Abstract
Older adults, compared to younger adults, focus on emotional well-being. While the lifespan trajectory of emotional processing and its regulation has been characterized behaviorally, few studies have investigated the underlying neural mechanisms. Here, older adults (range: 59–73 years) and younger adults (range: 19–33 years) participated in a cognitive reappraisal task during functional magnetic resonance imaging (fMRI) scanning. On each trial, participants viewed positive, negative or neutral pictures and either naturally experienced the image (‘Experience’ condition) or attempted to detach themselves from the image (‘Reappraise’ condition). Across both age groups, cognitive reappraisal activated prefrontal regions similar to those reported in prior studies of emotion regulation, while emotional experience activated the bilateral amygdala. Psychophysiological interaction analyses revealed that the left inferior frontal gyrus (IFG) and amygdala demonstrated greater inverse connectivity during the ‘Reappraise’ condition relative to the ‘Experience’ condition. The only regions exhibiting significant age differences were the left IFG and the left superior temporal gyrus, for which greater regulation-related activation was observed in younger adults. Controlling for age, increased performance on measures of cognition predicted greater regulation-related decreases in amygdala activation. Thus, while older and younger adults use similar brain structures for emotion regulation and experience, the functional efficacy of those structures depends on underlying cognitive ability.
Keywords: amygdala, executive function, affect, functional brain imaging, cognitive aging
INTRODUCTION
Older adults prioritize social and emotional well-being over the acquisition of new information (Lang and Carstensen, 2002), potentially because of the increasing salience of their limited lifespan (Carstensen et al., 1999). To optimize their emotional well-being, older adults report increased motivation to regulate emotion (Kennedy et al., 2004), both passively through selection of stimuli and situations that minimize negative emotions (Blanchard-Fields et al., 2004), and also actively through the use of regulatory strategies (Gross et al., 1997). These age-related changes in emotion regulation lead to robust changes in overall mood; most typically reported are decreased negative affect and increased or stable positive affect (Carstensen et al., 2000, but see Mroczek and Almeida, 2004). Collectively, these results point to the critical role emotion regulation plays in the lives of many older adults.
One common strategy for active emotion regulation is cognitive reappraisal. When engaging in reappraisal, an individual attempts to dampen or alter their emotional response to a stimulus by changing their interpretation of it (Gross, 1998). For example, viewing a photograph of a crowd of mourners might normally evoke feelings of empathy and sadness. Reappraisal of this stimulus could involve instructions to distance oneself from the scene (e.g. ‘you are documenting this event as a photographer, but are not involved personally’) or to change the meaning of the event to lessen or alter its emotional impact (e.g. ‘the mourners are in fact commemorating the long life of a beloved family member’). Whether an explicit part of therapy, or a strategy for dealing with everyday life, effective reappraisal can be an important contributor to mental health (Gross and John, 2003).
Recent functional neuroimaging studies have mapped the brain systems that support active attempts to reappraise emotional stimuli. Converging results in younger adults indicate that reappraisal increases activation in executive control systems and decreases activation in emotional evaluation and response systems (Ochsner and Gross, 2005). Successful reappraisal engages areas of the lateral and medial prefrontal cortex (PFC) implicated in cognitive control (Beauregard et al., 2001; Ochsner et al., 2002; Lévesque et al., 2003; Ochsner et al., 2004; Phan et al., 2005; Urry et al., 2006). Conversely, activation within the amygdala and other regions mediating affective states, such as the insula, decreases or increases consistent with the goal of regulation. That is, reappraisal to decrease negative affect leads to a diminished amygdalar response, while reappraisal to increase negative affect leads to an increased amygdalar response (Ochsner et al., 2004). Other emotion regulation strategies may involve different neural mechanisms. For comparison, expressive suppression in response to negative emotional content leads to slower responses in prefrontal cortex and increased activation in the insular cortex and amygdala (Goldin et al., 2008).
The engagement of PFC during cognitive reappraisal—as heretofore demonstrated in younger adults—raises important questions for applications to aging. Lateral and orbital PFC are among the regions exhibiting the greatest structural and functional decline in healthy aging (Raz et al., 2004). Yet, older adults report frequent and effective emotion regulation. Only a handful of previous functional magnetic resonance imaging (fMRI) studies have explored cognitive reappraisal in older adults (Urry et al., 2006; van Reekum et al., 2007; Urry et al., 2009); however, none of these studies included a comparison group of young adults, thereby precluding the identification of age effects. Urry and colleagues (2006) instructed a sample of older adults to reappraise, experience naturally or increase their emotional response to negatively valenced photographs. Consistent with data from previous studies in younger adults, decreasing negative affect led to attenuated activation in the amygdala and increased activation in the dorsomedial PFC. Yet, participants showing the greatest reappraisal-related decrease in amygdala activation also showed greater activation in the ventromedial PFC (not the medial and lateral regions more typically reported). Such ventromedial PFC activation may reflect regulatory control processes that rely on outcome-based processes responsible for creating stimulus–response or action–outcome contingencies, in contrast to description-based processes (e.g. semantic elaborations of emotional states or stimuli) that are implemented in dorsolateral PFC (Ochsner and Gross, 2007). If supported by direct group comparisons, such a shift in regulatory control would have important implications for understanding how older adults alter their emotional experience. Other recent investigations have shown reappraisal-related increases in activation of ventral and dorsal PFC (van Reekum et al., 2007; Urry et al., 2009), instead suggesting that neural mechanisms for regulation in older adults may be similar to those observed in younger adults.
Here we compared the neural mechanisms of cognitive reappraisal in younger and older adults. A first question explored whether older and younger adults would use similar brain structures to regulate and experience emotions. That is, would successful regulation be associated with increased lateral PFC and decreased amygdalar activation for both groups? A second question investigated whether individual differences in cognitive abilities would influence the activation in these regions. Behavioral research has demonstrated that older adults with superior cognitive abilities also exhibit the strongest positivity effect in a subsequent memory task for emotional pictures, but that this relationship dissipates under conditions of high cognitive load (Mather and Knight, 2005). Furthermore, recent data suggest that improved executive functioning in older adults predicts successful inhibition of startle responses (Gyurak et al., 2009) and lateral prefrontal activation to emotional images (Krendl et al., 2009). Generalizing this perspective, do cognitive changes themselves drive changes in brain function, independently of age-related effects?
METHODS
Participants
The subject sample comprised 20 older adults between the ages of 59 and 73 years (M = 69 years) and 22 younger adults between the ages of 19 and 33 years (M = 23 years). Participants reported no history of neurological or psychiatric disorder and scored a 27 or above on the Mini-Mental State Examination (Folstein, Folstein and McHugh, 1975). The age groups were approximately matched on demographic variables including education (Table 1). Each person participated in two sessions on different days. The first session included a psychometric testing battery and training on our emotional reappraisal paradigm, and the second session involved fMRI scanning. Participants were paid an average of approximately $55. All participants provided written consent under a protocol approved by the Institutional Review Board of Duke University Medical Center.
