Abstract
Localized cell and drug delivery to the cochlea and central auditory pathway can improve the safety and performance of implanted auditory prostheses (APs). While generally successful, these devices have a number of limitations and adverse effects including limited tonal and dynamic ranges, channel interactions, unwanted stimulation of non-auditory nerves, immune rejection, and infections including meningitis. Many of these limitations are associated with the tissue reactions to implanted auditory prosthetic devices and the gradual degeneration of the auditory system following deafness. Strategies to reduce the insertion trauma, degeneration of target neurons, fibrous and bony tissue encapsulation, and immune activation can improve the viability of tissue required for AP function as well as improve the resolution of stimulation for reduced channel interaction and improved place-pitch and level discrimination. Many pharmaceutical compounds have been identified that promote the viability of auditory tissue and prevent inflammation and infection. Cell delivery and gene therapy have provided promising results for treating hearing loss and reversing degeneration. Currently, many clinical and experimental methods can produce extremely localized and sustained drug delivery to address AP limitations. These methods provide better control over drug concentrations while eliminating the adverse effects of systemic delivery. Many of these drug delivery techniques can be integrated into modern auditory prosthetic devices to optimize the tissue response to the implanted device and reduce the risk of infection or rejection. Together, these methods and pharmaceutical agents can be used to optimize the tissue-device interface for improved AP safety and effectiveness.
Keywords: Drug delivery, Auditory prostheses, Cochlear implant, Auditory Brainstem Implant, Hearing loss, Pharmaceuticals
1. Auditory prosthetic devices and drug delivery
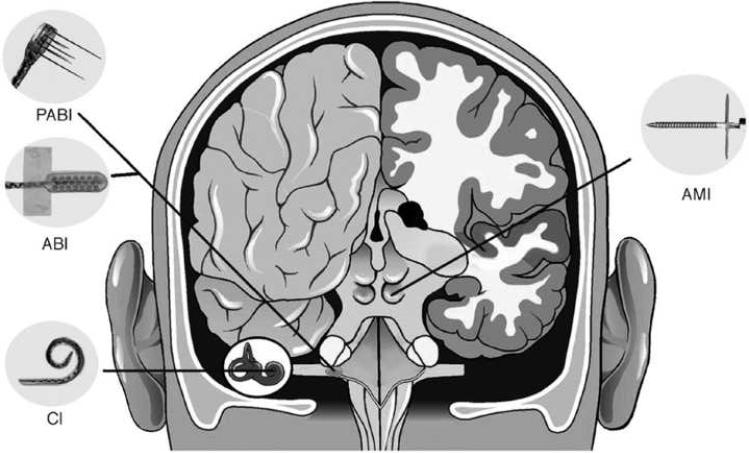
Auditory prostheses (APs) are neural prosthetic devices that help patients with hearing loss regain auditory function by stimulating the auditory nervous system. A wide variety of currently available and experimental APs serve to treat a broad range of clinical conditions. They can improve the hearing of patients with severe hearing loss or hearing loss specific to certain frequencies, and can restore auditory function in patients with complete deafness. The success and effectiveness of AP therapy depends on many factors including electrode placement, device design, stimulus waveform, proximity of electrodes to neurons, auditory system pathology, integrity of neural pathways, and extent of fibrous tissue (Blamey et al., 1992; Incesulu et al., 1998; Kawano et al., 1998). A number of APs are available for patients with different physiological sources and degrees of hearing loss. Hearing aids, middle ear implants, and bone-anchored hearing aids offer some functional recovery for patients with conductive hearing loss, but when sensorineural hearing loss (SNHL) is severe, direct stimulation of the auditory nerve is required. Cochlear implants (CIs) can restore hearing to patients with extensive SNHL at the level of the cochlea (Cervera-Paz et al., 2005; Deggouj et al., 2007; Di Girolamo et al., 2007; Wilson, This issue). CIs, shown in Figure 1, are placed directly into the cochlea and electrically stimulate the auditory nerve. When the nerve is damaged or otherwise inoperable, auditory brainstem implants (ABIs), penetrating auditory brainstem implants (PABIs), and auditory midbrain implants (AMIs), also shown in Figure 1, can be used to stimulate targets of the central auditory pathway including the cochlear nucleus (CN) and the inferior colliculus (IC) (Lim et al., This issue; McCreery et al., 2007; McCreery, This issue; Middlebrooks et al., This issue; Seki et al., 2007).
Figure 1.
Schematic of auditory prostheses in the cochlea and central auditory pathway. The cochlear implant (CI) is used to stimulate cochlear nerve processes from within the cochlea. The auditory brainstem implant (ABI) is composed of a grid of surface electrodes to stimulate the cochlear nucleus. The penetrating auditory brainstem implant (PABI) is better able to access the tonotopic structures of the cochlear nucleus that lie parallel to the surface of the cochlear nucleus. The target of the auditory midbrain implant (AMI) is the inferior colliculus. Reproduced from Lenarz et al. (2006b) with permission from Lippincott Williams & Wilkins.
In order for APs to send rich acoustic information covering broad tonal and dynamic ranges, they must be closely coupled to a large, healthy population of neurons (Fayad et al., 1991; Incesulu et al., 1998; Khan et al., 2005; Leake-Jones et al., 1983; Nadol et al., 2001; Pfingst et al., 1983; Sutton, 1983). Animal studies have shown that the number of intact nerve fibers is an important indicator of CI success (Pfingst et al., 1983; Pfingst et al., 1981), although successful cochlear implant treatment has been achieved in human patients with few surviving neurons (Fayad et al., 1991; Fayad et al., 2006; Linthicum et al., 1991). Other studies suggest that the location of intracochlear damage and distance between electrodes and spiral ganglion neuron (SGN) cell bodies are more directly related to clinical cochlear implant performance (Ketten et al., 1998; Nadol, 1997; Skinner et al., 2002). Degeneration of auditory neurons and their processes also occurs as hearing loss progresses and less neural input is received (Dodson et al., 2000; Nadol, 1990; Nadol, 1997; Shepherd et al., 2002a; Spoendlin, 1975). In addition, surgical implantation of CIs and devices in the central nervous system can cause trauma, inflammation, infection and reduction in the health and availability of target neurons near the implant (Leake-Jones et al., 1983; Lenarz et al., 2007; Li et al., 2007). In the majority of cases, CIs are effective and reliable in treating hearing loss, with overall failure rates of 3.8 % (Battmer et al., 2007)- 8.3 % (Maurer et al., 2005). Improvements in the tissue response to APs using cell and drug delivery can help reduce the device failures caused by immune rejection, infection, encapsulating tissue response, device migration, and degeneration of the primary auditory neurons. In addition, improvements in the tonal and dynamic resolution, place-pitch discrimination, and power consumption can also be made to improve device function.
The delivery of therapeutic compounds to the cochlea and central auditory pathway in conjunction with implanted devices may help to overcome many limitations associated with APs. Improvements in the direct coupling of healthy neurons to small electrode sites can increase the resolution of electric stimulation while reducing channel interactions. A number of medications and drug delivery techniques have been developed through the treatment of inner ear diseases and research on implantable neural prostheses. These methods have the potential to improve the health and accessibility of auditory neural structures. Growth factors and other biomolecules can be delivered to promote the survival of neurons and may be able to induce neurite extension towards the implants. Various anti-inflammatory steroids can reduce the degree of insertion trauma and subsequent fibrous tissue growth around the implant. The release of antibiotics near the implant can prevent infection and meningitis. Surface modification can also be used to direct the adhesion of desirable tissue to the implant surface. Cell delivery and gene therapy aimed at the regeneration of auditory neurons and sensory hair cells may partially restore natural hearing to improve auditory function with any type of AP. Together these approaches may improve the tonal range and resolution, dynamic range and resolution, power consumption, and safety of APs.
In this article, we will explore the use of localized cell and drug delivery to the cochlea and central auditory pathway to improve the integration and function of APs. We will discuss the drug delivery targets found in the cochlea and central auditory pathway that are relevant to APs. The pharmaceutical agents, biomolecules and biochemical compounds that may improve the function, integration, safety, and efficacy of AP devices will be presented. The delivery techniques described include currently available clinical methods as well as experimental methods. We will focus on the cell and drug delivery techniques and cellular targets relevant to CIs, ABIs, and AMIs since these are the most demanding applications with the broadest potential impact. Finally, we will suggest areas of future research that may benefit the further treatment potential of APs.
2. Drug delivery targets and tissue reactions
2.1. Cochlear implants
2.1.1. Device and drug delivery targets
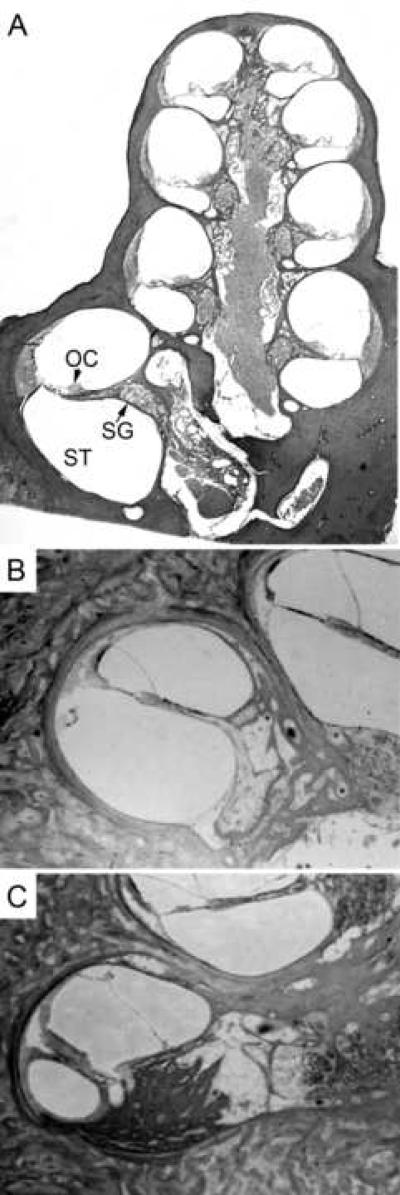
CIs have been used extensively since the 1970s for patients with severe SNHL. These devices have been largely successful at restoring speech perception to profoundly deaf patients (Middlebrooks et al., 2005; Tyler et al., 1997; Waltzman et al., 1993; Wilson, This issue). As a result, the benefits of CIs have become available to an increasingly larger patient group. Currently CIs are available for adults and for children less than 1 year old with moderate to profound hearing loss (Cervera-Paz et al., 2005; Deggouj et al., 2007). CIs electrically stimulate the remaining SGNs in the cochlea using an array of up to 24 electrodes implanted within one of the fluid-filled chambers of the cochlea, the scala tympani, as shown in figure 2A. Electrodes are typically made of inert metals such as platinum, immobilized on an elastomer carrier that can be shaped to contour to the curving shape of the cochlea. These contoured implants are intended to reduce the distance between the electrode and the modiolus, where the spiral ganglion cell bodies are found. Reviews of cochlear physiology related to cochlear implantation are available elsewhere (Dallos, 1996; Nadol et al., 2006; Raphael, 2002; Raphael et al., 2003; Stjernholm, 2003).
Figure 2.
2A. Anatomical section of guinea pig cochlea showing the scala tympani (ST), the chamber in which cochlear implants are inserted, the Organ of Corti (OC) where the sensory hair cells are found and enervated by spiral ganglion processes, and the spiral ganglion (SG) cell bodies. Electrical stimulation of the spiral ganglion processes or cell bodies can result in neuronal activation. 2B. Section of human cochlea with cochlear implant showing minimal tissue reaction. Small amounts of fibrous tissue were found around the electrode tip along the lateral wall. The implant had been in place for 5 years. 2C. Cochlear sections from another human patient with extensive new bone growth in the scala tympani between the electrode array and Rosenthal's canal, located at the right of the image. In addition, the osseous spiral lamina was fractured due to the implanted electrode. This cochlear implant had been in place for 8 years. Images 2B and 2C have been adapted from Kawano et al. (1998) with permission from Informa Healthcare.
The viability and proper function of SGNs is crucial for both acoustic hearing and hearing assisted by cochlear implants. SGNs transmit acoustic information from inner hair cells in the form of neural impulses to the CN, for further processing. They are also the targets of electrical stimulation from CIs. Unfortunately, degeneration of the SGN is known to occur after loss of sensory input from hair cells (Nadol, 1997; Nadol et al., 2006; Spoendlin, 1975). For CI operation, the proximity and availability of SGN to the implant may also be an important factor enhancing the success of the prosthesis. Methods to reduce the distance between the CI and the SGNs include the delivery of growth factors to induce neurite extension towards the electrode and the delivery of anti-inflammatory agents and other compounds to reduce the fibrous and bony tissue that forms between the implant and SGNs.
The most common cause of cochlear-level SNHL in humans is the loss of sensory hair cells found in the organ of Corti (Strenzke et al., 2008). These delicate cells detect vibrations of the basilar membrane, and without them, sound cannot be processed. A number of etiologies can contribute to the loss of sensory hair cells, including heredity disease, ototoxic medications, mechanical trauma, overstimulation and aging. In mammals, cochlear hair cell loss is permanent. As such, strategies to promote the survival and regeneration of sensory hair cells are some of the primary goals of drug delivery for improving prosthetic and natural hearing. The supporting cells in the organ of Corti are also targets for drug delivery systems, as these cells are crucial for the protection, survival, and function of hair cells. Supporting cells are also a prime target for gene therapy strategies to regenerate sensory hair cells (Raphael et al., 2007).
