Abstract
The purpose of these analyses was to explore whether physical activity score, leg power or grip strength were associated with tibia and radius estimates of bone strength, cortical density, or total bone area. Peripheral quantitative computed tomography (pQCT) was used to compare tibial and radial bone volumetric density (vBMD, mg/cm3), total (ToA, mm2) and cortical (CoA, mm2) bone area, and estimates of bone compressive strength (bone strength index, BSI) and bending strength (polar strength strain index, SSIp) in a subset (n=1171) of men (≥ 65 years) who participated in the multi-site Osteoporotic Fractures in Men (MrOS) study. Physical activity was assessed by questionnaire (PASE), leg power by Nottingham Power Rig, and grip strength by a hand-held Dynamometer. Participants were categorized into quartiles of PASE, grip strength or leg power. The model was adjusted for age, race, clinic, weight, and limb length. In the tibia, BSI (+7%) and SSIp (+4%) were highest in the most active physically quartile compared to the least active (p<0.05). At the 4% site of the tibia, men with the greatest leg power had both greater ToA (+5%, p<0.001) and BSI (+5.3%, p=0.086) compared to men with the least leg power. At the 66% site of the tibia, the men with the highest leg power, compared to the men with the lowest leg power, had greater ToA (+3%, p=0.045) SSIp (+5%, p=0.008). Similar results were found at both the distal and midshaft of the radius. The findings of this study suggest the importance of maintaining levels of physical activity and muscle strength in older men to prevent bone fragility.
Keywords: Older men, Physical activity, Bone strength, Bone geometry, Peripheral quantitative computed tomography
Introduction
Osteoporosis and related fractures are major public health and economic burdens. While men account for 29% of all osteoporotic fractures and 25% of osteoporosis-related costs in the United States [1], the majority of studies identifying determinants of bone density and strength have been conducted in female populations [2]. Therefore, it is important to identify determinants of bone strength in older men.
According to the mechanostat hypothesis, bones adapt their strength to mechanical loads generated from voluntary mechanical usage [3]. Animal studies of mechanical loading support this theory of bone functional adaptation by consistently showing significant increases in the strength of loaded bones [4,5]. How the force from physiologic mechanical loading is generated on bone has been debated [6]. However, studies have demonstrated that muscle force plays an important role in generating bending moments on bone [7]; and therefore, alteration in bone strength have been shown to follow alterations in muscle activity [8]. Given the important role of muscle force in bone functional adaptation, variables representing muscle mass or strength should be associated with bone strength. However, as recently summarized by Barry and Kohrt [9] exercise effects on bone mineral density (BMD) in human studies are remarkably modest when evident at all, and some clinical studies in cyclists suggest that extreme exercise levels may have negative effects on BMD [9,10].
Most studies exploring the relationship between physical activity and bone health have used DXA-based bone mineral content or areal density (aBMD) outcomes [11–13]. However, animal studies show that bone adapts its strength to changes in mechanical loading by preferentially increasing bone size rather than mass/aBMD [14]. Technology such as peripheral quantitative computed tomography (pQCT) allows for assessment of volumetric bone density, bone geometry and estimates of mechanical strength at the tibia and radius—outcomes that may be more sensitive for assessing the bone response to mechanical loading [15].
Therefore, the primary objective of this study was to examine the association between measures of physical activity, muscle strength and power and estimates of bone strength in community dwelling older men. We hypothesized that estimates of bone strength would be higher in men with higher levels of reported physical activity, muscle strength, and muscle power. We also hypothesized that the higher bone strength among more active men or those with greater muscle strength and power would be due to differences in bone geometry rather than bone volumetric density.
Methods
Participants
Men who were at least 65 years of age were recruited from six communities in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego California) to participate in the prospective Osteoporotic Fractures in Men (MrOS) study [16]. From March 2000 through April 2002, 5995 men with no history of bilateral hip replacement and who were able to walk without assistance of another person were enrolled in the baseline examination. The study design and recruitment methods used by the study have been published elsewhere [17]. The Institutional Review Boards at each center approved the study protocol, and written consent was obtained from all study participants.
Men who returned for their second exam an average of 4.7±0.3 years later were invited to participate in an ancillary study involving pQCT at the Minneapolis and Pittsburgh clinical centers. Of the 1550 men who attended the second exam at the Pittsburgh and Minneapolis sites, 1171 (76%) completed the clinic visit and agreed to participate in the pQCT ancillary study and are included in this analysis. The Institutional Review Boards at Minneapolis and Pittsburgh sites approved this ancillary study and written informed consent was obtained from all participants for the pQCT substudy.
