Abstract
There are complex reciprocal relationships between health and social, emotional and economic factors in aging populations. Social and affective neurosciences are rapidly developing an understanding of the mechanisms underlying these phenomena using sophisticated behavioural, neuroimaging and psychophysiological methods. These techniques are often complex and expensive, so are generally used in relatively small selected samples rather than in large-scale cohort studies. However, an understanding of the significance of these processes in health and well-being depends on integrating findings from social and affective neuroscience into population-level studies. The aim of this article is to describe how a population perspective on the determinants of health and well-being in old age articulates with the agenda of social, affective and economic neuroscience, particularly through the application of psychosocial biomarker research. Social and affective neuroscience and epidemiological approaches provide complementary research strategies for understanding the mechanisms linking social, emotional and economic factors with health risk. This will be illustrated primarily from findings from two studies conducted at University College London, the Whitehall II Study and the English Longitudinal Study of Ageing.
Keywords: aging, psychosocial, well-being, population studies, biomarkers
INTRODUCTION
A major goal in aging research is to understand what factors contribute to the maintenance of health and well-being as people grow older. Much of our knowledge is underpinned by longitudinal observational epidemiological population studies (Elwood, 1998). Such studies have a number of components. First, the recruitment of a large, preferably representative, population of individuals who are screened to ensure that they do not already suffer from the endpoint under investigation (coronary heart disease, dementia, disability, etc.). Second, the measurement of exposure to the risk factors or biological factors being tested (e.g. loneliness, C-reactive protein levels and habitual physical activity), along with other factors known to influence the outcome. Third, the tracking of the population over time, assessing the development of the endpoints under investigation. Finally, multivariate analysis to test whether exposure to the putative risk factor is associated with the endpoint after covariates have been taken into account.
This basic study design has been used over the last century to identify many risk factors such as hazardous chemical exposures in specific occupational groups and the impact of smoking on lung cancer and coronary heart disease. It is also being used to identify many of the risk factors for diseases of old age, including high blood pressure, elevated cholesterol levels and excessive adiposity. Observational epidemiology is central to understanding the impact of psychosocial risk factors as well. For example, we recently meta-analysed observational cohort studies of the association between anger and hostility and future coronary heart disease (Chida and Steptoe, 2009a). These studies involved more than 75 000 individuals with follow-up periods ranging from 2 to 33 years. The combined hazard ratio was 1.19 [95% confidence interval (CI) 1.05–1.35] for an association between anger or hostility and increased coronary heart disease in initially healthy population studies. Another recent meta-analysis from our research group highlighted the protective effect of regular physical activity on the development of dementia and Alzheimer’s disease (Hamer and Chida, 2009). Such epidemiological studies are more powerful than small scale investigations for convincing the research, policy and clinical communities of the importance of psychosocial and behavioural factors.
Observational epidemiological studies do not prove causality, since underlying factors may be associated both with risk exposure and health outcomes. For example, some common genetic factors underlying the tendency to exercise regularly and low depression levels have been identified, so relationships between physical activity and reduced risk of depression may not be causal (De Moor et al., 2008). Nevertheless, this approach remains among the strongest methods available for assessing the possible psychosocial and economic determinants of health and well-being. Unfortunately, such studies typically need to be of large scale and long duration, since major disease events are rare. Retention of participants in longitudinal studies, and an ability to trace health outcomes in those who have attrited from the sample is essential.
Social and affective neurosciences operate on a different scale, investigating the links between life experience, brain function and biology at a detailed within-person level. Many of the methods of social neuroscience and economic neuroscience are difficult to implement in population-level studies involving several thousand participants, since the cost is currently prohibitive. But developments in psychosocial biomarker research provide opportunities to integrate some of the more detailed methodologies and findings described elsewhere in this special issue into population studies. Social and affective neuroscience and psychosocial biomarker research are complementary strategies for identifying the mechanisms relating the environment with health risk. The relevance of psychosocial biomarker research will be illustrated in this article with particular reference to studies of aging and the life course.
