 14 nt), specific and efficient annealing could occur (reviewed in ref. 3). Collectively, these results indicated that while homology length is important for minus-strand transfer, it is not always the primary determinant.
More recent work has revealed that the structure and degree of thermostability of the nucleic acid substrates are the major factors that influence NC-facilitated minus-strand transfer. Several studies have pointed to the importance of maintaining the bulges in the TAR DNA structure of (−) SSDNA.139,141,159–162 Interestingly, a detailed investigation of the structural requirements in minus-strand transfer demonstrated that NC's ability to destabilize the TAR stem-loop structure in (−) SSDNA alone is not sufficient to promote successful strand transfer.146 The stability of acceptor RNA is also critical.120,127,146,163–166 (For additional citations, see ref. 3). In fact, minus-strand transfer is most sensitive to RNA secondary structure and little or no strand transfer occurs if the RNA is too stable.137,146
These results are consistent with NC's moderate helix destabilizing activity and demonstrate that productive strand transfer requires a delicate thermodynamic balance between the structures of (−) SSDNA and acceptor RNA as well as formation of a stable strand transfer duplex.146 If both nucleic acids are unstructured, NC has no effect on strand transfer.132,146,150,151 Similar effects were also observed for NC-promoted internal strand transfer events during recombination.167,168
Further studies led to the discovery that the stability of the local structure of acceptor RNA, rather than the overall structure, is critical for dictating the outcome of NC-mediated minus-strand transfer.132 These findings are in agreement with the mechanism proposed for NC-facilitated annealing of tRNALys3 to the PBS,94 RT-catalyzed primer extension using TAR RNA mutant templates,161 and HIV-1 recombination in vivo.168,169 Thus, the presence of less stable structures, containing ss regions, provides “hot spots” for NC binding and nucleation of annealing.
NC function and RNase H cleavage during minus-strand DNA synthesis.
During minus-strand DNA synthesis (initially, during elongation of (−) SSDNA), RT encounters different types of RNA-DNA hybrid substrates and uses three distinct cleavage modes for degradation of the RNA strand: (1) 3′-end directed cleavage, in which the 3′-OH of the DNA primer is recessed over a long RNA template and cleavage is coupled to DNA synthesis (polymerase-dependent cleavage); (2) RNA 5′-end directed cleavage, in which the 5′-end of an RNA is recessed over a long minus-strand DNA; and (3) internal cleavage. Both (2) and (3) represent polymerase-independent cleavage events.170
In polymerase-dependent cleavage, the distance between the polymerase and RNase H active site (18 nt) determines the site of primary RNase H cleavage, although the actual cleavage is reported to be ∼15 to 20 nt from the 3′ end of the DNA.170 However, since many of the initial RNA fragments are as long as 100 to 120 nt,171 polymerase-independent cleavages, which are not linked to DNA synthesis, are also required to achieve complete degradation of the vRNA genome. For a detailed description of these cleavages, see references 53 and 170. In addition, the role of nt sequence as a determinant for cleavage specificity can be found in references 170 and 172.
Mechanistic studies of RNase H cleavage have revealed that the RNA-DNA substrate contacts both the polymerase and RNase H sites at the same time.173 Moreover, recent experiments using a SM FRET assay have demonstrated that there is a dynamic interaction between RT and its nucleic acid substrates. RT is bound to nucleic acid in two opposite orientations, depending on whether the primer is DNA (polymerase-competent mode, RT bound close to the 3′-end of the DNA) or RNA (RNase H cleavage mode, RT bound to the 3′-end of the RNA)174–177 (reviewed in ref. 53 and 170). RT shuttles between the two ends by sliding along the nucleic acid duplex.177 Surprisingly, when RT synthesizes DNA, it locates the 3′-polymerase end by the sliding mechanism and if necessary, “flips” from the RNase H cleavage orientation to the polymerase-competent orientation without dissociation from the nucleic acid substrate.177,178 Whether the presence of NC impacts these events awaits further investigation.
However, there is evidence that during the course of polymerase-independent cleavages, NC helps to remove vRNA fragments resulting from primary cleavage events179,180 (also, see below). Thus, as RNase H cleavage proceeds during minus-strand DNA synthesis, the remaining hybrids become shorter and their thermodynamic stability is reduced. When this occurs, the helix destabilizing activity of NC displaces the RNA strand in the duplex and the RNA is released.180 It has also been suggested that NC collaborates with RNase H by forming an NC-RT complex,181–184 possibly through a zinc finger-dependent mechanism.184
Mechanisms of minus-strand transfer and roles of NC and RNase H.
In one scenario, minus-strand transfer occurs by endterminus transfer from the 3′-end of full-length (−) SSDNA to the 3′-end of genomic RNA, in a reaction facilitated by the base pairing of the complementary R regions (Fig. 2, step 3).185 As synthesis of (−) SSDNA proceeds, genomic RNA is degraded by the RNase H activity of RT (see above). However, small 5′ RNA fragments, 14 to 18 nt, remain annealed to the 3′-end of (−) SSDNA, since they form a blunt-end substrate that is cleaved very poorly by RNase H (reviewed in ref. 3). In a recent study using a model system, it could be shown that 5′ terminal fragments (up to 20 nt) are readily removed by the combined action of RNase H and the helix destabilizing activity of NC (C.B. Hergott, J. Guo, J.G. Levin, unpublished observations). Support for the end-terminus transfer pathway comes from early cell-based studies with genomic RNA mutants showing that most of the minus-strand transfers occur following completion of (−) SSDNA synthesis (reviewed in ref. 3).
There is also another pathway for minus-strand transfer that occurs by an acceptor invasion-driven mechanism.186,187 This pathway is particularly relevant when there is a long homology between the RNA and DNA substrates.187 The acceptor invasiondriven mechanism for strand transfer has been studied in detail in reconstituted RT assay systems and has been shown to occur as follows (reviewed in ref. 53). Briefly, RNase H cleavage at internal sites in the donor RNA, upstream of the 3′-end of the extended DNA primer, create gaps (“invasion sites”), the most prominent of which is in the region at the base of the TAR stem-loop. These gaps, often occurring at RT pause sites on the donor, allow acceptor RNA to displace the RNA fragments and subsequently anneal to complementary sites in the DNA with the help of NC chaperone activity. The acceptor-DNA duplex propagates to the 3′-end of the DNA, most commonly by branch migration and the acceptor then serves as the template for further extension of (−) SSDNA.
Propagation of the duplex can also occur by a proximity mechanism,134,188,189 which is facilitated by long range interactions between the nucleic acids. In one study, it was reported that nt 38 to 46 in the anticodon stem of human tRNALys3 are complementary to a 9 nt sequence in the U3 region of the HIV-1 genome.190 Base pairing between these two sets of sequences stimulates minus-strand transfer in reactions using the tRNA primer to initiate (−) SSDNA synthesis.190,191 In a recent development, it was found that the homology is more extensive and includes a region upstream of the 9-nt sequence, termed segment 1 and a sequence in R, which together further stimulate minus-strand transfer beyond what is observed with the 9-nt sequence alone. The larger sequence resembles a tRNA gene-like sequence (complementary to the tRNALys3 primer) with a 29 nt intron. The extended sequence is thought to be derived from an ancient tRNA gene embedded in HIV-1 U3-R, which was maintained during HIV-1 evolution as a means to promote efficient transcription and minus-strand transfer.192 In another recent report, it was shown that circularization of the HIV-1 genome due to interactions between the 5′ gag and 3′ U3-R regions facilitates minus-strand transfer by bringing the 5′- and 3′-ends close together.193
Elongation of Minus-strand DNA
Following minus-strand transfer, RT catalyzes elongation of minus-strand DNA, which is accompanied by further degradation of genomic RNA by RNase H (Fig. 2, steps 4 and 5). Since the RNA template is highly structured,55 when RT encounters a secondary structure, it pauses at that site or in some cases, prematurely terminates polymerization. The nucleic acid chaperone activity of NC by virtue of its ability to transiently destabilize secondary structures alleviates pausing, thereby allowing RT to continue to traverse the template unimpeded161,194–199 (see ref. 3 for additional citations).
With MLV200,201 and RSV,202 it was possible to synthesize full-length vDNA in endogenous RT (ERT) assays, which are performed with permeabilized virions in the presence of added dNTPs and Mg2+; indeed, in the case of MLV, the DNA was infectious.201 However, it has been known for many years that a full-length DNA copy of the HIV-1 viral genome (∼9 kb) cannot be detected in such assays. Moreover, in reactions with purified components, the largest DNA products that are synthesized are generally no longer than 1 to 2 kb (reviewed in ref. 199). Interestingly, it was shown that large aggregates are formed in the presence of NC, RT and nucleic acids.130,131,183,203,204 Such complexes are able to synthesize vDNA, since the aggregates effectively bring NC, RT and nucleic acids in close proximity203,204 (also, see the review by G. Mirambeau et al. in this issue).
The question of whether long HIV-1 DNA products can be synthesized in vitro and if so, under what conditions, was specifically addressed in two studies. Using an 874-nt vRNA template, one group showed that in the presence of high concentrations of NC, almost all of the synthesized DNA products were full-length, although the yields were low.196 Interestingly, the relative positions of the NC zinc finger domains were not important for this effect. A subsequent study investigated the influence of NC on primer extension reactions with long vRNA templates (1.9 or 4 kb in length).199 High concentrations of NC and template switching were required for synthesis of long products (e.g., 4 kb). Importantly in reactions containing NC, high molecular mass aggregates, similar to those reported by others (see above), were formed. Analysis of the NC-induced aggregates showed the presence of full-length DNA, suggesting the importance of these complexes for efficient RT-catalyzed polymerization. The absence of the N-terminal or C-terminal zinc fingers or switching their relative positions had no effect on DNA synthesis, in agreement with the earlier finding.196 These results demonstrate that with the templates used in this study,199 the aggregation activity of NC was more important than its duplex-destabilization activity for generation of long DNA products.
The influence of NC on RT-catalyzed polymerization was also studied at the molecular level by monitoring single nt incorporation under single turnover conditions.205 Two different effects of NC were observed. Thus, NC was shown to increase the amplitude of primer extension (i.e., the percentage of primers extended by 1 or more nt) by 3-fold, although it also drastically reduced the rate of transient nt incorporation (kpol). Further studies demonstrated that NC enhanced the stability of the RT-nucleic acid substrate complexes, since it decreased the dissociation rate constants of the complex. This effect was able to compensate for the reduction in kpol. The authors suggested that a direct physical interaction between NC and RT could account for these findings, although their efforts to map the sites of interaction on the RT polymerase domain have thus far been unsuccessful.
Plus-strand DNA Synthesis
Selection of the polypurine tract (PPT) RNA primer and initiation of plus-strand DNA synthesis.
Although almost the entire vRNA genome is ultimately degraded by RNase H during the course of minus-strand DNA synthesis, there are two 15-nt regions containing only purine bases, which are resistant to RNase H cleavage and function as the primers for plus-strand DNA synthesis: (i) the 3′ PPT, which abuts the 5′ boundary of U3; and (ii) the central PPT (cPPT), which is found in the center of the IN coding region. The sequence of the two PPTs (5′-AAA AGA AAA GGG GGG) is identical (reviewed in ref. 170, 206 and 207). (Priming activity of the cPPT will be discussed in a later section). The 3′ PPT is generated by a precise cut at the PPT-U3 junction. This ensures formation of integration-competent vDNA with the correct 5′ LTR.170,206 In contrast, cleavage at the 5′ end to remove the PPT once it is utilized as a primer, is less precise (reviewed in ref. 170).
As discussed above, RT has different orientations on the nucleic acid substrate, depending on whether the enzyme is extending a DNA primer (polymerase-competent mode) or degrading RNA in an RNA-DNA duplex (RNase H cleavage mode). However, the PPT is a special case since it is an RNA primer. In this case, both RNA cleavage and DNA extension occur at the 3′ end of the PPT primer, although each of these events requires a different RT orientation.175 SM FRET studies177 showed that the same RT molecule could flip from the polymerase to RNase H orientation and vice versa without dissociation from the duplex. A significant portion of RT assumed a polymerase-competent orientation with the polymerase domain of RT positioned over the 3′-end of the PPT primer. Under conditions where PPT duplexes were extended by a few deoxynucleotides to model elongation from the PPT primer, RT orientation was solely in the DNA polymerase-competent mode.