Table 1.
Demographic information about participant samples
| Younger | Older | |||
|---|---|---|---|---|
| M | (s.d.) | M | (s.d.) | |
| Characteristics by age group | ||||
| Age | 23.1 | (4) | 69 | (3.9) |
| Education (years) | 15.2 | (2.6) | 17.1 | (2.6) |
| MMSE | 29.6 | (0.5) | 29.3 | (0.7) |
| BDI | 2.5 | (2.7) | 3.8 | (2.7) |
| ERQ-R | 31.6 | (4.4) | 32.7 | (4.6) |
| ERQ-S | 12.7 | (3.8) | 14.6 | (5.2) |
| AIM | 57.9 | (18.3) | 55.3 | (20.2) |
MMSE, mini mental-state examination; BDI, Beck depression inventory; ERQ-R, ERQ-S, emotion regulation questionnaire, reappraisal and suppression subscales; AIM, affect intensity measure.
Psychometric tasks
To provide a measure of cognitive functioning, participants completed a battery of tasks drawn from a concurrently conducted study of aging, cognition and decision making (Henninger et al., in press).
As tests of memory, they viewed a series of 16 words on a computer screen and read each word aloud. At the end of the series, they verbally recalled as many of those words as possible (‘Immediate Memory’ task). After a 20-min delay, participants again recalled as many words as they could (‘Delayed Memory’ task). Thereafter, participants completed a ‘Recognition Memory’ task in which the 16 target words and 16 lure words were presented on a computer screen. Participants evaluated whether each word was ‘old’ (e.g. from the original list) or ‘new’.
Tests of executive function included measures of response time and working memory. In the ‘Simple Reaction Time’ task, participants were instructed to press the spacebar every time a white square appeared in the middle of the screen. In the ‘Choice Response Time’ task, participants were shown arrows pointing either to the left or to the right and were instructed to press the corresponding left or right button on the keyboard when the arrow appeared. Participants also completed a computerized ‘Stroop’ task in which they were shown the words ‘art’, ‘game’, ‘red’ and ‘blue’ in red and in blue text. Participants were instructed to press one button if the word was printed in blue text and another if it was printed in red text. Finally, participants performed ‘Forward Digit Span and Backward Digit Span’ tasks. In each, the participant listened to strings of numbers and then recited each string back to the experimenter in forward (or backward) order. Following two correct responses, the length of the string increased on the next trial. The task continued until the participant made errors on two consecutive trials.
For the memory tasks, performance was indexed by the number of words recalled or recognized in each condition. In the digit span tasks, performance was based on the number of correct inverse repetitions of number strings of increasing length. Performance on the simple and choice reaction time tasks was calculated as mean reaction time for correct choices. Stroop task performance included two elements, a value for accuracy on congruent trials minus accuracy on incongruent trials, and a value for mean reaction time difference between incongruent and congruent trials. Subjects were assigned z scores for each of these measures, relative to the mean and standard deviation of the younger subjects, and a single composite score was derived based on mean performance across all measures.
Participants also provided self-report measures of emotional functioning: the Beck Depression Inventory, the Emotion Regulation Questionnaire, and the Affect Intensity Measure (Gross and John, 2003; Beck, 1978; Larsen et al., 1987).
Cognitive reappraisal paradigm
Following cognitive testing, participants were trained in our emotion regulation task (Figure 1). Participants learned a reappraisal strategy that involved thinking of themselves as an emotionally detached and objective third party. During the training session, subjects verbalized how they were thinking about the image to the experimenter to ensure task compliance, but were instructed not to speak during the scanning session. All subjects reported that they understood the reappraisal strategy, and could implement that strategy for a set of novel photographs presented with the same timing as the fMRI experiment.
Fig. 1.
Cognitive reappraisal task. Participants were trained in the use of a reappraisal strategy for emotion regulation. (A) On ‘Experience’ trials, participants viewed an image then received an instruction to experience naturally the emotions evoked by that image. The image then disappeared, but participants continued to experience their emotions throughout a 6-s delay period. At the end of the trial, the participants rated the perceived affective valence of that image using an 8-item Likert scale. (B) ‘Reappraise’ trials had similar timing, save that the cue instructed participants to decrease their emotional response to the image by reappraising the image (e.g. distancing oneself from the scene). Shown are examples of the negative (A) and positive (B) images used in the study.
Each trial of this task began with a single photographic image that was negative, positive, or neutral in valence. All images were drawn from the International Affective Picture System (Lang et al., 2005). Images were categorized according to their standardized valence ratings: positive (M = 7.3), neutral (M = 5.1), negative (M = 2.5). The positive and negative images were matched for arousal (M = 5.6 and 5.4, respectively) and both categories were higher in arousal than the neutral images (M = 3.3). The image remained on screen by itself for 2 s, whereupon a 2-s verbal cue indicated whether the participant was to experience his emotions naturally (‘Experience’ condition) or to decrease his emotional response to the image (‘Reappraise’ condition). The picture was then removed, to minimize confounding effects of eye movements associated with scanning of a visual image (van Reekum et al., 2007). The fixation cross and instruction cue appeared on the screen for 6 s. Subjects were instructed to continue implementing the cued strategy for the entire 6-s block. At the end of each trial, participants rated how negative or positive the image was using an 8-option non-verbal Manikin scale that remained on the screen for 4 s. The ordering of the scale (e.g. very positive to very negative or very negative to very positive) was counterbalanced between participants. A random inter-trial interval (0–8 s, uniformly distributed over 2-s intervals) was imposed between trials. The task was created using Psychtoolbox (Brainard, 1997; Pelli, 1997).