Physical activity, strength and power
Physical activity was assessed with the Physical Activity Scale for the Elderly (PASE) [18] with higher scores indicating a greater level of activity. The Nottingham Power Rig was used to measure leg extension power (W) [19,20]. Participants were given five trials for each leg on the rig and the maximum value from either leg, regardless of whether or not the participant completed all 10 trials, was used in this analysis. Grip strength (kg) was measured twice by a hand held Dynamometer (Jamar) in both the right and left arms [21]. The maximum grip strength from either arm from two trials was utilized.
Health history, lifestyle and demographic data
Height was measured using a Harpenden stadiometer (DyFed, UK) and weight was measured in indoor clothing without shoes using a calibrated beam scale. Body mass index (BMI=kg/m2) was calculated from participant's height and weight. Tibia and forearm length were measured to the nearest millimeter with an anthropometric tape measure. Tibial length was measured from the tibial plateau to the medial malleolus and forearm length was measured from the ulnar styloid process to the olecranon process. The mean of two measurements for each variable was used for the analysis.
Information on demographics, medical and family history and lifestyle were obtained by questionnaire and interview by trained clinical staff at each site. Information from the baseline exam was used to assess race/ethnicity.
Dual energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) scans (QDR 4500 W, Hologic Inc., Bedford, MA) were performed to measure areal bone mineral density of the femoral neck, total body lean mass and total body fat mass. Standardized procedures for participant positioning and scan analysis were used for all scans. All DXA operators were centrally certified on the basis of an evaluation of scanning and analysis techniques. A daily phantom scan was completed at each site to monitor machine performance [17]. To adjust for inter-clinic differences, statistical models include indicator variables for the individual scanners. Each clinic scanned a Hologic whole body phantom throughout the study to monitor longitudinal changes, and correction factors were applied to participant data as appropriate [17].
Peripheral quantitative computed tomography measurements
Peripheral quantitative computed tomography (pQCT) was used to obtain slices (2.3±0.2 mm) at the 4% and 66% sites of the left tibia and at 4% and 33% of the non-dominant forearm (radius). Slices are taken as a percentage of limb length from the distal end of the relevant bone. The XCT 2000 device (Stratec Inc., Pforzheim, Germany) and the XCT-3000 (Stratec Inc., Pforzheim, Germany) were used to obtain the scans in Pittsburgh and Minneapolis respectively. The only difference between the 2000 and 3000 scanners is the gantry size. The same acquisition and analysis software (version 5.5) was used to analyze scans at both sites. We performed a precision study using a European forearm phantom scanned 3 times at each site at 200, 100, and 50 mg/cc respectively. Values on the two instruments were similar and within <0.5% for total area at all mg/cc, and from 0.5% to 1.0% for total density.
Voxel size was 0.5 mm and the scan speed was 25 mm/s. The anatomic reference line (distal edge of the tibial plafond and proximal point of the distal radial joint surface) was determined by acquisition of a 30-mm planar scout view of the joint line. Data were analyzed according to the manufacturer specifications. At the trabecular 4% sites, Contour mode 2 (169 mg/cm3) and Peel mode 1 (45% area) were used. Distal sites were assessed for total bone cross-sectional area (ToA, mm2) and total density (ToD, mg/mm3). Bone strength index (BSI, mg/mm4) was calculated as [ToA * ToD2]/1,000,000 as an index of bone compressive strength. At the more cortical 33% radius and 66% tibia sites, we used Contour mode 2 (169 mg/cm3) to determine whole bone properties and Cortmode 1 (710 mg/cm3) for cortical bone properties. A threshold of 280 mg/cm3 was used to determine the polar strength strain index (SSIp). At these cortical sites, we assessed total bone cross-sectional area (ToA, mm2), cortical area (CoA, mm2), and cortical density (CoD, mg/mm3). Polar strength strain index (SSIp, mm3) and section modulus (mm3) were calculated as estimates of bone bending strength [22]. SSIp is a “density weighted” section modulus value while section modulus includes only geometric properties. For the Minneapolis site, precision with repositioning was determined in adults (women n = 11, men n = 4, age 28.5 ± 6.5 years) as a coefficient of variation (CV, %) and varied from 0.28 (TotBMD) to 1.20 (TrabArea) at the distal tibia and from 0.31 (CortBMD) to 0.41 (TotArea) at the shaft [23]. Similar precision values were reported at the Pittsburgh site [24]. An anthropomorphic phantom was scanned daily for quality assurance at both sites.
Statistical analyses
Differences in characteristics by quartiles of physical function measures were analyzed by ANOVA for continuous variables and chi-square for categorical variables. Multiple regression analysis was used to determine the association between quartiles of grip strength, leg power and PASE score with measures of bone strength, geometry and volumetric density. All analyses were adjusted for age, limb length, weight, race (non-Hispanic white/not) and clinic site due to between group differences and established relationships between these factors and bone outcomes. The 106 men who were physically unable or refused to complete the leg power test were placed into a separate category. The 19 men that were unable or refused to complete the grip strength test were not included in the analysis. Data presented are least squares means with 95% confidence intervals. A trend test was also completed. Statistical significance was set at p<0.05. All statistical analyses were conducted using SAS version 9.13 (SAS Institute, Cary, NC, USA).