The nearly exclusive use of the PPT as the plus-strand primer is achieved because unlike all other RNA-DNA hybrids that form during reverse transcription, only the PPT duplex is resistant to RNase H cleavage. Several factors contribute to this unusual property. Mutational analysis of the HIV-1 primer has established that the unique sequence of the PPT and especially the stretch of six Gs at the 3′ end are required for proper RNase H cleavage and primer extension by RT.170,206–209
In addition, studies utilizing different biochemical and biophysical techniques including X-ray crystallography, chemical probing alone or combined with mass spectrometry, and NMR revealed that the structure of the PPT is also crucial (for a review of earlier work, see ref. 3, 206, and 210; also see ref. 207). Recently, a high resolution NMR study showed that the PPT duplex consists of conventional, albeit weakened, Watson-Crick base pairs (bp) and assumes an A-form-like helical conformation, although certain structural anomalies were detected.211 However, the distortion seen in the PPT duplex was relatively small, in contrast to the major structural anomalies observed in the PPT-RT crystal structure.210 Results from the NMR study and earlier work212 are consistent with an inherent flexibility in the HIV-1 PPT duplex and with limited long-range structural effects.211 In view of this conformational flexibility, Yi-Brunozzi et al.211 have suggested that upon binding to RT, structural perturbations throughout the PPT sequence provide access to residues present in different subdomains of RT, thereby achieving optimal binding and precise cleavage at the PPT/U3 junction (reviewed in ref. 207). Thus, the unique interactions between RT and the PPT duplex are a major factor in efficient propagation of plus-strand DNA synthesis from the PPT primer.
Despite the strong bias toward specific plus-strand priming, it has been reported that HIV-1 plus-strand DNA synthesis is discontinuous, suggesting initiation from multiple upstream sites.123,209,213,214 Thus, plus-strand DNA synthesis could be initiated from non-PPT primers generated during minus-strand DNA elongation by incomplete removal of RNA fragments still hybridized to minus-strand DNA.206 Nevertheless, “mispriming”, i.e., initiation by a non-PPT RNA, appears to occur at very low levels in vivo,209,214 implying that HIV-1 has a mechanism to block non-specific plus-strand priming.
Studies undertaken to investigate this possibility demonstrated that in addition to RNase H-mediated cleavage, the nucleic acid chaperone activity of NC was also crucial for removal of non-PPT RNAs.179,180 In fact, NC also inhibited mispriming in the absence of RNase H activity, although inhibition was greatest when RNase H and NC were both available.180 Using SSHS NC,99 a mutant that is unable to coordinate zinc (see above) and a WT zinc-less NC, it was shown that NC's helix destabilizing activity is required to dissociate duplexes containing non-PPT RNAs.180 However, since NC is a moderate helix destabilizer, it is not surprising that NC had no effect on the highly stable PPT duplex.179,180 Taken together, these findings demonstrated a new function for the chaperone activity of NC, i.e., maintaining the fidelity of plus-strand DNA initiation.
Synthesis of (+) SSDNA.
As minus-strand DNA synthesis proceeds, the 3′ PPT primer is extended by RT, using a template consisting of minus-strand DNA still attached to the 5′-terminus of the tRNALys3 primer (Fig. 2, steps 4 and 5). The resulting DNA extension product is termed (+) SSDNA. Elongation is expected to terminate when RT has copied the base preceding the methyl A at position 58 at the 3′-end of the tRNA primer, which reconstitutes the PBS sequence in (+) SSDNA (Fig. 2, step 5) (reviewed in ref. 3).
In reconstituted systems, termination also occurs at two other sites: a pseudouridine at position 55; and at a position in the anticodon loop of tRNALys3 , which results in a dead-end product.215–217 Termination at positions 55 and 58 was also observed in an HIV-1 ERT assay.216 Interestingly, in some HIV-1 strains, e.g., NL4-3,218 nt 56–58 in tRNALys3 are complementary to the first three bases downstream of the PBS in vRNA, so that termination at position 55 in the tRNA does not interfere with subsequent plus-strand transfer.216 Later, it was shown that this complementarity is not unique to HIV-1. Thus, a strong consensus sequence (“primer overextension sequence”) was also identified immediately downstream of the PBS in spumaviruses and other lentiviruses.217 It is likely that bypass of the methyl A at position 58 could result from undermodification of some of the molecules at this position.215–217
Plus-Strand DNA Transfer
Overview of plus-strand DNA transfer.
Plus-strand transfer is required for synthesis of a full-length copy of plus-strand vDNA. This event is mediated by base pairing of the 18-nt PBS region at the 3′-terminus of (+) SSDNA, termed (+) PBS and the complementary region, termed (−) PBS, at the 3′-terminus of minus-strand DNA (Fig. 1B), resulting in the formation of a circular intermediate (Fig. 2, step 7) (reviewed in ref. 3 and 53). NC stimulates overall plus-strand transfer, although in some reconstituted systems, the effect is small.215–217 However, NC's nucleic acid chaperone activity has been shown to be crucial for efficient removal of the tRNA primer and for facilitating the annealing reaction.
tRNA primer removal.
Plus-strand transfer cannot proceed unless the tRNA primer is first removed from the 5′-end of minus-strand DNA (Fig. 2, step 6). This step is necessary so that the (−) PBS sequence can be annealed to the (+) PBS sequence in (+) SSDNA. Once annealing is complete, RT-catalyzed elongation of (+) SSDNA proceeds(Fig. 2, step 7).
Removal of the primer involves primary and secondary RNase H cleavages. Primary cleavage does not occur at the tRNA-DNA junction, but rather between the 3′-terminal rA of the tRNA and the penultimate rC. This results in the attachment of an rA residue to the 5′-end of minus-strand DNA and formation of a 17 nt hybrid consisting of sequences from the 3′ end of the tRNA and the (+) PBS in (+) SSDNA (reviewed in ref. 170).
Using reconstituted systems that model the steps in plus-strand transfer, it was shown that the initial cleavage event was not sufficient to completely remove the tRNA, presumably because the 17 nt hybrid is relatively stable.215,216,219 Thus, secondary cleavages are also required.216,219 Interestingly, the nucleic acid chaperone activity of NC could mediate tRNA removal by dissociation of the 17 nt hybrid, but not as efficiently as RNase H.216 Removal was actually most efficient when both RNase H and NC were present. Subsequent studies demonstrated that the native zinc fingers are required for NC function in primer removal, indicating that NC's duplex destabilization activity is critical for this step.99,143
Annealing in plus-strand transfer.
As mentioned above, plus-strand transfer involves annealing of the 18-nt (+) and (−) PBS sequences (Figs. 1B and 2, step 7). An NMR study of the (−) PBS structure showed that it folds into a stable hairpin, containing a structured pentanucleotide loop, a stem with a bulged base and a 4-nt ss overhang220 (Fig. 1B). Addition of NC led to spectral shifts consistent with destabilization of the stem-loop structure: (1) broadening and reduction of signals from Watson-Crick imino protons as the NC concentration was increased; and (2) reduction by 20°C of the highest temperature at which these signals were still visible. These findings suggested that NC-induced conformational changes in the (−) PBS facilitate annealing to the (+) PBS. A subsequent NMR study performed with NC(12–55) or full-length NC with a (−) PBS construct lacking the 3′ overhang (to reduce the size of the complex) showed that binding is mediated by hydrophobic residues in the zinc fingers.221
Fluorescence studies demonstrated that NC only weakly destabilizes the PBS hairpins.222 Three NC molecules bind to one molecule of the (+) and (−) PBS structures, respectively. It was proposed that each of the three NC molecules is bound to a different portion of the structure i.e., to the loop, stem and overhang regions. Annealing of the 18-nt (−) and (+) PBS hairpins in reactions with or without NC was shown to follow second-order kinetics.223 Increasing the length of the (−) PBS constructs (at the 3′ end) to 33 or 58 nt had little effect on the kinetic parameters, indicating that folding of the (−) PBS does not change in the context of a longer molecule. In the absence of NC, annealing was shown to occur via two pathways: (1) nucleation mediated by ss stem overhangs in both structures (major pathway); and (2) nucleation by loop-loop kissing interactions (minor pathway). The data also indicated that formation of the extended duplex from a transient intermediate is the rate-limiting step in annealing and requires destabilization of the G:C-rich stems. Addition of NC(11–55), which lacks the basic N-terminal domain, stimulated annealing kinetics by 6-fold, primarily by activating the loop-loop kissing pathway. Compared with the result with truncated NC, the annealing kinetics with full-length NC were further increased by ∼10-fold. This shows that nucleic acid aggregation also plays a role in the PBS annealing reaction, but its contribution is less than for the TAR hairpin annealing step.127,128
Biochemical studies with longer nucleic acid constructs that model the donor and acceptor DNA substrates in plus-strand transfer and contain the complementary PBS sequences showed that NC stimulates the level of annealing.216 However, unlike annealing of the complementary R sequences, NC's zinc-coordinating activity is not required for productive annealing in plus-strand transfer.99 Moreover, NC has a greater stimulatory effect on the annealing rates of the complementary R sequences than is seen for annealing of the (−) and (+) PBS sequences in the minus-strand DNA and (+) SSDNA substrates, respectively.99 This difference presumably reflects the fact that in biophysical assays (e.g., fluorescence spectroscopy or NMR), the TAR DNA structure is more susceptible to NC destabilization than the G:C-rich stems in the PBS hairpins.138–140,220,222
Priming from the cPPT.
In addition to the 3′ PPT, HIV-1 and other lentiviruses have an additional PPT primer, which as mentioned above, is referred to as the cPPT (Fig. 5, step 1) (reviewed in ref. 206). During plus-strand transfer, the DNA replication intermediate is circularized (Fig. 5, step 2). Priming from both PPTs involves discontinuous DNA synthesis, which generates the ∼99 nt central DNA flap at the center of the unintegrated ds vDNA product (Fig. 5, steps 3 and 4).224 The flap is part of a triple-stranded structure containing two overlapping plus-strand DNA segments and a complementary minus-strand.224 The downstream segment is initiated from the cPPT, whereas the upstream segment is initiated from the 3′ PPT (Fig. 5, step 1). Flap removal and repair of the gap in ds DNA have not been demonstrated in vivo, although it is assumed that cellular enzymes are involved.206 Interestingly, in an in vitro HIV-1 system, flap removal was catalyzed by FEN1, a human replication enzyme with RNA and DNA nuclease activity and the gap was repaired by a human DNA ligase.225
Figure 5.
cPPT priming and formation of the central flap in plus-strand DNA synthesis. Step 1. Plus-strand DNA synthesis is initiated from two identical RNA primers: cPPT and 3′ PPT (shown in blue). The template consists of minus-strand DNA (in black) attached to tRNA (in green) at its 5′-end. The short segments of plus-strand DNA extended from both primers are shown in red. Step 2. RT extends the 3′PPT primer until the 3′ 18-nt of the tRNA (complement of PBS) is copied leading to the formation of (+) SSDNA with a reconstituted PBS sequence (also see Fig. 2). Concomitant with extension, RNase H removes the two PPT primers. With the assistance of NC, RNase H also removes the tRNA primer so that plus-strand synthesis may proceed. Plus-strand transfer occurs when the PBS sequence at the 3′-end of (+) SSDNA anneals to the PBS sequence at the 3′-end of minus-strand DNA. This leads to circularization of vDNA. Strand displacement synthesis of minus-strand DNA begins (see step 3). Extension of plus-strand DNA originating from the cPPT primer is also shown. Step 3. Left side. Minus-strand DNA is extended (shown by black arrow) by copying the (+) SSDNA sequence and in the process, the 5′-end of minus-strand DNA (originally annealed to (+) SSDNA) is displaced. (+) SSDNA is extended using the minus-strand DNA template until the cPPT sequence is copied. Further elongation proceeds by strand displacement of plus-strand DNA primed by the cPPT primer. Termination occurs when the end of the CTS is reached. This results in the formation of a triple-stranded structure containing a flapping strand (flap) in the middle of the dsDNA product (see step 4). Right side. Elongation of minus-strand DNA (black arrow) and plus-strand DNA (from the cPPT primer) (red arrow) by strand displacement synthesis continues. Step 4. This process ultimately leads to formation of LTR (U3-R-U5) regions at both ends of the linear dsDNA product. The flap region and gap shown at the center of the dsDNA are thought to be repaired by cellular enzymes.