BIOLOGICAL MEASURES IN AGING POPULATION SURVEYS
There is a long tradition in medical science of assessing biological measures in clinical epidemiological investigations but their use in studies of social, emotional and economic factors is relatively new. The NIH Biomarkers Working Group defined a biomarker as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention’ (Atkinson et al., 2001). This definition includes parameters such as body weight along with the measures of physiological functions (blood pressure and respiratory function) and biochemical measures such as cholesterol, C-reactive protein and blood glucose. Psychosocial biomarker research is a component of this larger enterprise, focusing particularly on relationships between biological indicators and social, psychological and economic factors. A thorough review of biomarkers, their measurement and availability in different aging surveys has been published by Crimmins et al. (2008), and further description of their use can be found in a recent publication from the National Academy of Sciences (Weinstein et al., 2008).
The inclusion of biological measures in large-scale general purpose aging surveys is relatively a new development. The MacArthur studies of successful aging pioneered psychosocial biomarker research some 20 years ago by measuring a range of biological parameters in a cohort of high functioning older individuals. They operationalized the construct of allostatic load, the cumulative physiological toll exacted by efforts to adapt to life experience, and demonstrated that higher allostatic load predicted future impairment in cognitive and physical function (Seeman et al., 1997). More recently the Health and Retirement Survey (HRS) included the series of biomarkers in a subsample in 2006, with measurement of anthropometry, blood pressure, peak flow, cheek cell collection for DNA extraction and blood spots for analysing glycated haemoglobin (HbA1c), total and high density lipoprotein cholesterol, C-reactive protein and other variables (Weir, 2008). The English Longitudinal Study of Ageing (ELSA) is a multidisciplinary study of health, economic, cognitive and social factors and quality of life in a representative sample of non-institutionalized men and women aged 50 and older in England, and was designed to explore the unfolding dynamic relationships between health and functioning, social participation and networks, and economic position and well-being, as people grow older (Marmot et al., 2003). The initial sample of just over 11 000 was recruited in 2002, and biological measures were added at wave 2 in 2004, with a second phase of biological data collection in wave 4 (2008/09). The Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan assessed a range of biomarkers in around 1000 individuals, including APOe genotypes, interleukin 6 (IL-6), insulin-like growth factor 1 (IGF-1) and urinary catecholamines and cortisol. Other more focused studies collect biological measures in the context of specific aspects of aging, such as the Dynamics of Health, Aging and Body Composition (Health ABC) Study.
These surveys vary greatly in the richness of their economic, psychosocial and cognitive data. For example, ELSA has an unusually comprehensive economic dataset with detailed questions about income, savings, pensions and wealth so as to characterize the complex financial profile of older individuals. Other studies have more limited economic data but more comprehensive assessments of physical function. Very few aging surveys have yet collected more than one wave of biomarker data. Repeated measures are vital, since single assessments can only provide evidence of association, and provide little idea about temporal trajectories. Studies of changes in biological function in relation to change in health and functional outcome provide vital additional information about likely causal pathways. Additionally, assessment at one point in time may provide an imprecise measure of long-term levels, and fail to reflect the cumulative impact of economic, social and psychological factors on biological processes and preclinical disease outcomes.
Biomarkers are important in population aging studies for a number of reasons. First, they provide objective data about health and functioning. Much survey work is based on self-report, and this may have limited accuracy in some older individuals because of failures in recall or self-presentation bias. Secondly, biomarkers provide information about important health outcomes that may not have been clinically diagnosed, and are not therefore effectively managed. Thirdly, these measures help our understanding of the mechanisms and pathways through which psychosocial and economic factors influence health and well-being.
BIOLOGICAL INDICATORS OF DISEASE STATE
The different biological measures assessed in aging population cohorts fall into two broad groups. The first group is related to specific disease outcomes. Measures include blood cholesterol as a cardiovascular risk factor, blood pressure as an indicator of hypertension and as a risk factor for coronary heart disease and stroke, lung function measures such as peak flow for the identification of obstructive pulmonary disease and glycated haemoglobin or blood glucose for the assessment of diabetes. These measures provide direct information about health states that can be related to social, emotional and economic experience. In some cases, the information is valuable because it may help identify serious problems that have not been diagnosed clinically, so allowing researchers to estimate the ‘clinical iceberg’. For example, in wave 2 of ELSA, 77% of men and 84% of women had total cholesterol levels above the recommended level of 5.00 mmol/l (193 mg/dl). A significant proportion of respondents (18% men and 16% women) had blood pressure levels in the hypertensive range, even though hypertension had never been diagnosed (Pierce et al., 2006). These findings point to failures in primary prevention and unmet clinical needs. Other results have been more reassuring. An analysis of undiagnosed diabetes compared self-reported doctor diagnosed disease with measures of fasting plasma glucose that exceeded the threshold of ≥7.00 mmol. The weighted prevalence of diabetes was 9.1%, with only 1.7% having undiagnosed diabetes (Pierce et al., 2009).
