Summary
Anti-CD3 monoclonal antibodies can modulate graft rejection and attenuate autoimmune diseases but their mechanism(s) of action remain unclear. CD8+ T cells with regulatory function are induced in vitro by Teplizumab, a humanized anti-CD3 antibody and inhibit responses of autologous and allogeneic T cells. They inhibit CD4+ T cell proliferation by mechanisms involving TNF and CCL4, and by blocking target cell entry into G2/M phase of cell cycle but neither kill them, nor compete for IL-2. CD8+ Tregs can be isolated from peripheral blood following treatment of patients with Type 1 diabetes with Teplizumab, but not from untreated patients. The induction of CD8+ Tregs by anti-CD3 mAb requires TNF and signaling through NF-κB cascade. The CD8+ Tregs express CD25, GITR, CTLA-4, FoxP3, and TNFR-2, and the combined expression of TNFR-2 and CD25 identifies a potent subpopulation of CD8+ Tregs. These studies have identified a novel mechanism of immune regulation by anti-CD3 mAb and markers that may be used to track inducible CD8+ Tregs in settings such as chronic inflammation or immune therapy.
Keywords: CD8+, Tregs, anti-CD3 mAb, TNF
Introduction
Adaptive or inducible (iTregs) have been described in humans and in mice in settings such as immunization, tumor progression, or following infection. These cells have been found in the spleen, thymus, and in the peripheral blood of healthy subjects, and they were inhibitory to autologous cells that were activated with a variety of stimuli [1–3]. Walker et al described expression of Foxp3, a transcription factor associated with regulatory T cells and acquisition of suppressive activity in human CD4+CD25+ T cells activated with anti-CD3 mAb OKT3 [4]. Pathways for differentiation of T cells with regulatory function have been described that are dependent on TGFβ, the expression of Foxp3, and suppression of RORγt[5]. Many studies have focused on subpopulations of CD4+CD25+ T cells but others have described inducible CD8+Tregs (iCD8+Tregs) in mice and humans in chronic viral infections or allograft tolerance that can inhibit responses by direct cytotoxicity to targets or by other mechanisms [6, 7] [8][9–13]
Immune therapies may induce and/or improve the function and survival of Tregs. Intravenous Ig has been shown to increase the suppressive function of CD4+CD25+ T cells in vitro [14]. Anti- TNF therapy was reported to increase the number of mucosal Foxp3+CD4+CD25+ Tregs in children with inflammatory bowel disease [15]. Anti-CD154 mAb was also shown to prevent diabetes in the NOD mouse model by inducing non-deletional tolerance and expanding regulatory T cells [16]. Rapamycin monotherapy of patients with Type 1 diabetes mellitus (T1DM) who received islet allotransplants resulted in improved potency of naturally occurring CD4+CD25+ Tregs although the number of the Tregs did not increase [17]. In a study of human islet allograft recipients with Type 1 diabetes mellitus (T1DM) treated with tacrolimus and sirolimus, Hering et al reported that humanized anti-CD3 mAb (Teplizumab, formerly hOKT3γ1(Ala-Ala)) increased the number and function of CD4+CD25+ T cells [18]. However, the cause for the appearance of such cells was not clear since the therapy involved a combination of drugs.
In previous studies, we reported that treatment of patients with new onset T1DM with Teplizumab attenuated the loss of insulin production during the first years of the disease [19, 20]. The humanized mAb is non-FcR binding but does cause activation of T cells in vitro and in vivo. Clinical responders to drug treatment were identified by a relative increase in the proportion of circulating CD8+ T cells. It has been shown that the mAb was more mitogenic for CD8+ T cells than for CD4+ cells [21, 22]. However, when CD8+ T cells were depleted from cultures, CD4+ T cells were shown to proliferate in response to anti-CD3 mAb, suggesting that the CD8+ T cells, particularly those that expressed CD25 following culture with anti-CD3 mAb were inhibitory to the CD4+ cells[22]. However, the mechanism whereby the mAb induced these regulatory cells, and functional evidence for the induction of these cells in patients was not available.
Herein, we show that treatment of patients with new onset T1DM may induce iCD8+ Tregs in vivo. In vitro induced CD8+ Tregs have phenotypic features characteristic of other Tregs including expression of Foxp3, CTLA-4, and GITR, but their mechanism of inhibition is independent of TGF-β or IL-10. Like other Treg populations, they can inhibit allogeneic CD4+ effector cells. The induction of these cells by anti-CD3 mAb is dependent on TNF, is associated with up-regulation of TNFRs, and requires signaling through NF-κB. In addition, TNFR-2 expression identifies the most potent regulatory CD8+ cells. Thus, our studies identify a new mechanism for generation of human iCD8+Tregs by TCR engagement.
Results
Culture of normal human PBMC with Teplizumab induces CD8+ Tregs
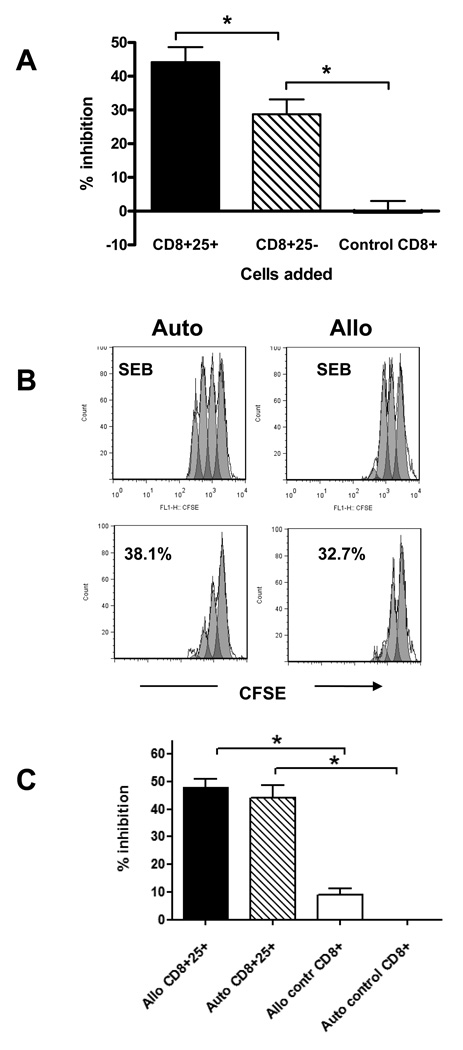
In our previous studies we found that hOKT3γ1(Ala-Ala) (Teplizumab) induced CD8+ T cell proliferation in vitro and that the activated cells inhibited proliferative responses to a nominal antigen, tetanus toxoid [22]. Our previous studies had suggested that following culture with the anti-CD3 mAb, the CD8+CD25+ subpopulation was more inhibitory than CD8+CD25− cells. In this study, we tested the ability of CD8+ T cells cultured with anti-CD3 mAb, to inhibit polyclonal CD4+ T cell responses to staphylococcal enterotoxin B (SEB) and confirmed that CD8+CD25+ T cells were more potent inhibitors compared to CD8+CD25− T cells (Figure 1A). In data from 12 independent experiments, the CD8+CD25+ T cells isolated from Teplizumab treated cultures showed 44.2±4.4 % inhibition compared to 28.7±4.4 % inhibition by CD8+CD25− cells from the same cultures (P=0.0005), or to −0.3±3.3 % inhibition by CD8+ cells that were cultured with control IgG (P=0.0005). CD25 was not detectable on CD8+ T cells cultured in control Ig. To exclude the possibility that the inhibitory effect of the CD8+ T cells was due to carry over of anti-CD3 mAb that had been bound to membranes of these cells, we added Teplizumab to control CD8+ T cells at ten-fold concentrations from 1 ng/mL to 1 µg/mL in the presence of CD4+ effector cells and SEB. No inhibition of proliferation to SEB was seen in the presence of anti-CD3 suggesting that coating with anti-CD3 mAb per se was not responsible for the inhibitory effect (data not shown).