Mechanistic studies on formation of the flap showed that during plus-strand synthesis, the 3′ PPT primer is extended through the cPPT sequence. To continue DNA synthesis beyond the cPPT, RT displaces the downstream DNA strand initiated from the cPPT until a site known as the central termination sequence (CTS) is reached (Fig. 5, step 3).224 The duplex at the CTS site has curvature that leads to a highly distorted structure with a compressed minor groove.226,227 This unique structure causes dissociation of RT, thereby terminating strand displacement synthesis. Synthesis of the complete flap was demonstrated in an in vitro study and NC was shown to increase the rate of flap formation.45 It was suggested that the ss nature of the flap might allow it to bind specific proteins such as NC, which could play a role in conversion of an RTC to a PIC. In other work, short oligonucleotides (17 and 24 nt) derived from the central flap sequence were shown to form G-quartets to which NC could bind with high affinity.228,229 However, in an independent study, it was reported that using a longer flap-derived sequence (89 nt), a canonical duplex with a “flapping” third strand was formed in the presence or absence of NC, but this structure did not fold into a G-quadruplex, as was shown for the shorter sequences.230
An important question to consider is the functional significance of having a second PPT primer and the central DNA flap. As already discussed, there is evidence for a possible role of the flap in nuclear import of PICs, although a number of studies have questioned this conclusion. Early studies using a mutational approach demonstrated that cPPT mutations cause a delay in HIV-1 replication and it was suggested that the presence of a second primer increases the rate as well as the overall efficiency of reverse transcription.231,232
More recently, two independent studies have presented evidence indicating that the cPPT protects HIV-1 from the activity of the human APOBEC3 (A3) restriction factors. Presumably, by initiating from two PPTs, minus-strand DNA exists for a shorter time as ssDNA. (As will be discussed below, the substrate for deamination by A3 proteins is ssDNA). In the initial study, using a vif-deficient virus, the authors showed that when a functional cPPT was present, editing by either A3G or A3B was reduced for a distance of 400 bp downstream of the cPPT.233
Subsequent work demonstrated that mutations in the cPPT and/or the CTS did not affect HIV-1 replication in assays with most T cell lines and non-dividing macrophages.50 However, replication was blocked in nonpermissive cells that express A3G. Moreover, when the Δvif and the double cPPT/CTS mutations were combined, restriction by A3G was enhanced, in a synergistic manner. These authors concluded that the most important aspect of cPPT priming is the protection of unpaired minus-stand DNA from attack by A3G.
Final steps in production of integration-competent vDNA.
Formation of a circular intermediate during plus-strand transfer allows RT to extend both the plus- and minus-strands of DNA (Fig. 2, step 7). Following circularization, RT displaces 637 nt to generate the LTR sequence (U3-R-U5) at the ends of both DNA strands (Fig. 2, step 8); (Fig. 5, step 3). Since HIV-1 NC has strand exchange activity,9 it was of interest to determine whether NC could stimulate strand displacement synthesis. Curiously, in one study, NC was found to have a positive effect on this process,234 whereas in another study, no stimulation could be detected.235 Indeed, in the latter work, high concentrations of NC actually inhibited strand displacement, possibly because it led to dissociation of the primer from the template. However, both studies demonstrated stimulatory activity with cellular ss binding proteins. The reason for the discordant results regarding the role of NC in the final step in HIV-1 reverse transcription is not known. Strand displacement activity and a possible role for NC in other retrovirus systems are discussed in reference 3.
Inhibition of HIV-1 Reverse Transcription by Human A3G
Overview of human A3G's molecular properties and antiviral activity.
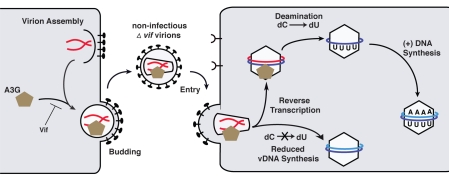
Human A3G is a host cytidine deaminase, which was first identified in 2002 by Sheehy et al.236 as the cellular protein that restricts the replication of HIV-1 virions lacking the viral protein Vif (Fig. 6). In the absence of Vif, newly synthesized A3G in the producer cell is packaged into virions during virus assembly by interaction with RNA and the NC domain in Gag (reviewed in ref. 21). A3G is present in the viral core and is thought to be associated with ribonucleoprotein complexes in the target cell.237–239 In contrast, Vif targets A3G for degradation via the ubiquitination/proteasome pathway or inactivates it by other independent mechanisms (reviewed in ref. 240 and 241, also see ref. 242).
Figure 6.
Effect of A3G on HIV -1 replication. During virus assembly at the plasma membrane of the producer cell, packaging of A3G proteins into newly formed virions occurs in the absence of the HIV-1 Vif protein. A3G-containing virions enter the target cell (on the right) and undergo reverse transcription. During this process, the deaminase activity of A3G causes C to U mutations on exposed ssDNA regions generated during reverse transcription (deaminase-dependent mechanism). These mutations are detrimental to the virus and therefore lead to inhibition of virus replication. A3G may also interfere with virus replication in a deaminase-independent manner (no C to U mutations) by acting as a “roadblock” to the movement of RT during the primer extension step (see Fig. 4). Adapted from reference 21 with permission from the Royal Society (UK).
A3G has two zinc-binding domains, each containing the conserved HXE(X)23–28 CXXC motif. The NTD is responsible for binding to nucleic acids and Vif and is required for A3G encapsidation; the CTD contains the catalytic center for deamination. The glutamic acid serves as a proton shuttle in deamination, whereas the histidine and two cysteine residues coordinate zinc. A3G binds ssRNA and ssDNA with similar affinity, but the sole substrate for deamination is ssDNA (reviewed in ref. 21).
Following initiation of reverse transcription, A3G deaminates the ss minus-strand DNA, which leads to G to A hypermutation in the positive sense strand of vDNA (Fig. 6). This ultimately results in inactivation of viral functions. In addition to an effect on vDNA synthesis, inhibition of integration has also been observed.239,243 The question of whether deamination is solely responsible for A3G's inhibitory activity is still not resolved. The field is divided between those whose work indicates that deamination is the exclusive mechanism for A3G's antiviral function and others who have evidence that A3G can also exert deamination-independent effects (reviewed in ref. 21 and 244).
Inhibition of HIV-1 reverse transcription by a non-editing mechanism.
Several groups have investigated the possibility that a non-editing mechanism might contribute to A3G inhibition of reverse transcription (Fig. 6). For example, it was reported that although A3G does not inhibit GagΔp6-promoted annealing of tRNALys3 to the PBS,87 A3G inhibits NC-mediated annealing by ∼2-fold.87,245 This result is consistent with a similar reduction in (−) SSDNA synthesis in cells cotransfected with A3G and a Δvif viral clone.246 Moreover, it was found that a decrease in late HIV-1 DNA synthesis was correlated with inhibition of the minus- and plus-strand transfer steps in vivo and with NC-facilitated minus-strand transfer in vitro.247 The authors also presented data suggesting that A3G can inhibit minus-strand transfer in the absence of deaminase activity, although not as effectively as WT A3G.
In other work with infected cells, A3G had no effect on minus-strand transfer and only a small effect on plus-strand transfer.243 However, analysis of 2-LTR circle junction clones amplified from unintegrated DNA demonstrated that some of these clones had a 6-nt sequence derived from the 5′-end of the PBS, strongly suggesting that A3G inhibits proper tRNA cleavage from the 3′-end of vRNA and the ability to complete integration.243
A study of A3G's molecular properties performed with highly purified, catalytically active protein expressed in a baculovirus system revealed that A3G and NC do not compete for binding to RNA.248 This suggested that A3G might not inhibit the nucleic acid chaperone activity of NC in reverse transcription. To test this hypothesis, the effect of A3G on individual steps in vDNA synthesis was investigated in well-defined reconstituted assay systems with purified A3G, NC and RT.249
The results demonstrated that the kinetics of tRNALys3 annealing to a vRNA transcript containing the PBS were the same whether NC alone or NC plus A3G was added. A similar result was obtained for annealing of (−) SSDNA to acceptor RNA. In addition, A3G (with or without NC) did not inhibit RNase H cleavage of RNA in a preformed RNA-DNA duplex. In contrast, all RT-catalyzed polymerization reactions were strongly inhibited in a dose-dependent manner and in the absence of deaminase activity.
Interestingly, SM force-induced melting studies (DNA stretching) that measure nucleic acid binding kinetics250 demonstrated that in contrast to NC's rapid nucleic acid binding off-rate, A3G dissociates from bound nucleic acid very slowly.249 Thus, like Gag,90 A3G also blocks movement of RT along ss nucleic acid templates and consequently, NC is unable to facilitate polymerization reactions (Fig. 4). However, since Gag155 has even slower nucleic acid binding kinetics than A3G,249 it is not surprising that A3G has no effect on Gag-facilitated tRNALys3 tRNALys3 annealing.87 Additionally, since A3G does not inhibit RNase H activity per se,249 the aberrant cleavage of tRNALys3 during primer removal243 may be indirectly caused by the roadblock mechanism, i.e., by A3G binding to sites on the tRNA and/or the ss DNA template, which would prevent RNase H from accessing the proper cleavage site.243,249
ERT assays with mellitin-treated vif-deficient HIV particles produced in cells transfected with A3G also demonstrated that A3G does not inhibit primer placement.251 However, RT-catalyzed elongation of the tRNA primer was reduced in a dose-dependent manner. Interestingly, the polymerization reaction was inhibited to an increasing extent as the tRNA primer was progressively elongated. These observations are in excellent agreement with the findings of Iwatani et al.249
Collectively, these studies demonstrate that A3G severely inhibits HIV-1 reverse transcription in a dose-dependent manner in reconstituted and ERT assay systems by blocking RT-catalyzed polymerization and has little or no effect on tRNALys3 or TAR annealing. Differences in the effect of NC on primer placement in vitro or on strand transfer events in vivo (see above) could be due to variations in the experimental conditions. However, it is clear from all of these investigations that the observed inhibition of reverse transcription occurs in the absence of editing activity, suggesting that multiple mechanisms are likely to be involved in A3G antiviral activity (reviewed in ref. 21).
Conclusion and Perspective
NC is essential for HIV-1 replication and plays a critical role in almost every step of reverse transcription including tRNA primer annealing and initiation, minus- and plus-strand transfer and RT-catalyzed DNA elongation. Many of these functions rely on the nucleic acid chaperone activity of NC, i.e., the ability to mediate nucleic acid conformational rearrangements that lead to the most thermodynamically stable structure. Due to pleiotropic effects of NC mutations in cell culture-based studies, ERT assays and reconstituted in vitro systems have been crucial for gaining insights into NC function. Over the past decade, remarkable progress has been made in our mechanistic understanding of the nucleic acid chaperone properties of mature HIV NC, aided by recent development of powerful SM techniques. Recent studies have also examined the chaperone function of NC in the context of the Gag precursor protein. These studies have revealed that unlike NC, Gag forms a roadblock to DNA elongation. This finding provides a greater understanding of why mature NC has evolved as the major cofactor for specific and efficient vDNA synthesis.
The central role of NC in reverse transcription together with its remarkable nucleic acid chaperone properties and high genetic barrier to mutation make it an attractive target for new HIV therapeutics. However, the small size of mature NC also makes the development of NC-targeted therapeutics a challenging task. Nevertheless, at least four different classes of anti-NC molecules have been described in the literature, including zinc-ejector agents, peptidomimetics, RNA aptamers and non-zinc-ejecting NC binders.252 A recent class of NC zinc-ejecting agents, the S-acyl-2-mercaptobenzamide thioesters (SAMTs), are promising new drug candidates for further development in anti-HIV-1 topical microbicide applications.253
Although our understanding of the many roles of NC in the virus life cycle has improved greatly, many challenging and critical open questions remain. How does the NC domain of Gag recognize the packaging element with such exquisite specificity and where does this interaction initially take place? What is the relationship between virus assembly, C-terminal Gag processing and reverse transcription? Where does tRNA primer annealing occur in the assembly pathway? How is premature primer annealing and reverse transcription normally avoided? Are there other host restriction factors, in addition to A3G, which interfere with NC-mediated steps in reverse transcription? The next decade will likely provide answers to these and other intriguing questions, which should bring us closer to the development of new highly effective therapeutic strategies to combat AIDS.
Acknowledgements
We thank Robert J. Gorelick, Alan Rein and James A. Thomas for critical reading of the manuscript and Ioulia Rouzina for valuable discussion.
This work was supported by the Intramural Research Program of the NIH (Eunice Kennedy Shriver National Institute of Child Health and Human Development) [J.G.L.] and NIH grant GM065056 [K.M.F.].
