Abstract
Animal and human research indicates that the early environment can exert effects on hypothalamic pituitary adrenal (HPA) axis functioning across the lifespan. Using data from the National Study of Midlife Development in the United States and the National Study of Daily Experience substudy, we identified curvilinear relations between adult reports of parental affection in childhood and adult diurnal cortisol rhythms. Reports of both very affectionate and very unaffectionate parental relations in childhood were associated with flatter diurnal rhythms, suggesting potential dysregulation of the HPA axis at both extremes of family environment. Participants in the bottom tertile showed more signs of HPA axis dysregulation than those in the top tertile. We discuss processes that may underlie these effects, with reference to the theory of allostatic load.
Keywords: early environment, stress, HPA axis
INTRODUCTION
Both animal and human research indicates that early life experiences can affect health trajectories across the lifespan. Converging evidence suggests that the hypothalamic pituitary adrenal (HPA) axis may be critically involved in the pathways that explain these relations. The HPA axis plays a central role in managing stress. Corticotropin-releasing hormone, produced in the paraventricular nuclei of the hypothalamus, stimulates the secretion of adrenocorticotropic hormone (ACTH) by the anterior pituitary, resulting in the release of glucocorticoids from the adrenal glands (e.g. cortisol in humans). Although these processes characterize the normative functioning of the system, frequent and long-lasting elevations in glucocorticoids, as occur in chronically or recurrently stressful environments or which may occur in organisms that have developed highly stress-reactive biological stress regulatory systems, can compromise HPA axis functioning and ultimately health; moreover, as the system loses its resiliency in response to chronic or recurring stress, hypocortisolism, reflecting a reduced ability of the system to respond to challenges, may occur (McEwen, 1998). In addition to alterations in reactivity, the diurnal rhythm of cortisol may change in response to repeated challenge. Cortisol normally rises upon awakening, declines over the course of the day, flattening out in the late afternoon and evening, with a rise in the later evening. Dysregulation of the HPA axis in response to repeated challenge may be manifested in a flatter diurnal pattern.
Animal research indicating life-long beneficial effects of early environment, in particular maternal licking and grooming following separation stress in infant rats, suggests that these beneficial effects are mediated through the HPA axis. Studies conducted with clinical samples in humans are also supportive of this pathway. In a review of the effects of childhood trauma on the neurobiology of mood and anxiety disorders, Heim and Nemeroff (2001) concluded that early life stress can induce long-term hyperreactivity of the CRH system. De Bellis and colleagues (De Bellis et al., 1994) found attenuated plasma ACTH but robust plasma cortisol responses to CRH stimulation in sexually abused girls, suggesting a dysregulation of the HPA axis at the level of the pituitary. Studies have also documented hypocortisolism in highly stressed populations as well, perhaps as a downstream consequence of long-term exposure to maltreatment (Gunnar and Vasquez, 2001; Gunnar and Donzella, 2002). That is, people exposed to the chronic stress of maltreatment may initially respond with hypercortisolism, which eventually gives way to hypocortisolism, as the HPA axis loses its resiliency (McEwen, 1998).
Much of the research conducted to date has examined these processes in clinical populations or in populations at risk for psychopathology. However, evidence is mounting that similar, if lesser, compromise of HPA axis pathways may be implicated in ‘risky families’, that is, families characterized by conflict, cold non-nurturant behavior, or neglect, but not by trauma and abuse (Repetti et al., 2002). For example, a study of 264 offspring from families characterized by few positive interactions and a high level of negative interactions found an association between a poor family environment and abnormal cortisol profiles, which led to diminished immunity and frequent illness (Flinn and England, 1997). Bugental et al. (2003) reported that infants who experienced frequent maternal withdrawal had elevated basal levels of cortisol. Granger et al. (1998) reported that children’s basal cortisol levels were associated with a family environment high in aggression, anger and conflict. Spangler et al. (1994) found that maternal insensitivity to her child during play was tied to an increase in the child’s cortisol during free play. In a study of responses to laboratory stress challenges, Taylor et al. (2004) reported that young adult offspring from risky families were more likely to show a flat cortisol trajectory across the stress tasks compared to people from more nurturant families, who showed a lower baseline cortisol level, an elevation in response to the stress task, and a return to baseline following completion of the stress tasks. In a review of the evidence, Chorpita and Barlow (1998) reported that families characterized by low levels of warmth and/or high levels of restrictive, controlling parenting had offspring with disruptions in HPA axis functioning in response to stress, leading to increased CRH and hypercortisolism. As noted, the long-term pattern may be hypocortisolism, reflected in a muted response to stress or a flatter diurnal rhythm in response to chronic activation of the HPA system (McEwen, 1998; see also Gunnar and Vasquez, 2001; Gunnar and Donzella, 2002). Thus, the effects of early environment on HPA stress responses may occur across the spectrum of early environment quality and involve multiple parameters of the HPA axis, as well as alterations in its functioning over time.
