Abstract
The present study aims to investigate the dependence CaM kinase IV cascade activation during hypoxia and tests the hypothesis that hypoxia-induced tyrosine phosphorylation of CaM and CaM kinase IV, activation of of CaM kinase IV and phosphorylation of CREB protein during hypoxia increases as a function of increase in cerebral tissue hypoxia as measured by decrease in tissue ATP and phosphocreatine (PCr). 3-5 days old newborn piglets were divided into normoxic (Nx, FiO2 of 0.21 for 1 hr) and hypoxic (Hx, FiO2 of 0.07 for 1 hr) groups. Cerebral tissue hypoxia was documented by determining the levels of high energy phosphates ATP and phosphocreatine (PCr). Cerebral cortical neuronal nuclei were isolated and purified, and tyrosine phosphorylation of calmodulin (Tyr99), the activator of CaM kinase IV, and CaM kinase IV determined by Western blot using anti-phospho-(pTyr99)-calmodulin, anti-pTyrosine and anti-CaM kinase IV antibodies. The activity of CaM kinase IV and its consequence the phosphorylation of CREB protein at Ser133 were determined. The levels of ATP (μmoles/ g brain) ranged between 3.48 to 5.28 in Nx, and 0.41 to 2.26 in Hx. The levels of PCr (μmoles/ g brain) ranged between 2.46 to 3.91 in Nx and 0.72 to 1.20 in Hx. The pTyr99 calmodulin (ODxmm2 ) ranged from 20.35 to 54.47.60 in Nx, and 84.52 to 181.42 in Hx (r2= 0.5309 vs ATP and . r2= 0.6899 vs PCr). Expression of tyrosine phosphorylated CaM kinase IV ranged from 32.86 to 82.46 in Nx and 96.70 to 131.62 in Hx(r2= 0.5132 vs ATP and . r2= 0.4335 vs PCr). The activity of CaM kinase IV (pmoles/mg protein/min) ranged from 1263 to 3448 in Nx and 3767 to 6633 in Hx (r2= 0.7113 vs ATP and . r2= 0.6182 vs PCr). The expression of p-CREB at Ser133 ranged from 44.26 to 70.28 in Nx and 82.70 to 182.86 in Hx (r2= 0.6621 vs ATP and . r2= 0.5485 vs PCr). The data show that hypoxia results in increased tyrosine phosphorylation of calmodulin(Tyr99), increased tyrosine phosphorylation of CaM.kinase IV, increased activity of CaM kinase IV and increased phosphorylation of CREB at Ser133 as an inverse function of cerebral concentration of high energy phosphates, ATP and PCr. We conclude that the hypoxia-induced increased activation of CaM kinase IV cascade increases with the increase in the degree of cerebral tissue hypoxia as measured by cerebral tissue high energy phosphates in a curvilinear manner. The tyrosine kinases (Src kinase and EGFR kinase) mediated activation of CaM kinase IV cascade potentially results in increased CREB phosphorylation that triggers transcription of proapoptotic proteins during hypoxia.
Keywords: Calmodulin, Tyr99, CaM kinase IV, Tyrosine phosphorylation, CREB Phosphorylation, Hypoxia
Cerebral hypoxia in the newborn occurs due to antepartum or perinatal hypoxia/asphyxia with an incidence ranging from 1-5% of all live births. Intrauterine hypoxia and birth asphyxia are associated with increased neonatal morbidity and mortality, as well as the long term sequelae of mental retardation, seizure disorders and cerebral palsy. Previously, we have shown that hypoxia results in increased expression and phosphorylation of apoptotic proteins and increased fragmentation of nuclear DNA. Studies also demonstrated that these indices increase as an inverse function of cerebral tissue high energy phosphates, an index of tissue hypoxia.. Furthermore, hypoxia resulted in increased activation of calcium /calmodulin-dependent protein kinase IV (CaM kinase IV) in neuronal nuclei of the cerebral cortex of newborn piglets [22]. In the present study, we focus on investigating the relationship between the level of high energy phosphates in the cerebral tissue and activation of CaM kinase IV cascade..
Ca++/calmodulin dependent protein kinase IV (CaMK IV), the key enzyme of the CaM kinase cascade, is enriched in the brain and predominantly localized in cell nuclei [11,19]. Cyclic AMP response element binding (CREB) protein is phosphorylated by CaMK IV at serine133 which initiates transcription. CREB protein is a transcription factor that mediates responses to a number of physiological and pathological signals [8,13].