In the fMRI session, participants completed 60 positive image trials (30 ‘Experience’ and 30 ‘Reappraise’), 60 negative image trials (30 ‘Experience’ and 30 ‘Reappraise’) trials, and 30 neutral image trials (all ‘Experience’). Within each condition one-half of all images contained people, and the other half did not. Images were not matched for other visual parameters; however, stimuli were randomly selected from a larger stimulus pool and then assigned to conditions (e.g. Reappraise vs Experience) for each participant. This assignment was counterbalanced between participants within each age group to minimize the effects of other factors (e.g. visual complexity). Images were presented through goggles with corrective lenses matched to each participant’s visual acuity.
fMRI methods
We acquired data on a GE 4T scanner using a gradient-echo inverse-spiral pulse sequence with standard parameters for blood-oxygenation-level-dependent (BOLD) fMRI: repetition time (TR) = 2000 ms; echo time (TE) = 31 ms; field of view (FOV) = 240 mm; flip angle = 90°; 34 axial slices parallel to the AC–PC plane; voxel size: 3.75 × 3.75 × 3.8 mm. Functional data were collected across six runs, each containing 238 imaging volumes. The first six volumes from each run were removed to allow stabilization of the T2 signal. An initial high-resolution inversion-recovery-prepared SPGR anatomical scan was acquired to aid with co-registration and normalization (TR = 12.3 ms; TE = 5.4 ms; whole-brain coverage with ∼1 mm3 voxel size).
fMRI analysis
Analysis of brain data was performed using FEAT Version 5.98, part of FSL (FMRIB's Software Library) package (Smith et al., 2004). The following pre-statistics processing steps were applied: motion correction using MCFLIRT, slice-timing correction, removal of non-brain voxels using BET, spatial smoothing with a Gaussian kernel of FWHM 6 mm, and high-pass temporal filtering (>100 s). To minimize the potential contribution of head motion to our results, we excluded runs with more than five volumes with greater than 1 mm of movement in any direction (younger adults: 14.4% discarded; older adults: 14.2% discarded). All participants had at least two runs included in the final dataset, and 35 out of 42 subjects had four or more runs included. Registration to high-resolution anatomical images and normalization to the Montreal Neurologic Institute (MNI) template image were carried out using FLIRT. All analyses described below use whole-brain-corrected thresholds for significance: voxel-significance thresholds of z > 2.3 and cluster-significance threshold corrected to P < 0.05.
We used two regression models for our analyses, one for condition effects and the other for interactions between regions. Our first model created five regressors of interest, one for each of the trial types (‘Experience-Positive, Experience-Negative, Experience-Neutral, Reappraise-Positive and Reappraise-Negative’). These regressors modeled the 8-s period when participants either reappraised or experienced the emotional content of the stimuli using a unit amplitude function. Additional nuisance regressors modeled the 2-s visual presentation of each stimulus and the response period. All regressors were convolved with a double-gamma hemodynamic response function. To determine brain regions contributing to emotion regulation regardless of age, we collapsed across age groups to reveal condition-specific activations.
We then determined age-related effects (using the same model) using both region-of-interest (ROI) and whole-brain comparisons. For the ROI analyses, we focused on the amygdala and PFC, with the latter subdivided into the inferior, middle and dorsomedial frontal gyri. Within each of these anatomical regions, we constrained our functional ROIs to voxels activated in any contrast of Experience vs Reappraisal (in either direction, for any valence condition), collapsing across age groups. We note that this ROI-creation procedure provides an unbiased prior for subsequent analyses of interactions among valence, regulation condition and age group. Parameter estimates were extracted using FSL’s Featquery tool and were converted into percent signal change for each subject. For the whole-brain comparisons, we introduced age group as a covariate at the third-level (i.e. across-subjects) analysis.
Our second model identified psychophysiological interactions of emotion regulation upon functional connectivity with the amygdala. This model included two regressors consisting of activation time courses in the left and right amygdala, a task regressor that was a boxcar waveform weighted according to the regulation conditions (collapsing across valence), and two regressors of interest created from the convolution of amygdala activation and the task regressor. We extracted time courses from functionally defined ROIs in each amygdala, using voxels defined by the conjunction of the ‘Reappraise > Experience’ contrasts for both positive and negative stimuli.
Finally, to explore the relation between individual differences in cognitive functioning and brain activation during reappraisal, we performed a partial correlation analysis (using JMP; SAS Business Analytics). We examined the relationship between activation changes in each ROI (in the contrast of Reappraisal vs Experience) and the cognitive composite score, while controlling for the effects of age upon both cognition and ROI activation.
RESULTS
Behavior
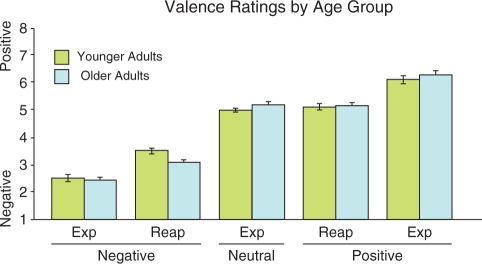
A three-way analysis of variance examined effects of age group (younger, older), regulation condition (‘Reappraise, Experience’), and stimulus category (positive, negative) upon self-reported valence. We found a main effect of condition (P < 0.001), but no main effect of age, nor any significant age-by-condition or age-by-condition-by-valence interaction. During the ‘Experience’ condition, both age groups judged nominally positive images as significantly more positive than neutral images, and negative images as significantly more negative than neutral images (all Ps < 0.001). During the ‘Reappraise’ condition, positive images were judged as less positive and negative images less negative, compared to ‘Experience’, for both age groups (all Ps < 0.001; Figure 2).
Fig. 2.
Valence ratings across task conditions. Both older and younger adults reported significant emotion regulation: the ‘Experience’ (Exp) trials led to more extreme valence ratings than the ‘Reappraise’ (Reap) trials, for both groups.
Because the raw data suggested that effects of age upon reappraisal success might be limited to the negative stimuli, post hoc tests examined age differences within each stimulus category, independently. In the ‘Reappraise’-negative trial type, older adults reported significantly more negative affect than did younger adults (two-tailed t-test, P = 0.01). No significant effects of age group were found for other trial types. There were no significant correlations, across individuals, between cognitive composite scores and success of emotion regulation (i.e. the average valence change from ‘Experience to Reappraise’ conditions) in either valence condition independently or when averaging across them.
Regions supporting emotion regulation
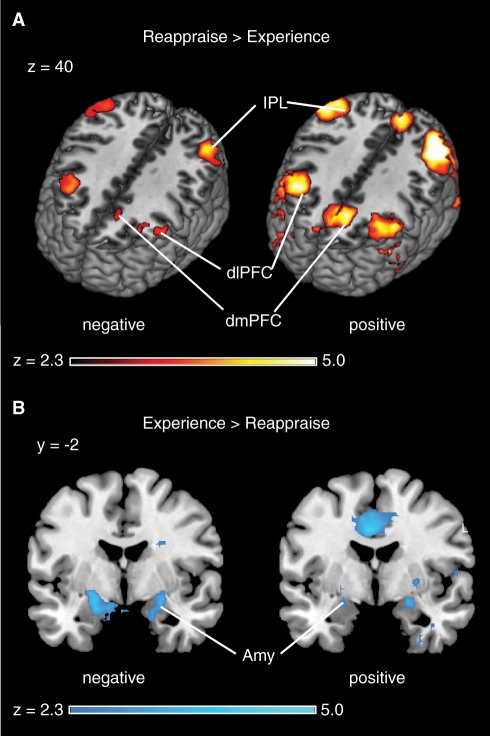
We first examined the main effect of condition, ‘Reappraise vs Experience’ (Figure 3; Table 2), collapsing across age groups, for positive and negative valence separately. We found that reappraisal of positive images evoked increased activation in the dorsolateral prefrontal cortex (dlPFC) and dorsomedial prefrontal cortex (dmPFC), along with the inferior parietal lobule (IPL), all bilaterally. The dlPFC activation included separate foci within the anterior inferior frontal gyrus (IFG) and adjacent insula, along with more posterior activation in the middle frontal gyrus (MFG). A similar pattern of activation was found when examining trials involving negative images.