2.1.2. Tissue reaction to cochlear implants
The key to developing appropriate pharmacological strategies to improve APs integration and function is an understanding of the tissue reaction to implanted devices. The insertion of CI electrodes into the scala tympani can cause trauma to the basilar membrane, spiral ligament, osseous spiral lamina, and lateral wall of the scala tympani, leading to acute inflammation. The long-term effects of implantation include chronic inflammation, fibrous encapsulation, new bone growth, and further hearing loss (James et al., 2008; Nadol et al., 2006). Figure 2B shows an implanted human cochlea with minimal tissue reaction, while figure 2C shows extensive fibrous and bony tissue between the implant and the modiolus. The amount of trauma to the lateral cochlear wall during the initial insertion has been shown to correlate with the amount of fibrous tissue and bone growth seen later (Li et al., 2007). In turn, the extent of fibrous tissue and bony growth has been associated with higher stimulation thresholds, reduced dynamic ranges, and decreased SGN viability (Hanekom, 2005; Kawano et al., 1998), although there is some debate on the subject (Li et al., 2007). It is widely agreed upon, however, that new bony and fibrous tissue in the cochlea limits subsequent treatment options including reimplantation and regeneration strategies (Somdas et al., 2007). The administration of electrical stimulation to the cochlea can increase the inflammation and encapsulation around the electrode in the scala tympani (Shepherd et al., 1994), although some studies have shown that electrical stimulation can help preserve SGNs (Hartshorn et al., 1991; Leake et al., 1991; Leake et al., 1992; Lousteau, 1987). Strategies to reduce the initial implantation trauma, including “soft” surgical approaches, steerable implants, and lubricants to facilitate insertion, have demonstrated decreased effects on hearing (Gantz et al., 2005; Kiefer et al., 2004; Laszig et al., 2002; Rogowski et al., 1995). Anti-inflammatory steroids have also been shown to reduce the inflammation and subsequent effects associated with electrode insertion (James et al., 2008).
2.2. Auditory brainstem and midbrain implants
2.2.1. Devices and drug delivery targets
For patients with profound hearing loss who are not eligible for CIs, APs in the central nervous system provide an alternative treatment option. The CN and IC are currently the primary locations for AP stimulation in the central nervous system using surface and penetrating microelectrode arrays. Detailed anatomical and physiological information regarding the central auditory pathway is available elsewhere (Cant et al., 2003; Demanez et al., 2003; Moore et al., 2007). ABIs have been used since 1979 to stimulate the CN (Edgerton et al., 1982; Schwartz et al., 2008). The majority of ABI users are patients with neurofibromatosis 2, a disease characterized by bilateral Schwannomas on the cochlear nerves that often require deafness-causing surgical removal. ABIs have been successful in providing some environmental auditory information to patients, but their effectiveness at restoring speech perception is quite limited (Colletti et al., 2005; Lenarz et al., 2001; Otto et al., 2002; Shannon et al., 1993). More recently, the IC has been used as the target of electrical stimulation with AMIs (Lenarz et al., 2007; Lim et al., 2007). Preliminary results in animals have shown that stimulation of the IC by AMIs is possible and may provide some benefits over CIs and ABIs (Lenarz et al., 2006a; Lim et al., 2006). Human pilot studies are currently underway, although encoding strategies for communicating with this higher processing center are still in development. While these technologies can circumvent areas of extreme damage in the auditory system, their ultimate success depends on the viability and availability of target neurons and the ability to stimulate these in a safe and useful manner. The target structures of the central auditory pathway, including the dorsal and ventral nuclei of the CN and the central nucleus of the IC, are composed of various types of neurons, glia, and vasculature depending on their location. In both the brainstem and midbrain, a number of nearby processes include both sensory and motor neurons, so stimulation must be localized within the auditory tract to prevent unwanted neuronal activation. To improve the performance of APs in the central auditory pathway, drug delivery strategies should focus on promoting the viability, function, accessibility and selectivity of target neurons near the device electrodes. By reducing the extent of inflammation and fibrous encapsulation, electrodes in the central nervous system can interact more directly with target neuronal structures.
2.2.2. Tissue reaction to implants in central auditory system
Studies on the tissue reaction of implants in the CN and IC in animals have shown similar tissue reactions as microelectrodes implanted into other soft structures of the brain. In the brain, there is a multi-stage response to implanted microelectrodes (Szarowski et al., 2003). The initial insertion of an electrode or array into the soft tissue causes trauma including tearing of neurons, glia and blood cells. The early tissue response typically lasts 2–4 weeks and includes inflammation, increased astrocyte proliferation, the presence of reactive astrocytes and growth of astrocytes within 100–200 μm of the implant. The severity of the early response is related to the magnitude of insertion trauma, which correlates with the device size and geometry. The early response is followed by a sustained tissue response that is independent of the implant size or shape. The sustained response is characterized by the formation of a dense and compact fibrous sheath, composed of reactive astrocytes and reactive microglia. The dense fibrous tissue, which is typically in place 6 weeks after insertion, reduces electro-ionic conduction between the electrode and neurons. In addition, a neuronal “kill zone” with decreased neuronal viability, extends approximately 100–200 μm from the implant (Biran et al., 1999; Biran et al., 2005; Cheung, 2007).
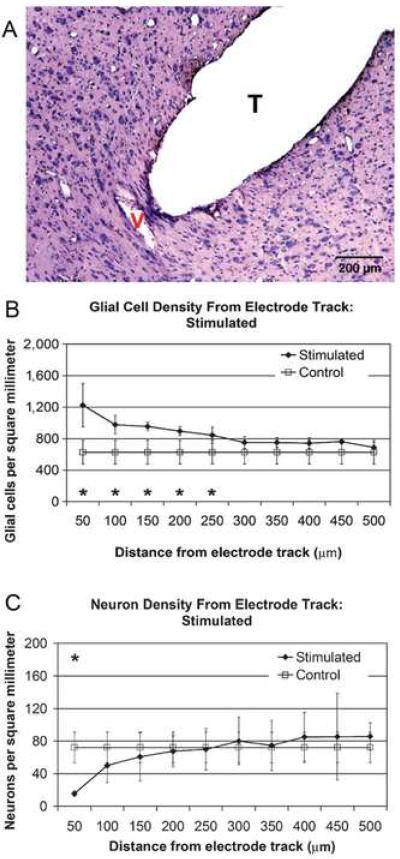
The tissue reaction in cats implanted with an AMI for 2 months in the IC is shown in Figure 3. The reaction included a fibrillary sheath, approximately 60 μm thick, around the implant, and reactive glial cells were present in both stimulated and non-stimulated animals within 500 μm from the implant; the glial cell density near the implant is represented in Figure 3B. A decrease in the number of viable neurons within 100 μm of the implant accompanies the glial cell proliferation, shown in Figure 3C (Lenarz et al. (2007). A human brainstem implant removed due to infection showed a dense, collagen-rich sheath after 22 months of use (Terr et al., 1989). The leads of the device were also covered by a sheath of connective tissue, which ranged from 150 to 430 μm thick. A study by Quester et al. (2002) examined the local tissue reaction in rats to polyethylene terephthalate meshes used as electrode carriers in ABIs. The study again demonstrated the presence of fibrous sheath around the meshes beginning 2 weeks after implantation, but also noted the importance of fibrous tissue in the prevention of implanted device migration. In addition, some adverse effects of implanting the pliable polymer mesh were seen, including fatal lesioning of brainstem structures and meningitis. While cellular encapsulation is often necessary to prevent migration of the implant and to sequester the foreign materials from the tissue, it can also block critical device components such as electrodes and retard charge transfer at the electrode interface. The results from Quester et al.'s study highlight the importance of using soft materials that are less likely to cause lesioning once implanted, and the use of anti-inflammatory, anti-bacterial, and possibly other drug treatments in conjunction with implants to reduce the risk of infection and cell death.
Figure 3.
3A. Histological section of inferior colliculus showing the implant track, T, from an auditory midbrain implant that had been in place in a cat for 3 months and electrically stimulated for the final 2 months. Dense tissue around implant track is fibrous encapsulation of electrode and shows increased staining for glial fibrillary acidic protein suggesting the presence of astrocytes around the implant. 3C. Average glial cell density around the probe show elevated glial populations for up to 500 μm from the implant site. The difference is statistically significant for 50–250 μm (*) (4 animals). 3B. The average density of neurons in the tissue surrounding the implant site is lower than in the control, unimplanted inferior colliculus. At 50 μm the difference in neuronal density is statistically significant (*), and around 200 μm from the implant no difference is seen (4 animals). Reproduced from Lenarz et al. (2007) with permission from Lippincott Williams & Wilkins.
3. Pharmaceuticals for improved auditory prosthesis therapy
Localized delivery of pharmacological agents is crucial in both the inner ear and the brain in order to reach necessary drug concentrations without causing adverse effects. Pharmacokinetic studies show that systemic administration of medications by injection or infusion leads to poorly controlled concentrations in the inner ear, and exposes the rest of the body to the effects of the drug (Chen et al., 2003; Imamura et al., 2003). The uptake of systemically administered compounds into the central nervous system is very low due to the protective blood-brain-barrier which prevents entry of many useful medications (Persidsky et al., 2006). Similarly, the blood-labyrinth barrier prevents compounds in the blood supply from entering the inner ear (Inamura et al., 1992; Juhn, 1988; Juhn et al., 1981; Juhn et al., 2001). The development of minimally invasive methods for localized and sustained delivery of precise quantities of medication near the implant site will circumvent these barriers and enable improvements in the safety, efficacy, cost, and reliability of APs while reducing adverse effects.
Clinical and experimental efforts have yielded numerous pharmacological agents that promote the survival of targets for electrical stimulation and cells necessary for processing of auditory signals. Medications and delivery methods developed to treat inner ear disorders such as SNHL, sudden SNHL, tinnitus, and infections have provided many useful approaches that can be harnessed to reduce hearing loss and prevent the degeneration of AP target cells. This research has identified many compounds including antibiotics, antioxidants, anti-inflammatory agents, neurotransmitters and their agonists, cytokine inhibitors, apoptosis blockers, and growth factors that protect and preserve the hair cells, neurons and supporting cells of the cochlea (Breuskin et al., 2008; Crumling et al., 2006; Darlington et al., 2007; Fritzsch et al., 2006; Gillespie et al., 2005; Guitton et al., 2004; Holley, 2002; Lalwani et al., 2002; Patel et al., 2004; Pettingill et al., 2007; Raphael, 2002; Richardson et al., 2006; Rybak et al., 1999; Rybak et al., 2005; Seidman et al., 2003; Seidman et al., 2004; Tang et al., 2006; Weber, 2002). Similar research on the central nervous system has yielded compounds including anti-apoptotic agents, amino acids, neurotransmitters and their agonists, antioxidants, anti-inflammatory agents, calcium, hormones, and growth factors which are useful in promoting the survival of neurons in many traumatic and neurodegenerative states (Chin et al., 2005; Diem et al., 2007; Friedman, 2006; Hara, 2007; Hoffman et al., 2006; Lescot et al., 2006; Sweeney, 1997). Cell delivery, gene therapy and tissue engineering approaches to repairing traumatic injury and diseases of the brain and central nervous system have also produced promising results for the regeneration of lost neurons (Buch et al., 2007; Mochizuki, 2007; Shen et al., 2007).
Many studies have been carried out to investigate the pharmacokinetics of therapeutic compounds in the cochlea. Sampling the perilymph of humans and animals has been used to measure concentrations of therapeutic compounds, although this method is susceptible to contamination by cerebrospinal fluid (Hara et al., 1989; Oertel et al., 2007; Salt et al., 2003). Microdialysis has been used to monitor the levels of compounds, such as steroids in the inner ear, without altering fluid levels or compositions (Hahn et al., 2006). Empirical measurements of intracochlear drug concentrations have been used to create computational models that can be used to predict spatial and temporal distribution of compounds in the cochlea (Salt, 2002; Salt et al., 2006). This finite element model is available for free download (oto.wustl.edu/cochlea/model.htm). While these studies have helped to elucidate cochlear pharmacokinetics, their predictions are prone to deviations due to inflammation, injury, and differences between individuals (Banerjee et al., 2004).
The diffusion of compounds in the brainstem and midbrain is limited due to the dense cellular structure. Lower molecular weight compounds tend to diffuse farther and faster while large compounds remain concentrated near the site of delivery. In some cases, measuring the extent of migration in animals can be achieved by fixing the compounds in place prior to tissue sectioning (Neeves et al., 2007). Staining and serial sectioning can elucidate the 3-D drug distribution.
4. Localized cell and drug delivery techniques
Techniques for localized drug delivery to the cochlea and central auditory pathway can be used to improve AP therapy by promoting the health and accessibility of target auditory structures. By delivering pharmaceutical agents closer to where they are needed, the adverse effects of systemic administration can be avoided and the concentrations can be better controlled. Sustained release methods that can extend delivery over longer times can provide tighter control of drug concentrations to prevent exceeding or falling below the therapeutic window as is common with discreet drug delivery methods. Ideally, these methods should also be minimally invasive and/or integrated with the AP device to avoid unnecessary surgery, trauma, or infection.
