Abstract
Studies on aging and emotion suggest an increase in reported positive affect, a processing bias of positive over negative information, as well as increasingly adaptive regulation in response to negative events with advancing age. These findings imply that older individuals evaluate information differently, resulting in lowered reactivity to, and/or faster recovery from, negative information, while maintaining more positive responding to positive information. We examined this hypothesis in an ongoing study on Midlife in the US (MIDUS II) where emotional reactivity and recovery were assessed in a large number of respondents (N = 159) from a wide age range (36–84 years). We recorded eye-blink startle magnitudes and corrugator activity during and after the presentation of positive, neutral and negative pictures. The most robust age effect was found in response to neutral stimuli, where increasing age is associated with a decreased corrugator and eyeblink startle response to neutral stimuli. These data suggest that an age-related positivity effect does not essentially alter the response to emotion-laden information, but is reflected in a more positive interpretation of affectively ambiguous information. Furthermore, older women showed reduced corrugator recovery from negative pictures relative to the younger women and men, suggesting that an age-related prioritization of well-being is not necessarily reflected in adaptive regulation of negative affect.
Keywords: aging, emotion reactivity, emotional recovery, positivity effect, psychophysiology
INTRODUCTION
As we age, our cognitive and physical performance generally decline, yet it seems that effects of advancing age on emotional experience and expression is mixed. Socio-emotional selectivity theory (e.g. Carstensen and Lockenhoff, 2003) suggests that increasing age is paired with a shift in motivation to prioritize current well-being and life satisfaction over anticipating the future. In line with this notion, behavioral studies have shown that older adults, relative to their younger counterparts, are biased to memorize affectively positive stimuli (Mather and Carstensen, 2005) and display an attentional bias towards positive information (Isaacowitz et al., 2006). The prioritization of well-being likely extends to the biasing of appraising emotion-relevant situations in a less negative light. Indeed, survey studies indicate increased general positive affect with increasing age (Mroczek and Kolarz, 1998), decreased reactivity to interpersonal stressors (Birditt et al., 2005) paired with more passive regulatory strategies in response to emotional situations (Blanchard-Fields et al., 2004; Coats and Blanchard-Fields, 2008), and a greater investment in and an improved ability to regulate one’s response in socio-emotional situations (Carstensen and Lockenhoff, 2003). These results suggest that aging is associated with more adaptive responding to potentially negative events, possibly engaging more with positive events.
However, findings concerning aging and emotional reactivity to laboratory-based mood inductions are mixed. Laboratory studies on emotion often present pictures selected from the International Affective Picture System (IAPS, Lang et al., 2005), a picture set that is widely used in neuroscientific research on emotion. Presenting a large number of pictures from this set to older (63–77 years) and younger (18–31 years) participants, Grühn and Scheibe (2008) demonstrated that older participants rated negative pictures as more negative but positive and neutral pictures as more positive than the younger participants. The older group also rated negative and neutral pictures as more arousing and positive pictures as less arousing than their younger counterparts.
The more intense reporting of valence and arousal experiences by older-aged participants is not necessarily reflected in other response systems. Studies in which physiological indicators of emotional reactivity were measured suggest lower autonomic nervous system (ANS) activity for older compared to younger participants in response to the emotion inductions, despite a lack of age differences in self-reported responses to relived emotions (Levenson et al., 1991), emotional film clips (Tsai et al., 2000), or despite higher arousal ratings of pictures (Gavazzeni et al., 2008). Smith and colleagues (2005) measured various indicators of peripheral and central nervous system changes in response to IAPS pictures. Apart from the older relative to the younger aged group displaying stronger response magnitudes to negative pictures in one of the measurements (that of eyeblink startle), no other effects of age on physiological responding to emotional pictures were found. This relative lack of age-related differences in physiological responding contradicted the more extreme valence and higher overall arousal ratings by the older group. Furthermore, Kunzmann and Grühn (2005) demonstrated that while older participants (60–70 years) relative to younger participants (20–30 years) reported stronger feelings of sadness in response to film clips selected to elicit sadness in the older-aged group, there were no substantial age-related differences in physiological responding to these clips. In summary, while most studies have shown relatively consistent age differences in subjective reporting, these differences are not necessarily reflected in other components of emotional responding.
As alluded to above, increasing age has been associated with improved emotion regulation capacity. In a cross-cultural study with a large age range, Gross and colleagues (1997) found increased self-reported emotional control with age for all sampled cultures. In addition, older adults endorse passive emotion-focused or inward-focused coping strategies for handling stressful or anger-eliciting encounters more than do younger adults (Blanchard-Fields et al., 2004; Folkman et al., 1987; Phillips et al., 2008). Assessing emotional reactivity and regulation in a laboratory setting, Kliegel and colleagues (2007) found that older individuals reacted more strongly to negative film clips but also showed stronger ‘mood repair’ following the films relative to younger participants. However, when explicitly instructed to suppress expressions to film clips, Kunzmann and colleagues (2005) did not find age differences in the ability to suppress expression nor in the physiological or subjective concomitants of emotion. Importantly, recent findings by Scheibe and Blanchard-Fields (2009) suggest that the instructed down-regulation of disgust takes less effort in older people, given the finding that the impact of down-regulation on the performance of a subsequent working memory task was reduced in the older (60–75 years) relative to the younger (20–30 years) group. Thus, emotion regulation may require less effort, likely reflected in the employment—implicit or explicit—of more adaptive coping or regulatory strategies. If adaptive regulatory, or coping, strategies are invoked more readily with increasing age, older-aged individuals should show a stronger recovery from emotional events with advancing age, as suggested by Kliegel et al.’s findings of better mood repair in the older, relative to the younger, participants.