Fig. 3.
Neural correlates of cognitive reappraisal and emotional experience. (A) The contrast of ‘Reappraise’ >‘Experience’, collapsed over conditions and age groups, revealed that activation in dlPFC, dmPFC and the IPL increased when participants engaged in reappraisal. (B) Conversely, the contrast of ‘Experience’ >‘Reappraise’ revealed that activation in the amygdala was decreased by reappraisal. Similar effects were observed for both negative-valenced (left images) and positive-valenced (right images) stimuli.
Table 2.
Regions of activation associated with cognitive reappraisal or emotional experience
| Positive |
Negative |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Max Z | BA | x | y | z | Max Z |
| Group activations for main effects of condition | ||||||||||
| Reappraise > experience | ||||||||||
| Middle frontal gyrus | R9 | 40 | 44 | 30 | 4.04 | L6, 8, 9 | 38 | 20 | 44 | 4.09 |
| Middle frontal gyrus | L46, 9 | −46 | 26 | 22 | 5.18 | |||||
| Superior frontal gyrus | L8 | −12 | 40 | 46 | 3.65 | |||||
| Superior frontal gyrus | L8 | 0 | 24 | 50 | 5.75 | L6 | −6 | 16 | 58 | 4.08 |
| Insula | L47 | −38 | 22 | −4 | 4.51 | |||||
| Insula | R47 | 40 | 20 | −4 | 3.91 | R13 | 46 | 16 | 6 | 4.28 |
| Precentral gyrus | R9 | 42 | 22 | 36 | 5.59 | R44 | 48 | 14 | 0 | 3.76 |
| Precentral gyrus | L44 | −54 | 20 | 2 | 5.28 | |||||
| Inferior frontal gyrus | L45 | −56 | 20 | 2 | 4.70 | L47, 13 | −39 | 20 | −3 | 4.41 |
| Inferior frontal gyrus | R45 | 44 | 18 | 4 | 4.02 | R47 | 41 | 20 | −4 | 3.38 |
| Middle frontal gyrus | L6 | −42 | 14 | 46 | 5.55 | L6 | −42 | 12 | 50 | 4.86 |
| Middle frontal gyrus | R6 | 48 | 10 | 50 | 4.08 | |||||
| Cingulate gyrus | L23 | −2 | −8 | 24 | 3.14 | |||||
| Cingulate gyrus | R31 | 0 | −32 | 36 | 2.89 | |||||
| Cingulate gyrus | L23, 31 | −6 | −34 | 24 | 3.36 | |||||
| Middle temporal gyrus | L21, 37 | −62 | −46 | −2 | 4.46 | L39 | −38 | −62 | 30 | 3.58 |
| Middle temporal gyrus | R39 | 58 | −64 | 26 | 3.58 | |||||
| Supramarginal gyrus | R40 | 60 | −52 | 34 | 5.70 | |||||
| Inferior temporal gyrus | L37 | −64 | −56 | −10 | 4.23 | |||||
| Angular gyrus | B39 | −42 | −58 | 44 | 5.89 | B39, 40 | 50 | −60 | 42 | 3.68 |
| Superior temporal gyrus | L39 | −52 | −60 | 26 | 4.43 | B39 | −52 | −60 | 34 | 3.74 |
| Inferior parietal lobule | L7 | −40 | −62 | 54 | 4.21 | B40 | 62 | −52 | 40 | 3.69 |
| Superior parietal lobule | L7 | −38 | −68 | 54 | 4.62 | |||||
| Precuneus | L31, 7 | −6 | −72 | 32 | 4.29 | L39, 19 | −40 | −66 | 42 | 4.55 |
| Experience > Reappraise | ||||||||||
| Medial frontal cortex | L10, 9 | −8 | 56 | 2 | 5.35 | |||||
| Medial frontal cortex | R10 | 4 | 56 | −6 | 4.72 | R25 | 14 | 8 | −26 | 3.18 |
| Medial frontal cortex | L32 | −4 | 44 | −6 | 4.71 | |||||
| Inferior frontal gyrus | L47 | −20 | 10 | −28 | 3.91 | |||||
| Putamen | R | 26 | 0 | −16 | 3.83 | |||||
| Cingulate gyrus | L24 | −4 | −2 | 36 | 4.42 | |||||
| Cingulate gyrus | R24 | 4 | −4 | 42 | 3.88 | |||||
| Para-central lobule | L31 | −6 | −6 | 46 | 4.84 | |||||
| Globus pallidus | B | −24 | −6 | −8 | 4.08 | |||||
| Amygdala | B | −22 | −8 | −20 | 3.20 | B | −19 | −2 | −18 | 3.90 |
| Pre-central gyrus | R4 | 34 | −18 | 62 | 3.61 | |||||
| Cingulate gyrus | L31 | −10 | −20 | 36 | 3.41 | |||||
| Post-central gyrus | R40 | −64 | −20 | 20 | 4.1 | |||||
| Caudate | R | 20 | −22 | 28 | 4.32 | L | −4 | 6 | −6 | 3.89 |
| Post-central gyrus | B2 | −46 | −22 | 28 | 5.03 | B2 | 48 | −22 | 26 | 4.27 |
| Insula | R41, 13 | 50 | −24 | 14 | 4.11 | R13 | 38 | −20 | 24 | 3.56 |
| Inferior parietal lobule | L2 | −44 | −26 | 34 | 4.56 | L40 | −56 | −24 | 26 | 4.29 |
| Insula | L13 | −48 | −28 | 24 | 4.13 | L13 | −40 | −22 | 22 | 3.27 |
| Cingulate gyrus | R31 | 26 | −36 | 26 | 4.02 | |||||
| Cuneus | R17 | 16 | −88 | 12 | 3.8 | R18 | 28 | −100 | 8 | 4.09 |
| Middle occipital gyrus | R18 | 32 | −90 | 2 | 4.08 | R19, 18 | 34 | −94 | 22 | 3.87 |
| Inferior occipital gyrus | R18 | 24 | −96 | −6 | 4.11 | L19 | −36 | −80 | 4 | 4.16 |
| Lingual gyrus | R17, 18 | 24 | −96 | 8 | 4.66 | R17 | 22 | −92 | 10 | 3.69 |
BA, estimated Brodmann’s Area; L, left; R, right; B, bilateral; x, y, z, coordinates of peak voxel, shown in MNI space; Max Z, z-statistic of peak voxel.