Much animal and human evidence suggests a linear relationship, such that a nurturant familial environment fosters good health and wellbeing and, with increasing conflict, neglect, cold and non-nurturant behavior or abuse, poor mental and physical health trajectories. The human clinical literature, however, suggests that overly solicitous and protective maternal or familial behavior can have adverse effects on offspring, including a low threshold for fear, limited exploratory behavior, a risk of anxiety disorders, and a potential exacerbation of psychopathology (e.g. Chorpita et al., 1998; Whaley et al., 1999; Hudson and Rapee, 2001). Findings such as these suggest that there may be a curvilinear relationship between early childhood environment and adult stress responses, with signs of compromise evident at both extremes. The HPA axis may be implicated in these relations.
HPA axis regulation is important for understanding trajectories of aging across the lifespan. Dysregulation of the HPA axis is one of the signs of allostatic load (McEwen, 1998), which is believed to represent an accelerated aging process. That is, older adults show many of the changes identified early in offspring from poor functioning families, presumably because of the cumulative effects of stress across the lifespan. To the extent that accelerated trajectories of aging can be identified early in life and from specific early-life indicators, such as family environment, risk factors for accelerated aging may be better understood and health disorders associated with allostatic load may be anticipated, with appropriate diagnostic and preventive efforts undertaken with relevant populations.
The National Study of Midlife Development in the United States (MIDUS) investigations (Midlife in the US) provide an unparalleled opportunity to pursue the potential role of the early environment in the functioning of the HPA axis in adulthood. The sample size is substantial, which permits assessment of potential curvilinear relationships; the childhood family environments, as reported by respondents, range from very warm and nurturant to abusive; and, in contrast to much of the previous literature, the diurnal rhythm of cortisol was assessed, making possible a fine-grained examination of HPA functioning. Accordingly, we analyzed data from the MIDUS, which included salivary cortisol assessments in a sub-study, the National Study of Daily Experiences (NSDE).
METHODS
The MIDUS study, initiated in 1995, was designed to determine how social, psychological and behavioral factors inter-relate to influence mental and physical health. The first wave (MIDUS I) collected socio-demographic and psychosocial data on 7108 Americans, aged 25–74 years, from a representative sample of English-speaking, non-institutionalized adults residing in the contiguous 48 states, whose household included at least one telephone (recruited by random digit dialing), with oversampling of five metropolitan areas, twin pairs and siblings. Eighty nine percent of the sample (N = 6329) completed both a phone interview and a detailed self-administered questionnaire (Brim et al., 2004).
In the second wave of data collection (MIDUS II), a random sub-sample also completed short telephone interviews about their daily experiences over eight consecutive days and collected saliva (for cortisol assessments) on four of the eight days, as part of the second wave of the NSDE. Of the initial NSDE sample of 1605 participants, 1589 participants had usable cortisol and sampling time data for 6071 days. Missing data brought the final sample for these analyses to 1547.