Results
Descriptive characteristics
A total of 1171 participants underwent pQCT scans and examinations at either Minnesota (n = 540) or Pittsburgh (n = 631) MrOS sites. Their mean age was 77.2 ± 5.1 years old and 98% of the men were non-Hispanic white. Characteristics of men by quartile of leg power are presented in Table 1. Men with greater leg power (Table 1) and grip strength (data not shown) were in general, younger, taller and heavier (p<0.001). They also had a higher PASE score, greater total body lean mass and a higher body mass index (p<0.001 for all). The most physically active men by PASE score tended to be younger and have a greater leg power and grip strength (p<0.001) as compared with men who were less physically active. Height and weight did not differ significantly by quartile of PASE (data not shown).
Table 1.
Characteristics of participants by quartile of leg power.
| Unable/ refused N=106 | Quartile 1 25.0–133.6W N=213 | Quartile 2 134.7–170.2W N=215 | Quartile 3 171.8–214.9W N=210 | Quartile 4 217.2–452.8 N=220 | p | |
|---|---|---|---|---|---|---|
| Age (years) | 78.5±5.7 | 80.6±5.1 | 77.4±4.5 | 75.7±4.3 | 74.0±3.7 | <0.001 |
| Caucasian (%) | 97 | 98 | 99 | 98 | 98 | 0.759 |
| Height (cm) | 172.5±7.4 | 170.9±6.6 | 172.3±6.8 | 176.3±6.8 | 173.6±6.81 | <0.001 |
| Weight (kg) | 88.3 ± 17.8 | 78.5±11.9 | 82.0±11.8 | 83.2±12.2 | 89.4±12.8 | <0.001 |
| BMI (kg/m2) | 29.6±5.1 | 26.9±3.6 | 27.6±3.5 | 27.6±3.6 | 28.8±3.6 | <0.001 |
| Total body fat mass (kg) | 25.5±8.7 | 21.5±7.0 | 22.5±6.7 | 22.3±6.5 | 23.8±7.1 | <0.001 |
| Total body lean mass (kg) | 60.3±9.3 | 54.6±6.6 | 56.7±6.5 | 58.0±7.2 | 61.6±7.0 | <0.001 |
| PASE score | 105.5±56.7 | 131.0±64.9 | 154.1±69.0 | 153.0±63.8 | 152.5±61.6 | <0.001 |
| Maximum Nottingham leg power (watts) | 171.2±108.8 | 108.5 ±20.4 | 152.2 ± 10.9 | 192.3 ±12.1 | 254.5±34.1 | <0.001 |
| Maximum Grip Strength (kg) | 36.6±7.8 | 36.0±6.2 | 39.6±7.1 | 42.5±6.5 | 46.4±7.7 | <0.001 |
| Tibia length (mm) | 400.3 ± 24.1 | 397.4 ±25.0 | 397.4 ± 27.5 | 402.8 ±24.9 | 410.6 ± 23.5 | <0.001 |
Values are mean ± SD unless otherwise noted.
Bone outcomes
Tibia
Significant differences in pQCT bone parameters were observed across quartiles of physical activity and leg power in models adjusted for age, race, tibia length and weight (Tables 2 and 3). At the highly trabecular distal tibia (4%), lower levels of physical activity were associated with reduced compressive bone strength and reduced total area, but were not associated with total density. Compressive bone strength (BSI) was on average 7% higher in the most active compared with the least active quartile (p-trend=0.025) because total area (ToA, +3%, p-trend=0.008) was greater, despite no difference in total density (ToD) at this site across quartiles of physical activity (p-trend=0.174).
Table 2.
Tibial bone volumetric density, geometry and strength in older men by quartiles of leg power.