The present study focuses on investigating the relationship between the levels of cerebral tissue high energy phosphates, ATP and PCr, with the activation of CaM kinase cascade and specifically examines tyrosine phosphorylation of CaM, CaM kinase IV, CaM kinase IV activity and phosphorylation of CREB protein at serine133 in neuronal neuclei of the cerebral cortex of newborn piglets. The study also aims to determine the degree of cerebral tissue hypoxia, as determined by the level of cerebral energy metabolism, at which the activation of CaM kinaseIV cascade is triggered.
Studies were performed on 3-5 day old Yorkshire piglets obtained from the Willow Glenn Farm, Strausburg, PA. The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of Drexel University. Newborn piglets were randomly divided into two groups: normoxic (n = 5) and hypoxic (n = 5). The animals were ventilated for one hour under either normoxic condition (FiO2 = 0.21) or hypoxic condition. Hypoxia was induced by lowering the FiO2 to 0.06-0.08 for 60 min. At the end of the experimental period, the animal was sacrificed; the cortical tissue was removed and placed either in homogenization buffer for isolation of neuronal nuclei or in liquid nitrogen, and then stored at -80°C for biochemical studies.
Cerebral cortical nuclei were isolated according to the method of Giuffrida et al. [6] as described before [5]. Purity of neuronal nuclei was assessed by phase contrast microscope. Neuronal nuclei were characterized by the presence of one nucleolus per nucleus, whereas, others have multiple nucleoli per nucleus. The final nuclear preparation was devoid of any microsomal, mitochondrial or plasma membrane contaminant with a purity of neuronal nuclei of 90%. Protein content was determined by the method of Lowry et al [10].
Brain tissue concentrations of ATP and phosphocreatine (PCr) concentrations were determined according to the method of Lamprecht et al [9] as described [5]. Cerebral tissue hypoxia was documented by determining the levels of high energy phosphates ATP and phosphocreatine (PCr)..
CaM kinase IV activity was determined as described by Park and Soderling [16], by 33P incorporation (2 min at 37 °C) into syntide-2 in a medium containing 50 mM HEPES (pH 7.5), 2 mM DTT, 40 μM syntide-2, 10 mM Mg acetate, 5 μM PKI 5–24 (protein kinase A inhibitor), 2 μM PKC 19–36 (protein kinase C inhibitor), 1 μM microcystin-LR (protein phosphatase 2A inhibitor), 200 μM sodium orthovandate (inhibitor of ATPase, alkaline phosphatase, protein tyrosine phosphatase), 0.2 mM ATP, 1 μCi 33P-ATP and either 1 μM calmodulin and 1 mM CaCl2 (for total activity) or 1 mM EGTA (for Ca2+/CaM independent activity) and 10 μl neuronal nuclei. The activity was expressed as pmol/mg of protein/min.
Western Blot Analysis of Tyrosine Phosphorylated Calmodulin (Tyr 99, CaM kinase IV and CREB protein(Ser133)
The nuclear protein was solubilized and brought to a final concentration of 1 μg/μl in a modified RIPA buffer (50 mM Tris–HCl, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and 1 μg/ml each of aprotinin, leupeptin and pepstatin). Then 5 μl of Laemmli buffer was added to each 20 μg of nuclear membrane protein mixture. The samples were heated for 5 min at 95 °C. Equal protein amounts of each sample was separated by using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). The proteins were electrically transferred to nitrocellulose membranes and probed with primary antibodies directed against anti-phospho (pTyr99)-calmodulin or anti-phospho (pSer133) CREB protein. Specific complexes were detected by enhanced chemiluminescence using the ECL system (Amersham, Buckinghamshire, UK) and analyzed by imaging densitometry (GS 800 Densitometer, Bio-Rad) using Quantity One Software (Bio-Rad). The data are expressed as optical density (OD)×mm2.
Data Analysis
The phosphorylation data was plotted against the levels of high energy phosphates. The curves were analyzed and r2 value (correlation coefficient) > 0.5 was considered as a significant correlation.
Cerebral cortical tissue hypoxia in newborn piglets was documented by the levels of ATP and PCr in the cerebral cortical tissue. The levels of ATP (μmoles/ g brain) ranged between 3.48 to 5.28 in Nx, and 0.41 to 2.26 in Hx. The levels of PCr (μmoles/ g brain) ranged between 2.46 to 3.91 in Nx and 0.72 to 1.20 in Hx. The results show that cerebral tissue high energy phosphates ATP and PCr, indices of cerebral tissue hypoxia, decreased indicating that varying degrees of tissue hypoxia was achieved in the experimental animals.