Abbreviations
- NC
nucleocapsid protein
- HIV-1
human immunodeficiency virus type 1
- Pr55gag
Gag precursor protein
- MA
matrix
- CA
capsid
- PR
protease
- vDNA
viral DNA
- PBS
primer binding site
- A3G
APOBEC3G
- Vif
virus infectivity factor
- ds
double-stranded
- ss
single-stranded
- vRNA
viral RNA
- RT
reverse transcriptase
- IN
integrase
- RTC
reverse transcription complex
- PIC
preintegration complex
- NTD
N-terminal domain
- CTD
C-terminal domain
- LTR
long terminal repeat
- Ψ
cisacting packaging signal
- LysRS
lysyl-tRNA synthetase
- MLV
murine leukemia virus
- FRET
Förster resonance energy transfer
- bp
base pair(s)
- UTR
untranslated region
- SHAPE
selective 2′-hydroxyl acylation analyzed by primer extension
- nt
nucleotide(s)
- AT-2
aldrithiol-2
- SD
splice donor
- (−) SSDNA
(−) strong-stop DNA
- IPs
inositol phosphates
- PAS
primer activation signal
- RSV
Rous sarcoma virus
- PR(−)
PR-minus
- TLE
tRNALys3-like element
- R
repeat
- TAR
transactivation response element
- SM
single-molecule
- SP
self-priming
- ERT
endogenous RT assay
- PPT
polypurine tract
- cPPT
central PPT
- CTS
central termination sequence
- A3
APOBEC3
- SAMTS
S-acyl-2-mercaptobenzamide thioesters
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/14115
References
- 1.Darlix J-L, Lapadat-Tapolsky M, de Rocquigny H, Roques BP. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J Mol Biol. 1995;254:523–537. doi: 10.1006/jmbi.1995.0635. [DOI] [PubMed] [Google Scholar]
- 2.Rein A, Henderson LE, Levin JG. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 3.Levin JG, Guo J, Rouzina I, Musier-Forsyth K. Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: critical role in reverse transcription and molecular mechanism. Prog Nucleic Acid Res Mol Biol. 2005;80:217–286. doi: 10.1016/S0079-6603(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JA, Gorelick RJ. Nucleocapsid protein function in early infection processes. Virus Res. 2008;134:39–63. doi: 10.1016/j.virusres.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson LE, Bowers MA, Sowder RC, II, Serabyn SA, Johnson DG, Bess JW, Jr, et al. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings and complete amino acid sequences. J Virol. 1992;66:1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanstrom R, Wills JW. Synthesis, assembly and processing of viral proteins. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 7.Vogt VM. Retroviral virions and genomes. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press; 1997. pp. 27–69. [PubMed] [Google Scholar]
- 8.Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release and maturation. Adv Pharmacol. 2007;55:347–387. doi: 10.1016/S1054-3589(07)55010-6. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchihashi Z, Brown PO. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1994;68:5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bampi C, Jacquenet S, Lener D, Décimo D, Darlix J-L. The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance. Int J Biochem Cell Biol. 2004;36:1668–1686. doi: 10.1016/j.biocel.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Carteau S, Gorelick RJ, Bushman FD. Coupled integration of human immunodeficiency virus type 1 cDNA ends by purified integrase in vitro: stimulation by the viral nucleocapsid protein. J Virol. 1999;73:6670–6679. doi: 10.1128/jvi.73.8.6670-6679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas JA, Gagliardi TD, Alvord WG, Lubomirski M, Bosche WJ, Gorelick RJ. Human immunodeficiency virus type 1 nucleocapsid zinc-finger mutations cause defects in reverse transcription and integration. Virology. 2006;353:41–51. doi: 10.1016/j.virol.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Kleiman L, Cen S. The tRNALys packaging complex in HIV-1. Int J Biochem Cell Biol. 2004;36:1776–1786. doi: 10.1016/j.biocel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Isel C, Ehresmann C, Marquet R. Initiation of HIV reverse transcription. Viruses. 2010;2:213–243. doi: 10.3390/v2010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darlix J-L, Gabus C, Nugeyre MT, Clavel F, Barré-Sinoussi F. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- 16.Feng YX, Campbell S, Harvin D, Ehresmann B, Ehresmann C, Rein A. The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site. J Virol. 1999;73:4251–4256. doi: 10.1128/jvi.73.5.4251-4256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafaie J, Song R, Abrahamyan L, Mouland AJ, Laughrea M. Mapping of nucleocapsid residues important for HIV-1 genomic RNA dimerization and packaging. Virology. 2008;375:592–610. doi: 10.1016/j.virol.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Jalalirad M, Laughrea M. Formation of immature and mature genomic RNA dimers in wild-type and protease-inactive HIV-1: Differential roles of the Gag polyprotein, nucleocapsid proteins NCp15, NCp9, NCp7 and the dimerization initiation site. Virology. 2010;407:225–236. doi: 10.1016/j.virol.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Dussupt V, Javid MP, Abou-Jaoude G, Jadwin JA, de La Cruz J, Nagashima K, et al. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009;5:1000339. doi: 10.1371/journal.ppat.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popov S, Popova E, Inoue M, Göttlinger HG. Divergent Bro1 domains share the capacity to bind human immunodeficiency virus type 1 nucleocapsid and to enhance virus-like particle production. J Virol. 2009;83:7185–7193. doi: 10.1128/JVI.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, et al. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herschhorn A, Hizi A. Retroviral reverse transcriptases. Cell Mol Life Sci. 2010;67:2717–2747. doi: 10.1007/s00018-010-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lori F, di Marzo Veronese F, de Vico AL, Lusso P, Reitz MS, Jr, Gallo RC. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992;66:5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- 27.Aiken C. Viral and cellular factors that regulate HIV-1 uncoating. Current opinion in HIV and AIDS. 2006;1:194–199. doi: 10.1097/01.COH.0000221591.11294.c1. [DOI] [PubMed] [Google Scholar]
- 28.Warrilow D, Tachedjian G, Harrich D. Maturation of the HIV reverse transcription complex: putting the jigsaw together. Rev Med Virol. 2009;19:324–337. doi: 10.1002/rmv.627. [DOI] [PubMed] [Google Scholar]
- 29.Bowzard JB, Wills JW, Craven RC. Second-site suppressors of Rous sarcoma virus CA mutations: evidence for interdomain interactions. J Virol. 2001;75:6850–6856. doi: 10.1128/JVI.75.15.6850-6856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang S, Murakami T, Agresta BE, Campbell S, Freed EO, Levin JG. Human immunodeficiency virus type 1 N-terminal capsid mutants that exhibit aberrant core morphology and are blocked in initiation of reverse transcription in infected cells. J Virol. 2001;75:9357–9366. doi: 10.1128/JVI.75.19.9357-9366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forshey BM, von Schwedler U, Sundquist WI, Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J Virol. 2002;76:5667–5677. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang S, Murakami T, Cheng N, Steven AC, Freed EO, Levin JG. Human immunodeficiency virus type 1 N-terminal capsid mutants containing cores with abnormally high levels of capsid protein and virtually no reverse transcriptase. J Virol. 2003;77:12592–12602. doi: 10.1128/JVI.77.23.12592-12602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Schwedler UK, Stray KM, Garrus JE, Sundquist WI. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J Virol. 2003;77:5439–5450. doi: 10.1128/JVI.77.9.5439-5450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arhel N, Genovesio A, Kim KA, Miko S, Perret E, Olivo-Marin JC, et al. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat Meth. 2006;3:817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Craigie R. The road to chromatin—nuclear entry of retroviruses. Nat Rev Microbiol. 2007;5:187–196. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- 38.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grewe C, Beck A, Gelderblom HR. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–9674. [PubMed] [Google Scholar]
- 40.Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita M, Perez O, Hope TJ, Emerman M. Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells. PLoS pathogens. 2007;3:1502–1510. doi: 10.1371/journal.ppat.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arhel NJ, Souquere-Besse S, Munier S, Souque P, Guadagnini S, Rutherford S, et al. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007;26:3025–3037. doi: 10.1038/sj.emboj.7601740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 45.Hameau L, Jeusset J, Lafosse S, Coulaud D, Delain E, Unge T, et al. Human immunodeficiency virus type 1 central DNA flap: dynamic terminal product of plus-strand displacement DNA synthesis catalyzed by reverse transcriptase assisted by nucleocapsid protein. J Virol. 2001;75:3301–3313. doi: 10.1128/JVI.75.7.3301-3313.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivière L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2010;84:729–739. doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dvorin JD, Bell P, Maul GG, Yamashita M, Emerman M, Malim MH. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J Virol. 2002;76:12087–12096. doi: 10.1128/JVI.76.23.12087-12096.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limón A, Nakajima N, Lu R, Ghory HZ, Engelman A. Wild-type levels of nuclear localization and human immunodeficiency virus type 1 replication in the absence of the central DNA flap. J Virol. 2002;76:12078–12086. doi: 10.1128/JVI.76.23.12078-12086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita M, Emerman M. The cell cycle independence of HIV infections is not determined by known karyophilic viral elements. PLoS Pathog. 2005;1:18. doi: 10.1371/journal.ppat.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu C, Saenz DT, Fadel HJ, Walker W, Peretz M, Poeschla EM. The HIV-1 central polypurine tract functions as a second line of defense against APOBEC3G/F. J Virol. 2010;84:11981–11993. doi: 10.1128/JVI.00723-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 52.Jacobo-Molina A, Ding J, Nanni RG, Clark AD, Jr, Lu X, Tantillo C, et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3. Å resolution shows bent DNA. Proc Natl Acad Sci USA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu VP, Song M, Gao L, Rigby ST, Hanson MN, Bambara RA. Strand transfer events during HIV-1 reverse transcription. Virus Res. 2008;134:19–38. doi: 10.1016/j.virusres.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Geigenmüller U, Linial ML. Specific binding of human immunodeficiency virus type 1 (HIV-1) Gag-derived proteins to a 5′ HIV-1 genomic RNA sequence. J Virol. 1996;70:667–671. doi: 10.1128/jvi.70.1.667-671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, et al. High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. PLoS Biol. 2008;6:96. doi: 10.1371/journal.pbio.0060096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grigorov B, Décimo D, Smagulova F, Péchoux C, Mougel M, Muriaux D, et al. Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers. Retrovirology. 2007;4:54. doi: 10.1186/1742-4690-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ott DE, Coren LV, Gagliardi TD. Redundant roles for nucleocapsid and matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J Virol. 2005;79:13839–13847. doi: 10.1128/JVI.79.22.13839-13847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aronoff R, Linial M. Specificity of retroviral RNA packaging. J Virol. 1991;65:71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levin JG, Grimley PM, Ramseur JM, Berezesky IK. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974;14:152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muriaux D, Mirro J, Harvin D, Rein A. RNA is a structural element in retrovirus particles. Proc Natl Acad Sci USA. 2001;98:5246–5251. doi: 10.1073/pnas.091000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rulli SJ, Jr, Hibbert CS, Mirro J, Pederson T, Biswal S, Rein A. Selective and nonselective packaging of cellular RNAs in retrovirus particles. J Virol. 2007;81:6623–6631. doi: 10.1128/JVI.02833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang M, Mak J, Ladha A, Cohen E, Klein M, Rovinski B, et al. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol. 1993;67:3246–3253. doi: 10.1128/jvi.67.6.3246-3253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mak J, Kleiman L. Primer tRNAs for reverse transcription. J Virol. 1997;71:8087–8095. doi: 10.1128/jvi.71.11.8087-8095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavon-Eternod M, Wei M, Pan T, Kleiman L. Profiling non-lysyl tRNAs in HIV-1. RNA. 2010;16:267–273. doi: 10.1261/rna.1928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cen S, Khorchid A, Javanbakht H, Gabor J, Stello T, Shiba K, et al. Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1. J Virol. 2001;75:5043–5048. doi: 10.1128/JVI.75.11.5043-5048.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Javanbakht H, Halwani R, Cen S, Saadatmand J, Musier-Forsyth K, Gottlinger H, et al. The interaction between HIV-1 Gag and human lysyl-tRNA synthetase during viral assembly. J Biol Chem. 2003;278:27644–27651. doi: 10.1074/jbc.M301840200. [DOI] [PubMed] [Google Scholar]