| Unable/refused (n=106) | Q1 25.0–133.6W (n=213) | Q2 134.7–170.2W (n=215) | Q3 171.8–214.9W (n=210) | Q4 217.2–452.8W (n=220) | p-trenda | |
|---|---|---|---|---|---|---|
| pQCT 4% Tibia | ||||||
| BSI | 106 (99–113)* | 114 (109–119) | 115 (110–120) | 117 (112–121) | 120 (115–125) | 0.059 |
| Total area (mm2) | 1273 (1240–1306)* | 1256 (1232–1280)** | 1281 (1259–1304)* | 1284 (1261–1307)* | 1320 (1296–1343) | <0.001 |
| Total density (mg/cm3) | 286 (277–295)* | 299 (292–306) | 299 (292–305) | 300 (294–307) | 301 (295–308) | 0.572 |
| Trabecular density (mg/cm3) | 220 (212–228)* | 231 (225–237) | 233 (227–238) | 232 (226–237) | 234 (228–239) | 0.534 |
| pQCT 66% Tibia | ||||||
| SSIp (mm3) | 3283 (3179–3387)* | 3300 (3225–3374)* | 3357 (3287–3427)* | 3408 (3337–3478) | 3471 (3397–3545) | 0.001 |
| Section modulus (mm3) | 3237 (3130–3345)** | 3277 (3200–3354)** | 3352 (3279–3424)* | 3431 (3358–3503) | 3474 (3397–3551) | <0.001 |
| Total area (mm2) | 752 (734–771) | 755 (742–768)* | 762 (750–775) | 764 (752–777) | 775 (762–788) | 0.070 |
| Cortical density (mg/cm3) | 1062 (1055–1070) | 1061 (1056–1067) | 1064 (1059–1069) | 1068 (1063–1074) | 1066 (1061–1072) | 0.080 |
| Cortical area (mm2) | 322 (311–333)* | 328 (320–336)* | 334 (327–341) | 341 (334–349) | 342 (334–350) | 0.003 |
| DXA femoral neck aBMDb | 0.758 (0.734–0.783)* | 0.787 (0.769–0.805) | 0.793 (0.776–0.809) | 0.801 (0.784–0.817) | 0.799 (0.781–0.817) |
Values are adjusted for age group, clinic site, race, tibia length and weight.
Values are means (95% confidence intervals). Significantly different from quartile four
p<0.05
p<0.001.
p for trend includes only quartiles 1–4.
Values are adjusted for age, clinic site, race, height and weight.
Table 3.
Tibial bone volumetric density, geometry and strength in older men by quartiles of physical activity.
| Q1 0–93.57 (n = 292) | Q2 93.68–138.25 (n = 292) | Q3 139–178.96 (n = 296) | Q4 179.25–434.36 (n = 291) | p-trend | |
|---|---|---|---|---|---|
| pQCT 4% Tibia | |||||
| BSI | 111 (107–115)* | 115 (111–119) | 116 (112–119) | 118 (114–122) | 0.025 |
| Total area (mm2) | 1268 (1249–1287)* | 1279 (1260–1298)* | 1273 (1254–1292)* | 1308 (1290–1327) | 0.008 |
| Total density (mg/cm3) | 294 (288–299) | 299 (293–304) | 300 (295–306) | 299 (294–304) | 0.174 |
| Trabecular Density (mg/cm3) | 228 (224–233) | 230 (225–235) | 232 (227–237) | 232 (228–237) | 0.185 |
| pQCT 66% Tibia | |||||
| SSIp (mm3) | 3330 (3269–3391)* | 3378 (3319–3438) | 3364 (3305–3424) | 3448 (3388–3508) | 0.014 |
| Section modulus (mm3) | 3322 (3259–3386)* | 3362 (3300–3424)* | 3373 (3311–3435) | 3451 (3389–3513) | 0.006 |
| Total area (mm2) | 761 (751–772) | 769 (759–780) | 763 (752–773) | 773 (763–784) | 0.225 |
| Cortical density (mg/cm3) | 1059 (1055–1064)* | 1060 (1056–1065)* | 1066 (1061–1070) | 1067 (1063–1071) | 0.004 |
| Cortical area (mm2) | 330 (324–337)* | 330 (324–337)* | 335 (329–341) | 342 (336–348) | 0.006 |
| DXA femoral neck aBMDa | 0.770 (0.756–0.784)* | 0.787 (0.773–0.801) | 0.799 (0.785–0.813) | 0.803 (0.789–0.817) |
Values are adjusted for age, clinic site, race, tibia length and weight.
Values are means (95% confidence intervals). Significantly different from quartile four
p<0.05
**p<0.001.
Values are adjusted for age, clinic site, race, height and weight.
Similarly, there was a significant association between reduced leg power and lower total area, but leg power was not associated with BSI or total density. More specifically, men in the highest leg power quartile tended to have a larger total bone area at the distal tibia (4%) (ToA, +5%, p-trend < 0.001) as compared with men in the lowest quartile, while total density (ToD) was similar across all four quartiles of leg power (p-trend=0.572). The difference between BSI in men with the greatest leg power (quartile 4) compared to that among men the least leg power (quartile 1) did not achieve statistical significance (BSI, +5%, p-trend=0.059). Table 2 includes the 106 men that were unable or refused to complete the leg power test. These results are not included in the p-trend tests.