4.1. Microfludic delivery
Microfluidic delivery is a method for continuous infusion of pharmaceuticals to the cochlea and central nervous system using a drug reservoir and small tubes or cannulas for delivery.
4.1.1. Pump-catheter systems
Pump-catheter systems have been used extensively in animal models to deliver numerous compounds including otoprotective compounds, drugs to promote regeneration, anti-bacterial compounds and transmitter agonist compounds. Early attempts used cannulas or microcatheters connected to skull-mounted infusion systems to deliver small discrete quantities of medications into the perilymph of the scala tympani (Kingma et al., 1992). Miller and colleagues developed techniques for applying mini-osmotic pumps connected to microcannulas for chronic sustained delivery to the cochlea (Brown et al., 1993). The advantage of the mini-osmotic pump is the constant infusion of solutions for days to weeks, while other methods offer discrete infusions followed by latent periods. For chronic delivery lasting more than 2 weeks, it is possible to exchange the empty drug reservoir for a full one by minor surgery. Continuous low volume infusion should provide the most stable concentrations of medications in the scala tympani (Hashimoto et al., 2007), because of the constant exchange of fluids within the cochlea and the degradation of exogenous compounds over time. Mini osmotic pumps have been used to promote neuronal viability by delivering neurotrophic factors including glial-derived neurotrophic factor (Shoji et al., 2000a; Yamasoba et al., 1999a), brain-derived neurotrophic factor (BDNF) (McGuinness et al., 2005; Miller et al., 1997; Shoji et al., 2000b), neurotrophic-factor 3 (NT-3) (Miller et al., 1997; Shoji et al., 2000b), as well as viral vectors to increase neurotrophic factor expression (Prieskorn et al., 2000; Yamasoba et al., 1999b). Other compounds including antioxidants (Ohinata et al., 2003; Yamasoba et al., 1999a), iron chelators (Yamasoba et al., 1999a), voltage-gated calcium channel agonists (Miller et al., 2003) and NMDA receptor agonists (Ohinata et al., 2003) have been delivered to protect hair cells and neurons and prevent degeneration. Takemura et al. demonstrated that dexamethasone, an anti-inflammatory steroid, delivered via mini osmotic pumps at a rate of 0.5 μl/hour for 11 days, prevented hair cell loss from noise-induced trauma in guinea pigs (Takemura et al., 2004). Previous work from this group showed that pump-infused dexamethasone also protected hair cells from ototoxic medications (Himeno et al., 2002). The mini-osmotic pump system offers a reliable platform for the sustained delivery of many compounds that can promote the survival of SGNs and hair cells in the cochlea, reduce inflammation due to insertion trauma, and possibly reduce the fibrous encapsulation and ossification around the implant. Unfortunately, these systems have had limited use clinically because of the risk of infection, the need to change drug reservoirs, and the difficulty of insertion and removal. Another problem with mini-osmotic pump delivery is the degradation of many therapeutic compounds in body temperature, limiting the selections of reagents that can be loaded into a pump and retain bioreactivity for many days.
Pump-catheter systems have also been used in the brain and central nervous system to infuse neurotrophins and other pharmacological compounds to protect neuronal structures from damage due to ischemia, excitotoxicity, and axotomy. However, compounds delivered into the brain experience slow diffusion, especially high molecular weight compounds and polar species. By applying a small but constant pressure to the fluid, it is possible to increase the diffusion of compounds through the interstitial spaces of the brain (Bobo et al., 1994). This process is termed convection-enhanced delivery (CED) and is essential for the increased and homogeneous distribution of therapeutic compounds in the brain.
A number of studies have made use of CED to deliver drugs, proteins, neurotrophins, and medications to various areas of the brain. Efforts to treat brain injury and deliver growth factors and steroids to the brainstem and midbrain offer the most utility for APs. Infusion of BDNF to the midbrain has been used in models of Parkinson's disease. The BDNF infusions reduce vestibular deficits, increase neuronal metabolism, and prevent degeneration of nigral neurons (Altar et al., 1994; Volpe et al., 1998). Intraventricular infusion of nerve growth factor (NGF) has been shown to promote the survival of cholinergic neurons, improve cognitive function, and reduce apoptosis in various rat models of traumatic brain injury (Dixon et al., 1997; Sinson et al., 1995; Sinson et al., 1997). The infusion of basic fibroblast growth factor (bFGF) following brain trauma also helped restore cognitive function (McDermott et al., 1997). Histologically, the neurons in both bFGF-treated and control animals appeared similar, although bFGF-treated animals had increased astrocytic proliferation, indicative of a neuro-immune response. Intramedullary infusion of BDNF had neuroprotective effects and promoted remyelination and regeneration after traumatic spinal cord injury (Namiki et al., 2000). Continuous intraventricular infusion of BDNF beginning prior to ischemic trauma in a rat model increased the number of surviving pyramidal cells (Beck et al., 1994) and reduced the volume of infarct tissue (Schabitz et al., 1997), even when administered directly after transient ischemia (Tsukahara et al., 1994; Yamashita et al., 1997). These results suggest that microfluidic delivery to the brainstem and midbrain is possible and may help promote the viability and function of target tissue, even after injury and degeneration have occurred, an important consideration for APs.
4.1.2. Drug delivery channels
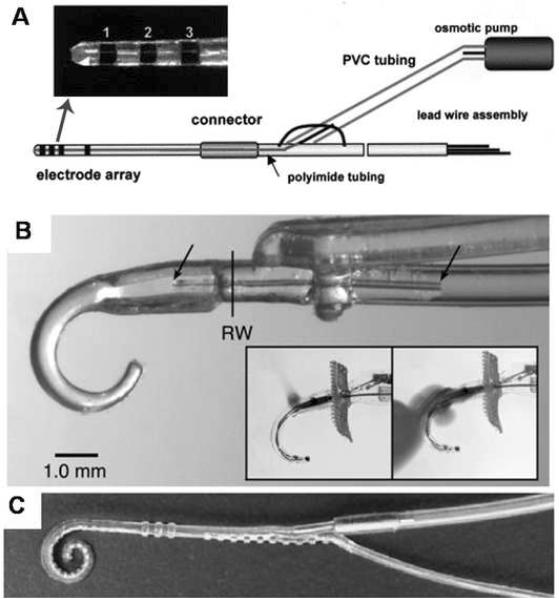
The clinical value of microfluidic delivery for APs may come from the integration of drug delivery channels onto implantable AP devices. In these cases, the fluid delivery system does not add more difficulty than implantation of the auditory prosthetic device, except for refilling of the drug reservoir. The drug delivery ports can be used to deliver compounds directly to the electrodes, one of the most important sites for directing the tissue reaction. Custom-fabricated devices demonstrate the feasibility and usefulness of hybrid devices capable of stimulating electrically and delivering drugs in animals models (Rebscher et al., 2007; Shepherd et al., 2002b) (Figures 4A and 4B). Paasche et al. (2003) modified existing commercial implants by removing the tip of a Nucleus 24 Contour lead manufactured by Cochlear Ltd. to expose the lumen of the clinical device (Figure 4C). The other end was coupled to either a mini osmotic pump or an infusion pump using a custom connector. This modification produced a robust CI capable of delivering drugs or other soluble compounds to the tip of the electrode. To further characterize the distribution of therapeutic compounds delivered through microfluidic ports on a CI, this modified implant was tested using dye and a model cochleae (Paasche et al., 2006). Simultaneous release from multiple sites on the electrode provided the most homogenous distribution of dye throughout the perilymph. Stover et al. examined the use of a femtosecond laser to precisely machine small ports for drug delivery in a Nucleus Contour 24 implant (Stover et al., 2007). Holes between 70 µm and 180 μm were rapidly machined in the silicone devices to release drugs from the internal lumen of the implant. This capability was demonstrated in vitro with the delivery of a dye compound in water (Stover et al., 2007).
Figure 4.
4A. Custom-built 4 channel cochlear implant with pharmacological delivery. A hole in the tip of the electrode, indicated by an arrow, is connected via polyimide microtubing to an osmotic pump to infuse drugs into the scala tympani. Reproduced from Shepherd et al. (2002b) with permission from Elsevier. 4B. A different custom-built contoured cochlear implant with integrated drug delivery microcannula for delivery of drugs 3into the basal portion of the cochlea using an osmotic pump. The delivery site and connection point are indicated with arrows. The implant is made of silicone molded to enter the cochlea through the RWM as indicated by “RW”. Reproduced from Rebscher et al. (2007) with permission from Elsevier. 4C. The Contour cochlear implant lead retrofitted with a drug delivery channel along its inner lumen. It can be attached to a mini-osmotic pump to deliver pharmacological agents through ports between electrodes along the implant or at the tip. Reproduced from Paasche et al. (2003) with permission from Lippincott Williams & Wilkins.
Microfabricated silicon and polymer probes with electrode sites for recording and stimulating the central nervous system have also been fabricated with integrated fluid delivery channels (Lee et al., 2004; Neeves et al., 2006; Retterer et al., 2004; Takeuchi et al., 2005; Ziegler et al., 2006). Most of these devices have demonstrated the ability to deliver fluids in vitro. Silicon devices with integrated microfluidic channels and shutters that enable delivery of 100–200 pl injections were implanted into the CN to study the effects of neuropharmacological delivery while electrically monitoring neuronal response. Injections of GABA into the dorsal CN could be detected using electrodes placed in the ventral CN to record the inhibitory effects (Papageorgiou et al., 2006). The dense brain matter limits the distribution of compounds in vivo, producing more localized distribution than in the fluid-filled cochlea. This can be beneficial when highly localized delivery is desired, but can also produce inhomogeneities in concentration due to tissue structure. One strategy to increase the distribution in vivo is to increase the permeability of the extracellular matrix (ECM). As the ECM is the primary pathway for fluid movement, Neeves et al (2007) implemented strategies to dilate and degrade the interstitial space, to promote the diffusion of 54 nm diameter polymer nanoparticles. They found that the most successful strategy was preinfusing the brain with isotonic buffer solution prior to nanoparticle delivery. This strategy increased the distribution volume by 123%. The pre-infusion of hyaluronidase, an enzyme that degrades the hyaluronan found in ECM, increased the volume of nanoparticle distribution by 64%. The delivery of enzymes to help degrade the ECM might also be a useful approach for reducing the insulating fibrous sheath that forms between implanted electrodes and auditory structures of the brain and impedes electrical transfer. This research shows that delivery of pharmaceutical compounds directly to the electrode sites in the central nervous system (CNS) can be used to direct the tissue reaction at this important interface.
There is a possibility that machining features onto auditory prosthetic devices may create suitable locations for drug-resistant, bacterial biofilms to adhere and propagate. In some cases, these infections can lead to meningitis. In vitro studies have been undertaken to understand the role of geometry and port design in biofilm formation (Johnson et al., 2007). Although larger holes in a device lead to significantly more biofilm colonization than smaller holes, even an unmodified silicone surface can be host to biofilm formation in vitro. The prevalence of biofilms on implant materials underscores the necessity of implementing drug delivery strategies on APs to reduce unwanted surface fouling and prevent infection.
4.2. Materials for sustained release
Many materials have been used as matrices for sustained drug delivery to the inner ear and brain. Some materials act as sponges to soak up therapeutic solutions and release them by diffusion once implanted. Other materials are biodegradable and release their payload as they erode or are digested by the body. These materials may benefit AP function by extending the timeframe of drug delivery, improving neuronal survival, and reducing inflammation related to AP implantation and drug delivery.
4.2.1. Non-degradable materials
The non-degradable polymer resin ethylene vinyl-acetate copolymer (EVAc) has been made into discs and used as a matrix for sustained delivery of compounds through the round window membrane (RWM) and into the perilymph. Pasic and Rubel demonstrated this technique by delivering tetrodotoxin, a reversible voltage-gated sodium channel blocker, to the cochlea using small discs they had made from solvent-cast solutions of EVAc and tetrodotoxin. The effects of tetrodotoxin on the auditory system were seen within 10 minutes of administration and lasted on average 24–46 hours and were dependent on drug loading concentration (Pasic et al., 1989).
The use of EVAc discs for intracranial drug delivery has also been investigated. EVAc discs, weighing on average 6.20 ± 0.05 mg, were each loaded with approximately 100 μg of recombinant human NGF (rhNGF) during fabrication and implanted into the brains of rats. Release of rhNGF was sustained for 4 weeks and provided levels near the implant of approximately 10,000 ng/ml after the first week. The concentration of rhNGF decreased exponentially with distance from the implant (Saltzman et al., 1999). EVAc has also been effectively used as a carrier for NT-3 to the rat cerebellum (Doughty et al., 1998).