- 67.Kovaleski BJ, Kennedy R, Hong MK, Datta SA, Kleiman L, Rein A, et al. In vitro characterization of the interaction between HIV-1 Gag and human lysyl-tRNA synthetase. J Biol Chem. 2006;281:19449–19456. doi: 10.1074/jbc.M601189200. [DOI] [PubMed] [Google Scholar]
- 68.Kovaleski BJ, Kennedy R, Khorchid A, Kleiman L, Matsuo H, Musier-Forsyth K. Critical role of helix 4 of HIV-1 capsid C-terminal domain in interactions with human lysyl-tRNA synthetase. J Biol Chem. 2007;282:32274–32279. doi: 10.1074/jbc.M706256200. [DOI] [PubMed] [Google Scholar]
- 69.Khorchid A, Javanbakht H, Wise S, Halwani R, Parniak MA, Wainberg MA, et al. Sequences within Pr160gag-pol affecting the selective packaging of primer tRNALys3 into HIV-1. J Mol Biol. 2000;299:17–26. doi: 10.1006/jmbi.2000.3709. [DOI] [PubMed] [Google Scholar]
- 70.Guo F, Cen S, Niu M, Javanbakht H, Kleiman L. Specific inhibition of the synthesis of human lysyl-tRNA synthetase results in decreases in tRNALys incorporation, annealing to viral RNA and viral infectivity in human immunodeficiency virus type 1. J Virol. 2003;77:9817–9822. doi: 10.1128/JVI.77.18.9817-9822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halwani R, Cen S, Javanbakht H, Saadatmand J, Kim S, Shiba K, et al. Cellular distribution of Lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 1 assembly. J Virol. 2004;78:7553–7564. doi: 10.1128/JVI.78.14.7553-7564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marquet R, Isel C, Ehresmann C, Ehresmann B. tRNAs as primer of reverse transcriptases. Biochimie. 1995;77:113–124. doi: 10.1016/0300-9084(96)88114-4. [DOI] [PubMed] [Google Scholar]
- 73.Harada F, Sawyer RC, Dahlberg JE. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975;250:3487–3497. [PubMed] [Google Scholar]
- 74.Harada F, Peters GG, Dahlberg JE. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979;254:10979–10985. [PubMed] [Google Scholar]
- 75.Zaitseva L, Myers R, Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4:332. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan R, Giedroc DP. Recombinant human immunodeficiency virus type 1 nucleocapsid (NCp7) protein unwinds tRNA. J Biol Chem. 1992;267:6689–6695. [PubMed] [Google Scholar]
- 77.Khan R, Chang HO, Kaluarachchi K, Giedroc DP. Interaction of retroviral nucleocapsid proteins with transfer RNAPhe: a lead ribozyme and 1H NMR study. Nucleic Acids Res. 1996;24:3568–3575. doi: 10.1093/nar/24.18.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan B, Weidemaier K, Yip W-T, Barbara PF, Musier-Forsyth K. Intra-tRNA distance measurements for nucleocapsid protein-dependent tRNA unwinding during priming of HIV reverse transcription. Proc Natl Acad Sci USA. 1999;96:459–464. doi: 10.1073/pnas.96.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hargittai MRS, Mangla AT, Gorelick RJ, Musier-Forsyth K. HIV-1 nucleocapsid protein zinc finger structures induce tRNALys,3 structural changes but are not critical for primer/template annealing. J Mol Biol. 2001;312:985–997. doi: 10.1006/jmbi.2001.5021. [DOI] [PubMed] [Google Scholar]
- 80.Tisné C, Roques BP, Dardel F. Heteronuclear NMR studies of the interaction of tRNALys3 with HIV-1 nucleocapsid protein. J Mol Biol. 2001;306:443–454. doi: 10.1006/jmbi.2000.4391. [DOI] [PubMed] [Google Scholar]
- 81.Rodríguez-Rodríguez L, Tsuchihashi Z, Fuentes GM, Bambara RA, Fay PJ. Influence of human immunodeficiency virus nucleocapsid protein on synthesis and strand transfer by the reverse transcriptase in vitro. J Biol Chem. 1995;270:15005–15011. doi: 10.1074/jbc.270.25.15005. [DOI] [PubMed] [Google Scholar]
- 82.Chertova E, Crise BJ, Morcock DR, Bess JW, Jr, Henderson LE, Lifson JD. Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins. Curr Mol Med. 2003;3:265–272. doi: 10.2174/1566524033479889. [DOI] [PubMed] [Google Scholar]
- 83.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darlix JL, Garrido JL, Morellet N, Mély Y, de Rocquigny H. Properties, functions, and drug targeting of the multifunctional nucleocapsid protein of the human immunodeficiency virus. Adv Pharmacol. 2007;55:299–346. doi: 10.1016/S1054-3589(07)55009-X. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y, Wang J, Shalom A, Li Z, Khorchid A, Wainberg MA, et al. Primer tRNALys3 on the viral genome exists in unextended and two-base extended forms within mature human immunodeficiency virus type 1. J Virol. 1997;71:726–728. doi: 10.1128/jvi.71.1.726-728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campbell S, Rein A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J Virol. 1999;73:2270–2279. doi: 10.1128/jvi.73.3.2270-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo F, Saadatmand J, Niu M, Kleiman L. Roles of Gag and NCp7 in facilitating tRNALys3 annealing to viral RNA in human immunodeficiency virus type 1. J Virol. 2009;83:8099–8107. doi: 10.1128/JVI.00488-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saadatmand J, Niu M, Kleiman L, Guo F. The contribution of the primer activation signal to differences between Gag- and NCp7-facilitated tRNALys3 annealing in HIV-1. Virology. 2009;391:334–341. doi: 10.1016/j.virol.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 89.Roldan A, Warren OU, Russell RS, Liang C, Wainberg MA. A HIV-1 minimal Gag protein is superior to nucleocapsid at in vitro tRNALys3 annealing and exhibits multimerization-induced inhibition of reverse transcription. J Biol Chem. 2005;280:17488–17496. doi: 10.1074/jbc.M501310200. [DOI] [PubMed] [Google Scholar]
- 90.Wu T, Datta SAK, Mitra M, Gorelick RJ, Rein A, Levin JG. Fundamental differences between the nucleic acid chaperone activities of HIV-1 nucleocapsid protein and Gag or Gag-derived proteins: Biological implications. Virology. 2010;405:556–567. doi: 10.1016/j.virol.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Quan Y, Arts EJ, Li Z, Preston BD, de Rocquigny H, et al. Human immunodeficiency virus type 1 nucleocapsid protein (NCp7) directs specific initiation of minus-strand DNA synthesis primed by human tRNALys3 in vitro: studies of viral RNA molecules mutated in regions that flank the primer binding site. J Virol. 1996;70:4996–5004. doi: 10.1128/jvi.70.8.4996-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iwatani Y, Rosen AE, Guo J, Musier-Forsyth K, Levin JG. Efficient initiation of HIV-1 reverse transcription in vitro. Requirement for RNA sequences downstream of the primer binding site abrogated by nucleocapsid protein-dependent primer-template interactions. J Biol Chem. 2003;278:14185–14195. doi: 10.1074/jbc.M211618200. [DOI] [PubMed] [Google Scholar]
- 93.Isel C, Westhof E, Massire C, Le Grice SFJ, Ehresmann B, Ehresmann C, et al. Structural basis for the specificity of the initiation of HIV-1 reverse transcription. EMBO J. 1999;18:1038–1048. doi: 10.1093/emboj/18.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hargittai MRS, Gorelick RJ, Rouzina I, Musier-Forsyth K. Mechanistic insights into the kinetics of HIV-1 nucleocapsid protein-facilitated tRNA annealing to the primer binding site. J Mol Biol. 2004;337:951–968. doi: 10.1016/j.jmb.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 95.Tisné C. Structural bases of the annealing of primer tRNALys3 to the HIV-1 viral RNA. Curr HIV Res. 2005;3:147–156. doi: 10.2174/1570162053506919. [DOI] [PubMed] [Google Scholar]
- 96.Tisné C, Roques BP, Dardel F. The annealing mechanism of HIV-1 reverse transcription primer onto the viral genome. J Biol Chem. 2004;279:3588–3595. doi: 10.1074/jbc.M310368200. [DOI] [PubMed] [Google Scholar]
- 97.Tisné C, Rigourd M, Marquet R, Ehresmann C, Dardel F. NMR and biochemical characterization of recombinant human tRNALys3 expressed in Escherichia coli: identification of posttranscriptional nucleotide modifications required for efficient initiation of HIV-1 reverse transcription. RNA. 2000;6:1403–1412. doi: 10.1017/s1355838200000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barraud P, Gaudin C, Dardel F, Tisne C. New insights into the formation of HIV-1 reverse transcription initiation complex. Biochimie. 2007;89:1204–1210. doi: 10.1016/j.biochi.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Guo J, Wu T, Anderson J, Kane BF, Johnson DG, Gorelick RJ, et al. Zinc finger structures in the human immunodeficiency virus type 1 nucleocapsid protein facilitate efficient minus- and plus-strand transfer. J Virol. 2000;74:8980–8988. doi: 10.1128/jvi.74.19.8980-8988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bolinger C, Boris-Lawrie K. Mechanisms employed by retroviruses to exploit host factors for translational control of a complicated proteome. Retrovirology. 2009;6:8. doi: 10.1186/1742-4690-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Isel C, Marquet R, Keith G, Ehresmann C, Ehresmann B. Modified nucleotides of tRNALys3 modulate primer/template loop-loop interaction in the initiation complex of HIV-1 reverse transcription. J Biol Chem. 1993;268:25269–25272. [PubMed] [Google Scholar]
- 102.Arts EJ, Stetor SR, Li X, Rausch JW, Howard KJ, Ehresmann B, et al. Initiation of (−) strand DNA synthesis from tRNALys3 on lentiviral RNAs: implications of specific HIV-1 RNA-tRNALys3 interactions inhibiting primer utilization by retroviral reverse transcriptases. Proc Natl Acad Sci USA. 1996;93:10063–10068. doi: 10.1073/pnas.93.19.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Isel C, Lanchy JM, Le Grice SFJ, Ehresmann C, Ehresmann B, Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNALys3. EMBO J. 1996;15:917–924. [PMC free article] [PubMed] [Google Scholar]
- 104.Liang C, Li X, Rong L, Inouye P, Quan Y, Kleiman L, et al. The importance of the A-rich loop in human immunodeficiency virus type 1 reverse transcription and infectivity. J Virol. 1997;71:5750–5757. doi: 10.1128/jvi.71.8.5750-5757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang C, Rong L, Gotte M, Li X, Quan Y, Kleiman L, et al. Mechanistic studies of early pausing events during initiation of HIV-1 reverse transcription. J Biol Chem. 1998;273:21309–1315. doi: 10.1074/jbc.273.33.21309. [DOI] [PubMed] [Google Scholar]
- 106.Beerens N, Groot F, Berkhout B. Initiation of HIV-1 reverse transcription is regulated by a primer activation signal. J Biol Chem. 2001;276:31247–31256. doi: 10.1074/jbc.M102441200. [DOI] [PubMed] [Google Scholar]
- 107.Aiyar A, Cobrinik D, Ge Z, Kung HJ, Leis J. Interaction between retroviral U5 RNA and the TΨC loop of the tRNATrp primer is required for efficient initiation of reverse transcription. J Virol. 1992;66:2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abbink TE, Berkhout B. HIV-1 reverse transcription initiation: a potential target for novel antivirals? Virus Res. 2008;134:4–18. doi: 10.1016/j.virusres.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Beerens N, Berkhout B. Switching the in vitro tRNA usage of HIV-1 by simultaneous adaptation of the PBS and PAS. RNA. 2002;8:357–369. doi: 10.1017/s1355838202028194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ooms M, Cupac D, Abbink TE, Huthoff H, Berkhout B. The availability of the primer activation signal (PAS) affects the efficiency of HIV-1 reverse transcription initiation. Nucleic Acids Res. 2007;35:1649–1659. doi: 10.1093/nar/gkm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roy BB, Hu J, Guo X, Russell RS, Guo F, Kleiman L, et al. Association of RNA helicase A with human immunodeficiency virus type 1 particles. J Biol Chem. 2006;281:12625–12635. doi: 10.1074/jbc.M510596200. [DOI] [PubMed] [Google Scholar]