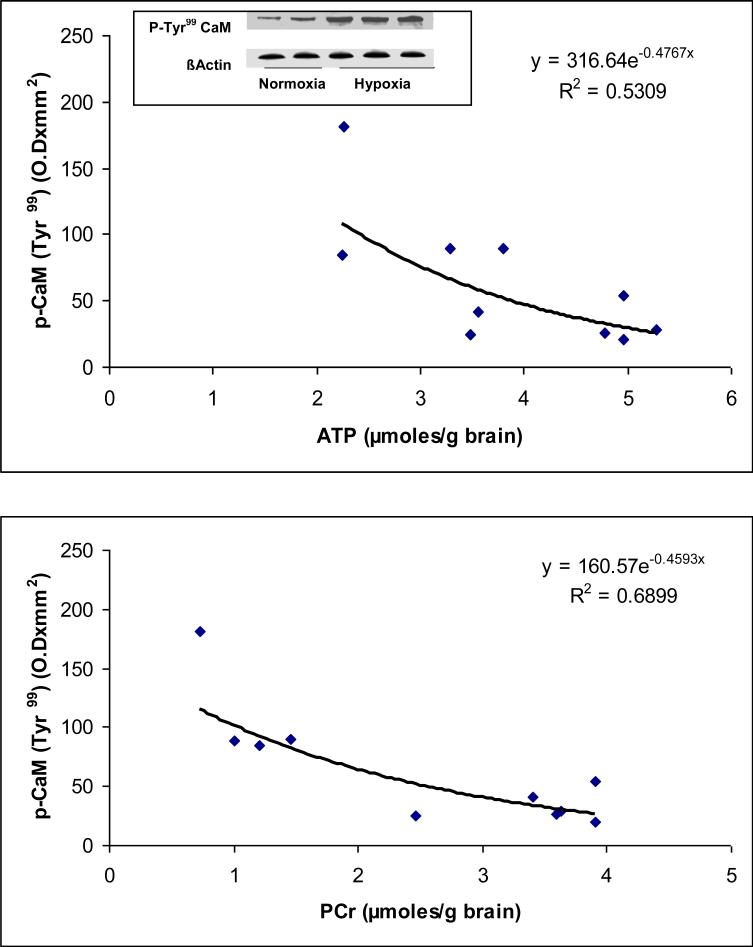
Representative Western blots of phospho (pTyr99)-calmodulin for normoxic and hypoxic groups are shown in Figure 1. The results show an increased expression of phoshorylated (p-Tyr99) calmodulin in the Hx group indicating increased level of phosphorylated (p-Tyr99) calmodulin in neuronal nuclei during hypoxia..
Figure 1.
(a) Representative western blots of phospho-(pTyr99)-calmodulin in neuronal nuclei of the cerebral cortex of normoxic and hypoxic piglets. Lanes 1 and 2 represent normoxic and lanes 3, 4 and 5 represent hypoxic piglets.
(b) Relationship between the level of cerebral high energy phosphates and phosphorylation of calmodulin at Tyr99.
The results (Fig 1) show that the density (expressed as optical density × mm2) of the phosphorylated (pTyr99 ) calmodulin (ODxmm2 ) ranged from 20.35 to 54.47.60 in Nx, and 84.52 to 181.42 in Hx (r2= 0.5309 vs ATP and . r2= 0.6899 vs PCr). The data show that hypoxia resulted in increased (pTyr99)-phosphorylation of calmodulin. A significant correlation was observed between the levels of (pTyr99)-phosphorylation of calmodulin with the levels of ATP and PCr. The results show that (pTyr99)-phosphorylation of calmodulin increases with an increase in degree of cerebral tissue hypoxia as an inverse function of ATP and PCr concentrations in the cerebral tissue.