4.2.2. Degradable particles
Degradable nanospheres made of poly(lactic-co-glycolic acid) (PLGA), a commonly used biodegradable polymer and drug carrier, have been used as a vehicle for the sustained release of vaccines, DNA, and small therapeutic molecules in the body (Jiang et al., 2005; Whittlesey et al., 2004). Tamura et al. compared the systemic delivery of fluorescently-tagged PLGA nanoparticles to local cochlear administration to the round window. Local administration to the RWM using 140–180 nm particles resulted in a significant number of fluorescent particles within the scala tympani of the basal portion of the cochlea while systemic administration resulted in high accumulation in the liver and only transient presence in the blood vessels of the cochlea (Tamura et al., 2005). Kim et al. delivered dexamethasone to the brain on a recording implant coated with a hydrogel containing many of these PLGA nanoparticles. They found that while the impedance of a control implant increased with time post-implantation, the impedance of the dexamethasone-PLGA particle/hydrogel coated implant remained steady (Kim et al., 2006), implying that the dexamethasone-particles helped to reduce the insulating tissue response. Saltzman et al. (1999) also explored the use of PLGA microparticles for rhNGF delivery in the brain. They found that using PLGA increased the concentrations of rhNGF up to 60,000 ng/ml, well above the therapeutic threshold required in neurodegenerative states, although release from PLGA was found to be less consistent than that of EVAc.
4.2.3. Hydrogels
Hydrogels are materials composed of at least 90 % w/v water held together by a lightly crosslinked network of polymer chains. They are an ideal scaffold material for tissue engineering due to their 3-D porosity and high water content. Many types of gels have been used in vitro and in vivo for a number of uses and are FDA approved (Potter et al., 2008). They can be made from either natural or synthetic sources and their biodegradability can be tailored from fully degradable to permanent (Babensee et al., 2000; Broder et al., 2006). Since they are predominantly made of water, hydrogels are suitable as vehicles for the delivery of water soluble drugs and can be tailored for delivering other compounds using emulsifying agents (Xiong et al., 2006). Hydrogels have been used extensively as surgical repair materials for otological and neural applications. For APs they may be loaded with pharmaceuticals and applied during surgery to the implant site to reduce inflammation and stimulate neuronal growth, applied as a coating to the AP to release their drug loads once the device is implanted (Williams et al., 2005), or used to deliver cells on AP devices (Rejali et al., 2007).
For cochlear and CNS drug delivery hydrogels are often used in the form of beads or sponges loaded with pharmaceutical compounds. A variety of growth factors have been delivered to the inner ear using hydrogels placed over the RWM. Noushi et al. (2005) used beads of alginate hydrogel to deliver NT-3 and found that release from the beads was nearly complete by 5 days. When these beads were implanted in guinea pig cochleae, there was minimal tissue inflammation or fibrous reaction. In deafened animals treated with 1.5 μg of NT-3 in alginate hydrogel beads placed on the round window, the density of SGNs surviving after 28 days was significantly higher than controls. Hydrogels loaded with BDNF have also been implanted in the middle ear to prevent SGN degeneration. After 1 week, animals implanted with BDNF-containing collagen hydrogels had significantly lower (improved) electrically-evoked auditory brainstem response thresholds, significantly higher density of surviving SGN, and perilymph levels of BDNF over 100 times higher than control animals treated with saline (Endo et al., 2005; Ito et al., 2005). Both collagen-glutaraldehyde hydrogels and gelatin hydrogels have been used to deliver recombinant human IGF-I to the inner ear to prevent noise-induced damage (Iwai et al., 2006; Lee et al., 2007). Animals with these gels had lower auditory brainstem response thresholds and higher SGN survival than animals that received control gels. Burdick et al. (Burdick et al., 2006) incorporated and delivered ciliary-neurotrophic factor (CNTF), NT-3, and BDNF into degradable hydrogels based on polyethylene glycol and polylactic acid to stimulate the outgrowth of sensory neurites. Neurotrophin release could be tailored by altering the concentration of the polymers, as well as the loading conditions and polymer degradability. Functionalization of hydrogels with ECM components or adhesion molecules such as collagen, polylysine and laminin-1 may also help to promote stable tissue fixation at the AP-tissue interface, while minimizing fibrous encapsulation (Zhong et al., 2001). Hydrogels have been shown to be effective for the delivery of cells, neurotrophins and other compounds to reduce inflammation and promote neuronal survival, and may also find uses as scaffolds to direct desirable tissue adhesion to the implant surface.
4.3. Surface modification
Surface coatings for APs can be used to deliver small quantities of biomolecules directly at the tissue-device interface. Therapeutic biomolecules can be adsorbed, chemically attached, or coated onto the implant surface using another material. Silicone tympanostomy tubes with adsorbed vitamin-E have been used to control reactive oxygen species in the inner ear and myringosclerosis in the middle ear of children (Uneri et al., 2006a; Uneri et al., 2006b). Coatings of albumin have been shown to reduce unwanted protein adhesions and occlusions of tympanostomy tubes in vitro and in vivo (Kinnari et al., 2005; Kinnari et al., 2001; Kinnari et al., 2004). While surface modification allows only small amounts of drugs to be delivered, this is a very important technique for directing the tissue reaction to implanted devices in the inner ear and central auditory pathway. Patterning of surface coatings can also be used to guide neurons towards the electrodes and to provide suitable points for tissue adhesion that do not interfere with the devices' ability to source electrical charge.
4.4. Iontophoresis
Iontophoresis is the movement of charged particles due to ionic gradients or brief electrical stimulation. This method of drug delivery has been used extensively in research applications for the delivery of molecules to the inner ear and central auditory pathway for pharmacological, physiological, and therapeutic purposes. Typically, glass micropipettes are loaded with ionically charged solutions that contain a soluble substance such as neurotransmitters, agonists, antagonists (Arnold et al., 1996; Ebert et al., 1992; Ebert et al., 1995; Ehrenberger et al., 1992; Felix et al., 1992; Hurley et al., 1999; Walsh et al., 1995), neurotrophins (Oestreicher et al., 2000), dyes, or anti-inflammatory agents. When current is applied to a metal electrode in the micropipette, small quantities of solution are expelled and drawn into the oppositely charged tissue. Iontophoresis has been used for studying the role of synaptic neurotransmitters in auditory processing (Felix et al., 1990; Hurley et al., 1999; Kleinlogel et al., 1999). Arnold et al. (1996) used microiontopheresis to apply glutamate channel agonists and antagonists to study the effects of lasers for inner and middle ear surgery. Oestreicher et al. (2000) delivered NT-3, NMDA and non-NMDA agonists by iontophoresis to the subsynaptic region of the inner hair cells to study the effects of acute administration on neuronal activity. They were able to modulate neuronal firing rates of clusters of neurons as soon as 5 minutes after administration as measured at afferent processes or at inner hair cells. While the delicacy of glass micropipettes limits iontophoresis to acute use, the use of electrical current to quickly drive small doses of neurotrophins or neurotransmitters to modulate neuronal activity is very promising as a means of controlled delivery in the inner ear and central auditory pathway. It is conceivable that a robust microiontopheretic system could replace electrical neuronal stimulation with biochemical stimulation.
4.5. Conducting polymer electrode coatings
Conducting polymers deliver compounds directly from AP electrodes and therefore offer a chance to direct the tissue response at the crucial tissue-electrode interface. Conducting polymers are a class of materials made from small organic molecules which when linked together conduct charge along the polymer backbone due to delocalized bonds. They include polyacetylene, polyaniline, polypyrrole (PPy), polythiophene, poly(3,4-ethylenedioxythiophene) (PEDOT) and their derivatives. PPy has been studied most extensively for biomedical applications due to its biocompatibility and electrical properties (Ateh et al., 2006; Ramanaviciene et al., 2007). PEDOT has been used for biomedical applications because of its chemical stability and conductivity (Cui et al., 2003; Kros et al., 2005). Research on the use of conducting polymers for neural engineering applications has demonstrated their biocompatibility, desirable electrical properties, including low impedance and high charge injection density, and ability to actuate upon application of electrical bias (Guimard et al., 2007; Smela, 2003). Upon application of charge, conducting polymers have been shown to expel minute quantities of ions, growth factors, corticosteroids or other compounds (Abidian, 2006; Massoumi et al., 2001). Release of charged species from the conducting polymer matrix is thought to occur by charge compensation during electrical stimulation. When an electrical bias is applied to the pharmaceutical-loaded conducting polymer, mobile compounds with the same charge are expelled in order to balance the applied charge (Kontturi et al., 1998; Pernaut et al., 2000; Wadhwa et al., 2006; Zhou et al., 1989). Conducting polymers have also been functionalized with enzymes and used for biosensing applications that may offer a useful method for monitoring the auditory system chemical environment (Geetha et al., 2006; Kros et al., 2005; Nien et al., 2006). Recent studies suggest that conducting polymers including PEDOT, PPy, polyaniline, and their derivatives may offer useful methods for controlled drug delivery to reduce insulating tissue formation and promote neuronal viability around AP electrodes.
NGF has been incorporated into conducting polymers for neural interactions. Kim et al. (2007) immobilized NGF in both PPy and PEDOT films using electrochemical co-deposition. The NGF in the films increased the attachment and neurite extension of PC12 (rat pheochromocytoma) cells. Covalent attachment of NGF to the surface of PPy also promoted neurite growth from PC12 cells (Gomez et al., 2007). Co-administration of electrical stimulation in addition to the tethered NGF provided the most significant neurite elongation. Thompson et al. (2006) also incorporated NT-3 into PPy films during electrochemical deposition. The films were electrically stimulated using a variety of methods including cyclic voltammetry, pulsed potential, and pulsed current to determine the optimum release protocol. During the first day, there was a burst of neurotrophin release from all of the samples, including un-stimulated samples. Subsequently, diffusion from the un-stimulated sample was extremely low, while the electrically stimulated films continued to secrete neurotrophin (Thompson et al., 2006). The effect of these NT-3-containing PPy films on cochlear cells was investigated by Richardson et al. (2007), where the incorporation and delivery of NT-3 in PPy and its effects on explanted SGNs was assessed. Spiral ganglion explants cultured on electrodes coated with an NT-3-containing PPy substrate exhibited 1.5 times more neurite outgrowth than explants grown on PPy without NT-3 or on tissue culture polystyrene. When biphasic pulses were applied to the NT-3/PPy coated electrodes for 1 hour, the cells responded with a 2.2 times higher level of neurite extension than unstimulated samples containing NT-3. The study showed that PPy substrates released NT-3 over 4 days and promoted neurite growth, and stimulation increased these effects.
It is possible to achieve repeatable, active release of pharmacological agents using conducting polymer actuators. One approach is to use bi-layer actuators built from conducting polymers to open small valves in order to release drugs from a reservoir (Tsai et al., 2007). Another approach is to coat drug-loaded fibers with conducting polymers that are stimulated to release the drug contents within. This process was shown to be effective at actively delivering dexamethasone for 2 months (Abidian, 2006). Actuators based on the expansion and contraction of conducting polymers due to the movement of ions can also be used to guide the placement of CIs. By attaching a conducting polymer actuator either into the lumen of a commercially available implant or onto the back of it, it is possible to create a steerable CI. This allows insertion and placement of the electrode closer to the target cells and with less damage to the outer wall of the scala tympani (Zhou et al., 2003).
4.6. Cell-based therapy
Future cell-based therapies, including the delivery of stem cells and other cell types, are of interest for the repair, regeneration and protection of neuronal and sensory tissue (Nakagawa et al., 2005). There are two primary cell transplant options. The first is to implant cells that produce protective or therapeutic molecules either naturally or through differentiation or genetic modification. With genetic modification, cells are transfected with a gene therapy vector and then secrete specific proteins that promote protection, repair, or regeneration. The second treatment modality is to deliver cellular replacements for damaged or permanently lost cells including hair cells, SGN, and neurons in the central nervous system. Cells can be implanted directly or within a support scaffold, such as hydrogel or other porous matrices, in order to rebuild damaged sections of the auditory system (Sekiya et al., 2007; Sekiya et al., 2006). The potential benefits of cell delivery for APs are to increase the viability of neurons and other auditory cells or to replace entirely the missing cells required for processing and transduction of auditory signals from implants to the brain. These techniques can affect not only prosthetic hearing, but may improve residual acoustic hearing as well.
Warnecke et al. (2007) demonstrated the ability of BDNF secreted by transfected fibroblast cell lines to improve the survival and neurite outgrowth of SGNs in culture. Rejali et al. (2007) confirmed the ability of BDNF-secreting fibroblasts transplanted into the scala tympani in a hydrogel scaffold on a cochlear implant to promote the survival of SGNs in vivo. Transfected fibroblasts have also been used to deliver BDNF to the central nervous system. The transplantation of transfected fibroblasts prevented degeneration of dopaminergic neurons in a rat model of Parkinson's Disease (Levivier et al., 1995). Transplanted BDNF-secreting fibroblasts also helped reduce neuronal degeneration following ischemia (Ferrer et al., 2001). These results suggest that the delivery of genetically modified cells can be used to prevent the degeneration associated with implantation trauma, lack of sensory input, or toxic conditions.
Olivius et al. (2003) transplanted dorsal root ganglion sensory neurons (DRGs) to the cochlea in order to restore damaged cochlear nerve pathways. Many cells survived transplantation into the scala tympani and attached near the organ or Corti. Infusions of BDNF and CNTF significantly increased the survival of transplanted cells in the cochlea. Further studies confirmed the survival of transplanted DRGs into the cochlea, as well as the survival of transplanted embryonic and adult stem cells (Ulfendahl et al., 2007). Transplanted cells required exogenous trophic factor support, and embryonic stem cells showed the best survival.