The aim of the present study is to assess age-related differences in reactivity to and recovery from emotion induced by pictures selected from the IAPS (Lang et al., 2005). The measurement of changes in emotional response systems in an objective fashion, using measures of ANS and expressive functioning in addition to self-report is imperative if we are to gain a fuller understanding of changes in emotion reactivity and regulation across the lifespan. It has been well established that the eyeblink reflex (EBR) magnitude to an acoustic startle probe shows a linear pattern with the valence of foreground stimuli, i.e. the magnitude is attenuated when the stimulus is positive, and enhanced when it is negative, relative to a neutral stimulus (Lang et al., 1990). Similarly, corrugator activity (COR) is not only an index of negative affect (Cacioppo et al., 1986) but has been shown to be reciprocally related to both positive and negative affect (Larsen et al., 2003), such that COR increases in response to negative and decreases in response to positive stimuli relative to neutral ones. Work from our laboratory has demonstrated that EBR and COR are useful indicators not only of reactivity to emotion-relevant stimuli but also of individual differences in the timeline of recovery (or return to baseline) after stimulus offset by measuring the EBR to an acoustic probe delivered after stimulus offset (Jackson et al., 2003) and aggregating COR after stimulus offset (Jackson, 2004). Indeed, Jackson and colleagues (2003) have shown that a positive emotional disposition—assessed with relative left frontal activation at rest—is associated with a faster recovery from negative information, but no effect on emotional reactivity was found. In an older population, however, van Reekum and colleagues (2007) provided evidence suggesting that higher reported psychological well-being biased the appraisal of negative information as less salient. We hypothesize that a motivation to prioritize (socio)emotional well-being with advancing age affects emotional responding due to a different appraisal of the situation, i.e., by focusing on a more positive outcome. Indeed, such positive (re)appraisal has been shown to be paired with a dampened emotion response profile (Foti and Hajcak, 2008; Lazarus and Alfert, 1964; Speisman et al., 1964) and likely underlies the observed faster recovery from negative events (Jackson et al., 2003).
Following up on our prior work, we presented positive, neutral and negative IAPS pictures to participants with a large age range (36–84 years) from the ‘Midlife in the US’ sample (MIDUS II, see http://midus.wisc.edu/) whilst recording COR and EBR magnitude to acoustic probes presented during the stimulus and after stimulus offset. The positive and negative stimuli were carefully matched on arousal, and across valences, pictures were matched for social content, luminosity and picture complexity. Based on the notion that older-aged individuals are motivated to prioritize well-being, positively biasing their (re)appraisals, we predicted lower reactivity to negative information and/or a faster recovery from negative information with increasing age, and a stronger maintenance of responses to positive stimuli.
METHOD
Participants
A total of 275 participants (aged 36–84 years, average age = 56 years, s.d. = 11.1, 157 or 57.1% females) of the national MIDUS II (http://midus.wisc.edu/) study who lived in the Midwest region of the USA agreed to participate in our experiment. For the purpose of this report, we included the data of all participants for whom data records on all three measures were complete.1 This inclusion criterion resulted in a final N = 159, of whom 90 or 56.6% were female, between 36 and 83 years with an average age of 54 years (s.d. = 10.43).
Stimuli
We presented a total of 90 digital color pictures (800 × 600) selected from the IAPS (Lang et al., 2005) in a randomized sequence. We identified 30 negative (M = 2.89, s.d. = 0.61), 30 neutral (M = 5.14, s.d. = 0.52) and 30 positive (M = 7.24, s.d. = 0.44) pictures according to the IAPS norms, of which the positive and negative pictures were matched on arousal (negative pictures M = 5.35, s.d. = 0.54; neutral M = 3.22, s.d. = 0.73; positive M = 5.23, s.d. = 0.73), and all valences were matched on picture luminosity, complexity and social content of the scene depicted.2
Procedure
Upon arrival in the laboratory, participants were informed about the general aim and procedures of the study. After obtaining informed consent, the participants completed questionnaires (data not included here) and performed a hearing test. Participants were then prepared for the collection of electromyogram (EMG) data—eyeblink startle reflex and corrugator—by cleaning the muscle and forehead sites over which the sensors would be placed. The participants were seated in a comfortable armchair in front of a computer monitor; the distance between the eyes and computer screen was ∼60 cm.
Instructions were provided on a 17-in. LCD flat screen monitor, and the experimenter read the instructions aloud. The participants then performed a brief practice session after which the actual experiment began. The participants watched the positive, neutral and negative pictures selected from the IAPS, and acoustic startle probes were presented. Acoustic startle probes consisted of 50 ms, 105 dB, white noise bursts with very rapid onset time which were presented through earbuds. Each picture either had a yellow or purple border around it. Participants were instructed to respond as quickly as possible to the color of the border by pressing one of two keyboard buttons marked with the color with either their index or middle finger of their dominant hand. They were also instructed to keep their gaze on the screen and avoid closing their eyes while the picture was displayed, and to avoid body and head movements during the task.3
Pictures were presented on the screen for 4 s and were preceded by a 1 s fixation screen. During the first 500 ms of the picture, the yellow or purple border was displayed. Acoustic startle probes were inserted at three time points (randomized across trials to maintain an average inter-probe interval of ∼16 s). One time point occurred during picture presentation (2900 ms following picture onset), a second probe occurred 400 ms after picture offset (4400 ms following picture onset), and a third probe occurred 1.9 s following picture offset (5900 ms following picture onset). A total of nine probes at each of the three time points were presented for each picture valence category, resulting in three non-probed trials for each picture valence. The inter-trial interval (ITI) varied randomly between 14 and 18 s.