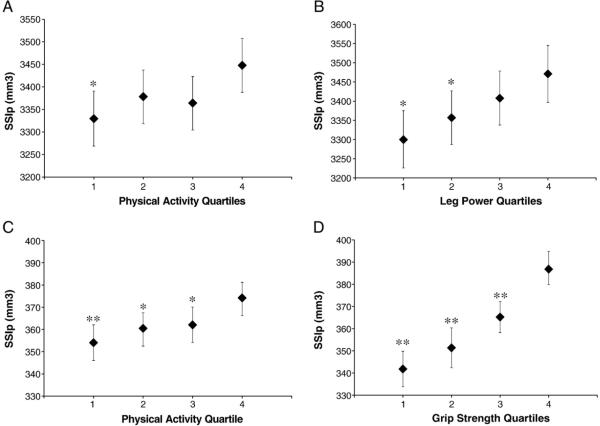
At the cortical 66% site of the tibia, Polar strength strain index (SSIp, mm3) and section modulus (mm3) were more robustly associated with leg power than physical activity (see Fig. 1 and Tables 2 and 3). Estimates were higher (+4%, p-trend=0.014 for SSIp; +4%, p-trend=0.006 for section modulus) in the most active quartile of men compared with the least active quartile, perhaps largely due to greater cortical area (+4%, p-trend=0.006) and cortical density (+1%, p-trend=0.004). Total bone area was not associated with physical activity at this site. SSIp (+5%) and section modulus (+6%) tended to be higher in the men with the greatest leg power compared with the least leg power (?p-trend=0.001 and p-trend<0.001, respectively). Men with the highest leg power (quartile 4) also tended to have larger total bone area (+3%, p-trend=0.070) and cortical area (+4%, p-trend=0.003) when compared with the men with the lowest leg power (quartile 1). Cortical bone density was not different between leg power quartiles at this site.
Fig. 1.
Estimated mean bone strength and 95% confidence intervals of the tibia 66% site by quartile of physical activity (A) and leg power (B). Estimated mean bone strength and 95% confidence intervals of the radius 33% site by quartile of physical activity (C) and grip strength (D). Values are adjusted for age, clinic site, race, limb length and weight. Significantly different from quartile four; *p<0.05, **p<0.001.
Radius
Parameters of bone strength at the distal trabecular site (4%) were significantly associated with both grip strength and physical activity in models adjusted for age, clinic site, race, radius length and weight (Tables 4 and 5). Greater BSI was found in the quartile with the highest activity level (+7%, p-trend=0.012) and the quartile with the highest grip strength (+6%, p-trend=0.016) when compared to the quartile with the lowest activity level or grip strength. This difference was primarily due to a greater total area between the highest and lowest quartiles of physical activity (+4%, p-trend=0.045) and grip strength (+11%, p-trend<0.001). No differences in total density were found in either the grip strength or activity quartiles.
Table 4.
Radius bone volumetric density, geometry and strength in older men by quartiles of grip strength.
| Q1 18–34 kg (n = 284) | Q2 36–38 kg (n = 211) | Q3 40–45 kg (n = 337) | Q4 46–78 kg (n = 320) | p-trend | |
|---|---|---|---|---|---|
| pQCT 4% Radius | |||||
| BSI | 47 (45–49)* | 48 (46–50) | 48 (47–50) | 50 (48–52) | 0.016 |
| Total area (mm2) | 368 (360–377)** | 379 (369–388)** | 390 (383–398)* | 408 (400–416) | <0.001 |
| Total density (mg/cm3) | 354 (346–363) | 356 (346–365) | 351 (344–358) | 351 (344–359) | 0.507 |
| Trabecular density (mg/cm3) | 197 (192–203) | 198 (192–205) | 198 (194–203) | 195 (190–200) | 0.592 |
| pQCT 33% Radius | |||||
| SSIp (mm3) | 342 (334–350)** | 351 (342–360)** | 365 (358–372)** | 387 (379–394) | <0.001 |
| Section modulus (mm3) | 335 (327–342)** | 343 (335–352)** | 356 (349–362)** | 378 (371–385) | <0.001 |
| Total area (mm2) | 140 (138–142)** | 144 (141–146)** | 144 (142–146)** | 151 (149–153) | <0.001 |
| Cortical density (mg/cm3) | 1152 (1148–1156)** | 1159 (1154–1163) | 1163 (1160–1167) | 1163 (1159–1167) | <0.001 |
| Cortical area (mm2) | 100 (99–102)** | 103 (101–105)** | 106 (104–107)** | 111 (109–112) | < 0.001 |
Adjusted for age, clinic site, race, radius length and weight.
Values are means (95% confidence intervals). Significantly different from quartile four
p<0.05
p<0.001.
Table 5.