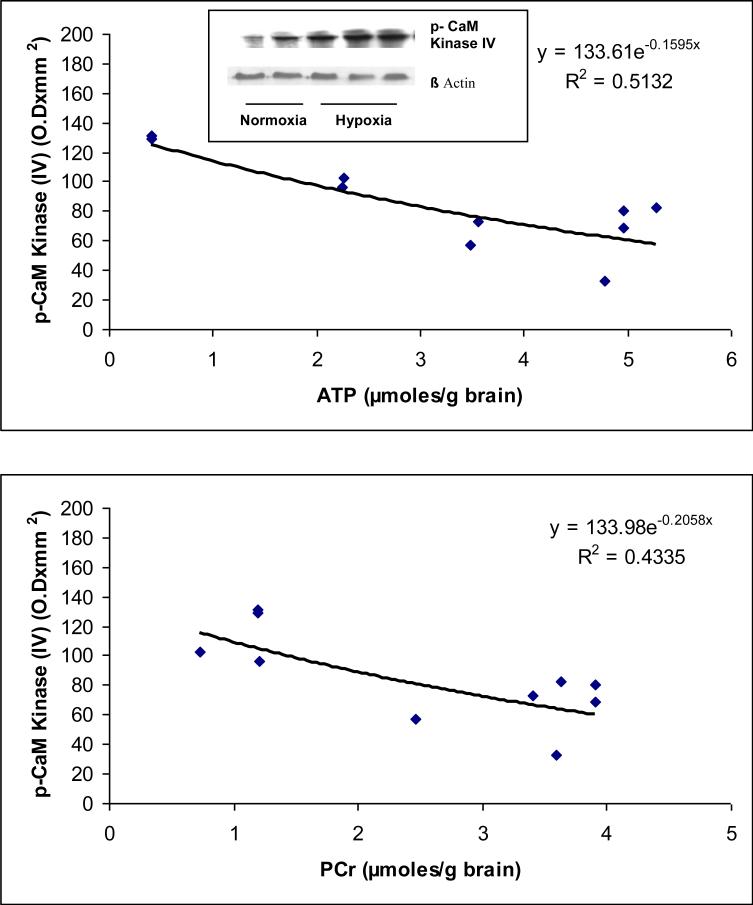
Representative Western blots of tyrosine phosphorylated CaM kinase IV for normoxic and hypoxic groups are shown in Figure 2 The results show an increased expression of tyrosine phosphorylated CaM kinase IV in the Hx group indicating increased level of Tyrosine phosphorylated CaM kinase IV in the cerebral cortex during hypoxia.
Figure 2.
(a) Representative western blots of tyrosine phosphorylated CaM kinase IV in neuronal nuclei of the cerebral cortex of normoxic and hypoxic piglets. Lanes 1 and 2 represent normoxic and lanes 3, 4 and 5 represent hypoxic piglets.
(b) Relationship between the level of cerebral high energy phosphates and tyrosine phosphorylation of CaM kinase IV.
The results (Fig 2) show that the density of tyrosine phosphorylated CaM kinase IV (OD × mm2) ranged from 32.86 to 82.46 in Nx and 96.70 to 131.62 in Hx (r2= 0.5132 vs ATP and . r2= 0.4335 vs PCr). The data show that hypoxia resulted in increased tyrosine phosphorylation of CaM kinase IV. A significant correlation was observed between the levels of tyrosine phosphorylated of CaM kinase IV with the levels of ATP and PCr. The results show that tyrosine phosphorylation of CaM kinase IV increases with an increase in degree of cerebral tissue hypoxia as an inverse function of ATP and PCr concentrations in the cerebral tissue.