Figure 1. Inhibition of proliferative responses by CD8+ Tregs induced from PBMC from healthy donors.
(A) PBMC from healthy control subjects were incubated with Teplizumab or control IgG for 5 days and CD8+ T cells were sorted on the basis of expression of CD25: CD8+CD25+ or CD8+CD25− cells from Teplizumab cultures or CD8+ cells from control IgG cultures. The cells were then incubated with autologous CD8-depleted PBMC and SEB for 72h, and inhibition of proliferation was calculated as described in Materials and Methods. Data are from 12 independent experiments (*P<0.01, ANOVA). (B) CD8+ 25+ cells were incubated with CD8-depleted autologous or allogeneic PBMC labeled with CFSE in the presence of SEB for 3 days and inhibition of proliferation measured. (a representative experiment of 3 is shown). (C) The pooled data from 3 independent experiments with 6 donors show a summary of the inhibition of anti-SEB responses by autologous or allogeneic CD8+CD25+ cells cultured with anti-CD3 or by CD8+ controls (*p<0.05 by ANOVA).
We then tested whether the iCD8+Tregs would inhibit allogeneic CD4+ T cells or whether their inhibitory function was limited to autologous cells. Analysis of pooled data with a total of 6 allogeneic donors tested in 3 separate experiments showed that CD8+25+ T cells inhibited proliferation by 48.1±3.0% (p<0.05 by ANOVA), suggesting that autologous MHC recognition is not required for the regulatory function (Figure 1B and C).
Phenotype of iCD8+ Tregs
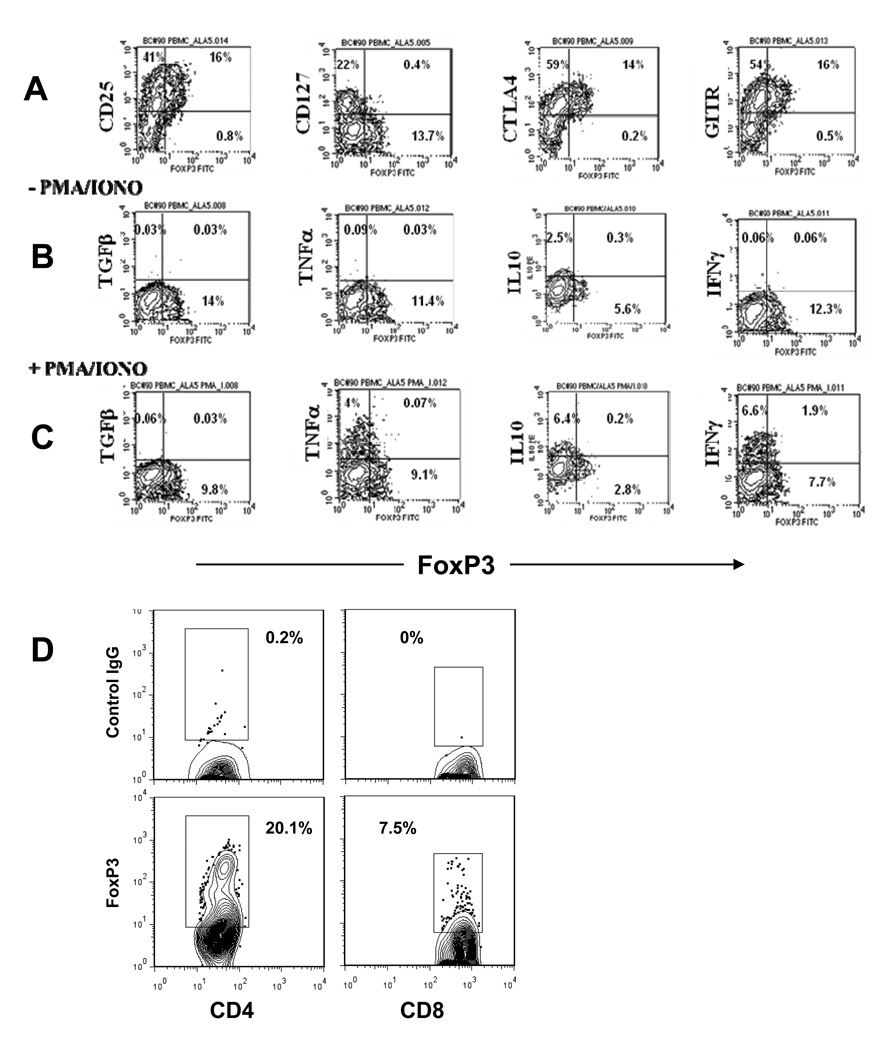
We characterized the phenotype of the CD8+CD25+ T cells following culture with the anti-CD3 mAb (Figure 2A). On day 5 of culture, Foxp3 was expressed in 2–10% of CD8+ T cells but about 30% of the CD8+CD25+ T cells. The Foxp3+CD8+ T cells expressed low levels of CD127 as well as CTLA-4 and GITR. Control IgG-treated CD8+ T cells were FoxP3−, CD25−, CD127+, GITR−. The CD8+Foxp3+ cells did not produce TGF-β or IL-10 before (Figure 2B) or after (Figure 2C) activation with PMA and ionomycin but did express low levels of IFN-γ and TNF. The relative expression of this transcription factor was lower on CD8+ T cells than on CD4+CD25+ T cells that had been cultured for an equivalent period of time with anti-CD3 mAb (Figure 2D).