Childhood psychosocial environment
Data on psychosocial conditions in childhood were obtained in MIDUS I, using a combination of telephone interview and self-administered questionnaires. This included information on family composition, parental divorce and death, family socio-economic status, quality of relationship with each parent and levels of affection received from each parent. Participants rated how much (1–4: for ‘not at all’, ‘a little’, ‘some’, ‘a lot’) each of the following six items was true separately for each parent: ‘how much did he/she understood your problems and worries’, ‘could you confide in him/her about things that were bothering you’, ‘how much love and affection did he/she give you’, ‘how much time and attention did he/she give you when you needed it’, ‘how much effort did he/she put into watching over you and making sure you had a good upbringing’ and ‘did he/she teach you about life’. Means over the six items were computed, separately for mother and father to create maternal affection and paternal affection scales (Rossi, 2001; Ryff et al., 2004). We then averaged the two to create a parental affection variable.
Cortisol
Assessments of diurnal cortisol derive from a saliva sampling protocol that included four samples per day (waking, +30 min, before lunch and before bedtime) on each of 4 days. Data on the exact time of each saliva sample were obtained from nightly telephone interviews by study staff and on a paper–pencil log sent with the collection kit. In addition, a quarter of the respondents receive a ‘Smart Box’ to store their salivettes, with a computer chip that records the time of box opening and closing. Salivettes were frozen (at –60°C) for storing and shipping. Cortisol concentrations (in nmol/l) were measured with a commercially available luminescence immunoassay (IBL, Hamburg, Germany).
We began with an initial NSDE sample of 1605 participants with 6383 days of cortisol data. We dropped data from 130 days when participants awoke before 4 a.m., 104 days when the third cortisol sample was 10 nmol or more higher than the second sample (since this might reflect a time-recording error for one of the saliva samples or saliva sample contamination with food), 49 days when respondents woke after 11 a.m., and an additional 28 days for respondents who were awake more than 20 h on a given day. This left us with 1589 participants with 6071 days of cortisol data and 24 284 saliva measurements. After excluding those with missing covariate data or cortisol values outside of the normal range (i.e. >60 nmol), we were left with a final analytic sample of 1547 people, 5698 days and 22 052 saliva measurements.
Covariates
Age at MIDUS II (in years), ethnicity and gender were self-reported. Body mass index (BMI) in kg/m2 was calculated from self-reported weight and height. Participants reported chronic conditions (from a list of 28 major conditions that included heart attack, stroke, hypertension, diabetes, cancer), and a simple count of chronic conditions was created. Depressed affect was measured in terms of a count of the number of ‘yes’ responses from a list of seven items (i.e. during two weeks in past 12 months, when you felt sad, blue or depressed; did you lose interest in most things; feel more tired out or low on energy than is usual; lose your appetite; appetite increased; have more trouble falling asleep than usual; have a lot more trouble concentrating than usual; feel down on yourself, no good, or worthless; think a lot about death).
Analysis
Because previous studies indicate that cortisol rhythms are driven by time elapsed since awakening and less by clock time (van Couter, 1990; Steptoe et al., 2003; Clow et al., 2004; Fries et al., 2009), we examined cortisol trajectories as a function of time since waking. Participants had been instructed to take the second saliva sample of the day 30 min after waking, but there was variability in actual timing of the second sample: 6% < 25 min after waking, 30% more than 35 min after waking and 14% more than 45 min after waking. There was more variability in the timing of the third (pre-lunch) and fourth saliva samples (bedtime); we exploited this variability in collection times to explore mean cortisol trajectories over the course of the day.
We first plotted mean cortisol and mean of log (cortisol + 1) over the study sample as a function of time since waking (in 15 min intervals) to examine the general shape of the daily cortisol trajectory. A visual comparison of the two plots indicated that the mean log (cortisol + 1) trajectory has less variability around straight-line segments (than the mean cortisol trajectory) and represented a better fit for piece-wise linear, growth curve modeling. Accordingly, we chose to model log (cortisol + 1). We examined mean trajectories over a series of demographic strata (including age, education, income, race and sex), waking time (before or after the sample median waking time, 6:40 a.m.), and bedtime (before or after the sample median bedtime, 10:30 p.m.), to confirm that the general shape of the diurnal trajectories did not vary by basic demographics (e.g. age, sex) and/or waking time and bedtime. The general form of the diurnal cortisol trajectories and the location of inflexion points were very consistent across these groups, with every group showing a morning peak 30 min after waking, then a rapid decline from the peak for 4 h, followed by a more gradual decline for 10.5 h, and a final upturn late in the day. Accordingly, we decided to model the diurnal cortisol trajectories by growth curves, using four linear splines with three knots, fixed at 0.5, 4.5 and 15 h.