- 112.Henriet S, Sinck L, Bec G, Gorelick RJ, Marquet R, Paillart J-C. Vif is a RNA chaperone that could temporally regulate RNA dimerization and the early steps of HIV-1 reverse transcription. Nucleic Acids Res. 2007;35:5141–5153. doi: 10.1093/nar/gkm542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Panganiban AT, Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988;241:1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- 114.Hu W-S, Temin HM. Retroviral recombination and reverse transcription. Science. 1990;250:1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 115.Jones JS, Allan RW, Temin HM. One retroviral RNA is sufficient for synthesis of viral DNA. J Virol. 1994;68:207–216. doi: 10.1128/jvi.68.1.207-216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Wamel JLB, Berkhout B. The first strand transfer during HIV-1 reverse transcription can occur either intramolecularly or intermolecularly. Virology. 1998;244:245–251. doi: 10.1006/viro.1998.9096. [DOI] [PubMed] [Google Scholar]
- 117.Hu W-S, Temin HM. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci USA. 1990;87:1556–15560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Negroni M, Buc H. Mechanisms of retroviral recombination. Annu Rev Genet. 2001;35:275–302. doi: 10.1146/annurev.genet.35.102401.090551. [DOI] [PubMed] [Google Scholar]
- 119.Andersen ES, Jeeninga RE, Damgaard CK, Berkhout B, Kjems J. Dimerization and template switching in the 5′ untranslated region between various subtypes of human immunodeficiency virus type 1. J Virol. 2003;77:3020–3030. doi: 10.1128/JVI.77.5.3020-3030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galetto R, Negroni M. Mechanistic features of recombination in HIV. AIDS Rev. 2005;7:92–102. [PubMed] [Google Scholar]
- 121.Onafuwa-Nuga A, Telesnitsky A. The remarkable frequency of human immunodeficiency virus type 1 genetic recombination. Microbiol Mol Biol Rev. 2009;73:451–480. doi: 10.1128/MMBR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Varmus HE, Heasley S, Kung HJ, Oppermann H, Smith VC, Bishop JM, et al. Kinetics of synthesis, structure, and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978;120:55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- 123.Thomas DC, Voronin YA, Nikolenko GN, Chen J, Hu W-S, Pathak VK. Determination of the ex vivo rates of human immunodeficiency virus type 1 reverse transcription by using novel strand-specific amplification analysis. J Virol. 2007;81:4798–4807. doi: 10.1128/JVI.02471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.You °C, McHenry CS. Human immunodeficiency virus nucleocapsid protein accelerates strand transfer of the terminally redundant sequences involved in reverse transcription. J Biol Chem. 1994;269:31491–1495. [PubMed] [Google Scholar]
- 125.Lapadat-Tapolsky M, Pernelle C, Borie C, Darlix J-L. Analysis of the nucleic acid annealing activities of nucleocapsid protein from HIV-1. Nucleic Acids Res. 1995;23:2434–2441. doi: 10.1093/nar/23.13.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo J, Wu T, Bess J, Henderson LE, Levin JG. Actinomycin D inhibits human immunodeficiency virus type 1 minus-strand transfer in in vitro and endogenous reverse transcriptase assays. J Virol. 1998;72:6716–6724. doi: 10.1128/jvi.72.8.6716-6724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vo M-N, Barany G, Rouzina I, Musier-Forsyth K. Mechanistic studies of mini-TAR RNA/DNA annealing in the absence and presence of HIV-1 nucleocapsid protein. J Mol Biol. 2006;363:244–261. doi: 10.1016/j.jmb.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 128.Vo M-N, Barany G, Rouzina I, Musier-Forsyth K. HIV-1 nucleocapsid protein switches the pathway of transactivation response element RNA/DNA annealing from loop-loop “kissing” to “zipper”. J Mol Biol. 2009;386:789–801. doi: 10.1016/j.jmb.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Godet J, de Rocquigny H, Raja C, Glasser N, Ficheux D, Darlix J-L, et al. During the early phase of HIV-1 DNA synthesis, nucleocapsid protein directs hybridization of the TAR complementary sequences via the ends of their double-stranded stem. J Mol Biol. 2006;356:1180–1192. doi: 10.1016/j.jmb.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 130.Stoylov SP, Vuilleumier C, Stoylova E, de Rocquigny H, Roques BP, Gérard D, et al. Ordered aggregation of ribonucleic acids by the human immunodeficiency virus type 1 nucleocapsid protein. Biopolymers. 1997;41:301–312. doi: 10.1002/(SICI)1097-0282(199703)41:3<301::AID-BIP5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 131.Le Cam E, Coulaud D, Delain E, Petitjean P, Roques BP, Gérard D, et al. Properties and growth mechanism of the ordered aggregation of a model RNA by the HIV-1 nucleocapsid protein: an electron microscopy investigation. Biopolymers. 1998;45:217–229. doi: 10.1002/(SICI)1097-0282(199803)45:3<217::AID-BIP4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 132.Wu T, Heilman-Miller SL, Levin JG. Effects of nucleic acid local structure and magnesium ions on minus-strand transfer mediated by the nucleic acid chaperone activity of HIV-1 nucleocapsid protein. Nucleic Acids Res. 2007;35:3974–3987. doi: 10.1093/nar/gkm375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vo M-N, Barany G, Rouzina I, Musier-Forsyth K. Effect of Mg2+ and Na+ on the nucleic acid chaperone activity of HIV-1 nucleocapsid protein: implications for reverse transcription. J Mol Biol. 2009;386:773–778. doi: 10.1016/j.jmb.2008.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berkhout B, Vastenhouw NL, Klasens BIF, Huthoff H. Structural features in the HIV-1 repeat region facilitate strand transfer during reverse transcription. RNA. 2001;7:1097–1114. doi: 10.1017/s1355838201002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanevsky I, Chaminade F, Ficheux D, Moumen A, Gorelick R, Negroni M, et al. Specific interactions between HIV-1 nucleocapsid protein and the TAR element. J Mol Biol. 2005;348:1059–1077. doi: 10.1016/j.jmb.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 136.Liu HW, Cosa G, Landes CF, Zeng Y, Kovaleski BJ, Mullen DG, et al. Single-molecule FRET studies of important intermediates in the nucleocapsid-protein-chaperoned minus-strand transfer step in HIV-1 reverse transcription. Biophys J. 2005;89:3470–3479. doi: 10.1529/biophysj.105.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bernacchi S, Stoylov S, Piémont E, Ficheux D, Roques BP, Darlix JL, et al. HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence. J Mol Biol. 2002;317:385–399. doi: 10.1006/jmbi.2002.5429. [DOI] [PubMed] [Google Scholar]
- 138.Azoulay J, Clamme J-P, Darlix J-L, Roques BP, Mély Y. Destabilization of the HIV-1 complementary sequence of TAR by the nucleocapsid protein through activation of conformational fluctuations. J Mol Biol. 2003;326:691–700. doi: 10.1016/s0022-2836(02)01430-4. [DOI] [PubMed] [Google Scholar]
- 139.Beltz H, Azoulay J, Bernacchi S, Clamme J-P, Ficheux D, Roques B, et al. Impact of the terminal bulges of HIV-1 cTAR DNA on its stability and the destabilizing activity of the nucleocapsid protein NCp7. J Mol Biol. 2003;328:95–108. doi: 10.1016/s0022-2836(03)00244-4. [DOI] [PubMed] [Google Scholar]
- 140.Hong MK, Harbron EJ, O'Connor DB, Guo J, Barbara PF, Levin JG, et al. Nucleic acid conformational changes essential for HIV-1 nucleocapsid protein-mediated inhibition of self-priming in minus-strand transfer. J Mol Biol. 2003;325:1–10. doi: 10.1016/s0022-2836(02)01177-4. [DOI] [PubMed] [Google Scholar]
- 141.Cosa G, Harbron EJ, Zeng Y, Liu HW, O'Connor DB, Eta-Hosokawa C, et al. Secondary structure and secondary structure dynamics of DNA hairpins complexed with HIV-1 NC protein. Biophys J. 2004;87:2759–2767. doi: 10.1529/biophysj.104.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo J, Henderson LE, Bess J, Kane B, Levin JG. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J Virol. 1997;71:5178–5188. doi: 10.1128/jvi.71.7.5178-5188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo J, Wu T, Kane BF, Johnson DG, Henderson LE, Gorelick RJ, et al. Subtle alterations of the native zinc finger structures have dramatic effects on the nucleic acid chaperone activity of human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 2002;76:4370–4378. doi: 10.1128/JVI.76.9.4370-4378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Beltz H, Clauss C, Piémont E, Ficheux D, Gorelick RJ, Roques B, et al. Structural determinants of HIV-1 nucleocapsid protein for cTAR DNA binding and destabilization and correlation with inhibition of self-primed DNA synthesis. J Mol Biol. 2005;348:1113–1126. doi: 10.1016/j.jmb.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 145.Golinelli MP, Hughes SH. Self-priming of retroviral minus-strand strong-stop DNAs. Virology. 2001;285:278–290. doi: 10.1006/viro.2001.0970. [DOI] [PubMed] [Google Scholar]
- 146.Heilman-Miller SL, Wu T, Levin JG. Alteration of nucleic acid structure and stability modulates the efficiency of minus-strand transfer mediated by the HIV-1 nucleocapsid protein. J Biol Chem. 2004;279:44154–44165. doi: 10.1074/jbc.M401646200. [DOI] [PubMed] [Google Scholar]
- 147.Gorelick RJ, Gagliardi TD, Bosche WJ, Wiltrout TA, Coren LV, Chabot DJ, et al. Strict conservation of the retroviral nucleocapsid protein zinc finger is strongly influenced by its role in viral infection processes: characterization of HIV-1 particles containing mutant nucleocapsid zinc-coordinating sequences. Virology. 1999;256:92–104. doi: 10.1006/viro.1999.9629. [DOI] [PubMed] [Google Scholar]
- 148.Williams MC, Rouzina I, Wenner JR, Gorelick RJ, Musier-Forsyth K, Bloomfield VA. Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proc Natl Acad Sci USA. 2001;98:6121–6126. doi: 10.1073/pnas.101033198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Williams MC, Gorelick RJ, Musier-Forsyth K. Specific zinc-finger architecture required for HIV-1 nucleocapsid protein's nucleic acid chaperone function. Proc Natl Acad Sci USA. 2002;99:8614–8619. doi: 10.1073/pnas.132128999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Derebail SS, Heath MJ, DeStefano JJ. Evidence for the differential effects of nucleocapsid protein on strand transfer in various regions of the HIV genome. J Biol Chem. 2003;278:15702–15712. doi: 10.1074/jbc.M211701200. [DOI] [PubMed] [Google Scholar]
- 151.Heath MJ, Derebail SS, Gorelick RJ, DeStefano JJ. Differing roles of the N- and C-terminal zinc fingers in human immunodeficiency virus nucleocapsid protein-enhanced nucleic acid annealing. J Biol Chem. 2003;278:30755–30763. doi: 10.1074/jbc.M303819200. [DOI] [PubMed] [Google Scholar]
- 152.Lee N, Gorelick RJ, Musier-Forsyth K. Zinc finger-dependent HIV-1 nucleocapsid protein-TAR RNA interactions. Nucleic Acids Res. 2003;31:4847–4855. doi: 10.1093/nar/gkg679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Narayanan N, Gorelick RJ, DeStefano JJ. Structure/Function mapping of amino acids in the N-terminal zinc finger of the human immunodeficiency virus type 1 nucleocapsid protein: residues responsible for nucleic acid helix destabilizing activity. Biochemistry. 2006;45:12617–12628. doi: 10.1021/bi060925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cruceanu M, Gorelick RJ, Musier-Forsyth K, Rouzina I, Williams MC. Rapid kinetics of protein-nucleic acid interaction is a major component of HIV-1 nucleocapsid protein's nucleic acid chaperone function. J Mol Biol. 2006;363:867–877. doi: 10.1016/j.jmb.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 155.Cruceanu M, Urbaneja MA, Hixson CV, Johnson DG, Datta SA, Fivash MJ, et al. Nucleic acid binding and chaperone properties of HIV-1 Gag and nucleocapsid proteins. Nucleic Acids Res. 2006;34:593–605. doi: 10.1093/nar/gkj458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stewart-Maynard KM, Cruceanu M, Wang F, Vo M-N, Gorelick RJ, Williams MC, et al. Retroviral nucleocapsid proteins display nonequivalent levels of nucleic acid chaperone activity. J Virol. 2008;82:10129–10142. doi: 10.1128/JVI.01169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Darugar Q, Kim H, Gorelick RJ, Landes C. Human T-cell lymphotropic virus type 1 nucleocapsid protein-induced structural changes in transactivation response DNA hairpin measured by single-molecule fluorescence resonance energy transfer. J Virol. 2008;82:12164–12171. doi: 10.1128/JVI.01158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qualley DF, Stewart-Maynard KM, Wang F, Mitra M, Gorelick RJ, Rouzina I, et al. C-terminal domain modulates the nucleic acid chaperone activity of human T-cell leukemia virus type 1 nucleocapsid protein via an electrostatic mechanism. J Biol Chem. 2010;285:295–307. doi: 10.1074/jbc.M109.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Driscoll MD, Hughes SH. Human immunodeficiency virus type 1 nucleocapsid protein can prevent self-priming of minus-strand strong stop DNA by promoting the annealing of short oligonucleotides to hairpin sequences. J Virol. 2000;74:8785–8792. doi: 10.1128/jvi.74.19.8785-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beltz H, Piémont E, Schaub E, Ficheux D, Roques B, Darlix J-L, et al. Role of the structure of the top half of HIV-1 cTAR DNA on the nucleic acid destabilizing activity of the nucleocapsid protein NCp7. J Mol Biol. 2004;338:711–723. doi: 10.1016/j.jmb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 161.Lanciault C, Champoux JJ. Effects of unpaired nucleotides within HIV-1 genomic secondary structures on pausing and strand transfer. J Biol Chem. 2005;280:2413–2423. doi: 10.1074/jbc.M410718200. [DOI] [PubMed] [Google Scholar]