Figure 2. Phenotypic characterization of CD8+ T cells from healthy control subjects after 5 days of culture with anti-CD3 mAb.
(A) PBMC were gated on CD8+ cells and stained for CD25, CD127, CTLA4, GITR, and FoxP3. A representative experiment of 4–8 independent experiments is shown. (B, C) PBMC cultured with anti-CD3 for 5 days were washed, rested in the absence of the mAb for three days, and then restimulated with PMA and ionomycin (C) or left untreated (B) for 6 hours in the presence of Golgi block. The cells were subsequently stained for intracellular cytokines and analyzed by flow cytometry. The plots show gated CD8+ populations with quadrant tool placed on the basis of isotype controls. The percentage of the gated cells is shown in each quadrant. A representative of 3 separate experiments with similar results is shown. (D) Comparison of relative numbers of FoxP3+ CD4+ (left) and CD8+ (right) cells 5 days after incubation with anti-CD3; a representative experiment out of 4 is shown. The percentage of cells within the indicated region (set based on isotype control) are shown.
Treatment with Teplizumab induces iCD8+Tregs in patients with T1DM
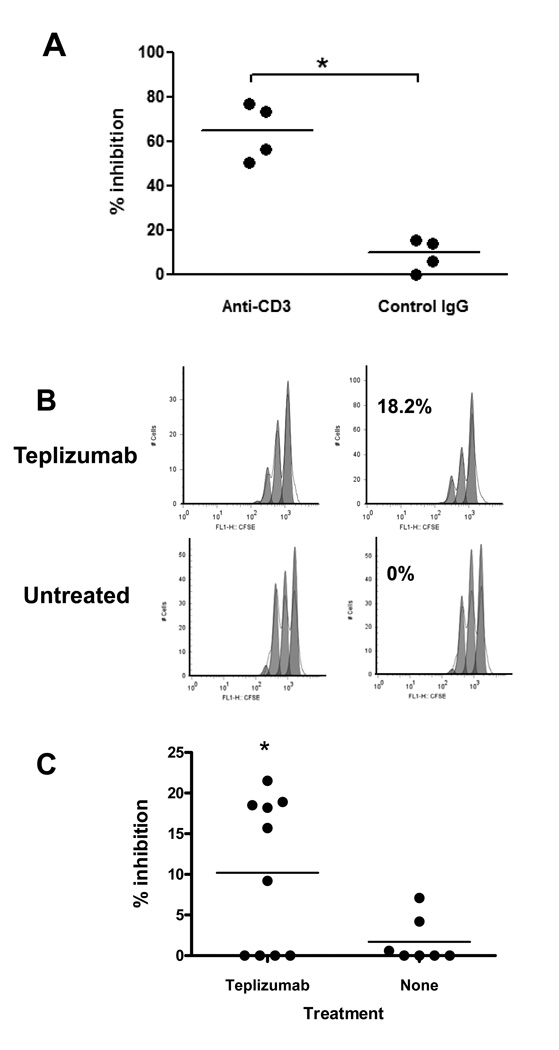
Our previous finding that clinical responses to anti-CD3 mAb correlated with an increased number of circulating CD8+ T cells raised the possibility that the mAb might induce iCD8+ Tregs in patients with T1DM who had been treated with the mAb. We first tested whether iCD8+Tregs may be induced in vitro from PBMC of T1DM patients. We cultured PBMC with anti-CD3 or control Ig for 5 days and isolated CD8+ T cells by negative magnetic selection. We then tested the inhibitory capacity of these cells to CD8-depleted allogeneic PBMC activated with SEB. Figure 3A shows that anti-CD3-treated CD8+ cells from patients were also able to inhibit proliferation.
Figure 3. Generation of iCD8+Tregs from patients with Type 1 diabetes in vitro and in vivo.
(A) PBMC from patients with T1DM were cultured with anti-CD3 for 5 days, at which time, CD8+ cells were magnetically separated. The cells were incubated with allogeneic CD8-depleted and CFSE labeled cells plus SEB for 72h and inhibition of proliferation was calculated as described in Materials and Methods. Data from 4 individuals are shown (*P<0.05 compared to cells incubated with control IgG; paired t-test). (B, C) Induction of CD8+Tregs in vivo after administration of Teplizumab to T1DM patients. Proliferation of CD8-depleted CFSE-labeled responder cells in the presence of CD8+ T cells isolated from a representative drug-treated or control subject at the 1st and 2nd visits corresponding to before and after the drug treated subjects received anti-CD3 mAb(B). (C) Data from all 10 drug treated and 7 untreated patients tested. The percentage of inhibition was calculated as described in Materials and Methods (*p<0.05, paired t-test).
To test directly whether administration of Teplizumab induces iCD8+Tregs in vivo, we isolated CD8+ T cells from patients with new onset Type 1 diabetes who were enrolled in clinical trials with Teplizumab and tested the ability of these cells to inhibit proliferative responses of allogeneic CD8-depleted PBMC to SEB. We used cryopreserved target cells from the same allogeneic donor to test the effects of the patients’ CD8+ cells in order to reduce the variability in anti-SEB responses between the patients. The CD8+ cells were negatively isolated from PBMC isolated from patients before and after Teplizumab treatment, or from patients who were not treated with the drug but in whom blood samples were obtained at the same time points. Here, we tested total CD8+ T cells without further sorting based on CD25 expression since in individuals not treated with anti-CD3, there were very low numbers of CD8+CD25+ T cells. The inhibition shown on Figure 3 B and C reflects the percentage reduction in proliferation when CD8+ cells from the 2nd draw (or after treatment) were added to the cultures, compared to CD8+ cells from the 1st draw (before treatment). It shows that CD8+ T cells isolated from patients on day 14 inhibited allogeneic CD4+ T cells proliferation in response to SEB by 9.23±2.83% whereas cells from the untreated control group inhibited proliferation of the same allogeneic responding CD4+ T cells by 1.54±0.95% (p<0.05). Five of 10 of the drug- treated patients showed the level of inhibition that was 3 SD greater than the level of inhibition seen in the control group. This data suggests that administration of anti-CD3 mAb resulted in the appearance of CD8+ T cells with inhibitory function in the peripheral blood of some T1DM patients.