Another valuable indicator of disease status is the haemoglobin level. Haemoglobin is the oxygen-carrying, iron-containing molecule in red blood cells. Low haemoglobin defines anaemia and is most commonly caused by iron deficiency that arises when iron requirements exceed supply either through excessive blood loss or inadequate dietary supply. Anaemia in older individuals is associated with a wide range of complications, including increased risk for mortality, cardiovascular disease, cognitive dysfunction, longer hospitalization for elective procedures, reduced bone density and falls or fracture (Eisenstaedt et al., 2006). ELSA shows a large increase in anaemia with age, from fewer than 2% in men <54 years to 20.9% in those aged 80 years or more (Pierce et al., 2006).
These disease indicators have important relationships with quality of life both cross-sectionally and longitudinally. For example, another ELSA analysis found cross-sectional relationships between poor lung function and quality of life, while longitudinal analyses over a 3–6-year period showed a stable long-term influence of impaired lung function on poor quality of life (Blane et al., 2008). However, many of the more interesting associations between biological measures and well-being have emerged from analysis of the second group of biomarkers detailed below.
BIOLOGICAL INDICATORS OF HEALTH AND FUNCTIONAL DECLINE
The second set of biological measures assessed in aging surveys are general indicators of health and risk of functional decline in older ages that are not so directly related to individual diseases. These indicators supply data about future risk but do not typically define a specific illness. Some are associated with the increase risk of future disease and frailty including C-reactive protein, IL-6, fibrinogen and cortisol, while others may have both protective and potentially damaging effects such as IGF-1.
C-reactive protein is a marker of acute inflammation, and has been extensively studied as a novel cardiovascular risk factor. Meta-analysis of longitudinal observational studies indicates that both high C-reactive protein and IL-6 are independent risk factors for coronary heart disease (Danesh et al., 2008, 2004), although the causality of these associations is debated. They are related to other health problems in old age such as autoimmune conditions and type 2 diabetes as well, and there is growing evidence that both are related to depression (Howren et al., 2009). For example, Bremmer et al. (2008) showed a positive association between raised IL-6 and C-reactive protein and depressive symptoms in a population-based study of men and women in the Netherlands aged 65 and over. However, most of the work on depression has been cross-sectional, making the direction of cause and effect uncertain. Theoretically, inflammation could contribute to depression through the activation of pathways related to sickness behaviour (Raison et al., 2006), or depression might stimulate low level inflammation as part of the stress response.
We recently used the longitudinal biomarker data available within the Whitehall II Study to examine the temporal relationship between depressive symptoms and both C-reactive protein and IL-6 (Gimeno et al., 2009). Over 3000 participants completed measures of depression and had blood drawn for the assessment of inflammatory markers at an 11-year interval. It was found that baseline levels of both C-reactive protein and IL-6 predicted the development of depressive symptoms at follow-up, independently of baseline depressive symptoms, age, gender and ethnicity. The relationship remained significant with a further adjustment for health behaviours (diet, physical activity, smoking and alcohol consumption), other biomarkers (adiposity, blood pressure and cholesterol), health status and medication. In contrast, depression at baseline did not predict C-reactive protein or IL-6 at follow-up, suggesting that inflammation is a predictor of depressive mood and not vice versa. The associations between C-reactive protein and future depressive symptoms have been confirmed over a shorter follow-up period in ELSA (Hamer et al., 2009).