We used random effects models to fit the cortisol growth curves and to study associations between the primary predictor (parental affection) and slopes of the four piece-wise linear segments, adjusted for age (<50, 50–64 and >64 years), gender, race (white vs non-white) and weekend day (if employed). To account for the correlation between repeated measures of cortisol in the same individual (between 1 and 16 measurements per person), we included random effects for the intercept (wakening value of cortisol) and all fours slopes. To allow for correlation between members of the same family (twin pairs and siblings), we included an additional hierarchical level with random intercept. Model-predicted intercept and slopes were used to estimate mean values for other trajectory parameters, such as the magnitude of the daily peak, the nightly nadir, and the total exposure over 20 h, or area under the curve (AUC). These were calculated as:
 |
Slope1, Slope2, Slope3 and Slope4 refer to the model-estimated mean slopes (per hour) for the four piece-wise linear segments of the trajectory. Stata version 10 was used for all analyses, with robust error variance estimation.
In order to account for possible non-linearity in relationships between parental affection and cortisol rhythms, we initially examined rhythm parameters by deciles of affection. Based on resulting evidence of non-linearity (with poorer rhythms among those in the top and bottom tertiles), tertiles of parental affection form the basis for results reported below.
RESULTS
Table 1 provides descriptive information about the participants. The age range of participants at the time of the MIDUS I data collection was 25–74 years, mean 48 years. By MIDUS II, mean age was 56.8 years. There were more women than men; the sample was largely White and relatively well-educated (see Table 1). There was substantial variance in the parental affection scale (range, 1–4): mean was 2.97, s.d.: 0.65. The cutoffs for the bottom and top tertiles were 2.58 and 3.50, respectively.
Table 1.
Descriptive statistics for analytic sample (n = 1547)
| Unit or category | Mean (s.d.) or % | |
|---|---|---|
| Age | Years | 56.8 (12.1) |
| Sex | Male | 44.4% |
| Female | 55.6% | |
| Race | White | 95.9% |
| Non-White | 4.1% | |
| Education | High school degree or less | 28.8% |
| Some college | 30.0% | |
| College degree or more | 41.2% | |
| Parental affection | Range, 1–4 | 2.97 (0.65) |
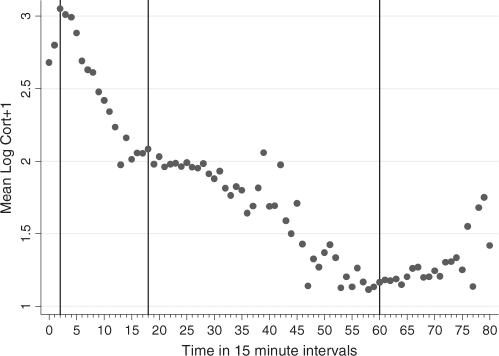
Figure 1 shows the mean of log (cortisol + 1) in the complete study sample, as a function of time since waking (in 15 min time-intervals). Inflexions occur at 0.5, 4.5 and 15 h. In the complete sample, mean awakening value of log (cortisol + 1) was 2.67, mean slope for the morning rise was +0.73 per hour, mean slope for the first rapid decline phase was –0.238 per hour, mean decline in the afternoon/evening phase was –0.089 per hour, and the mean slope in the late evening phase was +0.030 per hour. Corresponding mean values for peak, nadir and AUC, as predicted by the mean growth curve model, are listed in Table 2. After fitting the mean trajectory, the within-individual residual variance in log (cortisol + 1) was 0.189, between-individual random intercept variance was 0.091, and between-family variance was 0.043.
Fig. 1.
Mean log (cortisol+1) in 15-min intervals by time since waking.