After the task, EMG sensors were removed and the participants rated the pictures seen during the task using a computerized version of the evaluative space grid, above which the picture was displayed. For a full description of the grid, the reader is referred to Larsen and colleagues (Larsen et al., 2009). Briefly, the evaluative space grid allows the simultaneous measurement of both positive and negative feelings to pictures by positioning a cursor in a two-dimensional 5 × 5 grid space. The grid has been validated against unipolar and dichotomous-then-unipolar ratings (Larsen et al., 2009). The first 56 participants provided the valence and then their arousal rating on a nine-point Likert-scale using the wording by Lang et al. (2005) with 1 = very unpleasant and 9 = very pleasant for valence, and 1 = very calm and 9 = very excited for arousal. To allow the collapsing across the different ratings method, the grid data were recoded into nine-point valence ratings similar to the nine-point valence and arousal scales used.
EMG data acquisition and reduction
Raw EMG data from the orbicularis oculi were collected using two Sensormedics 4 mm Ag/AgCl sensors filled with electrogel (Electro-Cap International Inc, Eaton, Ohio) placed directly below the left or right eye (counterbalanced across participants) in accordance with guidelines provided by Blumenthal and colleagues (2005). A ground sensor was placed on the center of the participant’s forehead. The sensor regions were cleaned using distilled water and 70% isopropyl alcohol (Dynarex Inc, Orangeburg, NY), then slightly abraded using Mavidon Skin Prep (Mavidon Medical Products, Nailsea, Somerset) prior to sensor placement to reduce skin impedance to an acceptable level (below 20 kΩ). Eyeblink startle EMG were acquired and filtered using SAI Bioelectric amplifiers (SA Instrumentation Co., Encinitas, CA) with a gain of 10 000 and high-pass filter setting of 1 Hz. Startle eyeblink data was bandpass filtered at 30–800 Hz, integrated and rectified using a Coulbourn S76-01 contour-following integrator with a time constant of 20 ms. The EMG data were collected using a PC equipped with an analog-to-digital board (Analogic Corp., Wakefield, MA). The eyeblink startle signal was sampled at 1000 Hz, beginning 50 ms before the onset of each startle probe and ending 250 ms following probe onset. Eyeblink startle magnitude was calculated by subtracting the amount of integrated orbicularis oculi EMG at reflex onset from the maximum amount of integrated EMG between 20 and 120 ms following probe onset. Noise-free trials with no perceptible EBR (i.e. non-response) were given a magnitude of zero. Blink magnitudes were log-transformed to normalize the distribution, and z-transformed within-subjects to control for large individual differences—which can be due to extraneous variables such as skin permeability and other factors which may increase the surface impedance or decrease signal quality—in response amplitude and baseline EMG levels. Startle blink magnitudes were averaged within probe time and condition. Participants who did not respond with a perceptible EBR on 10 or more of 81-probed trials were excluded from analyses.
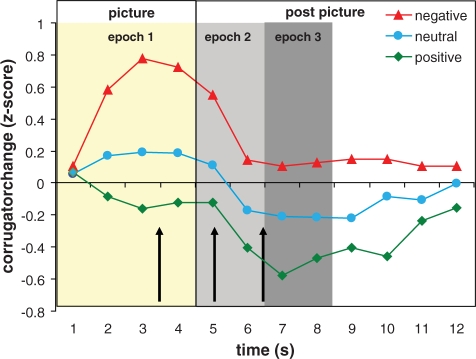
Raw EMG from the corrugator supercilii was recorded in similar fashion as the orbicularis EMG, with the following exceptions: sensors were placed over the left or right brow region (counterbalanced across participants) in accordance with guidelines provided by Tassinary and colleagues (1986). Corrugator EMG was low-pass filtered at 400 Hz, and data collection was continuous throughout the trial. We performed a fast Fourier transform (FFT) on all artifact-free 1 s chunks of data (extracted through Hanning windows with 50% overlap) to derive estimates of spectral power density (µV2/Hz) in the 45–200 Hz frequency band. These values were log-transformed to normalize the data and z-transformed within-subjects to control for large individual differences in response magnitudes. The first 1 s pre-picture (fixation) epoch was used as a baseline and subtracted from subsequent second-by-second data. COR was then divided into three distinct epochs for analysis, the first being the 4-s picture period (epoch 1, ‘emotion reactivity’). The second epoch is the 2 s following picture offset (epoch 2, ‘early emotion recovery’) and includes the time around the second startle probe. The last epoch consists of third and fourth second following picture offset (epoch 3, ‘late emotion recovery’), containing the last startle probe (see Figure 1 for second-by-second corrugator data of these 159 participants with the epochs indicated with graded areas). COR was then averaged for each epoch and condition separately.
Fig. 1.