iCD8+Tregs inhibit proliferation of target T cells
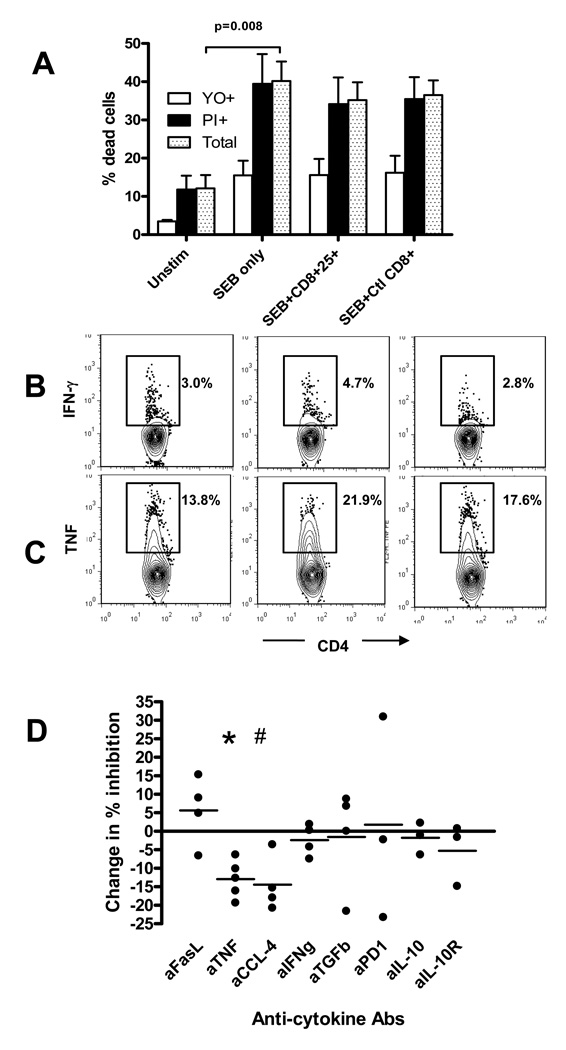
The inhibitory effect of CD8+ T cells might be due to a direct competition with CD4+ cells for IL-2. This was not the case, since the inhibitory effect of the CD8+ Tregs was not reversed by adding IL-2 to the co-cultures (not shown). A direct cytotoxic effect of Tregs on target cells has been suggested as a mechanism of inhibition by human CD8+ Tregs [23–25]. We therefore measured apoptotic and necrotic CD4+ T cells after 72h incubation in the presence of iCD8+CD25+T cells from cultures with Teplizumab and SEB. Figure 4A shows, that the SEB increased the proportion of dead CD4+ cells due to activation but the anti-proliferative properties of CD8+ Tregs were not associated with increased killing, since addition of the CD8+ Tregs cells did not increase the proportion of apoptotic (YO+) or necrotic (PI+) CD4+ T cells.
Figure 4. Analysis of potential mechanisms involved in inhibition by iCD8+Tregs generated from PBMCs of healthy donors.
(A) CD8-depleted target cells were activated with SEB for three days in the presence or absence of CD8+CD25+ T cells from Teplizumab or CD8+ cells from control Ig cultures and apoptosis (YO+) and necrosis (PI+) were analyzed on gated CD4+ cells as described in Materials and Methods. Pooled data from five independent experiments are shown. (B) Addition of iCD8+Tregs does not inhibit cytokine production by target cells in response to SEB as judged by staining for intracellular IFNγ or TNF. Gated CD4+ cells are shown with a region placed on staining with control IgG. A representative of two similar experiments show a proportion of cytokine+ CD4+ cells cultured with SEB only (left panels), SEB+ iCD8+Tregs (middle panels), or SEB+control CD8+ T cells (right panels). (C) The effects of the indicated neutralizing antibodies on inhibition of proliferation by iCD8+Tregs are shown. The change in % inhibition was calculated from the percentage of dividing cells as: (% dividing cells with neutralizing antibody-% dividing cells with control Ig)/%dividing cells with control IgG. (#p=0.005,*p=0.032; by t-test compared to control Ig, n=5 for anti-TNF, n=4 for anti-CCL4, anti-FasL, anti-IFNg, and anti-TGFb; n=3 for anti-PD1, anti-IL-10, and anti-IL-10R).
In addition, when measured by intracellular cytokine staining, the CD8+ T cells did not block secretion of cytokines such as TNF or IFNγ by CD4+ T cells in response to SEB (Figure 4B). Instead, using cell cycle analysis, we found that progression of CD4+ responder cells into G2/M phases, in response to SEB were inhibited when CD8+CD25+ Tregs were added to cultures (Table 1).
Table 1.
Cell cycle analysis of SEB-responding CD4+ T cells after 72h incubation with sorted CD8+ T cells pre-treated with either Teplizumab, or control human IgG. Cells were gated as CD4+Ki67+, and percent of these cells in each phase based on DNA content is shown for 3 separate experiments.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Anti-CD3 | Control hIgG |
Anti-CD3 | Control hIgG |
Anti-CD3 | Control hIgG |
|
| G1 | 56.5 | 42.5 | 45.6 | 43.6 | 68.4 | 65.8 |
| S | 40.3 | 47.1 | 46.6 | 53.7 | 29.7 | 27.7 |
| G2/M* | 3.6 | 5.2 | 4.8 | 8.1 | 1.75 | 3.05 |
P=0.04 by paired t test
Our previous data had shown that either cell contact and/or close proximity to target cells were required for the regulatory function of iCD8+Tregs, whereas studies by others have suggested a number of soluble mediators of inhibition. The inhibition of anti-SEB responses could not be transferred with supernatants from the CD8+- iTregs (not shown). Since it was still possible that soluble factors produced by the iCD8+Tregs could be responsible for the inhibition by working at high concentrations in close proximity, we used neutralizing antibodies to candidate mediators of Treg function, such as CCL-4 [11], IL-10 [26, 27], and others. Figure 4C shows that anti-TNF (in 5 out of 5 experiments) and anti-CCL-4 (in 4 out of 4 experiments) showed significant effects (12.9±2.3% (P=0.005), and 14.4±3.8% (P=0.032)) in partially reversing the inhibitory action of the iCD8+Tregs, respectively. This suggests that CCL4 and TNF may play a partial role in the cytostatic effect of the iCD8+Tregs.