Positive ‘Experience’ trials, compared to positive ‘Reappraise’ trials, evoked increased activation in regions including bilateral amygdala, mid-cingulate cortex, bilateral post-central gyrus and pregenual cortex. Negative ‘Experience’ trials, compared to negative ‘Reappraise’ trials, revealed activations in amygdala, post-central gyrus and putamen, all bilaterally. For both age groups, PFC activation increased and amygdala activation decreased under reappraisal (Figure 4). Collectively, these findings replicate prior reports from studies of emotion regulation.
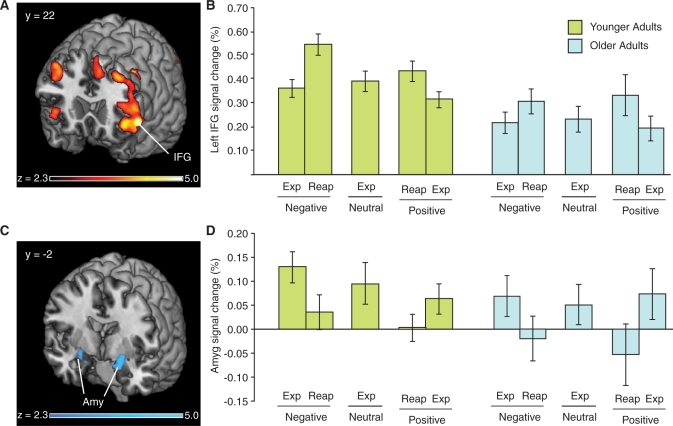
Fig. 4.
Modulation of prefrontal and amygdalar activation by emotion regulation. (A) Examination of a functional region of interest in the LIFG revealed a pattern of activation that followed subjects’ self-reports of emotion regulation. Shown here are voxels activated in the contrast between ‘Reappraise-Negative’ and ‘Experience-Negative’ conditions. (B) For both positive and negative stimuli, and for both younger and older adults, LIFG activation increased to ‘Reappraise’ trials compared to ‘Experience’ trials. (C) Conversely, a functional region of interest comprising the bilateral amygdala (Amy) revealed a systematic decrease in activation under emotion regulation. Shown are voxels activated in the contrast between ‘Experience-Negative’ and ‘Reappraise-Negative’ conditions. (D) For both positive and negative stimuli, and for both younger and older adults, amygdala activation increased to ‘Experience’ trials compared to ‘Reappraise’ trials.
Functional connectivity
To evaluate whether these opposing responses in PFC and the amygdala were driven by the functional demands of the task, we conducted psychophysical interaction (PPI) analyses using the left and right amygdala as seed regions and the regulation condition (‘Experience vs Reappraise’) as a modulatory variable. The time courses of amygdalar responses were regressors in the design matrix and convolved with a boxcar function using a positive weight for ‘Reappraise’ trials and a negative weight for ‘Experience’ trials to create the PPI regressor. Activity in both the left and right amygdala exhibited a regulation-modulated PPI with left PFC, specifically overlapping in left IFG (LIFG) (Figure 5, Table 3). This activation was observed in a more anterior region of the IFG than the IFG activation in the whole brain analysis of ‘Reappraise > Experience’. Interrogating connectivity for the each valence independently revealed that there were significant PPIs between each amygdala and the LIFG for the negative stimuli, but not for the positive stimuli, although direct comparison found no significant difference between the valence conditions. No significant age differences were observed in functional connectivity of these ROIs. Thus, we conclude that the functional connectivity between the LIFG and the amygdala was indeed modulated by the regulation demands of the task, leaving open the question of whether that modulation has valence dependence.
Fig. 5.
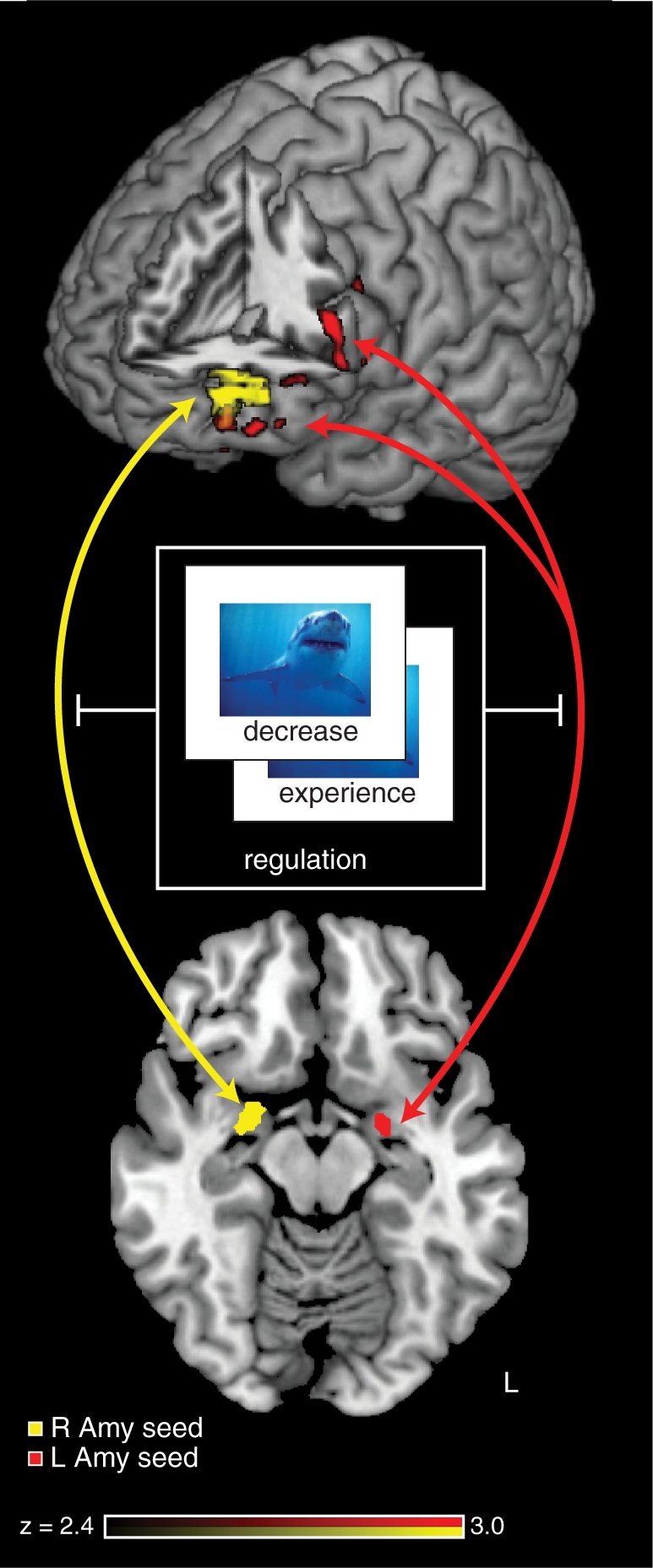
A PPI between amygdala and lateral prefrontal cortex. We conducted a PPI analysis using each of the left and right amygdala as seed regions, and the regulation condition (‘Reappraise’ or ‘Experience’) as a modulatory variable. Significant PPI effects were observed in the left prefrontal cortex, with a region of overlap in the LIFG. These results indicate that emotion regulation modulates the functional connectivity between the amygdala and the LIFG.