Baseline-corrected second-by-second EMG activity measured over the brow region (corrugator supercilii muscle, log-transformed, z-scored) for each of the picture valences across a 12 s period. Corrugator EMG was aggregated over three distinct epochs: the first 4 s denote the epoch during picture presentation marked in off-white, the first grey area marks the first 2 s epoch after picture offset, the second darker grey area marks the second 2 s epoch after picture offset. The three startle probe times are indicated by the black arrows.
After data from the first 56 participants were collected, a laboratory room change was made which resulted in a continuation of data collection using new hardware. EMG was from this moment onwards collected using Biopac software and hardware (BIOPAC Systems, Inc., Goleta, CA). Data acquisition details were similar as described above, with the following modifications: raw EMG signals were high-pass filtered at 20 Hz, then amplified (using ERS100C amplifiers) 5000 times prior to digitization at 1000 Hz with 16-bit precision.
RESULTS
Overview of analyses
We performed mixed-model multivariate analyses of variance (MANOVA, type III sum of squares) to test the predicted age effects on emotion reactivity and recovery separately. The advantage over analyzing data obtained from within-subjects (or mixed) designs with a multivariate test is that the multivariate tests are not sensitive to issues of compound symmetry or sphericity, which often plagues univariate repeated measures ANOVA tests (see O'Brien and Kaiser, 1985). We demeaned age to avoid changes of the main effects in a repeated measures ANCOVA if the mean of the covariate is not zero (see Thomas et al., 2009). To test whether age had the predicted effects on emotion reactivity and recovery, we entered the demeaned age as a covariate, together with gender (male vs female) and valence (negative vs neutral vs positive) as a within-subjects factor. We controlled for possible effects of differences in data collection methods (see ‘Methods’ section) on our variables by including recording method as an additional blocking factor. Moreover, effects of time (epoch 2 vs 3) were tested for emotion recovery. Effects of age were tested by examining interaction effects of age with valence, while interaction effects of age and gender with valence were explored. When interaction effects were significant across measures in the omnibus test (i.e. P < 0.05), follow-up tests were considered to further assess the source of the significant effect(s) with age.
For emotion reactivity, we assessed COR and eyeblink startle recorded during the time the picture was presented, as well as the ratings of these pictures obtained at a later stage. For emotional recovery, we included data acquired from a comparable time window across measures, i.e. COR from epoch 2 and 3, after picture offset, and eyeblink startle magnitude from the two probes following picture offset. To render the emotion recovery period independent from the emotion reactivity period, we residualized emotion recovery indices by performing regression analyses on each recovery measure (per condition), entering the emotion reactivity measure as a predictor and saving the residuals as a new variable.
Emotion reactivity
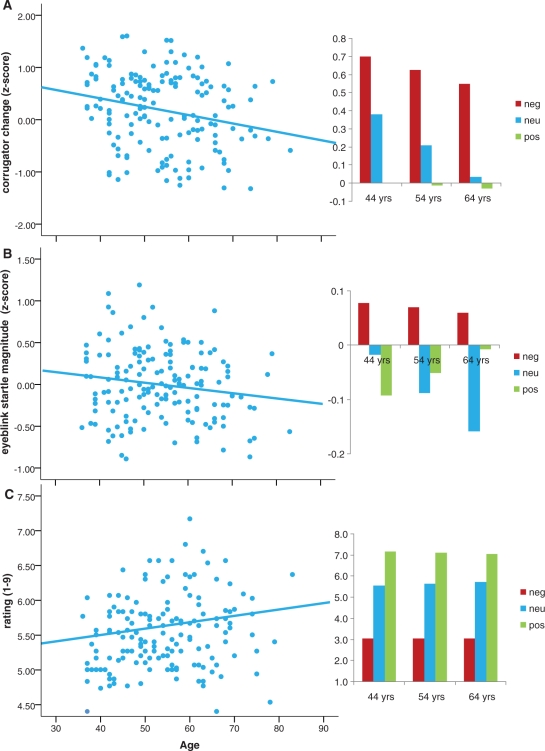
For emotion reactivity, as expected, the MANCOVA showed a strong main effect of valence, F(6, 146) = 58.86, P < 0.001, across all measures. As expected, linear effects of valence (negative > neutral > positive) were found for corrugator, Flinear (1, 51) = 19.25, P < 0.001 and ratings, Flinear (1, 151) = 353.26, P < 0.001, but surprisingly not for eyeblink startle, Flinear (1, 151) = 1.70, P = 0.19.4 Effects of age on valence were also significant, valence × age, F(6, 146) = 3.27, P = 0.005. Follow-up analyses indicated that age did not affect reactivity to negative pictures, all F < 1, nor to positive pictures, all F < 1, but instead there was an effect of age on reactivity to neutral pictures; with increasing age we observed lower COR, F(1, 155) = 3.70, P = 0.006, reduced eyeblink startle, F(1, 153) = 5.15, P = 0.043, and—although not quite reaching the conventional P < 0.05 level—more positive ratings, F(1, 155) = 3.778, P = 0.053, in response to the neutral pictures. Figure 2 depicts the age effects for each of the measures separately.
Fig. 2.
Scatterplots presenting age differences in (A) corrugator [r(157) = −0.23, top left] and (B) eyeblink startle [r(156) = −0.16, middle left] responses during the presentation of neutral pictures, and (C) in ratings of the neutral pictures [r(157) = 0.19, bottom left]. Bar graphs on the right of each scatter plot depict valence effects at the average age (54 years) and at ± 1 s.d. (10 years) of the average age. Neg, negative; neu, neutral; pos, positive pictures.