- 162.Liu HW, Zeng Y, Landes CF, Kim YJ, Zhu Y, Ma X, et al. Insights on the role of nucleic acid/protein interactions in chaperoned nucleic acid rearrangements of HIV-1 reverse transcription. Proc Natl Acad Sci USA. 2007;104:5261–5267. doi: 10.1073/pnas.0700166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berkhout B, van Wamel J, Klaver B. Requirements for DNA strand transfer during reverse transcription in mutant HIV-1 virions. J Mol Biol. 1995;252:59–69. doi: 10.1006/jmbi.1994.0475. [DOI] [PubMed] [Google Scholar]
- 164.Hanson MN, Balakrishnan M, Roques BP, Bambara RA. Effects of donor and acceptor RNA structures on the mechanism of strand transfer by HIV-1 reverse transcriptase. J Mol Biol. 2005;353:772–787. doi: 10.1016/j.jmb.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 165.Heath MJ, Destefano JJ. A complementary single-stranded docking site is required for enhancement of strand exchange by human immunodeficiency virus nucleocapsid protein on substrates that model viral recombination. Biochemistry. 2005;44:3915–3925. doi: 10.1021/bi0477945. [DOI] [PubMed] [Google Scholar]
- 166.Song M, Balakrishnan M, Chen Y, Roques BP, Bambara RA. Stimulation of HIV-1 minus strand strong stop DNA transfer by genomic sequences 3′ of the primer binding site. J Biol Chem. 2006;281:24227–24235. doi: 10.1074/jbc.M603097200. [DOI] [PubMed] [Google Scholar]
- 167.Derebail SS, DeStefano JJ. Mechanistic analysis of pause site-dependent and -independent recombinogenic strand transfer from structurally diverse regions of the HIV genome. J Biol Chem. 2004;279:47446–47454. doi: 10.1074/jbc.M408927200. [DOI] [PubMed] [Google Scholar]
- 168.Galetto R, Giacomoni V, Véron M, Negroni M. Dissection of a circumscribed recombination hot spot in HIV-1 after a single infectious cycle. J Biol Chem. 2006;281:2711–2720. doi: 10.1074/jbc.M505457200. [DOI] [PubMed] [Google Scholar]
- 169.Galetto R, Moumen A, Giacomoni V, Véron M, Charneau P, Negroni M. The structure of HIV-1 genomic RNA in the gp120 gene determines a recombination hot spot in vivo. J Biol Chem. 2004;279:36625–36632. doi: 10.1074/jbc.M405476200. [DOI] [PubMed] [Google Scholar]
- 170.Schultz SJ, Champoux JJ. RNase H activity: structure, specificity, and function in reverse transcription. Virus Res. 2008;134:86–103. doi: 10.1016/j.virusres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.DeStefano JJ, Buiser RG, Mallaber LM, Myers TW, Bambara RA, Fay PJ. Polymerization and RNase H activities of the reverse transcriptases from avian myeloblastosis, human immunodeficiency, and Moloney murine leukemia viruses are functionally uncoupled. J Biol Chem. 1991;266:7423–7431. [PubMed] [Google Scholar]
- 172.Schultz SJ, Zhang M, Champoux JJ. Preferred sequences within a defined cleavage window specify DNA 3′ end-directed cleavages by retroviral RNases H. J Biol Chem. 2009;284:32225–32238. doi: 10.1074/jbc.M109.043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Beilhartz GL, Wendeler M, Baichoo N, Rausch J, Le Grice S, Götte M. HIV-1 reverse transcriptase can simultaneously engage its DNA/RNA substrate at both DNA polymerase and RNase H active sites: implications for RNase H inhibition. J Mol Biol. 2009;388:462–474. doi: 10.1016/j.jmb.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Palaniappan C, Fuentes GM, Rodríguez-Rodríguez L, Fay PJ, Bambara RA. Helix structure and ends of RNA/DNA hybrids direct the cleavage specificity of HIV-1 reverse transcriptase RNase H. J Biol Chem. 1996;271:2063–2070. [PubMed] [Google Scholar]
- 175.Palaniappan C, Kim JK, Wisniewski M, Fay PJ, Bambara RA. Control of initiation of viral plus strand DNA synthesis by HIV reverse transcriptase. J Biol Chem. 1998;273:3808–3816. doi: 10.1074/jbc.273.7.3808. [DOI] [PubMed] [Google Scholar]
- 176.DeStefano JJ, Cristofaro JV, Derebail S, Bohlayer WP, Fitzgerald-Heath MJ. Physical mapping of HIV reverse transcriptase to the 5′ end of RNA primers. J Biol Chem. 2001;276:32515–32521. doi: 10.1074/jbc.M103958200. [DOI] [PubMed] [Google Scholar]
- 177.Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Le Grice SFJ, Zhuang X. Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature. 2008;453:184–189. doi: 10.1038/nature06941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu S, Abbondanzieri EA, Rausch JW, Le Grice SFJ, Zhuang X. Slide into action: dynamic shuttling of HIV reverse transcriptase on nucleic acid substrates. Science. 2008;322:1092–1097. doi: 10.1126/science.1163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jacob DT, Destefano JJ. A new role for HIV nucleocapsid protein in modulating the specificity of plus strand priming. Virology. 2008;378:385–396. doi: 10.1016/j.virol.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Post K, Kankia B, Gopalakrishnan S, Yang V, Cramer E, Saladores P, et al. Fidelity of plus-strand priming requires the nucleic acid chaperone activity of HIV-1 nucleocapsid protein. Nucleic Acids Res. 2009;37:1755–1766. doi: 10.1093/nar/gkn1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peliska JA, Balasubramanian S, Giedroc DP, Benkovic SJ. Recombinant HIV-1 nucleocapsid protein accelerates HIV-1 reverse transcriptase catalyzed DNA strand transfer reactions and modulates RNase H activity. Biochemistry. 1994;33:13817–13823. doi: 10.1021/bi00250a036. [DOI] [PubMed] [Google Scholar]
- 182.Cameron CE, Ghosh M, Le Grice SFJ, Benkovic SJ. Mutations in HIV reverse transcriptase which alter RNase H activity and decrease strand transfer efficiency are suppressed by HIV nucleocapsid protein. Proc Natl Acad Sci USA. 1997;94:6700–6705. doi: 10.1073/pnas.94.13.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lener D, Tanchou V, Roques BP, Le Grice SFJ, Darlix J-L. Involvement of HIV-I nucleocapsid protein in the recruitment of reverse transcriptase into nucleoprotein complexes formed in vitro. J Biol Chem. 1998;273:33781–33786. doi: 10.1074/jbc.273.50.33781. [DOI] [PubMed] [Google Scholar]
- 184.Druillennec S, Caneparo A, de Rocquigny H, Roques BP. Evidence of interactions between the nucleocapsid protein NCp7 and the reverse transcriptase of HIV-1. J Biol Chem. 1999;274:11283–11288. doi: 10.1074/jbc.274.16.11283. [DOI] [PubMed] [Google Scholar]
- 185.Telesnitsky A, Goff SP. Strong-stop strand transfer during reverse transcription. In: Skalka AM, Goff SP, editors. Reverse Transcriptase. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 49–83. [Google Scholar]
- 186.Negroni M, Buc H. Copy-choice recombination by reverse transcriptases: Reshuffling of genetic markers mediated by RNA chaperones. Proc Natl Acad Sci USA. 2000;97:6385–6390. doi: 10.1073/pnas.120520497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen Y, Balakrishnan M, Roques BP, Fay PJ, Bambara RA. Mechanism of minus strand strong stop transfer in HIV-1 reverse transcription. J Biol Chem. 2003;278:8006–8017. doi: 10.1074/jbc.M210959200. [DOI] [PubMed] [Google Scholar]
- 188.Balakrishnan M, Roques BP, Fay PJ, Bambara RA. Template dimerization promotes an acceptor invasion-induced transfer mechanism during human immunodeficiency virus type 1 minus-strand synthesis. J Virol. 2003;77:4710–4721. doi: 10.1128/JVI.77.8.4710-4721.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Song M, Basu VP, Hanson MN, Roques BP, Bambara RA. Proximity and branch migration mechanisms in HIV-1 minus strand strong stop DNA transfer. J Biol Chem. 2008;283:3141–3150. doi: 10.1074/jbc.M707343200. [DOI] [PubMed] [Google Scholar]
- 190.Brulé F, Bec G, Keith G, Le Grice SFJ, Roques BP, Ehresmann B, et al. In vitro evidence for the interaction of tRNALys3 with U3 during the first strand transfer of HIV-1 reverse transcription. Nucleic Acids Res. 2000;28:634–640. doi: 10.1093/nar/28.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Song M, Balakrishnan M, Gorelick RJ, Bambara RA. A succession of mechanisms stimulate efficient reconstituted HIV-1 minus strand strong stop DNA transfer. Biochemistry. 2009;48:1810–1819. doi: 10.1021/bi802149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Piekna-Przybylska D, DiChiacchio L, Mathews DH, Bambara RA. A sequence similar to tRNALys3 gene is embedded in HIV-1 U3-R and promotes minus-strand transfer. Nature structural & molecular biology. 2010;17:83–89. doi: 10.1038/nsmb.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beerens N, Kjems J. Circularization of the HIV-1 genome facilitates strand transfer during reverse transcription. RNA. 2010;16:1226–1235. doi: 10.1261/rna.2039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ji X, Klarmann GJ, Preston BD. Effect of human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein on HIV-1 reverse transcriptase activity in vitro. Biochemistry. 1996;35:132–143. doi: 10.1021/bi951707e. [DOI] [PubMed] [Google Scholar]
- 195.Wu W, Henderson LE, Copeland TD, Gorelick RJ, Bosche WJ, Rein A, et al. Human immunodeficiency virus type 1 nucleocapsid protein reduces reverse transcriptase pausing at a secondary structure near the murine leukemia virus polypurine tract. J Virol. 1996;70:7132–142. doi: 10.1128/jvi.70.10.7132-7142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Drummond JE, Mounts P, Gorelick RJ, Casas-Finet JR, Bosche WJ, Henderson LE, et al. Wild-type and mutant HIV type 1 nucleocapsid proteins increase the proportion of long cDNA transcripts by viral reverse transcriptase. AIDS Res Hum Retrovir. 1997;13:533–543. doi: 10.1089/aid.1997.13.533. [DOI] [PubMed] [Google Scholar]
- 197.Klasens BI, Huthoff HT, Das AT, Jeeninga RE, Berkhout B. The effect of template RNA structure on elongation by HIV-1 reverse transcriptase. Biochim Biophys Acta. 1999;1444:355–370. doi: 10.1016/s0167-4781(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 198.Zhang WH, Hwang CK, Hu W-S, Gorelick RJ, Pathak VK. Zinc finger domain of murine leukemia virus nucleocapsid protein enhances the rate of viral DNA synthesis in vivo. J Virol. 2002;76:7473–7484. doi: 10.1128/JVI.76.15.7473-7484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Anthony RM, Destefano JJ. In vitro synthesis of long DNA products in reactions with HIV-RT and nucleocapsid protein. J Mol Biol. 2007;365:310–324. doi: 10.1016/j.jmb.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rothenberg E, Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977;21:168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rothenberg E, Smotkin D, Baltimore D, Weinberg RA. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977;269:122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- 202.Junghans RP, Duesberg PH, Knight CA. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci USA. 1975;72:4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tanchou V, Gabus C, Rogemond V, Darlix J-L. Formation of stable and functional HIV-1 nucleoprotein complexes in vitro. J Mol Biol. 1995;252:563–571. doi: 10.1006/jmbi.1995.0520. [DOI] [PubMed] [Google Scholar]