Table 3.
Regions of activation exhibiting a PPI with seed regions in the left and right amygdale
| Region | BA | x | y | z | Max Z | |
|---|---|---|---|---|---|---|
| Activations for left and right amygdala PPI | ||||||
| Left amygdala | ||||||
| Middle frontal gyrus | L10, 11 | −44 | 60 | −4 | 3.52 | |
| Inferior frontal gyrus | L10 | −52 | 54 | −6 | 3.69 | |
| Anterior cingulate | L32 | −16 | 46 | −18 | 3.74 | |
| Medial frontal gyrus | L10 | −14 | 42 | −18 | 3.91 | |
| Middle frontal gyrus | R11 | 22 | 42 | −24 | 3.33 | |
| Inferior frontal gyrus | R47 | 18 | 34 | −24 | 3.51 | |
| Inferior frontal gyrus | L44 | −54 | 18 | 10 | 3.51 | |
| Middle frontal gyrus | L9, 46 | −48 | 18 | 22 | 3.82 | |
| Precuneus | B7 | −8 | −68 | 62 | 3.90 | |
| Right amygdala | ||||||
| Inferior frontal gyrus | L10 | −42 | 58 | −4 | 2.91 | |
| Middle frontal gyrus | L10 | −42 | 56 | 2 | 3.67 | |
| Middle frontal gyrus | L10 | −34 | 52 | 6 | 3.06 | |
| Superior frontal gyrus | L10 | −26 | 52 | 18 | 2.86 | |
| Middle frontal gyrus | L10 | −36 | 50 | 14 | 4.02 | |
| Middle frontal gyrus | L10 | −46 | 50 | 4 | 4.00 |
BA, estimated Brodmann’s Area; L, left; R, right; B, bilateral; x, y, z, coordinates of peak voxel, shown in MNI space; Max Z, z-statistic of peak voxel.
Effects of age
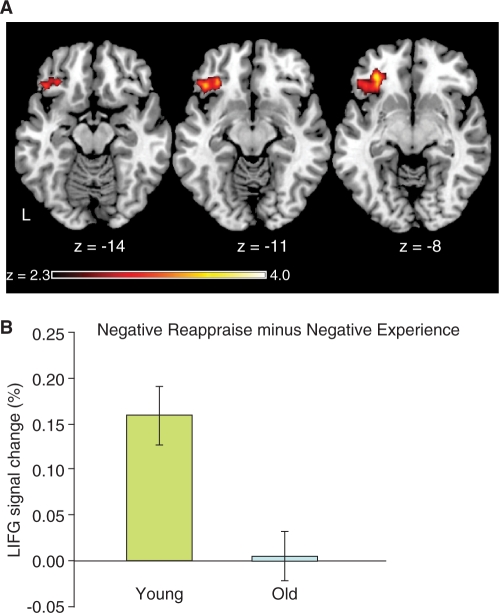
We next investigated differences in activation between the age groups, using both whole-brain and ROI analyses. We conducted an unbiased test that measured the percent signal change associated with each condition within PFC ROIs (IFG, MFG, dmPFC) and the amygdala. All ROIs were defined according to the procedures described above, and were interrogated separately for each hemisphere (except for dmPFC). Only one ROI showed a significant age-group difference: older adults exhibited decreased LIFG activation in the ‘Reappraisal’ minus ‘Experience’ contrast for negative stimuli (P < 0.05). To confirm this difference, we also performed a whole-brain analysis, which revealed that, for the contrast between negative ‘Reappraise’ and ‘Experience’ trials, the younger adults exhibited a greater change in activation than older adults in two regions: the LIFG and superior temporal gyrus (Figure 6, Table 4). The LIFG activation observed here was more ventral to the LIFG activation produced from our PPI analysis. Across our sample of older subjects, those individuals who showed greater neural effects of regulation (‘Reappraise’ minus ‘Experience’) evinced greater regulation success on negative trials (r = 0.48, P < 0.05). Younger participants did not show this relationship (r = 0.08, P = 0.70).
Fig. 6.
Age differences in the modulation of lateral prefrontal cortex by emotion regulation. A whole-brain analysis identified brain regions for which the contrast between ‘Reappraise’ and ‘Experience’ conditions was significantly different between younger and older adults. (A) We found one region for which there was greater activation for younger compared to older adults, for regulation of negative emotions: the LIFG. (B) Within this region, young adults exhibited significantly greater activation during ‘Reappraise’ compared to ‘Experience’ trials, whereas older adults showed no differences between those conditions.
Table 4.
Regions exhibiting a significant effect of age-group on the negative-reappraise > negative-experience contrast
| Region | BA | x | y | z | Max Z | |
|---|---|---|---|---|---|---|
| Age group difference for negative-reappraise > negative-experience | ||||||
| Superior temporal gyrus | L41,22 | −46 | −34 | 12 | 3.48 | |
| Middle temporal gyrus | L21 | −54 | −44 | 14 | 3.31 | |
| Inferior frontal gyrus | L47 | −34 | 38 | −6 | 3.74 | |
| Claustrum | −30 | 18 | −2 | 2.6 | ||
BA, estimated Brodmann’s Area; L, left; x, y, z, coordinates of peak voxel, shown in MNI space; Max Z, z-statistic of peak voxel.
Our whole-brain analyses of the ‘Reappraise’ minus ‘Experience’ contrast revealed no significant effects of age for positive stimuli. Post hoc analyses on the LIFG ROI identified above (i.e. the region found for age effects on the reappraisal of negative stimuli) revealed that the effect of age on the response to positive stimuli was non-significant and, moreover, significantly less than that to negative stimuli (age-group-by-condition interaction, P < 0.01). Thus, while we cannot exclude the possibility that this region contributes to reappraisal of positive stimuli in either age group—whether with a subthreshold response or in other task contexts—we found no evidence for such a relationship in the present data.