Radius bone volumetric density, geometry and strength in older men by quartiles of physical activity.
| Q1 (n = 292) | Q2 (n = 292) | Q3 (n = 296) | Q4 (n = 291) | p-trend | |
|---|---|---|---|---|---|
| pQCT 4% Radius | |||||
| BSI | 46 (44–48)* | 49 (47–51) | 49 (47–51) | 49 (47–51) | 0.012 |
| Total area (mm2) | 383 (375–391)* | 382 (374–390)* | 387 (379–396)* | 399 (391–407) | 0.045 |
| Total density (mg/cm3) | 345 (337–353) | 357 (349–364) | 357 (349–365) | 350 (343–358) | 0.348 |
| Trabecular density (mg/cm3) | 192 (186–197) | 199 (194–205) | 201 (195–206) | 196 (191–202) | 0.230 |
| pQCT 33% Radius | |||||
| SSIp (mm3) | 354 (346–362)** | 360 (353–368)* | 362 (354–370)* | 374 (367–382) | <0.001 |
| Section modulus (mm3) | 346 (339–354)** | 353 (345–360)* | 352 (345–360)* | 366 (359–374) | <0.001 |
| Total area (mm2) | 142 (140–145)** | 145 (143–147)* | 145 (143–147)* | 148 (146–150) | <0.001 |
| Cortical density (mg/cm3) | 1156 (1152–1160) | 1159 (1155–1162) | 1163 (1159–1167) | 1160 (1156–1164) | 0.080 |
| Cortical area (mm2) | 102 (101–104)** | 105 (103–107)* | 106 (104–107) | 108 (106–109) | <0.001 |
Adjusted for age, clinic site, race, radius length and weight.
Values are means (95% confidence intervals). Significantly different from quartile four
p<0.05
p<0.001.
At the 33% site of the radius, estimates of bone strength were again significantly higher for men in the highest quartile of physical activity (SSIp, +6%, p-trend<0.001; section modulus, +6%, p-trend<0.001) and grip strength (SSIp, +13%, p-trend<0.001; section modulus, +12%, p-trend<0.001) compared with the lowest quartile. Fig. 1 and Tables 4 and 5 illustrate these findings. A significantly larger total area and cortical area were found between the most active (ToA, +4%, p-trend<0.001; CoA, +6%, p-trend<0.001) or the highest grip strength (ToA, +8%, p-trend<0.001; CoA, 11%, p-trend<0.001) quartiles and the least active or lowest grip strength quartiles. Cortical density was significantly different between highest and lowest quartile of grip strength (p-trend<0.001) but not between the most and least physically active quartiles (p-trend=0.080).
DXA
DXA femoral neck aBMD results are presented in Tables 2 and 3. At the femoral neck, aBMD was not significantly different between the quartile of men with the highest leg power and the quartile of men with the lowest leg power. Men with the highest physical activity had 4% greater aBMD than men with the lowest physical activity.
Discussion
Our results suggest that older men with higher levels of reported physical activity and higher objective measures of leg power and grip strength have greater bone strength as estimated by pQCT measurements of the tibia and radius. Specifically, we found a 5% (p<0.05) difference in estimates of bone strength between the lowest and highest quartiles of leg power, and a 4% (p<0.05) difference between lowest and highest quartiles of physical activity. Furthermore, differences of 6% and 13% (p<0.001) were observed at the cortical site of the radius between the lowest and highest quartiles of physical activity and grip strength, respectively. Moreover, differences were found in bone cross-sectional geometry rather than in volumetric density at cortical sites across quartiles of physical activity, leg power and grip strength–highlighting the importance of assessing bone structure when evaluating these associations. These data suggest the importance for older men to maintain physical activity and muscle strength for prevention of bone fragility. We will discuss each of these points in more in detail below.
Physical activity (by questionnaire) tibial and radial bone strength
At both the midshaft and distal sites of the tibia and radius, estimates of bone strength were higher in the most active men as compared with that among least active men. These findings differ from those of a previous study that reported that PASE score was not significantly associated with areal bone mineral density (aBMD) in 1543 older adults after controlling for isokinetic knee extensor strength, age, race, and sex [25]. Another study conducted in 690 older men found the physical activity index to be positively associated with femoral neck areal bone mineral density values before adjustment of age, body mass index, quadriceps strength, and dietary calcium. However, after adjustment, the association no longer remained statistically significant [26]. For more direct comparison to these previously published studies, we added leg power to our PASE models. Although differences between groups decreased slightly, the highest PASE quartile still had a 2.5% greater bone strength than the first quartile (data not shown). Thus, it is likely that differences between these previous studies and ours is largely explained by use of different bone outcomes. Notably, an increase in bone diameter due to mechanical loading would show up as a lower aBMD by DXA if mineral content remains the same.
Leg power and tibial bone strength
Few studies have examined the association of leg power with bone strength in an older population. A recent study in older women found leg power explained 6.6% of the variance in bone strength-strain index and 8.9% of the variance in the section modulus at the tibial mid-shaft but that muscle strength did not significantly predict bone parameters [27]. Similarly, among our cohort of older men, men in highest quartile of leg power compared to those in the lowest quartile had a 5% greater SSIp and 6% higher section modulus at the cortical site of the tibia. Similar muscle/bone associations were also detected at the radius.