Cortisol is another general indicator of risk, since it is involved in immune and metabolic regulation, and elevated levels are associated with abdominal adiposity, insulin resistance, diabetes, coronary heart disease and depression (Dekker et al., 2008; Herbert et al., 2006; Raison et al., 2006). Cortisol also contributes to memory function across the life span, with evidence that long-exposure to high levels of glucocorticoids is associated with memory impairment and reduced hippocampal volume in the aging brain (Lupien et al., 2005).
Inflammatory markers and cortisol are highly sensitive to psychosocial factors, as demonstrated both in acute behavioural stress experiments and in cross-sectional population and clinical studies (Dickerson and Kemeny, 2004; Steptoe et al., 2007). They are therefore plausible pathways through which social and economic factors might influence health risk in older individuals.
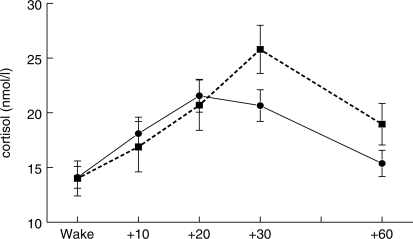
Cortisol is secreted in a pulsatile fashion from the adrenal cortex, and there is a pronounced diurnal rhythm, with relatively high levels on waking, an increase for the first 30–45 min of the day, then a progressive decline thorough the day and evening. The cortisol awakening response (CAR) has been investigated extensively in relation to psychosocial factors following the initial observation that people experiencing chronic life stress showed a larger CAR (Schulz et al., 1998). We recently meta-analysed 145 studies of the CAR, observing a consistent association between general life stress, job stress and an increased CAR, while people reporting fatigue or burnout show a reduced CAR (Chida and Steptoe, 2009b). A heightened CAR has also been related to lower socioeconomic position in old age. Figure 1 shows results from a study of community dwelling men and women aged 70 years or over (range 65–80 years), in which socioeconomic position was indexed by the subjective social status ‘ladder’ (Adler et al., 2000). It can be seen that the CAR was smaller and peaked earlier in the higher than lower status group, and this effect was independent of gender, body mass, abdominal adiposity, smoking and time of waking (Wright and Steptoe, 2005). The CAR has also been found to track changes in chronic adversity over time. In an analysis of a subgroup from the Whitehall II Study, we assessed the CAR twice over a 3-year interval, together with measures of financial strain (Steptoe et al., 2005a). Among men, those who reported a reduction in financial strain over the 3-year period showed a parallel diminution in the CAR.
Fig. 1.
Mean salivary cortisol on waking and 10, 20, 30 and 60 min after waking in older men and women in higher (solid line) and lower (dashed line) social status groups. Values are adjusted for gender, body mass, waist/hip ratio, smoking status and time of waking. Individuals who delayed between waking and taking the waking saliva sample were excluded. Error bars are standard error of the mean. Adapted from (Wright and Steptoe, 2005).
Other social, psychological and economic factors appear to be related to different components of the cortisol profile. The relationship between psychological coping and the overall output of cortisol over the day was investigated in a sample of 542 men and women from the Whitehall II Study who were aged an average 60.9 years at the time of testing (O'Donnell et al., 2008). Individuals who reported strong tendencies to cope with stress by engaging with the problem, or to cope by seeking social support, had lower cortisol output over the day, independently of age, gender, income, body mass, smoking, depression, self-related health or time of waking. This suggests that these types of coping may be adaptive from the biological perspective. Avoidant coping was unrelated to cortisol output. In contrast, negative psychosocial factors such as social isolation are associated with increased cortisol output over the day (Grant et al., 2009). Observations of this type highlight the critical importance of multiple samples of cortisol, since different psychosocial factors may be related to different components of the diurnal profile. Fortunately, the development of salivary measures has permitted the repeated assessment to be made over the day in several large-scale studies, including aging cohorts such as HRS and ELSA. However, the methodological challenges of collecting data from large cohorts should not be underestimated, and there are important issues of quality control, sample timing, confounding and statistical analysis that need to be resolved (Adam and Kumari, 2009).