Effects of cognition
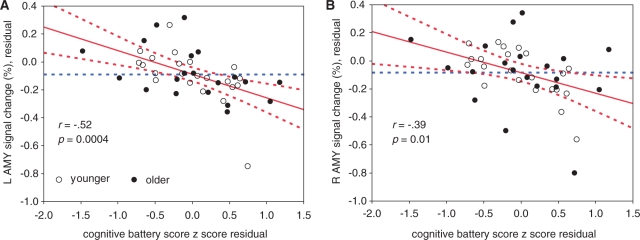
Finally, we investigated whether individual differences in cognitive functioning could explain the pattern of activation in the PFC observed during reappraisal, including the reduction in reappraisal-related IFG activation in older adults. We computed the partial correlation, across all subjects, between the cognitive composite score and relative activation in the ‘Reappraise vs Experience’ conditions, while controlling for age. For negatively valenced stimuli, there was a negative partial correlation between cognitive performance and activation in the left [r(40) = –0.52, P < 0.05] and right [r(40) = –0.39, P < 0.05] amygdala (Figure 7), with both correlations robust to removal of outlier subjects. For positively valenced stimuli, there was a positive partial correlation between activation in the LIFG and cognitive performance [r(40) = 0.32, P < 0.05]. We note, however, that post hoc analyses revealed that this correlation was no longer significant once the most extreme outlier was removed from the analysis. Collectively, these results indicate that increased cognitive abilities are associated with a greater decrease in amygdala activation, consistent with successful regulation. These results reveal that cognitive abilities are significant predictors of activation associated with successful reappraisal, above and beyond the effects of age itself.
Fig. 7.
Partial correlations between cognitive ability and regulation-related activation in the amygdala. For functional regions of interest in prefrontal cortex and the amygdala, we examined whether individual differences in cognitive abilities predicted regulation-induced changes in activation. We assessed cognition through a battery that tested aspects of memory and processing speed. Partial correlation analyses evaluated the relationship between composite cognitive scores and changes in activation between the ‘Reappraise’ and ‘Experience’ conditions, after controlling for any effects of age itself. Shown are leverage scatterplots of the relationships between age-controlled cognitive battery z scores and age-controlled activation in the left (A) and right (B) amygdala. For both regions, increased cognitive ability predicted greater decreases in activation under conditions of emotion regulation.
DISCUSSION
Despite substantial prior behavioral research on lifespan changes in emotional cognition, relatively little is known about the accompanying changes in the underlying brain mechanisms. In the current study, we investigated the neural substrates of emotion regulation within samples of younger and older adults. There were three main findings. First, we replicated the pattern of opposing activation in prefrontal cortex and the amygdala—here, expressed as a psychophysiological interaction—typically reported during conditions of emotion regulation. Second, we found that older adults exhibited decreased reappraisal-related activation in the lateral PFC, specifically the LIFG, compared to younger adults. Changes in activation in this region were predictive of reappraisal success, for older adults only, suggesting that the LIFG plays a critical role in older adults’ ability to regulate emotion. Third, we showed that the activation decrease in the bilateral amygdala in the negative condition was correlated with an independent measure of cognitive ability, even when controlling for age. We consider the implications of these results in the following sections.
The recent explosion of interest in social cognitive neuroscience has been accompanied by many explorations of cognition–emotion interactions. Of particular note have been studies of emotion regulation, which explore the neural underpinnings of common therapeutic strategies for minimizing experienced emotion. While the targeted aspects of emotion regulation have differed over many studies, leading to some diversity of results, some core findings have been consistently reported: active regulation of emotions leads to an increase in PFC activation, but a decrease in amygdalar activation (Ochsner and Gross, 2008). Moreover, recent studies have revealed that reappraisal modulates the functional connectivity between PFC and the amygdala (Banks et al., 2007), potentially among several pathways for cognitive control of emotional responses (Wager et al., 2008). These effects have been interpreted, naturally, as reflecting increased control demands but decreased emotional experience during reappraisal.
Of note, nearly all prior neuroimaging studies using cognitive reappraisal (or other emotion regulation strategies) have used young adult subjects (but see Urry et al., 2006, van Reekum et al., 2007 and Urry et al., 2009, each of whom studied only an older-adult sample). It has been long recognized that cognitive function declines with increasing age. Contributing to this decline are age-related changes within several brain regions, including PFC (Madden et al., 2005). With increasing age, there is reduced cortical volume within PFC (Raz et al., 1997) and diminished structural connectivity between PFC and other brain regions; for a review see (Sullivan and Pfefferbaum, 2006; Dennis and Cabeza, 2008; Madden et al., 2009). Moreover, a spate of studies have demonstrated differences between younger and older adults in observed patterns of prefrontal function (Hedden and Gabrieli, 2004). Given these robust age differences in the neural substrates of cognitive control, combined with the well-established changes in emotional processing with age, one might predict that emotion regulation would reflect a dramatically different set of neural contributors within older adults. That prediction was not supported by our data. Contrary to evidence suggesting that older adults show increased capacity to regulate emotion (Gross et al., 1997), our results indicated that older and younger adults show generally similar behavioral and neural consequences of cognitive reappraisal. One explanation for this finding is that most studies reporting age differences in emotional processing involve minimal engagement with stimuli. Recent data suggests that selective attention, which is thought to be highly important for emotional control in older adults (Isaacowitz et al., 2006), is not cognitively demanding (Allard et al., 2009). Reappraisal, in contrast, involves engaging a specific and explicit strategy, for which older adults may elicit substantial (and potentially compensatory) prefrontal control processes.
Despite the cognitively demanding nature of our task, older adults expressed the standard pattern of increased PFC activation as previously reported for younger adults. Furthermore, there were no age differences in reappraisal-related activation in the dorsolateral and dorsomedial PFC, or any changes in functional connectivity between the amygdala and the lateral PFC. Collectively, these results indicate that older and younger adults utilize a generally similar network for emotion regulation.
We did observe selective age-related differences within this network: older adults exhibited reduced LIFG activation to reappraisal of negative stimuli. As noted above, emotion regulation typically evokes activation in diverse aspects of lateral and medial prefrontal cortex, perhaps reflecting the breadth of strategies used by participants (Ochsner et al., 2004). One potential interpretation for the contribution of IFG comes from its role in goal-directed inhibition, as shown when people cancel a planned response based on a sudden cue (Aron et al., 2004) or a no-go stimulus (Liddle et al., 2001; Rubia et al., 2001). As one relevant example, the emotional oddball task requires inhibition of behavioral responses to unexpected and task-irrelevant emotional stimuli (i.e. infrequently presented photographs with negative valence). Those photographs evoke activation in the IFG, in contrast to the more dorsal activation observed to attended task-relevant stimuli (Dolcos et al., 2006; Dolcos and McCarthy, 2006). Though activations in the IFG during inhibition tasks are often right lateralized, damage to the LIFG also impairs performance on no-go tasks (Swick et al., 2008), suggesting that both left and right IFG play important roles in successful inhibition. Furthermore, greater increases in activation in the IFG during reappraisal of negative images predicted greater modulation of valence ratings in older but not younger adults. One possible explanation for our age differences in IFG activation, therefore, is that older and younger adults differ in their engagement of inhibitory control processes supported by IFG as involved in emotion regulation, particularly of negative affect.