Grip strength and radial bone strength
We also observed greater estimates of bone strength in both the midshaft and distal sites of the radius among men in the highest quartile of grip strength compared with men in the lowest quartile. This finding is in agreement with those reported by previous studies. One study assessing the relationship of grip strength and bone strength at the radius found a significant association with SSIp in adults ranging in age from 18 to 80 years of age [28]. Another study in men found a 7% increase in calcaneus BMD per standard deviation increase in grip strength [29]. These findings at both the tibia and radius highlight the site-specific association between muscle strength and power and bone outcomes.
Leg power and grip strength versus PASE score
In this study, we found a more consistent increase in bone strength between quartiles of leg power and grip strength than between quartiles of PASE score. Furthermore, PASE score was not strongly correlated with grip strength (Pearson r=0.153) or leg power (Pearson r=0.106). These findings suggest that evaluation of leg power and grip strength may be more helpful for identifying older men with lower bone strength than the PASE score when other clinical measures of bone strength have not been obtained or are not available. However, this remains to be empirically tested. Nevertheless, the significant difference in bone strength between the highest and lowest quartiles for physical activity, grip strength, and muscle power supports theories of functional adaptation of bone to implied mechanical demands [3,30] and supports other studies that suggest interventions in older male populations should focus on maintaining muscle strength and power as well as increase levels of physical activity [31,32]. Prospective randomized exercise intervention studies are needed in older male populations to test these theories and explore the relationship of exercise and fracture risk.
Bone density and geometry differences
In this study, we found that the association between bone strength and physical activity, muscle power and strength measures were attributable primarily to greater total bone area and not bone volumetric density at most sites. For example, we found a 3% difference in bone area, with no significant difference in bone volumetric density. Therefore, the 5% difference in estimated bone strength at this site is due primarily to a greater bone area, which is indicative of a greater periosteal diameter. Small differences in aBMD were found with DXA. These data highlight the importance of assessing true volumetric bone mineral density as compared to the two-dimensional areal bone mineral density as measured by dual X-ray density. Congruent with other studies assessing volumetric bone mineral density, we saw no difference in vBMD across quartiles of physical activity, leg power, or grip strength. Rather, by detecting differences in bone geometry, these data highlight the importance of measuring both volumetric bone density as well as the structural underpinnings of bone strength differences.
Strengths and limitations
The findings of this study suggest the importance of maintaining levels of physical activity and muscle strength in older men to prevent bone fragility. There are several strengths to this study including the unique focus on older men, large sample size, use of validated measures such as the use of leg power as a measure of load on bone and use of pQCT to assess volumetric BMD, bone geometry and structural strength estimates in older men. There are also several limitations to this study worth noting. First, the majority of the men in this sample were Caucasian and generally healthy; therefore, we are not able to generalize the results to other populations. Another limitation is that activity was measured by self-report at one time point rather than over a longer period of time. A further limitation is that in addition to being mechanically linked, muscle and bone are genetically linked; therefore, individuals with increased muscle mass genetically may have enhanced bone measures. Finally, this analysis is limited by the cross-sectional nature of the data. Future longitudinal analyses including repeat pQCT measurements and fracture ascertainment are needed to confirm these associations. If these results are confirmed, findings should be used to direct design of intervention studies aimed at maintaining bone strength and lowering fracture risk in older adults.
Conclusions
In conclusion, these data suggest that in older men higher grip strength, leg power and levels of physical activity are associated with higher estimates of bone strength in the tibia and radius. Our findings add to previous DXA studies of physical activity in older men by showing that differences in bone strength are generally attributable to greater bone area rather than greater bone volumetric density and may help explain discrepant findings in studies using aBMD as outcomes. These findings are congruent with findings in older women. They also suggest that it may be important for men to maintain muscle strength and power as well as physical activity with advancing age.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provided support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
References
- [1].Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- [2].Hamilton CJ, Swan VJ, Jamal SA. The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women: a systematic review of pQCT studies. Osteoporos Intl. 2009 doi: 10.1007/s00198-009-0967-1. [DOI] [PubMed] [Google Scholar]