iCD8+Tregs require TNF for induction
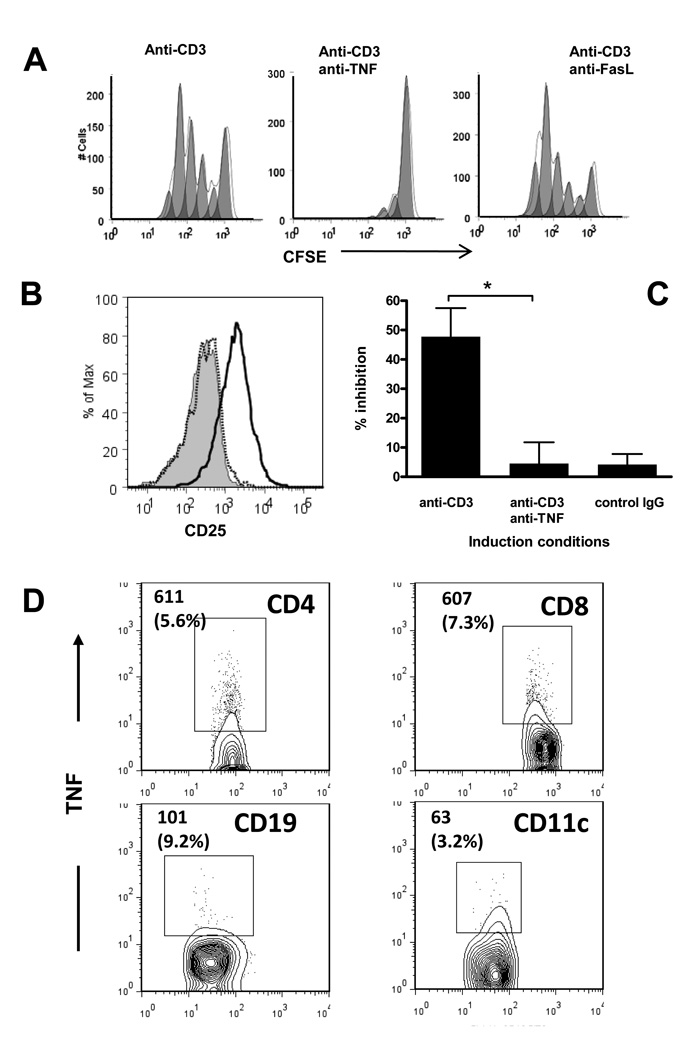
TNF is found in the supernatants of PBMC cultured with modified anti-CD3 mAb, and increased levels of TNF in circulation had been identified in patients with T1DM during treatment with Teplizumab [22]. We therefore tested the role of this cytokine for the development of the iCD8+Tregs. We added neutralizing anti-TNF antibody to normal human PBMC cultures in the presence of Teplizumab and studied proliferation, activation, and induction of CD8+ Tregs. Anti-TNF dramatically abrogated proliferation of CD8+ T cells in response to anti-CD3, whereas neutralization of another TNF family member, FasL did not (Figure 5A). Anti-TNF mAb prevented up-regulation of CD25 on CD8+ T cells (Figure 5B), and prevented induction of regulatory function (Figure 5C). The addition of normal human IgG at the equivalent molar concentrations as anti-TNF mAb (80 µg/ml) had no discernable effect on the expression of CD25 or the induction of CD8+ Tregs. To test which cells were the major producers of TNF, we stained anti-CD3-activated PBMC for intracellular TNF and counted absolute numbers of TNF producers per 30,000 total PBMC. As shown on Figure 5D, although non-T cells contributed to TNF production, the major cell populations secreting TNF in response to anti-CD3 were CD4+ and CD8+ T cells.
Figure 5. Effects of TNF neutralization on the generation of iCD8+Tregs from PBMC of healthy control donors by anti-CD3 mAb in vitro.
(A) CFSE-labeled PBMC were incubated with anti-CD3, with or without neutralizing anti-TNF or anti-FasL mAb for 5 days, and the proliferation and expression of CD25 on CD8+ cells was analyzed by FACS with a gate placed on CD8+ cells. A representative of three separate experiments is shown. (B) Up-regulation of CD25 on CD8+ T cells induced by anti-CD3 (bold histogram) on day 5 of culture is abrogated in the presence of anti-TNF (dotted histogram). A gate is placed on CD8+ T cells. The shaded peak shows CD25 expression on CD8+ cells incubated with normal human IgG. A representative of five separate experiments is shown. (C) Total CD8+ T cells isolated from cultures with Teplizumab with or without anti-TNF antibody were added to CD8-depleted PBMC labeled with CFSE and cultured with SEB. The inhibition of the target T cell proliferation was measured as described (*P=0.03 by ANOVA). Pooled data from 7 independent experiments are shown. (D) Analysis of the cell population responsible for TNF production after anti-CD3-induced activation by intracellular staining for TNF. Absolute numbers (and percent of the indicated subpopulation) of TNF+ cells counted during analysis of 30,000 PBMC with a gate placed on the lymphocyte subpopulation based on scatter. A representative of three independent experiments with similar results is shown. The region was set based on control IgG staining.
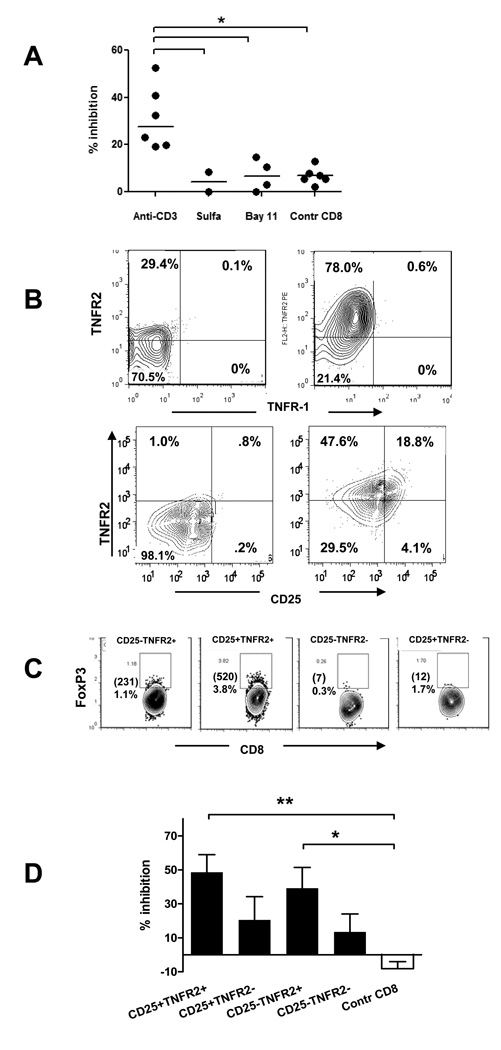
TNF activates several signal transduction pathways, the major of which is that of NF-κB [28]. We therefore tested whether blockade of the NF-κB pathway by sulfasalazine, or with Bay 11–7082, an inhibitor of IKK phosphorylation, [29–31] [32][33] affected the generation of CD8+ Tregs from PBMC. Both reagents blocked the induction of the activated cell phenotype and inhibitory function (Figure 6A). The effects of TNF are mediated via two distinct receptors, TNFR1 and TNFR2. We therefore stained PBMC for both receptors and compared the level of their expression after incubation of the cells with anti-CD3 or control human IgG. Figure 6B (upper panels) shows, that anti-CD3 treatment strongly up-regulated expression of TNFR2, whereas TNFR1 levels were relatively low. TNFR2 expression only partially overlapped with that of CD25 (Figure 6B, lower panels). After culture with anti-CD3 mAb, 9.5 ± 1.5 % (n=6) of CD8+ T cells were TNFR2+CD25+, and these cells were the major subset of CD8+ T cells that expressed Foxp3 (Figure 6C). We postulated, therefore, that TNFR2 may serve as a marker for a more potent subpopulation of CD8+ Tregs. To address this question, we sorted CD8+ T cells that had been cultured with anti-CD3 mAb based on TNFR2 expression and compared their potency as regulators. Figure 6D shows that both, CD8+ CD25+ and CD8+CD25− cells were better inhibitors of proliferation if they co-expressed TNFR2 suggesting that TNFR2 is a marker of human iCD8+Tregs.