Stem cells are of great interest for transplantation because they provide a source of cells that can both differentiate into numerous phenotypes for cell replacement and release trophic factors to promote the survival of existing cells (Altschuler et al., This issue). The transplantation of mesenchymal stem cells into the inner ear has been shown to promote hearing recovery in a rat model of SNHL (Kamiya et al., 2007). Recently, it has been shown that stem cells collected from the cochlea and vestibular organ can differentiate and show markers and behavior of auditory neurons (Martinez-Monedero et al., 2008). Parker et al. (2007) have shown that neural stem cells, following transplantation into the scala tympani, expressed markers for key auditory cell types, including hair cells and SGNs.
While promising, cell-based drug delivery presents many difficulties in both research and clinical applications. These include the reliability of donor cells sources, knowledge of the cell differentiation process, compatibility of the delivered cells with the target environment, and delivery of the appropriate cell type. Many of the critical factors for survival of implanted cells are often absent in diseased and degenerative states (Sekiya et al., 2007). Strategies to differentiate stem cells into appropriate cell types have made progress (Coleman et al., 2007; Shi et al., 2007), but more research is needed to reliably produce the desired phenotype, and the risk of stem cell implants forming tumors still exists (Matsui et al., 2005). However, cell-based therapies may be able to improve AP function by restoring or promoting survival of the components of the auditory pathway that are necessary for the processing of stimulation from the APs.
4.7. Gene therapy
Gene therapy offers the potential for longer time frame of effectiveness than the introduction of exogenous proteins (Lalwani et al., 1998). In addition, there is a reduced risk of infection from the external equipment associated with other cochlear drug delivery methods. In gene therapy, a gene of interest is delivered to a target cell, and an upregulation of the protein that the gene codes for is induced. The cochlea is a good candidate for transgene applications, due to its relatively isolated structure, and the fluid-filled spaces which allow for diffusion (Duan et al., 2002), but the need for surgical access for vector delivery is a complicating factor. The construction of vectors (gene carriers) with various promoters allows for some target cell choice. There are a number of viral and non-viral carriers currently in use, and several routes of introducing the gene of interest into the inner ear. Replication-deficient adenoviruses, adeno-associated viruses, herpes simplex virus, and lentiviruses have all been used in the inner ear, as well as non-viral liposomes (Chan et al., 2007; Chen et al., 2001; Crumling et al., 2006; Duan et al., 2004a; Kawamoto et al., 2004; Minoda et al., 2004; Nakaizumi et al., 2004; Staecker et al., 2004). Viral vectors have higher levels of cell transduction than nonviral methods, but also have a higher potential to induce an immune response. However, viral vectors have been shown to be safe vehicles for gene therapy into the cochlea in vivo as long as they are only used once (Raphael et al., 1996; Weiss et al., 1997). Infusions of vector suspensions into the cochlea can be through the RWM (Nakaizumi et al., 2004; Yagi et al., 1999) or through a cochleostomy into the scala tympani (Stover et al., 1999) or scala media (Ishimoto et al., 2002). RWM infusions are less invasive but offer less permeability than direct scalae infusions (Praetorius et al., 2003; Stover et al., 1999). RWM infusions have been shown to induce reporter gene expression in the mesothelial cells that line the scala tympani, throughout the length of the cochlea (Raphael et al., 1996; Yagi et al., 1999) and are therefore useful for inducing expression of secreted gene products.
One of the most promising results of gene therapy in the cochlea is the development of genes to promote hair cell viability in vivo. Viral-mediated introduction of the gene Atoh1 (previously Math1) has been used to restore lost hair cells and restore some level of function to both the cochlea and the vestibular apparatus (Izumikawa et al., 2005; Staecker et al., 2007a). These results show regeneration of mammalian sensory cells, a feat previously unachievable with other types of drug delivery. Gene therapy also has protective effects, as the expression of the gene bcl-2 has been shown to protect hair cells from damage due to oxidative stress in vitro (Staecker et al., 2007b). Bowers et al. (2002) used the herpes simplex virus as a vector to express NT-3/myc in vivo, which protected hair cells from ototoxic chemotherapy drugs. Gene therapy has also been used to introduce a number of neurotrophic factors into the cochlea and support survival of SGN in animal models of SNHL (Lalwani et al., 2002; Nakaizumi et al., 2004; Yagi et al., 2000). Gene therapy has the potential for combination with other drug delivery techniques. Rejali et al. (2007) transfected guinea pig fibroblast cells with an adenovirus containing a BDNF gene insert and then applied these transfected cells to a hydrogel-coated CI. This coated implant was able to not only release significant amounts of BDNF, but also significantly improve SGN survival, when compared to non-BDNF-treated controls. Okano et al. (2006) combined gene therapy with cell-based therapy by placing mouse fibroblast cells, which were non-virally transfected with BDNF into the inner ear of mice. A significant production of BDNF in vivo was seen using this technique.
4.8. Cochlear-specific methods
In addition to the aforementioned drug delivery techniques, a number of cochlear-specific techniques exist to improve neuronal viability and help retain residual hearing. These are based mostly on the clinical methods for treating inner ear diseases, which primarily consist of delivery to the middle ear space and rely on diffusion through the RWM for incorporation into the cochlea. The tri-layered RWM allows numerous therapeutic compounds, stains, and polymer nanoparticles to enter the perilymph of the basal turn of the cochlea (Goycoolea, 2001). Since diffusion through the RWM is slow and variable, facilitator compounds and changes in middle ear pressure have been used to increase cochlear uptake (Chandrasekhar et al., 2000; Salt et al., 2003; Selivanova et al., 2003).
Clinicians have used transtympanic injections to deliver compounds to the middle ear to treat SNHL, sudden SNHL and other diseases of the inner ear (Chandrasekhar et al., 2000; Coles et al., 1992; Itoh et al., 1991; Sakata et al., 1997; Sakata et al., 2001). Other techniques for middle ear delivery and subsequent inner ear uptake include tympanostomy tubes (Oxley et al., 2007; Sennaroglu et al., 1999) and Silverstein MicroWicks (Silverstein, 1999; Silverstein et al., 2004; Van Wijck et al., 2007), which provide longterm access to the middle ear and can permit patients to self-administer medications. Due to the variability of diffusion through the RWM and drainage through the Eustachian tubes, intracochlear concentrations of compounds are difficult to predict accurately and can vary significantly from one individual to the next (Plontke et al., 2002). While these techniques do not permit precise localization of medications within the auditory system, they are useful in treating infections and inflammation associated with AP implantation.
5. Summary and Conclusions
Localized drug delivery in the cochlea and central auditory system can help AP function by promoting the survival of cells required for auditory processing. By increasing the viability of neurons and supporting cells in the cochlea and central auditory pathway, reducing inflammation, reducing insulating fibrous tissue, and protecting against infection, drug delivery systems can improve AP performance and reduce adverse effects. In addition, many of these treatment techniques can protect and improve residual hearing in patients with or without APs.
Localized, sustained drug delivery directly to the target tissues has several advantages over systemic application, including fewer adverse effects, reduced ototoxicity, smaller quantities of drug used, and better therapeutic outcome. Many of these methods have been tested in animals and in some cases in human pilot studies. Among the tested delivery methods are hydrogels, degradable drug matrices for sustained release, microfluidic delivery, iontophoresis, conducting polymer coatings, and implant surface functionalization. Currently, commercial and custom-made CIs and implants for the CNS have been modified with microfluidic channels to deliver drugs or bioactive molecules in conjunction with electrical stimulation. Anti-fouling and lubricious coatings have also been shown to reduce biofilm adhesion and insertion trauma of CIs, respectively. Someday, cell delivery and gene therapy may be able to replace lost cells necessary for natural hearing and reduce the need for implantable APs.
Despite these advances in pharmaceutical interventions for APs, there is still room for improvement. Methods to reduce the electrically insulating properties of fibrous tissue encapsulation without allowing the implant to move freely are necessary. Optimal timing of pharmacological intervention and individualized treatment plans are required, and thus methods to characterize the ongoing tissue response in the cochlea and CNS can help provide this information (Duan et al., 2004b; Li et al., 2007; Williams et al., 2007). To fully harness the potential of APs, strategies for single cell communication may be required. Methods to target and maintain connection to individual neurons can enable this. Reductions in the electrode size and improvements in the encoding strategies must also occur. Currently, there is a wide portfolio of clinical and experimental methods that can provide controlled drug delivery to direct the tissue response near the implant site. These methods address the fundamental needs of drug delivery for APs: to deliver precisely controlled quantities of medications near the implant over the desired time course in order to improve the safety, reliability, and performance of AP therapy.
Acknowledgements
The authors thank Caitlyn Gertz for her comments on the manuscript, Lisa Beyer for assistance preparing images, and Bryan Pfingst for guidance throughout the preparation of the manuscript. The work of JAC, MAC, and YR was supported by the R. Jamison and Betty Williams Professorship, and NIH/NIDCD Grants F31-DC009134, R01-DC01634, R01-DC05401, and P30-DC05188. The work of JLH and DCM was supported in part by the National Science Foundation (DMR-0518079), the University of Michigan College of Engineering Translational Research (GAP) Program, Biotectix LLC, the National Academies Keck Futures Initiative on Smart Prosthetics, and an Army Research Office sponsored MURI on “Bio-Integrating Structural and Neural Prosthetic Materials”, grant number W911NF-06-1-0218. DCM and JLH are founders of Biotectix LLC. This conflict of interest is managed by the University of Michigan.