- [3].Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- [4].Hart KJ, Shaw JM, Vajda E, Hegsted M, Miller SC. Swim-trained rats have greater bone mass, density, strength, and dynamics. J Appl Physiol. 2001;91:1663–8. doi: 10.1152/jappl.2001.91.4.1663. [DOI] [PubMed] [Google Scholar]
- [5].Warner SE, Shea JE, Miller SC, Shaw JM. Adaptations in cortical and trabecular bone in response to mechanical loading with and without weight bearing. Calcif Tissue Int. 2006;79:395–403. doi: 10.1007/s00223-005-0293-3. [DOI] [PubMed] [Google Scholar]
- [6].Burr DR. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–51. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- [7].Lu T-W, O'Connor JJ, Taylor SJG, Walker PS. Influence of muscle activity on the forces in the femur: comparison between in vivo measurement and calculation. Trans Orthop Res Soc. 1997;22:721. [Google Scholar]
- [8].Sievanen H, Heinonen A, Kannus P. Adaptation of bone to altered loading environment: a biomechanical approach using x-ray absorptiometric data from the patella of a young woman. Bone. 1996;19:55–9. doi: 10.1016/8756-3282(96)00111-1. [DOI] [PubMed] [Google Scholar]
- [9].Barry DW, Kohrt WM. Exercise and the preservation of bone health. J Cardiopulm Rehabil Prev. 2008;28:153–62. doi: 10.1097/01.HCR.0000320065.50976.7c. [DOI] [PubMed] [Google Scholar]
- [10].Barry DW, Kohrt WM. BMD decreases over the course of a year in competitive male cyclists. J Bone Miner Res. 2008;23:484–91. doi: 10.1359/jbmr.071203. [DOI] [PubMed] [Google Scholar]
- [11].Devine A, Dhaliwal SS, Dick IM, Bollerslev J, Prince RL. Physical activity and calcium consumption are important determinants of lower limb bone mass in older women. J Bone Miner Res. 2004;19:1634–9. doi: 10.1359/JBMR.040804. [DOI] [PubMed] [Google Scholar]
- [12].Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci. 2002;57:M599–604. doi: 10.1093/gerona/57.9.m599. [DOI] [PubMed] [Google Scholar]
- [13].Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med. 2004;34:753–78. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- [14].Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- [15].Jarvinen TL, Kannus P, Sievanen H. Have the DXA-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res. 1999;14:634–5. doi: 10.1359/jbmr.1999.14.9.1634. [DOI] [PubMed] [Google Scholar]
- [16].Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [17].Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [18].Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fit. 1999;39:336–40. [PubMed] [Google Scholar]
- [19].Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–7. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- [20].Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–90. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- [21].Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6:259–62. doi: 10.1016/s0894-1130(12)80326-7. [DOI] [PubMed] [Google Scholar]
- [22].Ferretti JL, Capozza RF, Zanchetta JR. Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone. 1996;18:97–102. doi: 10.1016/8756-3282(95)00438-6. [DOI] [PubMed] [Google Scholar]
- [23].Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, et al. Ethnic differences in bone geometry and strength are apparent in childhood. Bone. 2009;44:970–5. doi: 10.1016/j.bone.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [24].Wang X, Kammerer CM, Wheeler VW, Patrick AL, Bunker CH, Zmuda JM. Genetic and environmental determinants of volumetric and areal BMD in multi-generational families of African ancestry: the Tobago Family Health Study. J Bone Miner Res. 2007;22:527–36. doi: 10.1359/jbmr.070106. [DOI] [PubMed] [Google Scholar]
- [25].Segal NA, Torner JC, Yang M, Curtis JR, Felson DT, Nevitt MC. Muscle mass is more strongly related to hip bone mineral density than is quadriceps strength or lower activity level in adults over age 50 year. J Clin Densitom. 2008;11:503–10. doi: 10.1016/j.jocd.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res. 2000;15:322–31. doi: 10.1359/jbmr.2000.15.2.322. [DOI] [PubMed] [Google Scholar]
- [27].Ashe MC, Liu-Ambrose TY, Cooper DM, Khan KM, McKay HA. Muscle power is related to tibial bone strength in older women. Osteoporos Int. 2008;19:1725–32. doi: 10.1007/s00198-008-0655-6. [DOI] [PubMed] [Google Scholar]
- [28].Hasegawa Y, Schneider P, Reiners C. Age, sex, and grip strength determine architectural bone parameters assessed by peripheral quantitative computed tomography (pQCT) at the human radius. J Biomech. 2001;34:497–503. doi: 10.1016/s0021-9290(00)00211-6. [DOI] [PubMed] [Google Scholar]
- [29].Aoyagi K, Ross PD, Hayashi T, Okano K, Moji K, Sasayama H, et al. Calcaneus bone mineral density is lower among men and women with lower physical performance. Calcif Tissue Int. 2000;67:106–10. doi: 10.1007/s00223001116. [DOI] [PubMed] [Google Scholar]
- [30].Skerry TM. One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J Musculoskelet Neuronal Interact. 2006;6:122–7. [PubMed] [Google Scholar]
- [31].Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, et al. Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol. 1993;74:2478–84. doi: 10.1152/jappl.1993.74.5.2478. [DOI] [PubMed] [Google Scholar]
- [32].Ryan AS, Treuth MS, Rubin MA, Miller JP, Nicklas BJ, Landis DM. Effects of strength training on bone mineral density: hormonal and bone turnover relationships. J Appl Physiol. 1994;77:1678–84. doi: 10.1152/jappl.1994.77.4.1678. [DOI] [PubMed] [Google Scholar]