Table 2.
Mean cortisol trajectory parameters in the study sample
| Awakening value | Peak | Nadir | AUC | |
|---|---|---|---|---|
| Log (cortisol + 1) | 2.67 | 3.03 | 1.14 | 34.62 |
| Cortisol (nmol/l) | 13.4 | 19.7 | 2.1 | – |
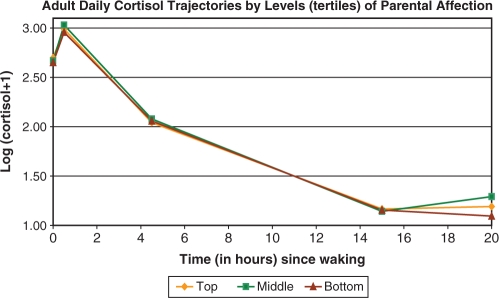
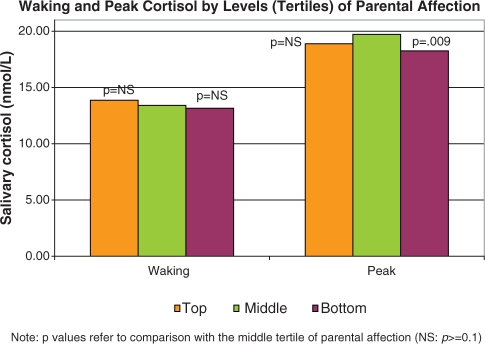
In the adjusted models, expected age, race, gender and workday differences were seen (i.e. higher nadirs and AUC in older compared to younger participants, non-whites compared to whites, and men compared to women, and higher waking and peak values on weekdays compared to weekend days if employed)—data not shown. In addition, both low and high levels of parental affection were associated with slower morning rise (less positive slope1), compared to average levels of affection (see Table 3 and Figure 2). This translated to significantly lower cortisol peaks in the bottom and top tertile of parental affection, compared to the middle tertile (Table 3 and Figure 3). In addition, those in the bottom tertile of parental affection also had marginally slower decline from the peak (more positive slope2) and significantly slower rises in the late evening (less positive slope4). Thus, diurnal cortisol rhythms were flatter (less morning rise, lower peak and less late evening rise) for those reporting the lowest levels of parental affection, with similar though less pronounced patterns also seen for those in the top tertile of parental affection. These findings were unchanged by additional controls for reports of parental divorce before age 17 and childhood socio-economic status (assessed by education level of mother and father) as well as measures of adult chronic conditions, depressive symptoms and BMI.
Table 3.
Adjusted parental affection associationsa with parameters of the log (cortisol + 1) trajectory
| Parental affectionb | Awakening value | Slope of morning risec | Slope of first rapid decline phase | Slope of afternoon/evening decline | Slope of late evening rise | Peak | Nadir | AUC |
|---|---|---|---|---|---|---|---|---|
| Bottom tertile | −0.02 (0.03) | −0.109 (0.044) | 0.013 (0.007) | 0.003 (0.004) | −0.042 (0.020) | −0.07 (0.03) | 0.01 (0.04) | −0.70 (0.62) |
| Top tertile | 0.03 (0.03) | −0.144 (0.046) | 0.001 (0.007) | 0.006 (0.004) | −0.025 (0.021) | −0.04 (0.03) | 0.02 (0.05) | −0.43 (0.65) |
Statistically significant associations (P < 0.05) are in bold font, and marginally significant associations (0.05 ≤ P < 0.075) are in italics.aAssociations presented as effect size (standard error).bReference group: middle tertile of parental affection.cAll slopes are per hour.
Fig. 2.
Adult daily cortisol trajectories by levels (tertiles) of parental affection.
Fig. 3.
Differences in adult daily cortisol rhythm parameters by levels of parental affection: waking and peak values.