A biological indicator that has a less consistent relationship with health in old age is IGF-1. This polypeptide plays an important role in growth, and low levels are associated with several disorders of old age including cardiovascular disease, cognitive decline, dementia, frailty and sarcopenia (Ceda et al., 2005). Longitudinally, low IGF-1 also predicts future coronary heart disease (Juul et al., 2002). IGF-1 levels typically decline with age, and higher levels appear to be neuroprotective, encouraging neural growth and synaptic formation in the central nervous system. It is interesting that in mid-life, levels are greater in people of higher socioeconomic status (Kumari et al., 2008). This might imply that high IGF-1 levels are advantageous, but unfortunately, circulating levels have been positively related to risk of several cancers (Lann and LeRoith, 2008; Rowlands et al., 2009). It has even been proposed that lifespan could be extended by lowering levels of IGF-1 and other growth factors, helping to guard against disorders involving cell proliferation (Sierra et al., 2009). It is possible that low levels are beneficial at earlier stages of life, but lead to impairment in later life. These issues will be clarified through longitudinal studies of biological measures in older age cohorts.
There is growing interest in biological indicators that are related to sustained health, rather than risk of disease and decline. One such may be vitamin D. Vitamin D has diverse effects on metabolism and immune function, and is crucial for bone health. It is thought that low levels may be anticarcinogenic and influence the risk of cardiovascular disease, diabetes and other conditions (SACN, 2007), although the causality of these associations has not been established. This notion is supported by various lines of investigation. For example, the known winter-time increases in cardiovascular disease, and the higher prevalence in more northern compared to southern locations, parallel known variations in the prevalence of vitamin D deficiency (Zittermann et al., 2005). Clinical studies report marked reductions in cardiovascular mortality following administration of active vitamin D or analogues to patients with end-stage renal disease, and low serum concentrations are associated with the risk of all cause and cardiovascular mortality (Teng et al., 2003, 2005). Vitamin D deficiency is a well established problem among the elderly, and randomized controlled trials indicate that vitamin supplementation reduces the risk of falls (Bischoff-Ferrari et al., 2009). The economic and social determinants of vitamin D have not been extensively studied; it is interesting, however, that vitamin D concentration partly accounted for the socioeconomic gradient in bone mineral density in one recent population-based study (Brennan et al., 2009).
BIOLOGICAL MEASURES AND POSITIVE WELL-BEING AT OLDER AGES
A key objective of cohort studies of aging populations is to understand the economic, social and psychological determinants of sustained positive well-being and quality of life. There are complex reciprocal relationships between positive well-being, physical and mental health, and well-being itself has several dimensions. One aspect focuses on the notion of capability pioneered by Amartya Sen that emphasizes how optimal capacity for well-being and development depends on capabilities or freedoms such as bodily integrity, affiliation and control over the environment (Anand et al., 2009). A second element is eudaimonic well-being, involving characteristics such as self-realization, sense of purpose, autonomy and personal growth. A third component is positive affect and happiness, which is linked with life satisfaction.
There is growing evidence that positive affect and well-being are associated with increased longevity in old age. Chida and Steptoe (2008) reported a meta-analysis of prospective observational cohort studies involving 35 studies of initially healthy populations and 35 studies of patient populations (coronary heart disease, cancer, renal failure, etc.). The follow-up periods ranged from 1 to 21 years. The combined hazard ratio for healthy population studies was 0.82 (95% CI 0.76–0.89), indicating a protective effect of psychological well-being on mortality. Effects in disease population were smaller (hazard ratio = 0.98, CI 0.95–1.00). Interestingly, rather comparable associations were observed for studies that measured recent or current positive affect (joy, happiness and positive mood) and more trait-like dispositions such as optimism and life satisfaction. It is notable that the effects were independent of negative affect and depression, so did not merely mirror the known adverse effects of negative emotional states. Relationships with reduced morbidity are also being identified, with various measures of positive affect being associated with reduced incident coronary heart disease (Kubzansky and Thurston, 2007), hypertension (Ostir et al., 2006) and resistance to infection (Cohen et al., 2006).
These relationships probably depend in part on lifestyle, since there is some evidence that positive well-being is linked with favourable health habits (Pressman and Cohen, 2005). Additionally, biological pathways are likely to contribute. Studies of aging cohorts are well suited to examining these biological processes. Most research to date has focused on positive affect rather than other aspects of well-being. This is no accident; there is growing evidence from affective neuroscience that negative emotional states stimulate autonomic and neuroendocrine pathways through activation of the limbic system and networked brain regions including areas in the brainstem and the anterior cingulate cortex (Gianaros et al., 2008). It is plausible therefore that positive affect will have the reverse effect.