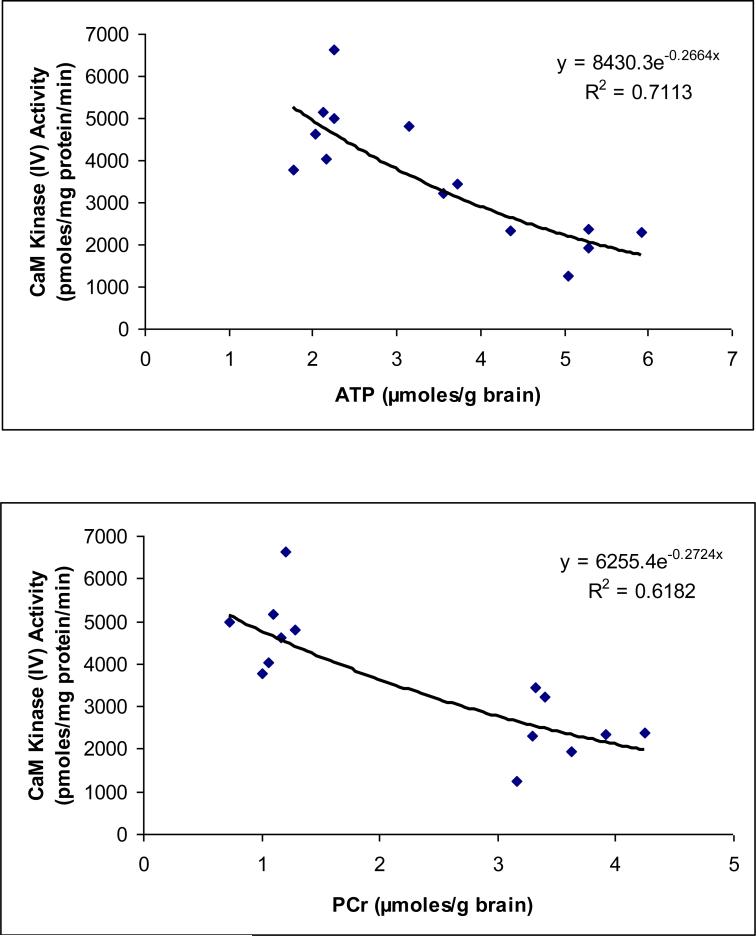
The results (Fig 3) show that the activity of CaM kinase IV (expressed as pmoles/mg protein/min) ranged from 1263 to 3448 in Nx and 3767 to 6633 in Hx (r2= 0.7113 vs ATP and . r2= 0.6182 vs PCr). The data show that hypoxia resulted in increased CaM kinase IV activity in neuronal nuclei of the cerebral cortex of newborn piglets (as shown previously by us). A significant correlation was observed between the activity levels of CaM kinase IV with the levels of ATP and PCr. The results show that CaM kinase IV activity increases with an increase in degree of cerebral tissue hypoxia as an inverse function of ATP and PCr concentrations in the cerebral tissue.
Figure 3.
Effect of hypoxia on CaM kinase IV activity in neuronal nuclei of normoxic and hypoxic piglets. The CaM kinase IV activity (pmoles/mg protein/min) is presented on Y-axis.
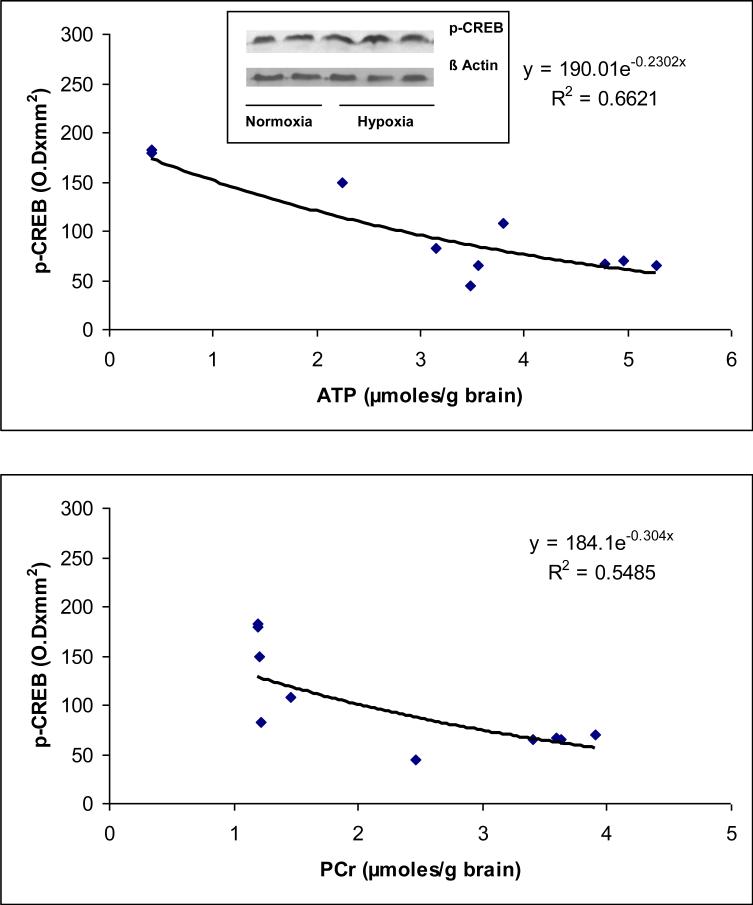
Representative Western blots of phospho (p-Ser133)-CREB protein for normoxic and hypoxic groups are shown in Figure 4. The results show an increased expression of phoshorylated (p-Ser133)-CREB protein in the Hx group indicating increased level of phosphorylated (p-Ser133) CREB protein in neuronal nuclei during hypoxia.