DISCUSSION
Analyses of the MIDUS and NSDE datasets suggest curvilinear relationships between levels of parental affection and several parameters of the diurnal cortisol rhythm, namely afternoon/evening decline and late evening rise. Specifically, respondents who reported either very high or very low affection in childhood had flatter cortisol rhythms, compared to those who reported moderate levels of affection. Although previous animal and human research had suggested a potential linear relationship between familial environment and adverse changes in HPA axis functioning, the suggestion of a curvilinear relationship is supported by the human clinical literature on overly solicitous, overly protective maternal hovering and its impact on fear, anxiety and exploratory behavior in offspring.
Despite the evidence for curvilinear relationships, evidence of HPA axis dysregulation is more evident in the lowest tertile of family affection than in the highest. Those participants reporting the lowest levels of parental affection showed diurnal cortisol rhythms that were flatter, marked by slower morning rise, a lower peak and less late evening rise, as well as marginally slower decline from the peak. These findings are suggestive of a sluggish diurnal rhythm, which may reflect hypocortisolism. Note that hypocortisolism in response to maltreatment in childhood has been reported by other researchers (Gunnar and Vasquez, 2001; Gunnar and Donzella, 2002). The present study departs from those samples in important ways, however. Specifically, the current sample is adults, whereas previous literature has focused on diurnal rhythms in children. In addition, the current measures focused on parental affection and support (the positive side of parental behavior), whereas previous studies from maltreated samples and from risky families (Taylor et al., 2004) have focused on degrees of maltreatment (the negative side of parental behavior). Accordingly, it is possible that the fine-grained analysis of the effects of negative parental behaviors is not well-captured in the present sample, just as a previous focus on risky families and maltreated children may not have focused sufficiently on variability in the positive side of parental behaviors, thus potentially explaining the failure to find curvilinear relationships. Nonetheless, the suggestion of HPA axis compromise, as reflected in alterations in diurnal rhythms in maltreated samples, risky families, and the present study is relatively consistent.
What are the pathways by which early family environment may alter the functioning of the HPA axis? McEwen (1998) proposed an allostatic load model, whereby repeated social challenges in a child’s environment disrupt basic homeostatic processes that may lead to cascading, potentially irreversible interactions between genetic predispositions and these environmental factors; over time, stress exposure can produce large individual differences in susceptibility to stress and in biological markers of the cumulative adverse effects of stress, including the HPA axis. As noted, these results suggest the possibility of accelerated aging, consistent with McEwen’s (1998) allostatic load model. Consistent with the fact that glucocorticoid receptors are found in the cells of almost all tissues in the body (resulting in cortisol’s having a range of physiologic effects; Munck et al., 1984; De Kloet, 2004), growing evidence links dysregulations in HPA axis regulation to a range of negative health outcomes. For example, excessive glucocorticoid action has been shown to have a role in the development of insulin resistance (Phillips et al., 1998; Reynolds and Walker, 2003), and is associated with other cardiovascular risk factors such as central obesity (Rosmond et al., 1998) and hypertension (Whitworth et al., 1995). Chronically higher levels of cortisol have also been postulated to result in a reduction of cortisol’s ability to inhibit the action of pro-inflammatory cytokines (Petrovsky et al., 1998; Kunz-Ebrecht et al., 2003). Evidence has also begun to link greater cortisol exposure to increased atherosclerosis (Dekker et al., 2008) with additional data linking higher wakening cortisol and morning cortisol rise among women with greater intima media thickness (cross-sectionally; Eller et al., 2001) and with greater 2-year progression (Eller et al., 2005).
It may be the case that intrusive and overly solicitous familial behavior has some of these same effects.
Limitations
A limitation of the present study is the fact that adult respondents reported on their childhood experiences retrospectively, raising concerns about the validity of reports. However, a number of studies have established the reliability and validity of retrospective reports of parental support and affection (Parker, 1989; Brewin et al., 1993) and child abuse (Dill et al., 1991). This evidence may mute concerns about the reliability and validity of childhood reports. In addition, we have demonstrated high levels of independent corroboration from 917 twin pairs in MIDUS for (i) reports of childhood events of major significance, such as divorce and death, (ii) reports of family poverty and residential stability and (iii) social class, with reliability in the 93–98% range (A.S.Karlamangla et al., unpublished data).