Figure 6. TNF signaling is needed to generate iCD8+Tregs from PBMC of healthy donors.
(A) PBMC were cultured with anti-CD3 mAb with or without the inhibitors of NF-kB cascade Sulfasalazine or Bay 11–7082 for 5 days, total CD8+ cells were sorted, and added to autologous CD8-depleted, CFSE-labeled PBMC activated with SEB. The percent inhibiton of proliferation was calculated on Day 3 as described in Materials and Methods (*P<0.05 by ANOVA). (B) Normal human PBMC were incubated with anti-CD3 mAb for 5 days (right panels) or with control human IgG (left panels), and gated CD8+ cells were analyzed for TNFR-2 and TNFR1 expression (upper panels), or for TNFR-2 and CD25 expression (lower panels). Quadrants are built on the basis of isotype control IgG staining. A representative of three separate experiments is shown. (C) Normal human PBMC were incubated with anti-CD3 mAb for 5 days and stained for CD8, TNFR2, CD25, and FoxP3. The panels show CD8+ cells gated on the basis of CD25 and TNFR2 expression. The numbers in the upper left corner indicate the percentage and absolute numbers of CD8+Foxp3+ cells from 100,000 total lymphocytes collected. (D) PBMC incubated with anti-CD3 and CD8+ T cells sorted based on the expression of CD25 and TNFR-2 were tested for their ability to inhibit proliferation of target cells. (pooled data of 5 separate experiments is shown) (*P<0.05; **P<0.01; ANOVA).
Discussion
The mechanism(s) whereby modified anti-CD3 mAbs affect human T cell responses are poorly understood. Preclinical and clinical studies from our group and others have indicated that T cell depletion alone is an unlikely mechanism. These and other studies have suggested that the mAbs may induce regulatory T cells [13, 22, 34]. However, the identity, function, and way in which iTregs are induced has not been clear.
Regulatory CD8+ T cells have been described in a number of immune settings[35]. Cantor and colleagues proposed that CD8+ Tregs, restricted by the minor Class I Qa-1 antigen may play a role in prevention of autoimmune responses by activated CD4+ T cells. [12, 36]. Likewise, Jiang et al described human CD8+ Tregs that inhibit alloresponses and were restricted by Class I human MHC molecule HLA-E presenting TCR Vbeta derived peptides [37, 38]. Our data would suggest that the regulation of CD4+ responses by the iCD8+ Tregs induced by anti-CD3 mAb is not HLA-restricted since the responses of allogeneic CD4+ cells were also inhibited. In tolerant heart allograft recipients, Suciu-Foca et al have described regulatory CD8+ T cells that interact with APC via ILT3 and ILT4 [39]. Similarly, we have found that APCs are needed to induce CD8-Tregs, but their role in mediating suppression has not been studied.
We have found that circulating CD8+ T cells isolated from patients after treatment with anti-CD3 mAb may have regulatory function ex vivo. To our knowledge, this is the first report showing functional human CD8+ Tregs in vivo after administration of anti-CD3 mAb. CD8+ T cells isolated from patients after anti-CD3 mAb treatment inhibited proliferation of allogeneic CD4+ T cells activated with superantigen. The degree of inhibition was less than we observed with CD8+ T cells that were cultured with the mAb in vitro, which cannot be explained by differences in T cells from healthy control subjects and patients with T1DM since CD8+ Tregs from the patients could be equally induced in vitro (Figure 3A). One possibility is that the Tregs induced in vivo were diluted in the total CD8+ fraction of cells that was tested. We did not study the function of other phenotypes of T cells, such as CD4+CD25+ cells that have been shown to have regulatory activity and have been reported to be induced by the anti-CD3 mAb, but we have not found an increase in the proportion of these cells in patients treated with Teplizumab unlike a previous report by Hering et al which included subjects treated with multiple immunologics [18]
Joosten et al reported that CD8+ T cells inhibited the proliferative responses of CD4+ cells to antigen through a mechanism that required CCL4 and LAG3 [11]. Our previous studies, which suggested that cell contact was required for inhibition, did not exclude an effect of a soluble mediator that might be inhibitory at high concentrations in a local environment. Indeed, our cytokine neutralization studies agree with these previous findings and suggest that there is a contributory role for TNF and CCL4 although the results from blockade of each of these molecules was modest.
TNF is responsible for induction of the CD8+Tregs since the effects of anti-CD3 mAb on functional responses of T cells were neutralized by anti-TNF mAb. The importance of TNF for the induction of human CD8+Tregs is interesting in the light of recent reports by Chen et al. demonstrating that TNF is needed for induction of murine CD4+25+ Tregs, and that TNFR2 in both mouse and human serves a marker of more potent CD4+ Tregs [40]. Our data reported here are in agreement with these observations, since TNFR2 was the major TNFR on T cells, TNFR2 + cells are the major expressors of FoxP3, and are more potent inhibitors of anti-SEB responses. Interestingly, in human CD4+ Tregs co-expression of CD25 and TNFR2 identifies a potent subpopulation of Tregs [41][42, 43][44], which is in agreement with our studies, but we also found that that TNFR2 is seemingly a more important marker than CD25 on CD8+Tregs.