Figure 4.
(a) Representative western blots of phospho-(p-Ser133)-CREB protein in neuronal nuclei of normoxic and hypoxic piglets. Lanes 1 and 2 represent normoxic and lanes 3, 4 and 5 represent hypoxic piglets.
(b) Relationship between the level of cerebral high energy phosphates and phospho-(p-Ser133)-CREB protein.
The results (Fig 4) show that the density of the phosphorylated (p-Ser133)-CREB protein (expressed as optical density × mm2) ranged from 44.26 to 70.28 in Nx and 82.70 to 182.86 in Hx (r2= 0.6621 vs ATP and . r2= 0.5485 vs PCr). The data show that hypoxia resulted in increased (p-Ser133) phosphorylation of CREB protein in neuronal nuclei of the cerebral cortex of newborn piglets. A significant correlation was observed between the levels of phosphorylated (p-Ser133)-CREB protein with the levels of ATP and PCr. The results show that phosphorylation of (p-Ser133)-CREB protein increases with an increase in degree of cerebral tissue hypoxia as an inverse function of ATP and PCr concentrations in the cerebral tissue.
DISCUSSION
Cerebral hypoxia results in increased nuclear Ca++ influx and increased activity of CaM kinase IV which is predominantly located in the nucleus [5,22]. We have shown that hypoxia results in increased phosphorylation of CREB protein and increased expression of proapototic proteins [13,23]. The present study investigates the relationship between the levels of cerebral tissue high energy phosphates, ATP and PCr, with the activation of CaM kinase IV cascade and specifically examines the relationship with tyrosine phosphorylation of CaM, tyrosine phosphorylation of CaM kinase IV, CaM kinase IV activity and phosphorylation of CREB protein in neuronal nuclei of the cerebral cortex of newborn piglets. The study aims to determine the degree of cerebral tissue hypoxia as assessed by the level of cerebral energy metabolism at which the activation of CaM kinase cascade is activated.
The results of the present study show that cerebral hypoxia results in increased tyrosine phosphorylation of calmodulin at Tyr99 and CaM kinase IV as well as CaM kinase IV activity and CREB phosphorylation (Ser133) in neuronal nuclei of the cortical membrane fraction of the cerebral cortex of newborn piglets and the data demonstrate an inverse exponential correlation with the cerebral tissue high energy phosphates ATP and phosphocreatine. The results also indicate that the activation of CaM kinase IV cascade is delayed until a 50% decrease in ATP and phosphocreatine indicating that there is a degree and duration of cerebral hypoxia beyond which the CaM kinase pathway is activated. In addition, the results also indicate that the relationship of high energy phosphates with CaM kinase activation may be mediated through activation of protein tyrosine kinases.
The results of the present study raise several important questions. First, these results indicate that there is a potential link between cerebral energy levels and activation of CaM kinase pathway that triggers transcription of proapoptotic proteins Bax and Bad which may activate procaspase-9 to active caspase-9 that subsequently activates caspase-3, the executioner of cell death. Second, what is the potential mechanism of activation of this pathway which is dependent on tyrosine phosphorylation. Thirdly, how the tyrosine phosphorylation of CaM and CaM kinase IV may lead to CaM kinase IV activation. Fourth, is this the pathway for the role of protein tyrosine kinases, Src kinase and EGFR kinase, in mediating cell death. Finally, the results indicate that there exists a cerebral tissue energy threshold beyond which the CaM kinase IV cascade is activated.
We have shown that administration of a selective Src kinase inhibitor prevents the hypoxia –induced increased Tyr99 phosphorylation of calmodulin and CaM kinase IV as well as the increased CaM kinase IV activity and CREB protein phosphorylation at Ser133 demonstrating that Src kinase mediates the activation of CaM kinase IV in the hypoxic brain.
There could be two potential mechanisms of Src kinase activation that results in increased tyrosine phosphorylation-dependent activation of CaM kinase. First- NO-free radical mediated inhibition of protein tyrosine phosphatases leading to increased activation of Src kinase, and second – NO free radical dependent oxidation of cysteine residue in Src kinase leading to Src kinase activation [7]. Therefore, free radicals mediated redox regulation of Src kinase is a potential mechanism of Src activation that leads to increased tyrosine phosphorylation of CaM and CaM kinase IV.