- 204.Mirambeau G, Lyonnais S, Coulaud D, Hameau L, Lafosse S, Jeusset J, et al. HIV-1 protease and reverse transcriptase control the architecture of their nucleocapsid partner. PLoS ONE. 2007;2:669. doi: 10.1371/journal.pone.0000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grohmann D, Godet J, Mély Y, Darlix J-L, Restle T. HIV-1 nucleocapsid traps reverse transcriptase on nucleic acid substrates. Biochemistry. 2008;47:12230–12240. doi: 10.1021/bi801386r. [DOI] [PubMed] [Google Scholar]
- 206.Rausch JW, Le Grice SFJ. ‘Binding, bending and bonding’: polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. Int J Biochem Cell Biol. 2004;36:1752–1766. doi: 10.1016/j.biocel.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 207.Fabris D, Marino JP, Le Grice SFJ. Revisiting plus-strand DNA synthesis in retroviruses and long terminal repeat retrotransposons: dynamics of enzyme:substrate interactions. Viruses. 2009;1:657–677. doi: 10.3390/v1030657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Powell MD, Levin JG. Sequence and structural determinants required for priming of plus-strand DNA synthesis by the human immunodeficiency virus type 1 polypurine tract. J Virol. 1996;70:5288–5296. doi: 10.1128/jvi.70.8.5288-5296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Miles LR, Agresta BE, Khan MB, Tang S, Levin JG, Powell MD. Effect of polypurine tract (PPT) mutations on human immunodeficiency virus type 1 replication: a virus with a completely randomized PPT retains low infectivity. J Virol. 2005;79:6859–6867. doi: 10.1128/JVI.79.11.6859-6867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sarafianos SG, Das K, Tantillo C, Clark AD, Jr, Ding J, Whitcomb JM, et al. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 2001;20:1449–1461. doi: 10.1093/emboj/20.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yi-Brunozzi HY, Brinson RG, Brabazon DM, Lener D, Le Grice SFJ, Marino JP. High-resolution NMR analysis of the conformations of native and base analog substituted retroviral and LTR-retrotransposon PPT primers. Chem Biol. 2008;15:254–262. doi: 10.1016/j.chembiol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kvaratskhelia M, Budihas SR, Le Grice SFJ. Preexisting distortions in nucleic acid structure aid polypurine tract selection by HIV-1 reverse transcriptase. J Biol Chem. 2002;277:16689–16696. doi: 10.1074/jbc.M109914200. [DOI] [PubMed] [Google Scholar]
- 213.Miller MD, Wang B, Bushman FD. Human immunodeficiency virus type 1 preintegration complexes containing discontinuous plus strands are competent to integrate in vitro. J Virol. 1995;69:3938–3944. doi: 10.1128/jvi.69.6.3938-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klarmann GJ, Yu H, Chen X, Dougherty JP, Preston BD. Discontinuous plus-strand DNA synthesis in human immunodeficiency virus type 1-infected cells and in a partially reconstituted cell-free system. J Virol. 1997;71:9259–9269. doi: 10.1128/jvi.71.12.9259-9269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Auxilien S, Keith G, Le Grice SFJ, Darlix J-L. Role of post-transcriptional modifications of primer tRNALys,3 in the fidelity and efficacy of plus strand DNA transfer during HIV-1 reverse transcription. J Biol Chem. 1999;274:4412–4420. doi: 10.1074/jbc.274.7.4412. [DOI] [PubMed] [Google Scholar]
- 216.Wu T, Guo J, Bess J, Henderson LE, Levin JG. Molecular requirements for human immunodeficiency virus type 1 plus-strand transfer: analysis in reconstituted and endogenous reverse transcription systems. J Virol. 1999;73:4794–4805. doi: 10.1128/jvi.73.6.4794-4805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Muthuswami R, Chen J, Burnett BP, Thimmig RL, Janjic N, McHenry CS. The HIV plus-strand transfer reaction: determination of replication-competent intermediates and identification of a novel lentiviral element, the primer over-extension sequence. J Mol Biol. 2002;315:311–323. doi: 10.1006/jmbi.2001.5205. [DOI] [PubMed] [Google Scholar]
- 218.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Smith CM, Smith JS, Roth MJ. RNase H requirements for the second strand transfer reaction of human immunodeficiency virus type 1 reverse transcription. J Virol. 1999;73:6573–6581. doi: 10.1128/jvi.73.8.6573-6581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson PE, Turner RB, Wu ZR, Hairston L, Guo J, Levin JG, et al. A mechanism for plus-strand transfer enhancement by the HIV-1 nucleocapsid protein during reverse transcription. Biochemistry. 2000;39:9084–9091. doi: 10.1021/bi000841i. [DOI] [PubMed] [Google Scholar]
- 221.Bourbigot S, Ramalanjaona N, Boudier C, Salgado GFJ, Roques BP, Mély Y, et al. How the HIV-1 nucleocapsid protein binds and destabilises the (−)primer binding site during reverse transcription. J Mol Biol. 2008;383:1112–1128. doi: 10.1016/j.jmb.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 222.Egelé C, Schaub E, Ramalanjaona N, Piémont E, Ficheux D, Roques B, et al. HIV-1 nucleocapsid protein binds to the viral DNA initiation sequences and chaperones their kissing interactions. J Mol Biol. 2004;342:453–466. doi: 10.1016/j.jmb.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 223.Ramalanjaona N, de Rocquigny H, Millet A, Ficheux D, Darlix J-L, Mély Y. Investigating the mechanism of the nucleocapsid protein chaperoning of the second strand transfer during HIV-1 DNA synthesis. J Mol Biol. 2007;374:1041–1053. doi: 10.1016/j.jmb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 224.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 225.Rumbaugh JA, Fuentes GM, Bambara RA. Processing of an HIV replication intermediate by the human DNA replication enzyme FEN1. J Biol Chem. 1998;273:28740–28745. doi: 10.1074/jbc.273.44.28740. [DOI] [PubMed] [Google Scholar]
- 226.Lavigne M, Roux P, Buc H, Schaeffer F. DNA curvature controls termination of plus strand DNA synthesis at the centre of HIV-1 genome. J Mol Biol. 1997;266:507–524. doi: 10.1006/jmbi.1996.0805. [DOI] [PubMed] [Google Scholar]
- 227.Lavigne M, Buc H. Compression of the DNA minor groove is responsible for termination of DNA synthesis by HIV-1 reverse transcriptase. J Mol Biol. 1999;285:977–995. doi: 10.1006/jmbi.1998.2367. [DOI] [PubMed] [Google Scholar]
- 228.Lyonnais S, Hounsou C, Teulade-Fichou MP, Jeusset J, Le Cam E, Mirambeau G. G-quartets assembly within a G-rich DNA flap. A possible event at the center of the HIV-1 genome. Nucleic Acids Res. 2002;30:5276–5283. doi: 10.1093/nar/gkf644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lyonnais S, Gorelick RJ, Mergny JL, Le Cam E, Mirambeau G. G-quartets direct assembly of HIV-1 nucleocapsid protein along single-stranded DNA. Nucleic Acids Res. 2003;31:5754–5763. doi: 10.1093/nar/gkg716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kankia BI, Musier-Forsyth K. The HIV-1 central DNA flap region contains a “flapping” third strand. Biophys Chem. 2007;127:64–68. doi: 10.1016/j.bpc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 231.Charneau P, Alizon M, Clavel F. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol. 1992;66:2814–2820. doi: 10.1128/jvi.66.5.2814-2820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hungnes O, Tjotta E, Grinde B. Mutations in the central polypurine tract of HIV-1 result in delayed replication. Virology. 1992;190:440–442. doi: 10.1016/0042-6822(92)91230-r. [DOI] [PubMed] [Google Scholar]
- 233.Wurtzer S, Goubard A, Mammano F, Saragosti S, Lecossier D, Hance AJ, et al. Functional central polypurine tract provides downstream protection of the human immunodeficiency virus type 1 genome from editing by APOBEC3G and APOBEC3B. J Virol. 2006;80:3679–3683. doi: 10.1128/JVI.80.7.3679-3683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Amacker M, Hottiger M, Mossi R, Hübscher U. HIV-1 nucleocapsid protein and replication protein A influence the strand displacement DNA synthesis of lentiviral reverse transcriptase. AIDS. 1997;11:534–536. [PubMed] [Google Scholar]
- 235.Fuentes GM, Rodríguez-Rodríguez L, Palaniappan C, Fay PJ, Bambara RA. Strand displacement synthesis of the long terminal repeats by HIV reverse transcriptase. J Biol Chem. 1996;271:1966–1971. doi: 10.1074/jbc.271.4.1966. [DOI] [PubMed] [Google Scholar]
- 236.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 237.Goila-Gaur R, Khan MA, Miyagi E, Kao S, Strebel K. Targeting APOBEC3A to the viral nucleoprotein complex confers antiviral activity. Retrovirology. 2007;4:61. doi: 10.1186/1742-4690-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Aguiar RS, Lovsin N, Tanuri A, Peterlin BM. Vpr.A3A chimera inhibits HIV replication. J Biol Chem. 2008;283:2518–2525. doi: 10.1074/jbc.M706436200. [DOI] [PubMed] [Google Scholar]
- 239.Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, et al. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J Virol. 2007;81:7238–7248. doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. doi: 10.1186/1742-4690-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Henriet S, Mercenne G, Bernacchi S, Paillart °C, Marquet R. Tumultuous relationship between the human immunodeficiency virus type 1 viral infectivity factor (Vif) and the human APOBEC-3G and APOBEC-3F restriction factors. Microbiol Mol Biol Rev. 2009;73:211–232. doi: 10.1128/MMBR.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Iwatani Y, Chan DS, Liu L, Yoshii H, Shibata J, Yamamoto N, et al. HIV-1 Vif-mediated ubiquitination/degradation of APOBEC3G involves four critical lysine residues in its C-terminal domain. Proc Natl Acad Sci USA. 2009;106:19539–19544. doi: 10.1073/pnas.0906652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, et al. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Aguiar RS, Peterlin BM. APOBEC3 proteins and reverse transcription. Virus Res. 2008;134:74–85. doi: 10.1016/j.virusres.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 245.Guo F, Cen S, Niu M, Yang Y, Gorelick RJ, Kleiman L. The interaction of APOBEC3G with human immunodeficiency virus type 1 nucleocapsid inhibits tRNALys3 annealing to viral RNA. J Virol. 2007;81:11322–11331. doi: 10.1128/JVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Guo F, Cen S, Niu M, Saadatmand J, Kleiman L. Inhibition of tRNALys3 -primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J Virol. 2006;80:11710–11722. doi: 10.1128/JVI.01038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li X-Y, Guo F, Zhang L, Kleiman L, Cen S. APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J Biol Chem. 2007;282:32065–32074. doi: 10.1074/jbc.M703423200. [DOI] [PubMed] [Google Scholar]
- 248.Iwatani Y, Takeuchi H, Strebel K, Levin JG. Biochemical activities of highly purified, catalytically active human APOBEC3G: correlation with antiviral effect. J Virol. 2006;80:5992–6002. doi: 10.1128/JVI.02680-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, et al. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007;35:7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Williams MC, Rouzina I, McCauley MJ. Peeling back the mystery of DNA overstretching. Proc Natl Acad Sci USA. 2009;106:18047–18048. doi: 10.1073/pnas.0910269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.de Rocquigny H, Shvadchak V, Avilov S, Dong CZ, Dietrich U, Darlix J-L, et al. Targeting the viral nucleocapsid protein in anti-HIV-1 therapy. Mini Rev Med Chem. 2008;8:24–35. doi: 10.2174/138955708783331603. [DOI] [PubMed] [Google Scholar]
- 253.Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Miller Jenkins LM, Hayashi R, et al. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J Virol. 2009;83:9175–9182. doi: 10.1128/JVI.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Berkhout B, Jeang K-T. Detailed mutational analysis of TAR RNA: critical spacing between the bulge and loop recognition domains. Nucleic Acids Res. 1991;19:6169–6176. doi: 10.1093/nar/19.22.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Baudin F, Marquet R, Isel C, Darlix J-L, Ehresmann B, Ehresmann C. Functional sites in the 5′ region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol. 1993;229:382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- 256.Jones CP, Datta SAK, Rein A, Rouzina I, Musier-Forsyth K. Matrix domain modulates HIV-1 Gag's nucleic acid chaperone activity via a conformational switch triggered by inositol phosphate binding. J Virol. 2011 doi: 10.1128/JVI.01809-10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]