Emotion recovery
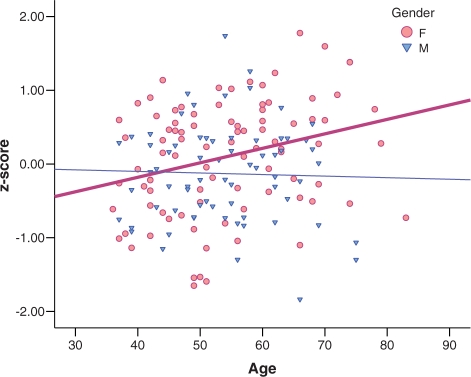
The MANCOVA on the corrugator and eyeblink startle measures (corrected for reactivity) indicated an interaction of age with valence and gender, F(4, 147) = 2.50, P = 0.045. Follow-up tests reveal that with increasing age, women recovered less after negative stimuli for corrugator, F(1, 86) = 4.88, P = 0.030. This effect was not observed for men, F(1, 65) = 2.14, n.s. (see Figure 3). No interactions including both valence and time were found.
Fig. 3.
Corrugator activity after offset of negative pictures (corrected for reactivity to negative pictures) plotted against age, separately for females [r(88) = 0.28] depicted in pink circles, and males [r(67) = −0.03] in blue triangles, collapsed across the post-stimulus epochs. F = female, M = male.
DISCUSSION
In this study, using objective psychophysiological measures of emotional responding in addition to subjective ratings, we assessed whether advancing age is related to reduced reactivity to negative events and whether increasing age is related to a faster recovery from such negative events. We further hypothesized that older-aged individuals would maintain positive responding to positive information relative to their younger peers. Our data suggest that aging effects in emotion reactivity are expressed not as reduced responding to negative stimuli, nor enhanced responding to positive stimuli, but by more positive responding to neutral stimuli. This age effect was observed in both EBR and COR, with the picture ratings suggesting a similar trend. With regards to recovery from emotional stimuli, we observed that women, but not men, maintain higher levels of COR with increasing age after the offset of negative events, suggesting reduced recovery from negative events. We discuss these findings further below.
The literature on emotion processing in aging suggests a positivity effect in older-aged individuals (Carstensen and Lockenhoff, 2003; Lockenhoff and Carstensen, 2004), where Carstensen and colleagues have postulated that older-aged individuals are motivated to prioritize socio-emotional goals over knowledge-based or information goals, to maintain well-being. Based on this theory, and combined with findings from others, we posited that this positivity effect could be reflected in a more positively biased (re)appraisal of information with increasing age, thereby dampening reactivity to, and accelerate recovery from negative events, and maintaining higher levels of positive affect after the offset from positive pictures. Instead, what we found is a more positive response to neutral information. This finding is in line with the conceptualization of the ‘positivity offset’ by Cacioppo and colleagues (Cacioppo et al., 1997; Ito and Cacioppo, 2005) which postulates that in the situation of relatively low levels of evaluative activation or affect, a positive motivational system maintains a level of activation which determines the formation of attitudes, behavior selection and action. In other words, the positivity offset biases the perception and evaluation of neutral states or stimuli as mildly positive. Consistent with this postulate, Ito and Cacioppo (2005) found that individuals characterized by a stronger positivity offset judge a fictional character that had been described in neutral terms more positively, an effect not found when the character was then described negatively.
Our finding of a ‘positivity offset’ with increasing age recurred in our different measures of reactivity, most prominently in COR and EBR. A number of studies have reported a linear effect of COR and EBR magnitude associated with the valence of the person’s state. For example, Larsen and colleagues (2003) illustrated how COR tracks variation in moderate to strong unpleasantness and pleasantness of IAPS pictures and reviewed other evidence supporting the notion that positive affect may inhibit COR. Our data are in line with their observations: COR increased after picture onset in response to negative and, to a lesser extent, neutral pictures, but displayed on average a decrease or deactivation for positive pictures relative to baseline. This suggests to us that lower COR observed with increasing age to affectively neutral or ambiguous stimuli is associated with a relative stronger output of an appetitive motivational system (Lang et al., 1990), inhibiting activation of the muscle group underlying furrowing of the brow. This interpretation is also in line with recent findings by Neta and colleagues (2009) where individuals who responded with relative decreased COR to emotionally ambiguous faces of surprise, at levels similar to their corrugator response to positive faces, rated the surprise faces as more positive than those showing levels of COR in response to surprise faces similar to their corrugator responses to angry faces.
Similar to the linear effect of valence on COR, a large number of studies (Bradley et al., 1993, 2001; Cuthbert et al., 1996; Vrana et al., 1988) have demonstrated that pleasant pictures attenuate the EBR to an acoustic probe compared to neutral and negative pictures. Lang and colleagues (e.g. 1990) have proposed that two opposing motivational brain systems, an appetitive and an aversive system, drive attention and facilitation of information intake and emotion-related behavior through the mobilization of energy to prompt action. They hypothesize that these systems are engaged by reward signaling or otherwise life sustaining information, or by threat-related information, carrying importance for survival. Moreover, the stronger one system is engaged, the more likely that actions associated with this system are elicited, thereby inhibiting the non-engaged system. The workings of these opposing systems underlie the emotional modulation of the EBR to a startling probe when the foreground stimulus is negative, and the relative inhibition of this reflex when the foreground is positive.