Anti-CD3 up-regulates both CD25 and TNFR2, however, the CD25+ TNFR2− cells represent a relatively minor subpopulation, so the majority of CD25+ cells are TNFR2 +. In contrast, CD25− population consists of comparable numbers of TNFR2+ and TNFR2 – cells Consistent with notion of relative potency of TNFR2+ and/or CD25+ cells is our finding that the FoxP3+ CD8 cells are found almost exclusively in the TNFR2+ cells (Figure 6C).
In vitro, TNF appears to be produced by T cells, but a role for APC-derived TNF cannot be excluded [22]. This possibility seems particularly attractive in the light of a recent report showing that TNFR2 is activated by the membrane-bound TNF [45]. Interestingly, our findings are in conflict with the findings in patients with Crohn’s disease, in which anti-TNF treatment increased mucosal CD4+ CD25+ (Foxp3+) T cells . This discrepancy may reflect the different culture conditions since we analyzed the role of TNF in the setting of TCR stimulation[15]. In addition, it is possible that there is a unique role for TNF in the setting of Crohn’s disease and the inflammatory setting in the gut.
The signs and symptoms of “cytokine release syndrome” associated with anti-CD3 administration and other biologics have been attributed to the actions of several cytokines including TNF [46]. These new findings suggest that both beneficial and harmful effects may arise from the actions of this cytokine. Therefore, careful consideration of dosing of the anti-CD3 mAb is needed to avoid the systemic toxicities while maintaining the immunologic effects of TNF.
We propose that iCD8+Tregs may be a common occurrence in humans in the settings of inflammation, TCR activation, and TNF production. Our studies show that the effect of these cells is to inhibit the division of CD4+ T cells which may serve to limit expansion of antigen specific cells. The duration of the CD8+ Tregs following anti-CD3 mAb is unknown, but the changes in CD4/CD8+T cell ratio that had identified clinical responders to drug treatment in our initial studies persisted for beyond 3 months[19]. Holding CD4+ cells at bay for an extended period of time may allow other inflammatory signals to resolve and avoid propagation of cells with autoreactive potential.
Materials and Methods
Human subjects and samples
The studies were done with peripheral blood mononuclear cells (PBMC) from healthy volunteers were received from the New York Blood Center (Long Island City, NY) and, in selected experiments, PBMC collected from patients with Type 1 diabetes, participating in open label portions of 2 clinical studies: "Study 1" [20, 22] or an open label segment of Protégé (Clinicaltrials.gov: NCT00385697). In addition, samples were collected from patients with Type 1 diabetes for > 2yrs. The patients were between the ages of 8–30. Teplizumab-treated subjects received a 12–14 day course of the mAb treatment as described [19, 20]. Control samples, from patients with T1DM who did not receive Teplizumab, were collected at the same time intervals. Isolated PBMC were stored in liquid nitrogen until use. The protocols received Institutional Review Board approval and informed consent was obtained from all participants or their guardians.
Culture with anti-CD3 mAb and sorting of CD8+ T cells
PBMC were separated using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). The cells were cultured in AIM-V medium (Invitrogen, Grand Island, NY) at 1×106/mL with 4µg/mL Teplizumab (hOKT3γ1(Ala-Ala) [47], or with normal human IgG (Sigma, St. Louis, MO). To block NF-κB signaling, Sulfasalazine (Sigma) or Bay 11–7082 (Calbiochem) were added at the final concentration 2 mM, or 2.5 µM, respectively, during the primary cultures of PBMC with anti-CD3 mAb [30][33][32]. In preliminary studies, we determined the maximal concentration of Bay 11–7082 that would not affect CD8+ T cell viability. On day 5, the cells were washed and used for studies. In some experiments the cells were stained with antibodies to cell surface and intracellular markers and analyzed by flow cytometry. For cell sorting, the cells were stained with anti-CD8, and anti-CD25 mAbs (BD Pharmingen, Franklin Lakes, NJ), and sorted as CD8+25+ and CD8+25−, or as a whole CD8+ (CD25−) population from control cultures using a FACSAria cell sorter (BD Biosciences). In some experiments, CD8+ T cells were sorted based on the expression of TNFR2 (CD120b, BD Pharmingen) and CD25.
Inhibitory assays
Autologous or allogeneic PBMC were depleted of CD8+ T cells using Dynabeads CD8 (Invitrogen), labeled with CFSE (Cell Trace Kit, Invitrogen), and used as responding cells in inhibitory assays. Responder cells and sorted CD8+ T cells (105 each per well) were incubated in the presence of SEB (1 µg/mL, Sigma) in AIM-V medium for 72h and the dilution of CFSE was analyzed by FACS. The percent divided cells was analyzed using the FlowJo software (TreeStar Inc, Ashland, OR), and inhibition of proliferation was calculated as [1-(%divided with added cells / %divided without added cells)]×100. For inhibitory assays, using samples from patients in clinical studies, we compared proliferation of allogeneic CD8-depleted cells with CD8+ T cells isolated by magnetic beads before and after treatment with anti-CD3 mAb. The % inhibition was calculated as [1-(%divided with added CD8+ cells from day 14 / %divided with added CD8+ cells from day 0)]×100.
Neutralizing cytokines with mAbs
The following neutralizing antibodies were used at a concentration 50 µg/mL: anti-IFNγ, anti-IL-10, anti-IL10 receptor (CDw210), anti-TNF and anti-FasL(BD Biosciences); anti-CCL4 (Sigma); anti-PD-1, anti-TGFβ1 (R&D Systems, Minneapolis, MN). Target cells activated by SEB in the presence of each neutralizing mAb served as controls. The effect of the blocking Abs was expressed as the difference in % inhibition by activated or control CD8+ T cells with and without addition of the blocking Ab. Blockade of TNF during induction of CD8+ Tregs by anti-CD3 mAb was performed using neutralizing mAb MAb11 (BD Biosciences) or, with Infliximab (Remicade, Centocor, Inc., Malvern, PA), both at 80µg/mL.
Apoptosis and necrosis assays
These studies were performed on day 3 after SEB stimulation of CD8-depleted targets cultured with or without sorted CD8+Tregs or control CD8+ cells. Gated CD4+ T cells were analyzed by flow cytometry using Vybrant® Apoptosis Assay Kit #4 (Molecular Probes, Inc. Eugene, OR) with YO-PRO®-1 stain for apoptotic and propidium iodide for necrotic cell discrimination. Since the CFSE stain emits in the same channel as YO stain, so we could not measure both in the same cells, but ran parallel wells with CFSE-labeled targets to ensure the integrity of the experiment.