We proposed that during hypoxia Src kinase-mediated increased phosphorylation of calmodulin at Tyr99 results in binding of the tyrosine phosphorylated calmodulin with increased affinity to the calmodulin binding domain of CaM kinase IV and leads to increased activation of CaM kinase IV. The phosphorylated calmodulin (negatively charged) will bind with a higher affinity to the calmodulin binding domain of CaM kinase IV, a positively charged domain (725-756) rich in basic amino acid residues, Lys and Arg. The sequence of the calmodulin binding domain of CaM kinase IV showing basic amino acid residues (in bold italics) is as follows: -Arg-Arg-Lys-Leu-Lys-Ala-Ala-Val-Lys-Ala-Val-Val-Ala-Ser-Ser-Arg-Leu-Ser-. Note the high presence of Arg and Lys residues in this domain. Similarly, the increased tyrosine phosphorylation of CaM kinase IV will facilitate binding of its substrate which is rich in basic amino acids. The amino acid sequence of the phosphorylated kinase-inducible-domain of CREB protein is as follows: -Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser133-Tyr-Arg-Lys-Ilu-Leu-Asn-Asp-. Note the high presence of Arg and Lys residues in the domain.
The increased tyrosine phosphorylation of calmodulin and CaM kinase IV may lead to increased activation of CaMK IV resulting in increased activation of Ca++-dependent nuclear mechanisms and activate cascade of post-hypoxic programmed cell death. We have demonstrated that hypoxia results in increased CaM kinase IV activity, increase in CREB phosphorylation, increase in the expression of pro-apoptotic protein Bax and increased fragmentation of nuclear DNA [13,22,23]. The expression of Bcl-2 did not increase in nuclei, mitochondria or cytosol. The increased ratio of Bax/Bcl-2 will increase the permeability of the mitochondrial membrane as well as increase caspase-9 activation leading to subsequent activation of caspase-3 and caspase-activated DNAse dependent degradation of nuclear DNA.
Hypoxia results in increased generation of nitric oxide (NO) free radicals [12]. The NO generated during hypoxia may result in activation of Src kinase by inactivating protein tyrosine phosphatases (SH-PTP-1 and SH-PTP-2) by oxidizing cysteine residue at their active site [1,7, 20]. Hypoxia-induced nitration of NMDA receptor subunits indicates formation of peroxynitrite [21]. Therefore, during hypoxia peroxynitrite-dependent inactivation of SH-PTP-1 and SH-PTP-2 may lead to activation of Src kinase which may further result in increased tyrosine phosphorylation (p-Tyr99) of calmodulin and CaM kinase IV, as demonstrated in the present study. Thus a cycle between nNOS activation and activation of Src kinase can continue in the hypoxic brain.
The increased phosphorylation of calmodulin leading to increased activation of Src kinase, via nNOS activation, may be a mechanism in cancers [2,4,18]. As shown here, hypoxia lead to increased tyrosine phosphorylation of calmodulin and CaM kinase IV. Hypoxia also results in activation of Src kinase and EGFR kinase in the cerebral cortex of newborn piglets [14,15]. Hypoxia also results in cell death. Therefore, the role of tyrosine phosphorylated calmodulin and CaM kinase IV via Src kinase/EGFR kinase leading to both the cell proliferation and cell death needs serious consideration. The role of CaM kinase IV is expanding in health and diseases [3,17].