However, it should be noted that we did not obtain the oft-reported valence modulation effect of the EBR in this study across all participants; as described in Footnote 4, a valence effect in EBR was moderated by age. The lack of a strong valence main effect for eyeblink startle is likely due to two factors: Heterogeneity of age in our sample and the on average relative low arousing nature of the positive and negative pictures presented. Past studies have overwhelmingly recruited participants from a university student population, and even then, low internal consistency of the emotion modulation of the EBR has been reported (Larson et al., 2000). Our data suggest that the valence modulation effect is observed in the younger participants, but this effect dissipates with increasing age. Furthermore, when considering the arousingness of the positive and negative pictures (see Footnote 4 and Supplementary Figure), we observed that younger participants specifically show EBR modulation in response to high arousing negative pictures (relative to high arousing positive). This valence modulation of eyeblink startle is evident in older participants in the low arousing pictures only. These findings notwithstanding, the overall effect of relative inhibited responding to neutral information remained reliable.
The mechanisms underlying affect modulation in COR and EBR are not necessarily the same. Evidence from Davis and colleagues (Davis, 2006; Hitchcock and Davis, 1986) suggests a crucial role of the central nucleus of the amygdala in the fear potentiation of the startle response in rats; lesion work in humans has supported a similar mechanism of startle modulation (Angrilli et al., 1996; Buchanan et al., 2004). The neural mechanisms underlying emotion modulation of COR are not yet clear. Given the multitude of influences on COR, including social context (Vrana and Rollock, 1998) and (in)voluntary display of expressions (Rinn, 1984), the neural pathways underlying emotion modulation of COR are likely more complex than those supporting emotion modulation of the startle response. These differences in neural pathways notwithstanding, our EBR and COR data both suggest a relative inhibition of the aversive system through a stronger engagement of an appetitive system with increasing age when confronted with affectively ambiguous stimuli. These findings are further supported by the ratings of the neutral pictures, with a more positive trend with increasing age.
During the recovery from negative pictures, we observed that the older-aged women maintained higher levels of COR relative to both the younger-aged women in this study, and the male participants. This effect was not predicted and is not in line with the literature reviewed earlier suggesting less effortful, more adaptive emotion regulation with increasing age (Kliegel et al., 2007; Scheibe and Blanchard-Fields, 2009). Asking long-term married couples to discuss conflict, Carstensen and colleagues observed more expressions of affection in older relative to middle-aged couples (Carstensen et al., 1995). However, old-aged individuals (average 69 years) were more facially expressive than younger adults (average 28 years) when reliving recent strong emotional experiences (Malatesta-Magai et al., 1992), while a more recent study did not detect age differences in ability to suppress the expression to negative film clips (Kunzmann et al., 2005). None of these studies measured facial EMG, with which even visually unnoticeable changes in expressions can be detected, nor did they examine the time course of emotional expressions. Our observed effect of extended corrugator responding in older women was not reflected in eyeblink startle. Given the discrepant results between sex differences in expressivity and experience, as well as the suggestion that aging is associated with adaptive ‘mood repair’ (Kliegel et al., 2007), it is possible that while the expression of negative affect may have persisted in older women, the time course of experiential recovery may have been similar across the ages (and gender). Further research should examine the time course of emotion reactivity and recovery measuring several indicators of emotional responding.
In conclusion, our findings suggest that aging is associated with an increasing strength in ‘positivity offset’ (cf. Cacioppo et al., 1997) reflected in a more positive appraisal of emotionally ambiguous situations. Whether this positivity offset affects processing at an early or late stage of information processing as well as the extent to which the positivity offset is associated with adaptive responding in daily life and potential better health outcomes is as yet unknown. Drawing from findings obtained in other projects, this aspect will be further explored within the MIDUS II study. While this age-related positivity effect was not reflected in more adaptive regulation as indexed by faster recovery from negative stimuli in our study, further research is needed to ascertain whether increased expressivity after the offset of a negative situation is predictive of persisting experiential state.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Gina Beguhn, Nicole Rute, David Bachhuber, Adam Koppenhaver, Chelsea Riley and a number of students and staff from the Laboratory for Affective Neuroscience for their help in data collection and reduction, and Heather Urry and Tom Johnstone for helpful comments and discussions. We also wish to thank Carol Ryff and staff from the Institute of Aging, University of Wisconsin, as well as staff and scientists involved in MIDUS II for their suggestions and contributions to the project. This research was supported by grants from the National Institute of Aging (P01-AG20166) to RJD and by a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme to CvR (#208572).
Footnotes
1 Of these 275 participants, eyeblink startle was recorded from 265 participants and EMG over the brow region (‘corrugator activity’) was recorded from 231 participants. For a variety of technical, responsivity and other data quality issues, 15.1% of the participants did not provide 10 or more quantifiable eyeblink startle responses. Due to a delayed integration of corrugator activity recordings in the study, 18.2% of the original 275 participants did not have corrugator data. Due to time constraints or technical issues, 19.3% of the participants did not complete the picture ratings data.
2 Of the original N = 275 participant group, the first N = 56 participants saw one of two picture sets, counterbalanced across participants. The negative and positive pictures were matched within and across sets for valence and arousal, and the ratio of social content was held constant across valences. The N > 56 participants saw a picture set selected from the two picture sets, in which half of the pictures within each valence contained social content (one or more persons were identifiable), and the other half did not. This final set matched the previous two on valence and arousal ratings. At the time of the design of the study, ratings of the IAPS pictures by older-aged individuals were not available. As summarized in the Introduction section, Grühn and Scheibe (2008) have published ratings performed by different age group on a subset of the IAPS pictures. These ratings can be found at: http://www4.ncsu.edu/∼dgruehn/page7/page8/page8.html .