Cell cycle analysis
Proliferation of CD4+ responders was determined using 7-Aminoactinomycin D (7-AAD) and anti-Ki-67-FITC (BD Biosciences). After co-culture with activated CD8+T cells, CD4+ cells were isolated using Dynabeads® Untouched Human CD4 T cells Kit (Invitrogen Dynal, Oslo, Norway), fixed and RNA removed by treatment with RNAse A (Sigma) prior to addition of 7-AAD. Gated Ki-67+ cells were analyzed for DNA content using FlowJo Cell Cycle platform.
Flow cytometry and intracellular staining
Cells were activated with SEB or PMA/ionomycin, treated with monensin, and stained for surface markers followed by fixation, permeabilization, and incubation with anti-cytokine mAbs (BD Biosciences) and anti-FoxP3 (eBioscience). To confirm that monensin did not inhibit iCD8+Tregs, CD4+ cells were stained for secreted TNF using TNF Secretion Assay Detection Kit (Miltenyi Biotec Inc., Auburn, CA). Anti-GITR and anti-CTLA-4 mAb were from BD Biosciences.
Statistical analysis
was performed using GraphPad Prizm® Version 4 software (GraphPad Software Inc., San Diego, CA). Unless indicated the data are presented as the mean ± SEM. One way ANOVA with Dunnett’s or Bonferroni multiple comparison post tests were used with P<0.05 considered significant. Where indicated, single column statistics, paired t test, Mann Whitney, and Fisher exact tests were used.
Acknowledgments
This work was supported by NIH grants DK057846 and UL1 RR024139, grants 2006-351, 2006-502, 2007-1059, and grant 2005-1168 from the Juvenile Diabetes Research Foundation, and a gift from the Brehm foundation. The authors are thankful to Paula Preston-Hulburt for technical assistance, Jeffrey Lyon for expert cell sorting, and Lesley Devine from Immune Monitoring Core Facility at Yale Cancer Center for her help with patients’ samples.
Abbreviations
- GITR
Glucocorticoid-induced TNF receptor family
- SEB
Staphylococcal enterotoxin B
- RORγt
retinoic acid-related orphan receptor γt
- 7-AAD
7-Aminoactinomycin D
Footnotes
Conflicts of interests
The authors declare that they do not have conflicts of interests.
References
- 1.Shou L, Schwartz SA, Good RA. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976;143:1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson D, Kauffman HM, Jr, Grotelueschen C, Metzig J. Suppressor activity of the human spleen and thymus. Surgery. 1976;79:393–397. [PubMed] [Google Scholar]
- 3.Hubert C, Delespesse G, Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976;26:95–98. [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 6.Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–198. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 7.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 8.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008;69:771–775. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Suciu-Foca N, Manavalan JS, Cortesini R. Generation and function of antigen-specific suppressor and regulatory T cells. Transpl Immunol. 2003;11:235–244. doi: 10.1016/S0966-3274(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule, Qa-1. Annu Rev Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 11.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebelc F, van der Wal A, de Heer E, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panoutsakopoulou V, Huster KM, McCarty N, Feinberg E, Wang R, Wucherpfennig KW, Cantor H. Suppression of autoimmune disease after vaccination with autoreactive T cells that express Qa-1 peptide complexes. J Clin Invest. 2004;113:1218–1224. doi: 10.1172/JCI20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ablamunits V, Bisikirska BC, Herold KC. Human regulatory CD8 T cells. Ann N Y Acad Sci. 2008;1150:234–238. doi: 10.1196/annals.1447.000. [DOI] [PubMed] [Google Scholar]
- 14.Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, Toubi E. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5575. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 15.Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology. 2008;125:178–183. doi: 10.1111/j.1365-2567.2008.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector t-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57:2672–2683. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, Nakano M, Sawada T, Matsumoto I, Zhang HJ, Sutherland DE, Bluestone JA. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 19.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 21.Glandt M, Hagopian W, Herold KC. Treatment of type 1 diabetes with anti-CD3 monoclonal antibody. Rev Endocr Metab Disord. 2003;4:361–368. doi: 10.1023/a:1027354129493. [DOI] [PubMed] [Google Scholar]
- 22.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 24.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 26.Battaglia M, Roncarolo MG. The role of cytokines (and not only) in inducing and expanding T regulatory type 1 cells. Transplantation. 2004;77:S16–S18. doi: 10.1097/01.TP.0000106468.96542.26. [DOI] [PubMed] [Google Scholar]
- 27.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 28.Wegener E, Krappmann D. Dynamic protein complexes regulate NF-kappaB signaling. Handb Exp Pharmacol. 2008:237–259. doi: 10.1007/978-3-540-72843-6_10. [DOI] [PubMed] [Google Scholar]
- 29.Cavallini L, Francesconi MA, Zoccarato F, Alexandre A. Involvement of nuclear factor-kappa B (NF-kappaB) activation in mitogen-induced lymphocyte proliferation: inhibitory effects of lymphoproliferation by salicylates acting as NF-kappaB inhibitors. Biochem Pharmacol. 2001;62:141–147. doi: 10.1016/s0006-2952(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 30.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- 32.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 33.Yabe T, Wilson D, Schwartz JP. NFkappaB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem. 2001;276:43313–43319. doi: 10.1074/jbc.M107831200. [DOI] [PubMed] [Google Scholar]
- 34.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 35.Siegmund K, Ruckert B, Ouaked N, Burgler S, Speiser A, Akdis CA, Schmidt-Weber CB. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J Immunol. 2009;182:2124–2130. doi: 10.4049/jimmunol.0802271. [DOI] [PubMed] [Google Scholar]
- 36.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware R, Jiang H, Braunstein N, Kent J, Wiener E, Pernis B, Chess L. Human CD8+ T lymphocyte clones specific for T cell receptor V beta families expressed on autologous CD4+ T cells. Immunity. 1995;2:177–184. doi: 10.1016/s1074-7613(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 38.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–180. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002 doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 40.Chen B, Kapturczak MH, Joseph R, George JF, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Tisher CC, Flotte TR, Agarwal A, Chen S. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007;7:1112–1120. doi: 10.1111/j.1600-6143.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Oppenheim JJ. TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr Dir Autoimmun. 11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauert H, Wicovsky A, Muller N, Siegmund D, Spindler V, Waschke J, Kneitz C, Wajant H. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 285:7394–7404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferran C, Bach JF, Chatenoud L. In vivo T cell activation properties of anti-T cell monoclonal antibodies. Exp Nephrol. 1993;1:83–89. [PubMed] [Google Scholar]
- 47.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]