Abbreviations
- ABI
Auditory brainstem implant
- AMI
Auditory midbrain implant
- AP
Auditory prosthesis
- BDNF
Brain-derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- CED
Convection-enhanced diffusion
- CI
Cochlear implant
- CN
Cochlear nucleus
- CNS
Central nervous system
- CNTF
Ciliary neurotrophic factor
- DRG
Dorsal root ganglion
- ECM
Extracellular matrix
- EVAc
Ethylene vinyl-acetate copolymer
- FDA
Food and drug administration
- GABA
Gamma-aminobutyric acid
- IC
Inferior colliculus
- IGF-1
Insulin-like growth factor 1
- NGF
Nerve growth factor
- NMDA
N-methyl-D-aspartic acid
- NT-3
Neurotrophic factor 3
- PABI
Penetrating auditory brainstem implant
- PC12
Pheochromocytoma
- PEDOT
Poly(3,4-ethylenedioxythiophene)
- PLGA
Poly(lactic-co-glycolic acid)
- PPy
Polypyrrole
- rhNGF
Recombinant human nerve growth factor
- RWM
Round window membrane
- SGN
Spiral ganglion neuron
- SNHL
Sensorineural hearing loss
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidian MR, Kim DH, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. Advanced Materials. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J Neurochem. 1994;63:1021–32. doi: 10.1046/j.1471-4159.1994.63031021.x. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, O'Shea KS, Miller JM. Stem cell transplantation for auditory nerve Replacement. Hear Res This issue. doi: 10.1016/j.heares.2008.06.004. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A, Ehrenberger K, Frenz M, Pratisto H, Weber HP, Altermatt HJ, Felix D. Experimental erbium laser surgery in the guinea pig cochlea: its use in the study of afferent cochlear neurotransmitters. Eur Arch Otorhinolaryngol. 1996;253:460–3. doi: 10.1007/BF00179950. [DOI] [PubMed] [Google Scholar]
- Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–52. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Parnes LS. The biology of intratympanic drug administration and pharmacodynamics of round window drug absorption. Otolaryngol Clin North Am. 2004;37:1035–51. doi: 10.1016/j.otc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Battmer RD, O'Donoghue GM, Lenarz T. A multicenter study of device failure in European cochlear implant centers. Ear Hear. 2007;28:95S–99S. doi: 10.1097/AUD.0b013e3180315502. [DOI] [PubMed] [Google Scholar]
- Beck T, Lindholm D, Castren E, Wree A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J Cereb Blood Flow Metab. 1994;14:689–92. doi: 10.1038/jcbfm.1994.86. [DOI] [PubMed] [Google Scholar]
- Biran R, Noble MD, Tresco PA. Characterization of cortical astrocytes on materials of differing surface chemistry. J Biomed Mater Res. 1999;46:150–9. doi: 10.1002/(sici)1097-4636(199908)46:2<150::aid-jbm3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Pyman BC, Gordon M, Clark GM, Brown AM, Dowell RC, Hollow RD. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol. 1992;101:342–8. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–8. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Breuskin I, Bodson M, Thelen N, Thiry M, Nguyen L, Belachew S, Lefebvre PP, Malgrange B. Strategies to regenerate hair cells: identification of progenitors and critical genes. Hear Res. 2008;236:1–10. doi: 10.1016/j.heares.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118:7S–14S. doi: 10.1097/01.prs.0000234900.26676.0b. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–72. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Buch PK, Ali RR, Maclaren RE. Neuroprotective gene therapy for the treatment of inherited retinal degeneration. Curr Gene Ther. 2007;7:434–45. doi: 10.2174/156652307782793531. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–9. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–74. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cervera-Paz FJ, Manrique MJ. Traditional and emerging indications in cochlear and auditory brainstem implants. Rev Laryngol Otol Rhinol (Bord) 2005;126:287–92. [PubMed] [Google Scholar]
- Chan DK, Lieberman DM, Musatov S, Goldfein JA, Selesnick SH, Kaplitt MG. Protection against cisplatin-induced ototoxicity by adeno-associated virus-mediated delivery of the X-linked inhibitor of apoptosis protein is not dependent on caspase inhibition. Otol Neurotol. 2007;28:417–25. doi: 10.1097/01.mao.0000247826.28893.7a. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar SS, Rubinstein RY, Kwartler JA, Gatz M, Connelly PE, Huang E, Baredes S. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521–8. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- Chen X, Frisina RD, Bowers WJ, Frisina DR, Federoff HJ. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol Ther. 2001;3:958–63. doi: 10.1006/mthe.2001.0334. [DOI] [PubMed] [Google Scholar]
- Chen Z, Duan M, Lee H, Ruan R, Ulfendahl M. Pharmacokinetics of caroverine in the inner ear and its effects on cochlear function after systemic and local administrations in Guinea pigs. Audiol Neurootol. 2003;8:49–56. doi: 10.1159/000067893. [DOI] [PubMed] [Google Scholar]
- Cheung KC. Implantable microscale neural interfaces. Biomed Microdevices. 2007;9:923–38. doi: 10.1007/s10544-006-9045-z. [DOI] [PubMed] [Google Scholar]
- Chin PC, D'Mello SR. Brain chemotherapy from the bench to the clinic: Targeting neuronal survival with small molecule inhibitors of apoptosis. Frontiers in Bioscience. 2005;10:552–568. doi: 10.2741/1551. [DOI] [PubMed] [Google Scholar]
- Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25:2685–94. doi: 10.1634/stemcells.2007-0393. [DOI] [PubMed] [Google Scholar]
- Coles RR, Thompson AC, O'Donoghue GM. Intra-tympanic injections in the treatment of tinnitus. Clin Otolaryngol Allied Sci. 1992;17:240–2. doi: 10.1111/j.1365-2273.1992.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–8. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- Crumling MA, Raphael Y. Manipulating gene expression in the mature inner ear. Brain Res. 2006;1091:265–9. doi: 10.1016/j.brainres.2006.01.075. [DOI] [PubMed] [Google Scholar]
- Cui XY, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensors and Actuators B-Chemical. 2003;89:92–102. [Google Scholar]
- Dallos P. Overview: cochlear neurobiology Springer. Berlin Heidleberg; New York: 1996. [Google Scholar]
- Darlington CL, Smith PF. Drug treatments for tinnitus. Prog Brain Res. 2007;166:249–62. doi: 10.1016/S0079-6123(07)66023-3. [DOI] [PubMed] [Google Scholar]
- Deggouj N, Castelein S, Gérard JM, Gersdorff M, Garin P. Today's indications for cochlear implantation. B-ENT. 2007;3:9–14. [PubMed] [Google Scholar]
- Demanez JP, Demanez L. Anatomophysiology of the central auditory nervous system: basic concepts. Acta Otorhinolaryngol Belg. 2003;57:227–36. [PubMed] [Google Scholar]
- Di Girolamo S, Ottaviani F, Saccoccio A, Giacomini PG. Functional outcome of auditory implants in hearing loss. Acta Neurochir Suppl. 2007;97:425–9. doi: 10.1007/978-3-211-33081-4_48. [DOI] [PubMed] [Google Scholar]
- Diem R, Sattler MB, Bahr M. Neurodegeneration and -protection in autoimmune CNS inflammation. JOURNAL OF NEUROIMMUNOLOGY. 2007;184:27–36. doi: 10.1016/j.jneuroim.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Flinn P, Bao J, Venya R, Hayes RL. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol. 1997;146:479–90. doi: 10.1006/exnr.1997.6557. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29:525–37. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Doughty ML, Delhaye-Bouchaud N, Mariani J, Lohof A, Campana A. Neurotrophin-3 promotes cerebellar granule cell exit from the EGL. Eur J Neurosci. 1998;10:3007–11. doi: 10.1111/j.1460-9568.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- Duan M, Venail F, Spencer N, Mezzina M. Treatment of peripheral sensorineural hearing loss: gene therapy. Gene Ther. 2004a;11(Suppl 1):S51–6. doi: 10.1038/sj.gt.3302369. [DOI] [PubMed] [Google Scholar]
- Duan ML, Ulfendahl M, Laurell G, Counter AS, Pyykko I, Borg E, Rosenhall U. Protection and treatment of sensorineural hearing disorders caused by exogenous factors: experimental findings and potential clinical application. Hear Res. 2002;169:169–78. doi: 10.1016/s0378-5955(02)00484-7. [DOI] [PubMed] [Google Scholar]
- Duan YY, Clark GM, Cowan RS. A study of intra-cochlear electrodes and tissue interface by electrochemical impedance methods in vivo. Biomaterials. 2004b;25:3813–28. doi: 10.1016/j.biomaterials.2003.09.107. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci Lett. 1992;145:51–4. doi: 10.1016/0304-3940(92)90201-h. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. GABA alters the discharge pattern of chopper neurons in the rat ventral cochlear nucleus. Hear Res. 1995;91:160–6. doi: 10.1016/0378-5955(96)83100-5. [DOI] [PubMed] [Google Scholar]
- Edgerton BJ, House WF, Hitselberger W. Hearing by cochlear nucleus stimulation in humans. Ann Otol Rhinol Laryngol Suppl. 1982;91:117–24. [PubMed] [Google Scholar]
- Ehrenberger K, Felix D. Caroverine depresses the activity of cochlear glutamate receptors in guinea pigs: in vivo model for drug-induced neuroprotection? Neuropharmacology. 1992;31:1259–63. doi: 10.1016/0028-3908(92)90054-s. [DOI] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, Iwai K, Tabata Y, Ito J. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–20. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- Fayad J, Linthicum FH, Jr., Otto SR, Galey FR, House WF. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–11. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Felix D, Ehrenberger K. A microiontophoretic study of the role of excitatory amino acids at the afferent synapses of mammalian inner hair cells. Eur Arch Otorhinolaryngol. 1990;248:1–3. doi: 10.1007/BF00634769. [DOI] [PubMed] [Google Scholar]
- Felix D, Ehrenberger K. The efferent modulation of mammalian inner hair cell afferents. Hear Res. 1992;64:1–5. doi: 10.1016/0378-5955(92)90163-h. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Ambrosio S, Martí E, Krupinski J, Goutan E, Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101:229–38. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- Friedman LK. Calcium - A role for neuroprotection and sustained adaptation. Molecular Interventions. 2006;6:315–329. doi: 10.1124/mi.6.6.5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–93. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Geetha S, Rao CRK, Vijayan M, Trivedi DC. Biosensing and drug delivery by polypyrrole. Analytica Chimica Acta. 2006;568:119–125. doi: 10.1016/j.aca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–33. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. Journal of Biomedical Materials Research Part A. 2007;81A:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–47. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Progress in Polymer Science. 2007;32:876–921. [Google Scholar]
- Guitton MJ, Wang J, Puel JL. New pharmacological strategies to restore hearing and treat tinnitus. Acta Otolaryngol. 2004;124:411–5. doi: 10.1080/00016480310000665. [DOI] [PubMed] [Google Scholar]
- Hahn H, Kammerer B, DiMauro A, Salt AN, Plontke SK. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hear Res. 2006;212:236–44. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanekom T. Modelling encapsulation tissue around cochlear implant electrodes. Med Biol Eng Comput. 2005;43:47–55. doi: 10.1007/BF02345122. [DOI] [PubMed] [Google Scholar]
- Hara A, Salt AN, Thalmann R. Perilymph composition in scala tympani of the cochlea: influence of cerebrospinal fluid. Hear Res. 1989;42:265–71. doi: 10.1016/0378-5955(89)90150-0. [DOI] [PubMed] [Google Scholar]
- Hara H. Molecular mechanism of neuroprotective drugs against oxidative stress-induced neuronal cell death. Yakugaku Zasshi-Journal of the Pharmaceutical Society of Japan. 2007;127:1199–1205. doi: 10.1248/yakushi.127.1199. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104:311–9. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Iwasaki S, Mizuta K, Arai M, Mineta H. Pattern of cochlear damage caused by short-term kanamycin application using the round window microcatheter method. Acta Otolaryngol. 2007;127:116–21. doi: 10.1080/00016480600794438. [DOI] [PubMed] [Google Scholar]
- Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, Doi T, Kuriyama H, Miller JM, Yamashita T. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. ENDOCRINE. 2006;29:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–69. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–82. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol. 2003;4:176–95. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Salt AN. Permeability changes of the blood-labyrinth barrier measured in vivo during experimental treatments. Hear Res. 1992;61:12–8. doi: 10.1016/0378-5955(92)90030-q. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr. Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1998;107:906–11. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–97. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Ito J, Endo T, Nakagawa T, Kita T, Kim TS, Iguchi F. A new method for drug application to the inner ear. ORL J Otorhinolaryngol Relat Spec. 2005;67:272–5. doi: 10.1159/000089407. [DOI] [PubMed] [Google Scholar]
- Itoh A, Sakata E. Treatment of vestibular disorders. Acta Otolaryngol Suppl. 1991;481:617–23. doi: 10.3109/00016489109131486. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim TS, Tabata Y, Ito J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–33. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- James DP, Eastwood H, Richardson RT, O'Leary SJ. Effects of round window dexamethasone on residual hearing in a Guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13:86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Loeffler KA, Burne RA, Jolly CN, Antonelli PJ. Biofilm formation in cochlear implants with cochlear drug delivery channels in an in vitro model. Otolaryngol Head Neck Surg. 2007;136:577–82. doi: 10.1016/j.otohns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Juhn SK. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP, Prado S. Nature of blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol Laryngol. 1981;90:135–41. doi: 10.1177/000348948109000208. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- Kamiya K, Okamoto Y, Kusano R, Kouike H, Fujinami Y, Hoya N, Satoh H, Komatsuzaki R, Nakagawa S. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–26. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–81. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118:313–26. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Ketten DR, Skinner MW, Wang G, Vannier MW, Gates GA, Neely JG. In vivo measures of cochlear length and insertion depth of nucleus cochlear implant electrode arrays. Ann Otol Rhinol Laryngol Suppl. 1998;175:1–16. [PubMed] [Google Scholar]