We note that age-related changes in functioning may be manifest in various changes in neural function, depending on task context. Previous research across many cognitive domains, from executive function to memory, has come to equivocal conclusions. In some settings, older adults demonstrate reduced prefrontal activation (Stebbins et al., 2002; Thomsen et al., 2004), while in other paradigms older adults show increased activation (Schiavetto et al., 2002; Langenecker et al., 2004; Madden et al., 2004; Grady et al., 2005; Gutchess et al., 2005). According to functional compensation theories (Cabeza, 2002), older adults recruit regions of prefrontal cortex to compensate for other deficits in processing, whether within prefrontal cortex itself (Cabeza et al., 2002) or in more posterior brain regions (Davis et al., 2007). Under these and similar perspectives, changes in PFC function during emotion regulation may reflect not age itself, but the cognitive decline that accompanies aging to greater or lesser degrees across individuals. Though it has been hypothesized that specific cognitive abilities support reappraisal (Ochsner and Gross, 2008), a relationship between explicit measures of cognitive functioning and neural activation during reappraisal has not been demonstrated.
Our results provide the novel demonstration that individual differences in cognitive ability are correlated with patterns of brain activation during reappraisal; specifically, we found that the negative reappraisal-related decrease in amygdala activation was correlated with the relative cognitive abilities of our participants. Our results converge with findings from both behavioral and neural investigations of the effects of cognitive ability on emotion regulation. Specifically, biases in emotional memory thought to result from emotion regulation during encoding correlate with executive functioning in older adults (Mather and Knight, 2005). Additionally, compared to younger adults and cognitively low-functioning older adults, high-functioning older adults show greater recruitment of the LIFG in a task thought to engage the inhibition of negative stereotypes (Krendl et al., 2009). Our results, therefore, support the hypothesis that cognitive functioning predicts neural markers, if not behavioral manifestations, of emotion regulation.
The correlation between cognitive functioning and the amygdala was only significant for the negative condition, consistent with recent findings that different pathways between the ventrolateral PFC and the amygdala and ventral striatum independently support successful regulation. Wager et al. (2008), suggest that the pathway between the PFC and the ventral striatum might be engaged by the process of generating more positive appraisals, whereas the pathway between the PFC and the amygdala may be engaged by generating less negative appraisals of emotional stimuli. Our reappraisal instructions encouraged subjects to minimize negative aspects of stimuli, not to emphasize positive aspects, supporting this interpretation. Strikingly, the observed relationships between cognition and activation (see Figure 7) held when collapsing over both age groups, suggesting that it reflects differences across individuals that are not specific to age-related change. We speculate that the neural consequences of emotion regulation might be best considered not as a direct effect of age, but as a secondary consequence of age-related cognitive decline. Accordingly, our results make the strong prediction that placing older adults in contexts that minimize cognitive demands would lead to improved emotion regulation.
Acknowledgments
The authors thank Mauricio Delgado for suggestions about our emotion regulation paradigm, Susanne Harris for assistance with data collection, Max Cohen and Jasmeet Pannu Hayes for assistance with data analysis, and McKell Carter, John Clithero and David Smith for manuscript comments. This project was supported by the National Institute of Aging (R21-30771).
REFERENCES
- Allard ES, Wadlinger HA, Isaacowitz DM. Positive Gaze Preferences in Older Adults: Assessing the Role of Cognitive Effort with Pupil Dilation. Aging, Neuropsychology, and Cognition. 2009 doi: 10.1080/13825580903265681. [Epub-ahead of print; 4 November 2009], doi:10.1080/13825580903265681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory. New York: Psychological Corporation; 1978. [Google Scholar]
- Blanchard-Fields F, Stein R, Watson TL. Age differences in emotion-regulation strategies in handling everyday problems. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:261–9. doi: 10.1093/geronb/59.6.p261. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–6. [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: a theory of socioemotional selectivity. American Psychologist. 1999;54:165–81. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–55. [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cerebral Cortex. 2007;18:1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik F.I.M., Salthouse TA, editors. Handbook of Aging and Cognition. 3rd. Mahwah, NJ: Erlbaum; 2008. pp. 1–54. [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. NeuroReport. 2006;17:1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26:2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–81. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2:271–99. [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Gotestam Skorpen C, Hsu AYC. Emotion and aging: experience, expression, and control. Psychology and Aging. 1997;12:590–9. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, et al. Aging and the Neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Madan A. Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective, and Behavioral Neuroscience. 2009;9:144–52. doi: 10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henninger DE, Madden D, Huettel SA. Processing speed and memory mediate age-related differences in decision making. Psychology and Aging. in press doi: 10.1037/a0019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006;21:40–8. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–14. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Heatherton TF, Kensinger EA. Aging minds and twisting attitudes: an fMRI investigation of age differences in inhibiting prejudice. Psychology and Aging. 2009;24:530–41. doi: 10.1037/a0016065. [DOI] [PubMed] [Google Scholar]
- Lang FR, Carstensen LL. Time counts: future time perspective, goals, and social relationships. Psychology and Aging. 2002;17:125–39. doi: 10.1037/0882-7974.17.1.125. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-6. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. NeuroImage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Affect intensity as an individual difference characteristic; a review. Journal of Research in Personality. 1987;21:1–39. [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–9. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA. Age-related changes in neural activity during visual perception and attention. In: Cabeza R., Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford; 2005. pp. 155–83. [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel S.A. Age-related changes in neural activity during visual target detection measured by fMRI. Cerebral Cortex. 2004;14:143–55. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19:415–35. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–70. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–78. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross J.J., Buck R, editors. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–42. [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–96. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Schiavetto A, Köhler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia. 2002;40:1428–42. doi: 10.1016/s0028-3932(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, et al. Aging effects on memory encoding in the frontal lobes. Psychology and Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen T, Specht K, Hammar Å., Nyttingnes J, Ersland L, Hugdahl K. Brain localization of attentional control in different age groups by combining functional and structural MRI. NeuroImage. 2004;22:912–9. doi: 10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36:1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-Subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]