Moreover, although recollections of childhood environment certainly include participants’ internal reconstructions of the family environment, it may be the psychological impact of the family environment that is most important for affecting biology. Previous research from our laboratories and those of other investigators indicate that recalled childhood variables are strongly related to adult neural responses to threat (Taylor et al., 2006a), biological variables (Luecken, 1998; Nicolson, 2004; Taylor et al., 2006b; Bloch et al., 2007; Pollitt et al., 2007; Tyrka et al., 2008; Williams et al., 2008), cardiovascular risk factors (Brunner et al., 1999; Karlamangla et al., 2005; Lehman et al., 2005, 2009), coronary heart disease (Dong et al., 2004; Singh-Manoux et al., 2004), other adult physical health conditions (Lundberg, 1997; Draper et al., 2008; Thurston et al., 2008) and mortality (Smith et al., 1997; Felitti et al., 1998; Tillin et al., 2008), after controlling for age, gender and adult socio-economic status. Thus, there is substantial evidence linking retrospective reports of childhood family environment to altered biological stress regulatory systems and to related health outcomes.
CONCLUSION
The early environment has effects on HPA axis functioning across the lifespan and on corresponding risks of illness. The present study reports evidence from the MIDUS and NSDE investigations to suggest that the relationship between parental affection and the diurnal cortisol rhythm may be curvilinear. Adult offspring from both harsh and highly solicitous families have flatter diurnal rhythms, suggesting that the diurnal rhythm of cortisol may be dysregulated at both extremes of family affection. Consistent with previous research, however, evidence at the lower levels of parental affection appears in this sample to be more consistently associated with evidence of HPA axis dysregulation than at the higher end of familial affection.
Acknowledgments
This work was supported by a grant from the National Institute on Aging of the National Institutes of Human and Health Services [P01-AG020166]. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Preparation of this manuscript was also supported by the National Institute on Aging [AG030309].
REFERENCES
- Bloch M, Peleg I, Koren D, Aner H, Klein E. Long-term effects of early parental loss due to divorce on the HPA axis. Hormones and Behavior. 2007;51:516–23. doi: 10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin. 1993;113:82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. The MIDUS national survey: an overview. In: Brim OG, Ryff CD, Kessler RC, editors. How Healthy Are We?: A National Study of Well-being at Midlife. Chicago: University of Chicago Press; 2004. pp. 1–36. [Google Scholar]
- Brunner E, Shipley MJ, Blane D, Smith GD, Marmot MG. When does cardiovascular risk start? Past and present socioeconomic circumstances and risk factors in adulthood. Journal of Epidemiology and Community Health. 1999;53:757–64. doi: 10.1136/jech.53.12.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–44. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Brown TA, Barlow DH. Perceived control as a mediator of family environment in etiological models of childhood anxiety. Behavior Therapy. 1998;29:457–76. doi: 10.1016/j.beth.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism. 1994;78:249–55. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Dekker M.J.HJ, Koper JW, van Aken MO, et al. Salivary cortisol is related to atherosclerosis of carotid arteries. Journal of Clinical Endocrinology & Metabolism. 2008;93:3741–7. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Annals of the New York Academy of Sciences. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dill DL, Chu JA, Grob MC, Eisen SV. The reliability of abuse history reports: a comparison of two inquiry formats. Comprehensive Psychiatry. 1991;32:166–9. doi: 10.1016/0010-440x(91)90009-2. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Draper B, Pfaff JJ, Pirkis J, et al. Long-term effects of childhood abuse on the quality of life and health of older people: results from the depression and early prevention of suicide in general practice project. Journal of the American Geriatrics Society. 2008;56:262–71. doi: 10.1111/j.1532-5415.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Allerup P. Progression in intima media thickness – the significance of hormonal biomarkers of chronic stress. Psychoneuroendocrinology. 2005;30:715–23. doi: 10.1016/j.psyneuen.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Hansen AM. Cortisol in urine and saliva: relations to the intima media thickness, IMT. Atherosclerosis. 2001;159:175–85. doi: 10.1016/s0021-9150(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many leading causes of death in adults: the adverse childhood experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress responses and health. American Journal of Physical Anthropology. 