- Khan AM, Handzel O, Damian D, Eddington DK, Nadol JB., Jr. Effect of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann Otol Rhinol Laryngol. 2005;114:381–5. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, Baumgartner W, Pok SM, Tillein J, Ye Q, von Ilberg C. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–80. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Kim D, Richardson-Burns SM, Hendricks JL, Sequera C, Martin DC. Effect of Immobilized Nerve Growth Factor on Conductive Polymers: Electrical Properties and Cellular Response. Advanced Functional Materials. 2007;17:79–86. [Google Scholar]
- Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Kingma GG, Miller JM, Myers MW. Chronic drug infusion into the scala tympani of the guinea pig cochlea. J Neurosci Methods. 1992;45:127–34. doi: 10.1016/0165-0270(92)90050-n. [DOI] [PubMed] [Google Scholar]
- Kinnari TJ, Jero J. Experimental and clinical experience of albumin coating of tympanostomy tubes. Otolaryngol Head Neck Surg. 2005;133:596–600. doi: 10.1016/j.otohns.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Kinnari TJ, Salonen EM, Jero J. New method for coating tympanostomy tubes to prevent tube occlusions. Int J Pediatr Otorhinolaryngol. 2001;58:107–11. doi: 10.1016/s0165-5876(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Kinnari TJ, Rihkanen H, Laine T, Salonen EM, Jero J. Albumin-coated tympanostomy tubes: prospective, double-blind clinical study. Laryngoscope. 2004;114:2038–43. doi: 10.1097/01.mlg.0000147944.20676.17. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S, Oestreicher E, Arnold T, Ehrenberger K, Felix D. Metabotropic glutamate receptors group I are involved in cochlear neurotransmission. Neuroreport. 1999;10:1879–82. doi: 10.1097/00001756-199906230-00015. [DOI] [PubMed] [Google Scholar]
- Kontturi K, Pentti P, Sundholm G. Polypyrrole as a model membrane for drug delivery. Journal of Electroanalytical Chemistry. 1998;453:231–238. [Google Scholar]
- Kros A, Sommerdijk NAJM, Nolte RJM. Poly(pyrrole) versus poly (3,4-ethylenedioxythiophene): implications for biosensor applications. Sensors and Actuators B-Chemical. 2005;106:289–295. [Google Scholar]
- Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, Mhatre A. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–81. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112:1325–34. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Laszig R, Ridder GJ, Fradis M. Intracochlear insertion of electrodes using hyaluronic acid in cochlear implant surgery. J Laryngol Otol. 2002;116:371–2. doi: 10.1258/0022215021910816. [DOI] [PubMed] [Google Scholar]
- Leake-Jones PA, Rebscher SJ. Cochlear pathology with chronically implanted scala tympani electrodes. Ann N Y Acad Sci. 1983;405:203–23. doi: 10.1111/j.1749-6632.1983.tb31634.x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res. 1991;54:251–71. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Lee K, He J, Clement R, Massia S, Kim B. Biocompatible benzocyclobutene (BCB)-based neural implants with micro-fluidic channel. Biosens Bioelectron. 2004;20:404–7. doi: 10.1016/j.bios.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lee KY, Nakagawa T, Okano T, Hori R, Ono K, Tabata Y, Lee SH, Ito J. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol Neurotol. 2007;28:976–81. doi: 10.1097/MAO.0b013e31811f40db. [DOI] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Patrick JF, Anderson DJ, Lenarz T. Electrophysiological validation of a human prototype auditory midbrain implant in a guinea pig model. J Assoc Res Otolaryngol. 2006a;7:383–98. doi: 10.1007/s10162-006-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Lenarz T, Reich U, Marquardt N, Klingberg MN, Paasche G, Reuter G, Stan AC. Auditory midbrain implant: histomorphologic effects of long-term implantation and electric stimulation of a new deep brain stimulation array. Otol Neurotol. 2007;28:1045–52. doi: 10.1097/MAO.0b013e318159e74f. [DOI] [PubMed] [Google Scholar]
- Lenarz T, Lim HH, Reuter G, Patrick JF, Lenarz M. The auditory midbrain implant: a new auditory prosthesis for neural deafness-concept and device description. Otol Neurotol. 2006b;27:838–43. doi: 10.1097/01.mao.0000232010.01116.e9. [DOI] [PubMed] [Google Scholar]
- Lenarz T, Moshrefi M, Matthies C, Frohne C, Lesinski-Schiedat A, Illg A, Rost U, Battmer RD, Samii M. Auditory brainstem implant: part I. Auditory performance and its evolution over time. Otol Neurotol. 2001;22:823–33. doi: 10.1097/00129492-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Lescot T, Marchand-Verrecchia C, Puybasset L. Anti-inflammatory modulators in traumatic brain injury. Annalles Francaises D'Anesthesie et de Reanimation. 2006;25:755–760. doi: 10.1016/j.annfar.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Levivier M, Przedborski S, Bencsics C, Kang UJ. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci. 1995;15:7810–20. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PMMC, Somdas MA, Eddington DK, Nadol JB. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Annals of Otology Rhinology and Laryngology. 2007;116:731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant. J Neurophysiol. 2006;96:975–88. doi: 10.1152/jn.01112.2005. [DOI] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Anderson DJ, Lenarz M. The auditory midbrain implant: Effects of electrode location. Hear Res This issue. doi: 10.1016/j.heares.2008.02.003. This issue. [DOI] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Joseph G, Battmer RD, Samii A, Samii M, Patrick JF, Lenarz M. Electrical stimulation of the midbrain for hearing restoration: insight into the functional organization of the human central auditory system. J Neurosci. 2007;27:13541–51. doi: 10.1523/JNEUROSCI.3123-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum FH, Jr., Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991;12:245–311. [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–42. [PubMed] [Google Scholar]
- Martinez-Monedero R, Glowatzki E, Oshima K, Edge ASB, Yi E. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008 doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- Massoumi B, Entezami A. Controlled release of sulfosalicylic acid during electrochemical switching of conducting polymer bilayers. European Polymer Journal. 2001;37:1015–1020. [Google Scholar]
- Matsui JI, Parker MA, Ryals BM, Cotanche DA. Regeneration and replacement in the vertebrate inner ear. Drug discovery today. 2005;10:1307–12. doi: 10.1016/S1359-6446(05)03577-4. [DOI] [PubMed] [Google Scholar]
- Maurer J, Marangos N, Ziegler E. Reliability of cochlear implants. Otolaryngol Head Neck Surg. 2005;132:746–50. doi: 10.1016/j.otohns.2005.01.026. [DOI] [PubMed] [Google Scholar]
- McCreery D, Lossinsky A, Pikov V. Performance of multisite silicon microprobes implanted chronically in the ventral cochlear nucleus of the cat. IEEE Trans Biomed Eng. 2007;54:1042–52. doi: 10.1109/TBME.2007.891167. [DOI] [PubMed] [Google Scholar]
- McCreery DB. Cochlear nucleus auditory prostheses. Hear Res This issue. doi: 10.1016/j.heares.2007.11.014. This issue. [DOI] [PubMed] [Google Scholar]
- McDermott KL, Raghupathi R, Fernandez SC, Saatman KE, Protter AA, Finklestein SP, Sinson G, Smith DH, McIntosh TK. Delayed administration of basic fibroblast growth factor (bFGF) attenuates cognitive dysfunction following parasagittal fluid percussion brain injury in the rat. J Neurotrauma. 1997;14:191–200. doi: 10.1089/neu.1997.14.191. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–72. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Intraneural stimulation for auditory prostheses: Peripheral and intra-cranial stimulation sites. Hear Res This issue. doi: 10.1016/j.heares.2008.04.001. This issue. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Bierer JA, Snyder RL. Cochlear implants: the view from the brain. Current Opinion in Neurobiology. 2005;15:488–493. doi: 10.1016/j.conb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Miller AL, Prieskorn DM, Altschuler RA, Miller JM. Mechanism of electrical stimulation-induced neuroprotection: effects of verapamil on protection of primary auditory afferents. Brain Res. 2003;966:218–30. doi: 10.1016/s0006-8993(02)04170-7. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Minoda R, Izumikawa M, Kawamoto K, Raphael Y. Strategies for replacing lost cochlear hair cells. Neuroreport. 2004;15:1089–92. doi: 10.1097/00001756-200405190-00001. [DOI] [PubMed] [Google Scholar]
- Mochizuki H. Gene therapy for Parkinson's disease. Expert Rev Neurother. 2007;7:957–60. doi: 10.1586/14737175.7.8.957. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH., Jr. The human auditory system: a timeline of development. Int J Audiol. 2007;46:460–78. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr. Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–54. doi: 10.1016/0378-5955(90)90101-t. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Eddington DK. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol. 2006;64:31–49. doi: 10.1159/000094643. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr., Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–91. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ito J. Cell therapy for inner ear diseases. Curr Pharm Des. 2005;11:1203–7. doi: 10.2174/1381612053507530. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–43. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000;17:1219–31. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Control Release. 2006;111:252–62. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Neeves KB, Sawyer AJ, Foley CP, Saltzman WM, Olbricht WL. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles. Brain Res. 2007;1180:121–32. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien PC, Tung TS, Ho KC. Amperometric glucose biosensor based on entrapment of glucose oxidase in a poly(3,4-ethylenedioxythiophene) film. Electroanalysis. 2006;18:1408–1415. [Google Scholar]
- Noushi F, Richardson RT, Hardman J, Clark G, O'Leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–33. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- Oertel R, Kirch W, Klemm E. Prednisolone concentration in the cochlea of patients with perilymph fistula. Pharmazie. 2007;62:239–40. [PubMed] [Google Scholar]
- Oestreicher E, Knipper M, Arnold A, Zenner HP, Felix D. Neurotrophin 3 potentiates glutamatergic responses of IHC afferents in the cochlea in vivo. Eur J Neurosci. 2000;12:1584–90. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Kita T, Endo T, Ito J. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol Ther. 2006;14:866–71. doi: 10.1016/j.ymthe.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Olivius P, Alexandrov L, Miller J, Ulfendahl M, Bagger-Sjoback D, Kozlova EN. Allografted fetal dorsal root ganglion neuronal survival in the guinea pig cochlea. Brain Res. 2003;979:1–6. doi: 10.1016/s0006-8993(03)02802-6. [DOI] [PubMed] [Google Scholar]
- Otto SR, Brackmann DE, Hitselberger WE, Shannon RV, Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96:1063–71. doi: 10.3171/jns.2002.96.6.1063. [DOI] [PubMed] [Google Scholar]
- Oxley KS, Thomas JG, Ramadan HH. Effect of ototopical medications on tympanostomy tube biofilms. Laryngoscope. 2007;117:1819–24. doi: 10.1097/MLG.0b013e3180d09ede. [DOI] [PubMed] [Google Scholar]
- Paasche G, Bogel L, Leinung M, Lenarz T, Stover T. Substance distribution in a cochlea model using different pump rates for cochlear implant drug delivery electrode prototypes. Hear Res. 2006;212:74–82. doi: 10.1016/j.heares.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Paasche G, Gibson P, Averbeck T, Becker H, Lenarz T, Stover T. Technical report: modification of a cochlear implant electrode for drug delivery to the inner ear. Otol Neurotol. 2003;24:222–7. doi: 10.1097/00129492-200303000-00016. [DOI] [PubMed] [Google Scholar]
- Papageorgiou DP, Wise KD, Shore SE, Bledsoe Jr SC. A shuttered neural probe with on-chip flowmeters for chronic in vivo drug delivery. Journal of Microelectromechanical Systems. 2006;15:1025–1033. [Google Scholar]
- Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007;232:29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic TR, Rubel EW. Rapid changes in cochlear nucleus cell size following blockade of auditory nerve electrical activity in gerbils. J Comp Neurol. 1989;283:474–80. doi: 10.1002/cne.902830403. [DOI] [PubMed] [Google Scholar]
- Patel NP, Mhatre AN, Lalwani AK. Biological therapy for the inner ear. Expert Opin Biol Ther. 2004;4:1811–9. doi: 10.1517/14712598.4.11.1811. [DOI] [PubMed] [Google Scholar]
- Pernaut JM, Reynolds JR. Use of conducting electroactive polymers for drug delivery and sensing of bioactive molecules. A redox chemistry approach. Journal of Physical Chemistry B. 2000;104:4080–4090. [Google Scholar]
- Persidsky Y, Kanmogne GD, Ramirez SH, Haorah J. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–36. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Richardson RT, Wise AK, O'Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1138–48. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D. Relation of cochlear implant function to histopathology in monkeys. Ann N Y Acad Sci. 1983;405:224–39. doi: 10.1111/j.1749-6632.1983.tb31635.x. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, Bohne BA. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol. 1981;92:1–13. doi: 10.3109/00016488109133232. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Plinkert PK, Plinkert B, Koitschev A, Zenner HP, Lowenheim H. Transtympanic endoscopy for drug delivery to the inner ear using a new microendoscope. Adv Otorhinolaryngol. 2002;59:149–55. doi: 10.1159/000059253. [DOI] [PubMed] [Google Scholar]
- Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–21. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Baker K, Weich CM, Plinkert PK, Staecker H. Hearing preservation after inner ear gene therapy: the effect of vector and surgical approach. ORL J Otorhinolaryngol Relat Spec. 2003;65:211–4. doi: 10.1159/000073117. [DOI] [PubMed] [Google Scholar]
- Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140:212–5. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Quester R, Klosterhalfen B, Stutzer H, Schroder R, Klug N. Polyester meshes and adhesive materials in the brain: comparative research in rats to optimize surgical strategy. J Neurosurg. 2002;96:760–9. doi: 10.3171/jns.2002.96.4.0760. [DOI] [PubMed] [Google Scholar]
- Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. Biocompatibility of polypyrrole particles: an in-vivo study in mice. J Pharm Pharmacol. 2007;59:311–5. doi: 10.1211/jpp.59.2.0017. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–41. doi: 10.1016/0304-3940(96)12499-x. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Kim YH, Osumi Y, Izumikawa M. Non-sensory cells in the deafened organ of Corti: approaches for repair. International Journal of Developmental Biology. 2007;51:649–654. doi: 10.1387/ijdb.072370yr. [DOI] [PubMed] [Google Scholar]