We have carried out a series of studies assessing the associations between positive affect and biological function in early old age using the Whitehall II cohort. In our first study, positive affect was inversely related to cortisol output over the day independently of age, gender, socioeconomic position, body mass, smoking and psychological distress (Steptoe et al., 2005b). There were also differences in ambulatory blood pressure and heart rate over the day, and in fibrinogen (an inflammatory marker) responses to psychological stress. The association between positive affect and low cortisol was confirmed in a larger study of 2873 men and women aged 60 years on average, again independent of covariates including depressed mood (Steptoe et al., 2008a). We also observed a relationship between higher positive affect and lower C-reactive protein and IL-6 concentration in women. A longitudinal study of an older age cohort of Wisconsin women found that positive affect predicted reduced glycated haemoglobin over a two-year period independently of age, education, income, marital status and baseline glycated haemoglobin levels (Tsenkova et al., 2007). Other studies suggest a relationship of positive affect with increased heart rate variability, indicative of a healthy pattern of autonomic regulation (Bhattacharyya et al., 2008). The implication is that low positive affect is accompanied by low level chronic activation of stress-related biological pathways.
Research on the biological correlates of eudaimonic well-being has been more limited. However, Ryff and colleagues have carried out studies relating six components of well-health—acceptance, purposes in life, personal growth, positive relationships with others, environmental mastery and autonomy—with biomarkers in the sample of 135 Wisconsin women (mean age 74 years) mentioned earlier. Positive relationship scores were positively associated with higher levels of urinary epinephrine but negatively with waist circumference and glycated haemoglobin, while autonomy was positively related to greater urinary norepinephrine. Women reporting higher personal growth had flatter diurnal cortisol slopes (Ryff et al., 2006). Some of these effects are not consistent with the notion that well-being is coupled with the favourable biological risk profile, since one would expect lower catecholamines and steeper cortisol slopes among individuals with greater well-being. In contrast, a later analysis showed that positive relationships were associated with lower plasma IL-6 after adjusting for age, education, marital status, medication, smoking and alcohol consumption (Friedman et al., 2007).
The scales developed by Ryff to assess eudaimonic well-being have not proved to be psychometrically robust in UK population samples (Abbott et al., 2006), so we have conceptualized well-being with different instruments. The CASP-19 is a quality of life measure based on a needs–satisfaction model, and has four scales: control, autonomy, pleasure and self-realization (Hyde et al., 2003). High scores on the CASP-19 are associated with good self-reported sleep independently of age, gender, income, employment status and self-rated health (Steptoe et al., 2008b). In new, unpublished analyses, we have tested the associations between psychological well-being assessed with the CASP-19 and two major inflammatory markers, C-reactive protein and IL-6, in 2574 members of the Whitehall II cohort. We found that CASP-19 scores were inversely associated with plasma IL-6 (β = −0.36, s.e. = 0.018, P = 0.050) after controlling for age, gender, socioeconomic position, ethnicity, body mass, waist/hip ratio and employment status. These effects were driven primarily by high scores on the autonomy subscale (β = −0.36, s.e. = 0.018, P = 0.046), although self-realization also contributed (β = −0.035, s.e. = 0.018, P = 0.054). High levels of autonomy are also correlated with lower C-reactive protein concentration after adjustment for these covariates plus depression (β = −0.51, s.e. = 0.022, P = 0.019). These effects are potentially meaningful at the population health level. Plasma C-reactive protein, e.g. was 16.2% higher on average in the highest compared with the lowest autonomy quartile, after adjustment for covariates.
Studies of the biological correlates of positive well-being have largely been cross-sectional, so the causal relationship has not been established. It is possible either that sustained positive affect and well-being lead to reduced neuroendocrine, autonomic and immune responses through deactivation of the prefrontal–limbic circuits responsible for stress reactivity, or that biological activation contributes to mood state. Interventions that target either the biological substrate or affective state are required to determine causal processes (Sin and Lyubomirsky, 2009). Longitudinal investigations in older age in cohort will also establish the role of these associations in predicting future adaptation and health outcome.