1997;102:33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, Ikeda S. Children’s salivary cortisol, internalizing behavior problems, and family environment: results from the Concordia longitudinal risk project. International Journal of Behavioral Development. 1998;22:707–28. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Developmental Psychopathology. 2001;13:515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hudson JL, Rapee RM. Parent-child interactions and anxiety disorders: an observational study. Behavior Research and Therapy. 2001;39:1411–27. doi: 10.1016/s0005-7967(00)00107-8. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer B, Williams D, et al. Impact of socioeconomic status on accumulation of cardiovascular risk factors in young adults: the CARDIA Study. Social Science and Medicine. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity. 2003;17:373–83. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846–54. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA Study. Health Psychology. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine. 1998;60:765–72. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Lundberg O. Childhood conditions, sense of coherence, social class and adult ill health: exploring their theoretical and empirical relations. Social Science and Medicine. 1997;44:821–33. doi: 10.1016/s0277-9536(96)00184-0. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Annals of the New York Academy of Sciences. 1998;84:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–8. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Parker G. The Parental Bonding Instrument: psychometric properties reviewed. Psychiatric Developments. 1989;7:317–35. [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Phillips D.IW, Barker D.JP, Fall C.HD, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? Journal of Clinical Endocrinology & Metabolism. 1998;83:757–60. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–66. [PubMed] [Google Scholar]
- Reynolds RM, Walker BR. Human insulin resistance: the role of glucocorticoids. Diabetes, Obesity and Metabolism. 2003;5:5–12. doi: 10.1046/j.1463-1326.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. Journal of Clinical Endocrinology & Metabolism. 1998;83:1853–9. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Rossi AS. Caring and Doing for Others: Social Responsibility in the Domains of Family, Work, and Community. Chicago: University of Chicago Press; 2001. [Google Scholar]
- Ryff CD, Singer BH, Palmersheim KA. Social inequalities in health and well-being: the role of relational and religious protective factors. In: Brim OG, Ryff CD, Kessler RC, editors. How Healthy Are We?: A National Study of Well-being at Midlife. Chicago, IL: University of Chicago Press; 2004. pp. 90–123. [Google Scholar]
- Singh-Manoux A, Ferrie JE, Chandola T, Marmot M. Socioeconomic trajectories across the life course and health outcomes in midlife: evidence for the accumulation hypothesis. International Journal of Epidemiology. 2004;33:1072–9. doi: 10.1093/ije/dyh224. [DOI] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Gillis C, Hawthorne V. Lifetime socioeconomic position and mortality: prospective observational study. British Medical Journal. 1997;314:547–52. doi: 10.1136/bmj.314.7080.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, Ackermann C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology. 1994;27:425–37. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine. 2003;65:461–70. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006a;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006b;60:819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72:1365–93. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Bromberger J, Chang Y, et al. Childhood abuse or neglect is associated with increased vasomotor symptom reporting among midlife women. Menopause. 2008;15:16–22. [PMC free article] [PubMed] [Google Scholar]
- Tillin T, Chaturvedi N, Forouhi NG, Smith GD, McKeigue PM. Cardiovascular disease mortality in relation to childhood and adulthood socioeconomic markers in British South Asian men. Heart. 2008;94:476–81. doi: 10.1136/hrt.2006.109165. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological Psychiatry. 2008;63:1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Couter E. Diurnal and ultradian rhythms in human endocrine function: a mini review. Hormone Research. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- Whaley SE, Pinto A, Sigman M. Characterizing interactions between anxious mothers and their children. Journal of Consulting and Clinical Psychology. 1999;67:826–36. doi: 10.1037//0022-006x.67.6.826. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Brown MA, Kelly JK, Williamson PM. Mechanisms of cortisol-induced hypertension in humans. Steroids. 1995;60:76–80. doi: 10.1016/0039-128x(94)00033-9. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Siegler IC, et al. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosomatic Medicine. 2008;70:32–9. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]