3 The border task was included to ensure engagement of the participant in the experiment, based on prior experience where individuals tend to get sleepy and distracted when presented with pictures with a relatively long ITI. The border task was designed to be completed within the first second of picture presentation (average response time was indeed 859 ms, SD = 20 ms). Due to the relative complexity of the IAPS pictures, which takes time to decode and appraise, we did not expect this simple task to have an effect on subsequent emotional responsivity—the primary process of interest. Indeed, the response times indicate no effect of picture valence (F < 1) nor a main or interaction effect with age (both F < 1).
4 This lack of a valence finding for eyeblink startle is likely due to the relatively low average of arousingness of the positive and negative pictures selected. As highlighted by Cuthbert et al. (1996), emotion modulation of the eyeblink startle occurs most strongly at high levels of picture arousal. In terms of the IAPS this would translate to pictures rated as six or higher on the arousal dimension. To assess the extent to which emotion modulation does occur across age, we divided the eyeblink startle responses to positive and negative pictures in high and low arousal, based on a median split on the normative arousal ratings, and analyzed these in a 2 (valence: positive vs negative) × 2 (arousal: high vs low) GLM with age, gender and recording method. The significant interaction between valence, arousal and age (F1, 137 = 11.52, P = 0.001) is carried by higher startle responses to negative relative to positive pictures when they are highly arousing for the younger (average 44 years: P = 0.000) and middle-aged (average 54 years, P = 0.005) participants. This effect is reversed with the older aged, where EBR to negative pictures are higher than to positive pictures when they are low in arousal only (P = 0.029). See Supplementary Figure for a depiction of this effect. When the main analysis is repeated with the high arousal eyeblink responses to negative and positive pictures included, the Valence × Age interaction remains significant (F6, 139 = 3.40, P = 0.004), and this age effect is still carried by the neutral condition. This analysis also illustrates that the linear effect of Valence for eyeblink startle is present in the younger participants (average 44 years, P = 0.016) but not in the older participants (lowest P > 0.112). See also Figure 2, middle-right panel for a depiction of these valence effects across age. (We thank an anonymous reviewer for highlighting the Cuthbert et al. findings to us).
REFERENCES
- Angrilli A., Mauri A., Palomba D., et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain: A Journal of Neurology. 1996;119(Pt 6):1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Birditt K.S., Fingerman K.L., Almeida D.M. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20(2):330–40. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F., Stein R., Watson T.L. Age differences in emotion-regulation strategies in handling everyday problems. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2004;59(6):261–9. doi: 10.1093/geronb/59.6.p261. [DOI] [PubMed] [Google Scholar]
- Blumenthal T.D., Cuthbert B.N., Filion D.L., Hackley S., Lipp O.V., van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Cuthbert B.N., Lang P.J. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–98. [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J., Cuthbert B.N. Emotion, novelty, and the startle reflex: habituation in humans. Behavioral Neuroscience. 1993;107(6):970–80. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Tranel D., Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behavioral Neuroscience. 2004;118(2):429–37. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Gardner W.L., Berntson G.G. Beyond bipolar conceptualizations and measures: the case of attitudes and evaluative space. Personality and Social Psychology Review. 1997;1(1):3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Petty R.E., Losch M.E., Kim H.S. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology. 1986;50(2):260–8. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- Carstensen L.L., Gottman J.M., Levenson R.W. Emotional behavior in long-term marriage. Psychology and Aging. 1995;10(1):140–49. doi: 10.1037//0882-7974.10.1.140. [DOI] [PubMed] [Google Scholar]
- Carstensen L.L., Lockenhoff C.E. Aging, emotion, and evolution: the bigger picture. Annals of the New York Academy of Sciences. 2003;1000:152–79. doi: 10.1196/annals.1280.008. [DOI] [PubMed] [Google Scholar]
- Coats A.H., Blanchard-Fields F. Emotion regulation in interpersonal problems: the role of cognitive-emotional complexity, emotion regulation goals, and expressivity. Psychology and Aging. 2008;23(1):39–51. doi: 10.1037/0882-7974.23.1.39. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Bradley M.M., Lang P.J. Probing picture perception: activation and emotion. Psychophysiology. 1996;33:103–11. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. The American Psychologist. 2006;61(8):741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Folkman S., Lazarus R.S., Pimley S., Novacek J. Age differences in stress and coping processes. Psychology and Aging. 1987;2(2):171–84. doi: 10.1037//0882-7974.2.2.171. [DOI] [PubMed] [Google Scholar]
- Foti D., Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20(6):977–88. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Gavazzeni J., Wiens S., Fischer H. Age effects to negative arousal differ for self-report and electrodermal activity. Psychophysiology. 2008;45(1):148–51. doi: 10.1111/j.1469-8986.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Carstensen L.L., Pasupathi M., Tsai J., Skorpen C.G., Hsu A.Y. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12(4):590–9. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gruhn D., Scheibe S. Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): do ratings become more extreme with age? Behavior Research Methods. 2008;40(2):512–21. doi: 10.3758/brm.40.2.512. [DOI] [PubMed] [Google Scholar]