- Rebscher SJ, Hetherington AM, Snyder RL, Leake PA, Bonham BH. Design and fabrication of multichannel cochlear implants for animal research. J Neurosci Methods. 2007;166:1–12. doi: 10.1016/j.jneumeth.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–7. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retterer ST, Smith KL, Bjornsson CS, Neeves KB, Spence AJ, Turner JN, Shain W, Isaacson MS. Model neural prostheses with integrated microfluidics: a potential intervention strategy for controlling reactive cell and tissue responses. IEEE Trans Biomed Eng. 2004;51:2063–73. doi: 10.1109/TBME.2004.834288. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Noushi F, O'Leary S. Inner ear therapy for neural preservation. Audiol Neurootol. 2006;11:343–56. doi: 10.1159/000095896. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Thompson B, Moulton S, Newbold C, Lum MG, Cameron A, Wallace G, Kapsa R, Clark G, O'Leary S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials. 2007;28:513–23. doi: 10.1016/j.biomaterials.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Rogowski M, Reiss G, Lehnhardt E. Morphologic study of the guinea pig cochlea after cochlear implantation using the “soft surgery” technique. Ann Otol Rhinol Laryngol Suppl. 1995;166:434–6. [PubMed] [Google Scholar]
- Rybak LP, Somani S. Ototoxicity. Amelioration by protective agents. Ann N Y Acad Sci. 1999;884:143–51. [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–21. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- Sakata E, Ito Y, Itoh A. Clinical Experiences of Steroid Targeting Therapy to Inner Ear for Control of Tinnitus. Int Tinnitus J. 1997;3:117–121. [PubMed] [Google Scholar]
- Sakata H, Kojima Y, Koyama S, Furuya N, Sakata E. Treatment of cochlear tinnitus with transtympanic infusion of 4% lidocaine into the tympanic cavity. Int Tinnitus J. 2001;7:46–50. [PubMed] [Google Scholar]
- Salt AN. Simulation of methods for drug delivery to the cochlear fluids. Adv Otorhinolaryngol. 2002;59:140–8. doi: 10.1159/000059251. [DOI] [PubMed] [Google Scholar]
- Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hear Res. 2003;182:24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006;153:121–9. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman WM, Duenas ET, Cleland JL, Mak MW, Mahoney MJ. Intracranial delivery of recombinant nerve growth factor: release kinetics and protein distribution for three delivery systems. Pharm Res. 1999;16:232–40. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–6. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE. Auditory brainstem implants. Neurotherapeutics. 2008;5:128–136. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD, Van De Water TR. Pharmacologic manipulation of the labyrinth with novel and traditional agents delivered to the inner ear. Ear Nose Throat J. 2003;82:276–80. 282–3, 287–8 passim. [PubMed] [Google Scholar]
- Seidman MD, Vivek P. Intratympanic treatment of hearing loss with novel and traditional agents. Otolaryngol Clin North Am. 2004;37:973–90. doi: 10.1016/j.otc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Seki Y, Samejima N, Komatsuzaki A. Auditory brainstem implants: current state and future directions with special reference to the subtonsillar approach for implantation. Acta Neurochir Suppl. 2007;97:431–5. doi: 10.1007/978-3-211-33081-4_49. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Kojima K, Matsumoto M, Holley MC, Ito J. Rebuilding lost hearing using cell transplantation. Neurosurgery. 2007;60:417–33. doi: 10.1227/01.NEU.0000249189.46033.42. discussion 433. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Kojima K, Matsumoto M, Kim TS, Tamura T, Ito J. Cell transplantation to the auditory nerve and cochlear duct. Exp Neurol. 2006;198:12–24. doi: 10.1016/j.expneurol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Selivanova O, Maurer J, Ecke U, Mann WJ. The effects of Streptolysin-O and sodium hyaluronate on the permeability of the round window membrane in guinea pigs--an electrophysiologic study. Laryngorhinootologie. 2003;82:235–9. doi: 10.1055/s-2003-38937. [DOI] [PubMed] [Google Scholar]
- Sennaroglu L, Dini FM, Sennaroglu G, Gursel B, Ozkan S. Transtympanic dexamethasone application in Meniere's disease: an alternative treatment for intractable vertigo. J Laryngol Otol. 1999;113:217–21. doi: 10.1017/s0022215100143610. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Fayad J, Moore J, Lo WW, Otto S, Nelson RA, O'Leary M. Auditory brainstem implant: II. Postsurgical issues and performance. Otolaryngol Head Neck Surg. 1993;108:634–42. doi: 10.1177/019459989310800603. [DOI] [PubMed] [Google Scholar]
- Shen F, Wen L, Yang XF, Liu W. The potential application of gene therapy in the treatment of traumatic brain injury. Neurosurgical Review. 2007;30:291–298. doi: 10.1007/s10143-007-0094-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 2002a;6:305–18. doi: 10.1159/000046843. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002b;172:92–8. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear Res. 1994;81:150–66. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shi FX, Corrales CE, Liberman MC, Edge ASB. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. European Journal of Neuroscience. 2007;26:3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Shoji F, Yamasoba T, Magal E, Dolan DF, Altschuler RA, Miller JM. Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res. 2000a;142:41–55. doi: 10.1016/s0378-5955(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000b;146:134–42. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Silverstein H. Use of a new device, the MicroWick, to deliver medication to the inner ear. Ear Nose Throat J. 1999;78:595–8. 600. [PubMed] [Google Scholar]
- Silverstein H, Thompson J, Rosenberg SI, Brown N, Light J. Silverstein MicroWick. Otolaryngol Clin North Am. 2004;37:1019–34. doi: 10.1016/j.otc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Sinson G, Voddi M, McIntosh TK. Nerve growth factor administration attenuates cognitive but not neurobehavioral motor dysfunction or hippocampal cell loss following fluid-percussion brain injury in rats. J Neurochem. 1995;65:2209–16. doi: 10.1046/j.1471-4159.1995.65052209.x. [DOI] [PubMed] [Google Scholar]
- Sinson G, Perri BR, Trojanowski JQ, Flamm ES, McIntosh TK. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J Neurosurg. 1997;86:511–8. doi: 10.3171/jns.1997.86.3.0511. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Ketten DR, Holden LK, Harding GW, Smith PG, Gates GA, Neely JG, Kletzker GR, Brunsden B, Blocker B. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3:332–50. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smela E. Conjugated polymer actuators for biomedical applications. Advanced Materials. 2003;15:481–494. [Google Scholar]
- Somdas MA, Li PM, Whiten DM, Eddington DK, Nadol JB., Jr. Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiol Neurootol. 2007;12:277–84. doi: 10.1159/000103208. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–75. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Staecker H, Brough DE, Praetorius M, Baker K. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37:1091–108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007a;28:223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Staecker H, Liu W, Malgrange B, Lefebvre PP, Van De Water TR. Vector-mediated delivery of bcl-2 prevents degeneration of auditory hair cells and neurons after injury. ORL J Otorhinolaryngol Relat Spec. 2007b;69:43–50. doi: 10.1159/000096716. [DOI] [PubMed] [Google Scholar]
- Stjernholm C. Aspects of temporal bone anatomy and pathology in conjunction with cochlear implant surgery. Acta Radiol Suppl. 2003;430:2–15. [PubMed] [Google Scholar]
- Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–30. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Stover T, Paasche G, Lenarz T, Ripken T, Breitenfeld P, Lubatschowski H, Fabian T. Development of a drug delivery device: using the femtosecond laser to modify cochlear implant electrodes. Cochlear Implants Int. 2007;8:38–52. doi: 10.1179/cim.2007.8.1.38. [DOI] [PubMed] [Google Scholar]
- Strenzke N, Pauli-Magnus D, Meyer A, Brandt A, Maier H, Moser T. Update on physiology and pathophysiology of the inner ear: pathomechanisms of sensorineural hearing loss. HNO. 2008;56:27–36. doi: 10.1007/s00106-007-1640-7. [DOI] [PubMed] [Google Scholar]
- Sutton D. Cochlear implant effects on the spiral ganglion. Ann Otol Rhinol Laryngol. 1983;92:316. doi: 10.1177/000348948309200325. [DOI] [PubMed] [Google Scholar]
- Sweeney MI. Neuroprotective effects of adenosine in cerebral ischemia, window of opportunity. Neuroscience and Biobehavioral Reviews. 1997;21:207–217. doi: 10.1016/s0149-7634(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K, Suzuki T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip. 2005;5:519–23. doi: 10.1039/b417497f. [DOI] [PubMed] [Google Scholar]
- Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T, Mizushima Y, Higaki M, Ito J. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–5. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- Tang LS, Montemayor C, Pereira FA. Sensorineural hearing loss: potential therapies and gene targets for drug development. IUBMB Life. 2006;58:525–30. doi: 10.1080/15216540600913258. [DOI] [PubMed] [Google Scholar]
- Terr LI, Mobley JP, House WF. Biocompatibility of the central electroauditory prosthesis and the human cochlear nuclei. Am J Otol. 1989;10:339–42. [PubMed] [Google Scholar]
- Thompson BC, Moulton SE, Ding J, Richardson R, Cameron A, O'Leary S, Wallace GG, Clark GM. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J Control Release. 2006;116:285–94. doi: 10.1016/j.jconrel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Tsai HKA, Ma KS, Wang C, Xu H, Wang CL, Zoval J, Kulinsky L, Madou M. Development of integrated protection for a miniaturized drug delivery system. Smart Materials & Structures. 2007;16:S295–S299. [Google Scholar]
- Tsukahara T, Yonekawa Y, Tanaka K, Ohara O, Wantanabe S, Kimura T, Nishijima T, Taniguchi T. The role of brain-derived neurotrophic factor in transient forebrain ischemia in the rat brain. Neurosurgery. 1994;34:323–31. doi: 10.1227/00006123-199402000-00016. [DOI] [PubMed] [Google Scholar]
- Tyler R, Parkinson AJ, Fryauf-Bertchy H, Lowder MW, Parkinson WS, Gantz BJ, Kelsay DM. Speech perception by prelingually deaf children and postlingually deaf adults with cochlear implant. Scand Audiol Suppl. 1997;46:65–71. [PubMed] [Google Scholar]
- Ulfendahl M, Hu ZQ, Olivius P, Duan ML, Wei DG. A cell therapy approach to substitute neural elements in the inner ear. Physiology & Behavior. 2007;92:75–79. doi: 10.1016/j.physbeh.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Uneri C, Baglam T, Yazici M. The effect of Vitamin E treatment on the development of myringosclerosis after ventilation tube insertion. Int J Pediatr Otorhinolaryngol. 2006a;70:1045–8. doi: 10.1016/j.ijporl.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Uneri C, Sari M, Akboga J, Yuksel M. Vitamin e-coated tympanostomy tube insertion decreases the quantity of free radicals in tympanic membrane. Laryngoscope. 2006b;116:140–3. doi: 10.1097/01.mlg.0000191460.32862.bf. [DOI] [PubMed] [Google Scholar]
- Van Wijck F, Staecker H, Lefebvre PP. Topical steroid therapy using the Silverstein Microwicktrade mark in sudden sensorineural hearing loss after failure of conventional treatment. Acta Otolaryngol. 2007:1–6. doi: 10.1080/00016480601126952. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Wildmann J, Altar CA. Brain-derived neurotrophic factor prevents the loss of nigral neurons induced by excitotoxic striatal-pallidal lesions. Neuroscience. 1998;83:741–8. doi: 10.1016/s0306-4522(97)00424-7. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. Journal of Controlled Release. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Fitzakerley JL. Activity-dependent responses of developing cochlear nuclear neurons to microionophoretically-applied amino acids. Hear Res. 1995;84:194–204. doi: 10.1016/0378-5955(95)00011-r. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Shapiro WH. The benefits of cochlear implantation in the geriatric population. Otolaryngol Head Neck Surg. 1993;108:329–33. doi: 10.1177/019459989310800404. [DOI] [PubMed] [Google Scholar]
- Warnecke A, Wissel K, Hoffmann A, Hofmann N, Berkingali N, Gross G, Lenarz T, Stover T. The biological effects of cell-delivered brain-derived neurotrophic factor on cultured spiral ganglion cells. Neuroreport. 2007;18:1683–1686. doi: 10.1097/WNR.0b013e3282f0b5d7. [DOI] [PubMed] [Google Scholar]
- Weber PC. Medical and surgical considerations for implantable hearing prosthetic devices. Am J Audiol. 2002;11:134–8. doi: 10.1044/1059-0889(2002/018). [DOI] [PubMed] [Google Scholar]
- Weiss MA, Frisancho JC, Roessler BJ, Raphael Y. Viral-mediated gene transfer in the cochlea. Int J Dev Neurosci. 1997;15:577–83. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190:1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Williams JC, Rousche P, Holecko Ii MM, Kipke DR, Massia SP. Multi-site incorporation of bioactive matrices into MEMS-based neural probes. Journal of Neural Engineering. 2005;2:L23–L28. doi: 10.1088/1741-2560/2/4/L03. [DOI] [PubMed] [Google Scholar]
- Williams JC, Shain W, Hippensteel JA, Kipke DR, Dilgen J. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. Journal of Neural Engineering. 2007;4:410–423. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- Wilson BS. Cochlear implants: A remarkable past and a brilliant future. Hear Res This issue. doi: 10.1016/j.heares.2008.06.005. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XY, Tam KC, Gan LH. Polymeric nanostructures for drug delivery applications based on Pluronic copolymer systems. J Nanosci Nanotechnol. 2006;6:2638–50. doi: 10.1166/jnn.2006.449. [DOI] [PubMed] [Google Scholar]
- Yagi M, Magal E, Sheng Z, Ang KA, Raphael Y. Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor. Human gene therapy. 1999;10:813–23. doi: 10.1089/10430349950018562. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–25. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Wiessner C, Lindholm D, Thoenen H, Hossmann KA. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab Brain Dis. 1997;12:271–80. doi: 10.1007/BF02674671. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999a;815:317–25. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999b;10:769–74. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Yu X, Gilbert R, Bellamkonda RV. Stabilizing electrode-host interfaces: a tissue engineering approach. J Rehabil Res Dev. 2001;38:627–32. [PubMed] [Google Scholar]
- Zhou D, Wallace GG, Spinks GM, Liu L, Cowan R, Saunders E, Newbold C. Actuators for the cochlear implant. Synthetic Metals. 2003;135:39–40. [Google Scholar]
- Zhou QX, Miller LL, Valentine JR. Electrochemically Controlled Binding and Release of Protonated Dimethyldopamine and Other Cations from Poly(N-Methylpyrrole) Polyanion Composite Redox Polymers. Journal of Electroanalytical Chemistry. 1989;261:147–164. [Google Scholar]
- Ziegler D, Suzuki T, Takeuchi S. Fabrication of flexible neural probes with built-in microfluidic channels by thermal bonding of Parylene. Journal of Microelectromechanical Systems. 2006;15:1477–1482. [Google Scholar]