PSYCHOSOCIAL BIOMARKER RESEARCH AND THE LIFE COURSE PERSECTIVE
The potential of psychobiological biomarker research to illuminate connections between the social and economic environment and health and well-being at older ages has been illustrated in this article by studies of contemporaneous exposures. But there is growing evidence for the value of a life course perspective, since earlier experiences impact substantially on biomarkers assessed at later time points. For example, studies of the Dunedin birth cohort have shown that harsh treatment in childhood is associated with the inflammatory marker C-reactive protein at age 32, independently of stress in adult life, adult health and lifestyle factors (Danese et al., 2007). Similarly, Taylor et al. (2006) showed that low socioeconomic position in early life and a risky (cold and unsupportive) early family environment predicted C-reactive protein at ages 32–47 years. Both child and adult socioeconomic positions are predictors of cortisol rhythms in middle age (Li et al., 2007), and are related to adult cardiovascular and metabolic risk factors such as abdominal adiposity, fibrinogen and high density lipoprotein cholesterol (Brunner et al., 1999). It is likely that exposures in middle age also affect biomarker levels in old age. As longitudinal population studies progress into older ages, the impact of earlier social environments and economic circumstances on the progression of biomarker risk and protective profiles will become better understood.
CONCLUSIONS
We are entering a new era of psychosocial biomarker research in population aging studies, when assessments of biological indicators are not confined to clinical and subclinical disease identification, but extend to measures of physiological processes that reflect psychological, social and economic experience. The focused, technically sophisticated studies of fundamental processes in social and affective neuroscience need to be coupled with larger scale work in which associations with health outcomes can be evaluated. Biological measures are relevant not only to risk of disease and frailty, but also to the maintenance of optimal functioning and well-being. Social and affective neuroscience and epidemiological psychosocial biomarker research are not only complementary research strategies, but also share important methodological issues. A good example is the area statistical modelling, since both disciplines are faced with large and complex multivariate datasets. Techniques such as multilevel and growth curve modelling, data imputation and structural equation modelling are evolving in both fields. The challenges for the next decade are to understand these processes more precisely, so that their implications for sustained well-being and healthy functioning in old age can be put into practice.
REFERENCES
- Abbott RA, Ploubidis GB, Huppert FA, Kuh D, Wadsworth ME, Croudace TJ. Psychometric evaluation and predictive validity of Ryff's psychological well-being items in a UK birth cohort sample of women. Health Quality of Life Outcomes. 2006;4:76. doi: 10.1186/1477-7525-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19:586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Anand P, Hunter G, Carter I, Dowding K, Guala F, Van Hees M. The development of capability indicators. Journal of Human Development Capabilities. 2009;10:125–52. [Google Scholar]
- Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosomatic Medicine. 2008;70:1020–27. doi: 10.1097/PSY.0b013e318189afcc. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. British Medical Journal. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blane D, Netuveli G, Montgomery SM. Quality of life, health and physiological status and change at older ages. Social Sciences Medicine. 2008;66:1579–87. doi: 10.1016/j.socscimed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. Journal of Affective Disorders. 2008;106:249–55. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Brennan SL, Henry MJ, Wluka AE, et al. BMD in population-based adult females is associated with socioeconomic status. Journal of Bone and Mineral Research. 2009;24:809–15. doi: 10.1359/jbmr.081243. [DOI] [PubMed] [Google Scholar]
- Brunner E, Shipley MJ, Blane D, Smith GD, Marmot MG. When does cardiovascular risk start? Past and present socioeconomic circumstances and risk factors in adulthood. Journal of Epidemiology and Community Health. 1999;53:757–64. doi: 10.1136/jech.53.12.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceda GP, Dall'Aglio E, Maggio M, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. Journal of Endocrinological Investigation. 2005;28:96–100. [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. Journal of American College of Cardiology. 2009a;53:936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychology. 2009b;80:265–78. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza a virus. Psychosomatic Medicine. 2006;68:809–15. doi: 10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Advanced Clinical Chemistry. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of theUnited States of America. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Medicine. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Archives of General Psychiatry. 2008;65:897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, et al. Salivary cortisol is related to atherosclerosis of carotid arteries. Journal of Clinical and Endocrinological Metabolism. 2008;93:3741–47. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenstaedt R, Penninx BW, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Reviews. 2006;20:213–26. doi: 10.1016/j.blre.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Elwood M. Critical Appraisal of Epidemiological Studies and Clinical Trials. 2nd , Oxford: Oxford University Press; 1998. [Google Scholar]
- Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychology. 2007;26:305–13. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28:990–99. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39:413–23. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N, Hamer M, Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Annals of Behavioral Medicine. 2009;37:29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological Medicine. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: the English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009;34:1050–55. doi: 10.1016/j.psyneuen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, et al. Do corticosteroids damage the brain? Journal of Neuroendocrinology. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hyde M, Wiggins RD, Higgs P, Blane DB. A measure of quality of life in early old age: the theory, development and properties of a needs satisfaction model (CASP-19) Aging & Mental Health. 2003;7:186–94. doi: 10.1080/1360786031000101157. [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–44. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Archives of General Psychiatry. 2007;64:1393–401. doi: 10.1001/archpsyc.64.12.1393. [DOI] [PubMed] [Google Scholar]
- Kumari M, Tabassum F, Clark C, Strachan D, Stansfeld S, Power C. Social differences in insulin-like growth factor-1: findings from a British birth cohort. Annals of Epidemiology. 2008;18:664–70. doi: 10.1016/j.annepidem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2008;13:371–79. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–33. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Marmot M, Banks J, Blundell R, Lessof C, Nazroo J, editors. Health, Wealth and Lifestyles of the Older Population in England. London: Institute of Fiscal Studies; 2003. [Google Scholar]
- O'Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–11. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Berges IM, Markides KS, Ottenbacher KJ. Hypertension in older adults and the role of positive emotions. Psychosomatic Medicine. 2006;68:727–33. doi: 10.1097/01.psy.0000234028.93346.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Tabassum F, Kumari M, Zaninotto P, Steel N. Measures of physical health. In: anks J, Breeze E, Lessof C, Nazroo J, editors. Retirement, Health and Relationships of the Older Population in England: The 2004 English Longitudinal Study of Ageing (Wave 2) London: Institute for Fiscal Studies; 2006. pp. 127–63. [Google Scholar]
- Pierce MB, Zaninotto P, Steel N, Mindell J. Undiagnosed diabetes-data from the English longitudinal study of ageing. Diabetic Medicine. 2009;26:679–85. doi: 10.1111/j.1464-5491.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. International Journal of Cancer. 2009;124:2416–429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Dienberg Love G, Urry HL, et al. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychotherapy and Psychosomatics. 2006;75:85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- SACN. Position Statement by the Scientific Advisory Committee on Nutrition. London: The Stationery Office; 2007. Update on Vitamin D. [Google Scholar]
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine. 1997;157:2259–68. [PubMed] [Google Scholar]
- Sierra F, Hadley E, Suzman R, Hodes R. Prospects for life span extension. Annual Reviews of Medicine. 2009;60:471–84. doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. Journal of Clinical Psychology. 2009;65:467–87. doi: 10.1002/jclp.20593. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Brydon L, Kunz-Ebrecht S. Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosomatic Medicine. 2005a;67:281–87. doi: 10.1097/01.psy.0000156932.96261.d2. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior and Immunity. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O'Donnell K, Badrick E, Kumari M, Marmot MG. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: Whitehall II study. American Journal of Epidemiology. 2008a;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O'Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and god sleep. Journal of Psychosomatic Research. 2008b;64:409–15. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:6508–12. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60:819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. New England Journal of Medicine. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of American Society of Nephrology. 2005;16:1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- Tsenkova VK, Love GD, Singer BH, Ryff CD. Socioeconomic status and psychological well-being predict cross-time change in glycosylated hemoglobin in older women without diabetes. Psychosomatic Medicine. 2007;69:777–84. doi: 10.1097/PSY.0b013e318157466f. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- Weir D. Elastic powers: the integration of biomarkers into the Health and Retirement Study. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys. Washington, DC: National Academies Press; 2008. pp. 78–95. [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–90. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. British Journal of Nutrition. 2005;94:483–92. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]