- Hitchcock J., Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100(1):11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Isaacowitz D.M., Wadlinger H.A., Goren D., Wilson H.R. Selective preference in visual fixation away from negative images in old age? an eye-tracking study. Psychology and Aging. 2006;21(1):40–8. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Ito T.A., Cacioppo J.T. Variations on a human universal: Individual differences in positivity offset and negativity bias. Cognition & Emotion. 2005;19(1):1–26. [Google Scholar]
- Jackson D.C. Relations Between Voluntary Emotion Regulation, Automatic Emotion Regulation, and Frontal EEG Asymmetry. Doctoral Thesis: University of Wisconsin, Madison; 2004. [Google Scholar]
- Jackson D.C., Mueller C.J., Dolski I., et al. Now you feel it, now you don't: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14(6):612–7. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Kliegel M., Jager T., Phillips L.H. Emotional development across adulthood: differential age-related emotional reactivity and emotion regulation in a negative mood induction procedure. International Journal of Aging & Human Development. 2007;64(3):217–44. doi: 10.2190/U48Q-0063-3318-1175. [DOI] [PubMed] [Google Scholar]
- Kunzmann U., Gruhn D. Age differences in emotional reactivity: the sample case of sadness. Psychology and Aging. 2005;20(1):47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Kunzmann U., Kupperbusch C.S., Levenson R.W. Behavioral inhibition and amplification during emotional arousal: a comparison of two age groups. Psychology and Aging. 2005;20(1):144–58. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–95. [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. International Affective Picture System (IAPS): Digitized Photographs, Instruction Manual and Affective Ratings. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Larsen J.T., Norris C.J., Cacioppo J.T. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40(5):776–85. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Larsen J.T., Norris C.J., McGraw A.P., Hawkley L.C., Cacioppo J.T. The evaluative space grid: a single-item measure of positivity and negativity. Cognition & Emotion. 2009;23(3):453–80. [Google Scholar]
- Larson C.L., Ruffalo D., Nietert J.Y., Davidson R.J. Temporal stability of the emotion-modulated startle response. Psychophysiology. 2000;37(1):92–101. [PubMed] [Google Scholar]
- Lazarus R.S., Alfert E. Short-circuiting of threat by experimentally altering cognitive appraisal. Journal of Abnormal & Social Psychology. 1964;69(2):195–205. doi: 10.1037/h0044635. [DOI] [PubMed] [Google Scholar]
- Levenson R.W., Carstensen L.L., Friesen W.V., Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6(1):28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Lockenhoff C.E., Carstensen L.L. Socioemotional selectivity theory, aging, and health: The increasingly delicate balance between regulating emotions and making tough choices. Journal of Personality. 2004;72(6):1395–424. doi: 10.1111/j.1467-6494.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- Malatesta-Magai C., Jonas R., Shepard B., Culver L.C. Type A behavior pattern and emotion expression in younger and older adults. Psychology and Aging. 1992;7(4):551–61. doi: 10.1037//0882-7974.7.4.551. [DOI] [PubMed] [Google Scholar]
- Mather M., Carstensen L.L. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mroczek D.K., Kolarz C.M. The effect of age on positive and negative affect: a developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75(5):1333–49. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Neta M., Norris C.J., Whalen P.J. Corrugator muscle responses to surprised facial expressions are associated with individual differences in positivity-negativity bias. Emotion. 2009;9(5):640–8. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R.G., Kaiser M.K. MANOVA method for analyzing repeated measures designs: an extensive primer. Psychological Bulletin. 1985;97(2):316–33. [PubMed] [Google Scholar]
- Phillips L.H., Henry J.D., Hosie J.A., Milne A.B. Effective regulation of the experience and expression of negative affect in old age. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2008;63(3):138–45. doi: 10.1093/geronb/63.3.p138. [DOI] [PubMed] [Google Scholar]
- Rinn W.E. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychological Bulletin. 1984;95(1):52–77. [PubMed] [Google Scholar]
- Scheibe S., Blanchard-Fields F. Effects of regulating emotions on cognitive performance: what is costly for young adults is not so costly for older adults. Psychology and Aging. 2009;24(1):217–23. doi: 10.1037/a0013807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.P., Hillman C.H., Duley A.R. Influences of age on emotional reactivity during picture processing. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2005;60(1):49–56. doi: 10.1093/geronb/60.1.p49. [DOI] [PubMed] [Google Scholar]
- Speisman J.C., Lazarus R.S., Mordkoff A., Davison L. Experimental reduction of stress based on ego-defense theory. Journal of Abnormal & Social Psychology. 1964;68(4):367–80. doi: 10.1037/h0048936. [DOI] [PubMed] [Google Scholar]
- Tassinary L.G., Geen T.R., Cacioppo J.T. Optimizing surface electrode placements for facial EMG recording: Guidelines for recording from the corrugator-supercilii and zygomaticus-major muscle regions. Psychophysiology. 1986;23(4):466. [Google Scholar]
- Thomas M.S., Annaz D., Ansari D., Scerif G., Jarrold C., Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research. 2009;52(2):336–58. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Tsai J.L., Levenson R.W., Carstensen L.L. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychology and Aging. 2000;15(4):684–93. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- van Reekum C.M., Urry H.L., Johnstone T., et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–48. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Vrana S.R., Spence E.L., Lang P.J. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–91. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Vrana S.R., Rollock D. Physiological response to a minimal social encounter: effects of gender, ethnicity, and social context. Psychophysiology. 1998;35(4):462–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.