In summary, the results of the present study demonstrate that hypoxia results in increased tyrosine phosphorylation of calmodulin and CaM kinase IV as an inverse function of cerebral energy levels of ATP and PCr. Hypoxia-induced increased activity of CaM kinase IV and CREB protein phosphorylation at Ser133 also increased as an inverse function with cerebral high energy phosphates. We propose that hypoxia induced activation of tyrosine kinases (Src kinase and EGFR kinase) lead to increased activation of CaM kinase IV cascade resulting in increased CREB phosphorylation that triggers increased transcription of proapoptotic proteins and initiates hypoxic neuronal death.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Health grant HD-20337. The authors thank Ms. Anli Zhu and Miss Hien Pham for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barret WE, Degnore JP, Keng YF, Zang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase IB: J. Biol. Chem. 1999;49:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 2.Cameron DA, Stein S. Drug insight: intracellular inhibitors of HER2-clinical development of lapatinib in breast cancer. Nat. Clin. Pract. Oncol. 2008;5:512–520. doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 3.Colomer J, Means AR. Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell Biochem. 2007;45:169–214. doi: 10.1007/978-1-4020-6191-2_7. [DOI] [PubMed] [Google Scholar]
- 4.Contessa JN, Hamstra DA. Revoking the privilege: Targeting HER2 in the central nervous system. Mol Phamacol. 2008;73:271–273. doi: 10.1124/mol.107.042986. [DOI] [PubMed] [Google Scholar]
- 5.Delivoria-Papadopoulos M, Akhter WA, Mishra OP. Hypoxia-induced Ca++-influx in cerebral cortical neuronal nuclei of newborn piglets. Neurosci. Lett. 2003;342:119–123. doi: 10.1016/s0304-3940(03)00256-8. [DOI] [PubMed] [Google Scholar]
- 6.Giuffrida AM, Cox D, Mathias AP. RNA polymerade activity in various classes of nuclei from different regions of rat brain during postnatal development. J. Neurochem. Res. 1975;26:821–827. [PubMed] [Google Scholar]
- 7.Giannoni E, Taddie ML, Chiarugi P. Src redox regulation: again in front line. Free Radic Biol. Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 9.Lamprecht W, Stein P, Heinz F, Weissner H. In: Creatine phosphate. Bergmeyer HU, editor. Vol. 4. Methods of Enzymatic Analysis Academic Press; New York: 1974. pp. 1777–1781. [Google Scholar]
- 10.Lowry O, Rosenbrough NJ, Farr A, Randall RJ. Protein measurement with Folin phenol reagent. J Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 11.Mathews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differently regulate CREB-dependent gene expression. Mol. Cell. Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra OP, Zanelli S, Ohnishi ST, Delivoria-Papadopoulos M. Hypoxia-induced generation of nitric oxide free radicals in cerebral cortex of newborn guinea pigs. Neurochem.Res. 2000;25:1559–1565. doi: 10.1023/a:1026610301978. [DOI] [PubMed] [Google Scholar]
- 13.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Phosphorylation of cAMP response element binding (CREB) protein during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibition. Neurosci. 2002;115:985–991. doi: 10.1016/s0306-4522(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 14.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. NO-mediated activation of Src kinase during hypoxia in the cerebral cortex of newborn piglets. Neurosci. Lett. 2009;460:61–65. doi: 10.1016/j.neulet.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. M, Hypoxia-induced activation of Epidermal growth factor receptor(EGFR) kinase in the cerebral cortex of newborn piglets: The role of nitric oxide. Neurochem. Res. 2010;35:1471–1477. doi: 10.1007/s11064-010-0208-1. [DOI] [PubMed] [Google Scholar]
- 16.Park IK, Soderling TR. Activation of Ca2+/calmodulin-dependent protein kinase (CaM-kinase) IV by CaM-kinase kinase in jurkat T lymphocytes. J. Biol. Chem. 1995;270:30464–30469. doi: 10.1074/jbc.270.51.30464. [DOI] [PubMed] [Google Scholar]
- 17.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveler. Trends Immunol. 2008;12:600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Sharma PS, Sharma R, Tyagi T. Receptor tyrosine kinase inhibitors as potent weapons in war against cancers. Curr Pharm Res. 2009;15:758–776. doi: 10.2174/138161209787582219. [DOI] [PubMed] [Google Scholar]
- 19.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 20.Takakure K, Beckman JS, MacMillan-Cron LA, Cron JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTPIB, CD45 and LAR by peroxynitrite. Arch. Biochem. Biophys. 1999;369:197–207. doi: 10.1006/abbi.1999.1374. [DOI] [PubMed] [Google Scholar]
- 21.Zanelli S, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neurosci. Lett. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 22.Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM, Fritz KI, Mishra OP. Nitric Oxide-Mediated Ca2+/Calmodulin-Dependent Protein Kinase IV Activity During Hypoxia in Neuronal Nuclei from Newborn Piglets. Neurosci. Lett. 2002;335:5–8. doi: 10.1016/s0304-3940(02)01138-2. [DOI] [PubMed] [Google Scholar]
- 23.Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM, Ballesteros JR, Fritz KI, Mishra OP. Nitric Oxide-Mediated Expression of Bax Protein and DNA Fragmentation During Hypoxia in Neuronal Nuclei from Newborn Piglets. Brain Res. 2002;954:60–67. doi: 10.1016/s0006-8993(02)03342-5. [DOI] [PubMed] [Google Scholar]